Abstract
We report a case of hypermagnesemia associated with the use of milk of magnesia in a male patient with end-stage renal disease. After experiencing nausea and vomiting, he developed severe bradycardia and then asystole. Resuscitation efforts were successful; however, he developed atrial fibrillation with severe widening of the QRS and diffuse ST elevation, hypothermia, hypotension and apnoea requiring intubation. Initial diagnoses included ST-elevation myocardial infarction, cardiogenic and/or septic shock and hyperkalaemia. However, serum magnesium was later found to be >4.1 mmol/L (equivalent to >10 mg/dL). He underwent haemodialysis (HD) to remove serum magnesium with remarkable overall improvement. Severe hypermagnesemia can manifest with severe bradycardia and asystole, shock, hypothermia and respiratory failure and can mimic acute coronary syndromes complicated with cardiogenic shock or septic shock. Therefore, clinicians should be aware of this life-threatening condition in patients with significant renal dysfunction. Timely treatment with HD is highly effective and lifesaving.
Background
Hypermagnesemia is a rare, fatal condition, which predominantly occurs in patients with kidney dysfunction after an oral, rectal or intravenous magnesium load.1 2 We report a case of hypermagnesemia associated with the use of a magnesium-containing laxative in a patient with end-stage renal disease (ESRD) complicated by sudden cardiac arrest, shock, hypothermia and apnoea.
Case presentation
A 73-year-old man with ESRD, diabetes mellitus, hypertension, peripheral vascular disease, coronary artery disease and past coronary artery bypass grafting was given multiple daily doses of milk of magnesia for constipation. After developing persistent nausea and vomiting, he was transported to the emergency department and during transit to the hospital, developed severe bradycardia followed by asystolic cardiac arrest. Advanced cardiac life support procedures were initiated; intravenous glucose, insulin and calcium were administered for suspected hyperkalaemic cardiac arrest. On arrival in the emergency room, he was afebrile with an irregular heart rate of 102 bpm and a blood pressure of 91/48 mm Hg. EKG showed atrial fibrillation with a wide QRS and diffuse ST elevation, especially in the anterolateral leads (figure 1). He also had respiratory distress prompting intubation. He was initially diagnosed with ST-elevation myocardial infarction complicated with cardiogenic shock and/or sepsis. Initial laboratory data showed serum potassium concentration of 4.2 mmol/L (normal 3.4–4.8 mmol/L) and calcium concentration of 2.60 mmol/L (normal 2.05–2.55 mmol/L). Initial troponin concentration was 0.019 μg/L (normal 0.00–0.034 μg/L). Thyroid stimulating hormone concentration was 0.605 mIU/L (normal 0.465–4.680 mIU/L).
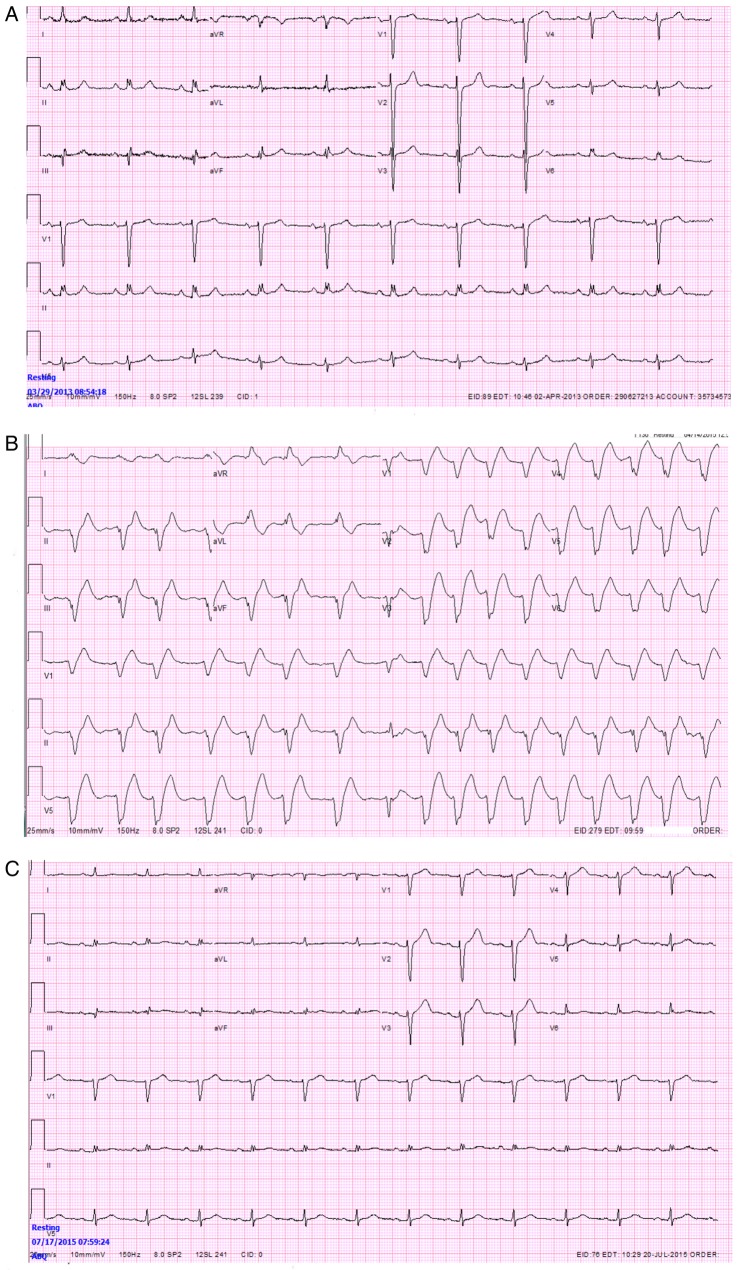
