Abstract
Variations in the signalling NRG1-ErbB4 pathway have been associated with genetic susceptibility for both bipolar disorder and schizophrenia, although the underlying neural mechanisms are still uncertain. Reduced integrity of the anterior limb of the internal capsule (ALIC) has been found in association with risk-associated genetic variation in the 5′ region of the NRG1 gene. We hypothesised that variation in the gene encoding the NRG1 receptor, ErbB4, would also be associated with reduced ALIC integrity and with cognitive impairments characteristic of individuals with bipolar disorder and schizophrenia. Using diffusion tensor imaging (DTI), we examined the white matter integrity associations of the ErbB4 polymorphism rs4673628, which resides within intron 12 of the gene encoding ErbB4, in 36 healthy individuals. We also sought to clarify the cognitive effects of any findings. We found that genetic variation at the rs4673628 locus in the ErbB4 gene was significantly associated with ALIC white matter integrity which was also significantly and positively associated with mnemonic function. These findings provide further evidence to support a key role of NRG1-ErbB4 signalling in the pathophysiology of major mental disorders.
Keywords: ErbB4, NRG1, Diffusion tensor imaging, Magnetic resonance imaging, Voxel based morphometry, Anterior limb of internal capsule
1. Introduction
Bipolar disorder and schizophrenia are highly familial, severe psychiatric disorders with a combined lifetime risk of greater than 2%. Although some ameliorative treatments are available, the disorders show a tendency to become recurrent or persistent and our limited mechanistic understanding of these disorders currently restricts opportunities for early detection and more effective treatment. Bipolar disorder and schizophrenia have been traditionally considered separate entities but evidence from family, twin and adoption studies together with the lack of symptoms, treatment or outcome specific to either disorder, support the hypothesis of a genetic overlap in the pathophysiology of these disorders (Crow, 1990; Maier et al., 2006; Purcell et al., 2009). Currently one of the strongest candidate genes is NRG1 (Stefansson et al., 2002; Green et al., 2005; Thomson et al., 2007), whose involvement has been supported by numerous genetic association studies, and also by structural and functional imaging studies (Hall et al., 2006; Winterer et al., 2008; Mechelli et al., 2008). A receptor for NRG1, the ErbB4 receptor, has also been implicated in