Figure 1.
(A) Normal preadmission ECG. (B) ECG on arrival in the Emergency Department demonstrating atrial fibrillation with severe widening of the QRS and diffuse ST elevation, especially in the anterolateral leads. (C) Normal ECG after resolution of hypermagnesemia.
The patient was taken for emergent cardiac catheterisation. A sequential saphenous vein graft to posterior descending, obtuse marginal and ramus intermedius arteries was found to be severely stenotic (95%), but without thrombosis. A drug eluting stent was successfully placed. His right heart catheterisation demonstrated normal pulmonary capillary wedge pressure of 12 mm Hg, moderate pulmonary hypertension, normal cardiac index (2.64 L/min/m2) and low systemic vascular resistance (810 dynes-sec-cm5) despite vasopressor therapy. After cardiac catheterisation, he was transferred to the medical intensive care unit and placed on a cooling blanket in an attempt to decrease body temperature to preserve cerebral function. However, the cooling blanket was quickly taken off due to a significant drop in temperature to <32°C. Rewarming was initiated with warmed intravenous and gastric fluids. He remained bradycardic, hypotensive and continued to require vasopressors. Despite his severe haemodynamic derangement, his troponin I peaked at only 0.220 μg/L and therefore it was thought that the patient had a small, likely type II myocardial infarction. Serum magnesium was initially reported as >4.1 mmol/L and believed to be in error (normal range 0.7–1.0 mmol/L) because serum magnesium measured 4 days prior was only 1.15 mmol/L. However, repeat measurement verified that serum magnesium was, indeed >4.1 mmol/L.
Investigations
Left and right cardiac catheterisation. A bedside ECG demonstrated normal left ventricular systolic function with normal wall motion, no significant valvular heart disease and no pericardial effusion.
Urine and blood cultures drawn at the time of admission were negative.
Differential diagnosis
ST-elevation myocardial infarction.
Cardiogenic and septic shock.
Treatment
The patient underwent emergency dialysis with reduction in magnesium to 2.59 mmol/L. After three consecutive days of dialysis, his serum magnesium concentration was 1.15 mmol/L.
Outcome and follow-up
The lowering of serum magnesium was accompanied by improvement in patient's blood pressure, resolution of atrial fibrillation, resolution of intermittent severe sinus bradycardia alternating with slow junctional escape rhythm, resolution of intraventricular conduction abnormalities consisting of alternating left and right bundle branch blocks, improvement in mental status and stabilisation of thermoregulation (figure 2). He was eventually weaned from the ventilator and discharged home 8 days after admission in stable clinical condition.
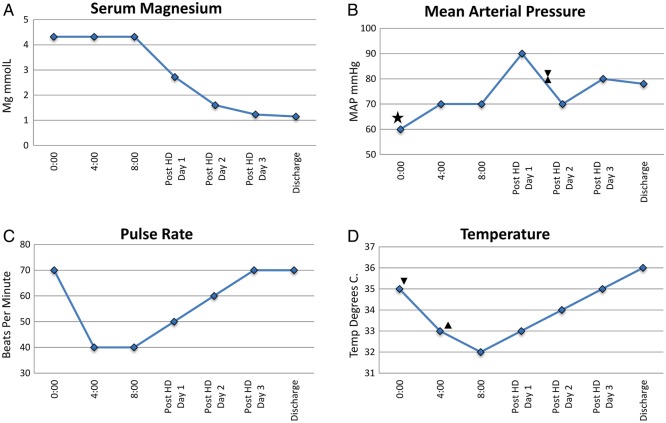
Figure 2.
Vital signs and magnesium concentration before and after dialysis. (A) Magnesium concentration above the level of detection (>4.1 mmol/L) for the first 24 hours of admission with subsequent progressively decreasing concentration over the course of haemodialysis (HD). (B) Low mean arterial blood pressure on admission with initiation (star) and discontinuation (hourglass) of vassopressors after HD. (C) Severe bradycardia with progressive resolution after HD. (D) Profound hypothermia despite discontinuation of cooling protocol (up triangle) with progressive resolution after initiation of HD (down triangle).
Discussion
Magnesium is the fourth most abundant mineral in the body and the second most common intracellular cation. It is found in soft tissues, bone and muscle. Hypermagnesemia is defined as a serum magnesium concentration >1.0 mmol/L. Symptoms of hypermagnesemia are usually not seen until concentrations are >1.77 mmol/L. Increased magnesium concentration inhibits neuromuscular signals and, at the presynaptic level, prevents acetylcholine release and the entry of calcium into nerve channels. Hypermagnesemia essentially functions as an extremely effective blocker of calcium channels. The most deleterious effects are seen in cardiac conduction, the neuromuscular system and central nervous system.3 4
Symptomatic hypermagnesemia is rare. It may occur with decreased elimination, as in renal insufficiency; magnesium overdose with oral administration of antacids or laxatives, or with intravenous magnesium sulphate infusion in the treatment of toxaemia of pregnancy.2 Clinical manifestations of hypermagnesemia include nausea, vomiting, flushing, headache, lethargy, drowsiness, loss of consciousness, diminished deep tendon reflexes, respiratory paralysis and abnormal cardiac conduction progressing from bradycardia to complete heart block, repolarisation abnormalities and intraventricular conduction disturbances (left and/or right bundle branch blocks) to asystole. Finally, hypermagnesemia can result in the loss of vasoregulation and thermoregulation.
Hypermagnesemia has previously been reported as a cause of bradycardia and cardiac arrest.5 6 Hypothermia is a less recognised consequence of magnesium toxicity, as only one other report of magnesium-induced hypothermia was found.7 In this case, a patient in premature labour developed hypermagnesemia that correlated inversely with temperature, similar to the patient presented here. Many reports reference the relationship between magnesium and thermal regulation3 4 8 9 attributed to magnesium's effect on the central thermoregulation centre or its role in inhibiting vasoconstriction. Either or both of these mechanisms may explain the hypothermia in our patient since his cardiac index was normal and he was not septic. Most cases of severe hypermagnesemia are unfortunately, fatal. As our patient survived, we are able to present the magnesium's effect on heart rate, mean arterial pressure and temperature before and after normalisation of magnesium (figure 2).
Hypermagnesemia can mimic other medical conditions such as ST-elevation myocardial infarction, cardiogenic or septic shock and respiratory failure.10 11 Clinicians should have a high degree of suspicion to diagnose or consider significant hypermagnesemia, a life-threatening condition, especially in patients with renal dysfunction.
Magnesium toxicity can develop quickly in patients with ESRD despite undergoing dialysis. In one study, moderate hypermagnesemia (>1.48 mmol/L) occurred in patients on haemodialysis (HD)whose magnesium intake was much less than the average amount consumed in the general population.12 In patients with impaired kidney function, extreme symptomatic hypermagnesemia can occur when therapeutic doses of contraindicated magnesium-containing laxatives or antacids are ingested.
Treatment of hypermagnesemia depends on renal function, magnesium concentration and the severity of clinical symptoms. In patients with normal to moderately impaired kidney function, stopping the magnesium-containing treatment with or without the addition of loop or thiazide diuretics (dosed according to the degree of renal dysfunction) to enhance elimination is usually adequate. One must be aware that diuresis can induce hypocalcaemia, which may potentiate magnesium toxicity. Because calcium antagonises the neuromuscular and cardiovascular effects of hypermagnesemia, cardiac dysfunction should be immediately treated with intravenous administration of calcium. In cases of severe neurological or cardiac abnormalities, HD is indicated, even in patients with only moderately impaired renal function.1 12 Respiratory, vasopressor and thermoregulatory supports may be needed until magnesium concentration normalises.
In summary, two mechanisms, decreased elimination as a consequence of ESRD and the ingestion of laxatives resulted in nearly fatal hypermagnesemia in our patient. He responded remarkably well to HD.
Prevention costs nothing. This case illustrates that clinicians should avoid prescribing magnesium-based laxatives to patients with renal dysfunction. Magnesium carbonate alone and in combination with calcium as a phosphate binder has been shown to be well tolerated in patients with ESRD when a low magnesium ≤0.5 mmoles/L dialysate is used. Hypermagnesemia is a valid concern though and magnesium levels should be routinely monitored.12 Patients should be educated regarding the safe use of magnesium containing over-the-counter products, such as milk of magnesia or Mylanta.
Finally, recognition of the constellation of symptoms and signs of severe hypermagnesemia is imperative. Timely treatment with diuresis, intravenous calcium and HD is highly effective in preventing significant morbidity and mortality associated with hypermagnesemia.
Learning points.
Severe hypermagnesemia is life-threatening.
The kidney is essential in maintaining magnesium homeostasis.
Normal doses of over-the-counter and prescribed magnesium-containing products can be dangerous for patients with kidney disease.
Hypermagnesemia can mimic ST-elevation myocardial infarction, cardiogenic or septic shock and respiratory failure.
Life-threatening hypermagnesemia requires high suspicion and immediate treatment with intravenous calcium and haemodialysis.
Acknowledgments
For additional assistance with manuscript preparation, Dr. Kavitha Ganta.
Footnotes
Contributors: Each of the above authors contributed significantly to the writing and editing of this manuscript. The finalised manuscript was approved by each author prior to submission.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Drueke TB, Lacour B. Disorders of calcium, phosphate and magnesium metabolism. Feehally J, Floege J, Johnson RJ, eds. Comprehensive clinical nephrology. 3rd edn Philadelphia, PA: Mosby, 2007:137–8. [Google Scholar]
- 2.Wyskida K, Witkowicz J, Chudek J et al. Daily magnesium intake and hypermagnesemia in hemodialysis patients with chronic kidney disease. J Ren Nutr 2012;22:19 10.1053/j.jrn.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Makrides M, Crosby DD, Baine E et al. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev 2014;4:CD000937 10.1002/14651858.CD000937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krendel DA. Hypermagnesemia and neuromuscular transmission. Semin Neurol 1990;10:42 10.1055/s-2008-1041252 [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama C, Kato T, Tomida K et al. Fatal hypermagenesemia induced by preoperative colon preparation in an elderly woman: report of a case. Clin J Gastroenerol 2013;2:105–10. 10.1007/s12328-012-0353-y [DOI] [PubMed] [Google Scholar]
- 6.Tatsumi H, Masuda Y, Imaizumi H et al. A case of cardiopulmonary arrest caused by laxatives-induced hypermagnesemia in a patient with anorexia nervosa and chronic renal failure. J Annesth 2011;25: 935–8. 10.1007/s00540-011-1220-6 [DOI] [PubMed] [Google Scholar]
- 7.Cardosi RJ, Chez RA. Magnesium sulfate, maternal hypothermia, and fetal bradycardia with loss of heart rate variability. Obstet Gynecol 1998;92:691–3. [DOI] [PubMed] [Google Scholar]
- 8.Durlach J. New data on the importance of gestational mg deficiency. J American College of Nutrition 2004;23:694S–700S. 10.1080/07315724.2004.10719411 [DOI] [PubMed] [Google Scholar]
- 9.Rizzo MA, Fisher M, Loack JP. Hypermagnesemic pseudocoma. Arch Intern Med 1993;153:1130 10.1001/archinte.1993.00410090072009 [DOI] [PubMed] [Google Scholar]
- 10.Weng YM, Chen SY, Chen HC et al. Hypermagnesemia in a constipated female. J Emerg Med 2013;44:e57 10.1016/j.jemermed.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Cholst IN, Steinberg SF, Tropper PJ et al. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984;310:1221 10.1056/NEJM198405103101904 [DOI] [PubMed] [Google Scholar]
- 12.Hutchison AJ, Wilkie M. Use of magnesium as a drug in chronic kidney disease. Clin Kidney J 2012;5(Suppl 1):i62–70. 10.1093/ndtplus/sfr168 [DOI] [PMC free article] [PubMed] [Google Scholar]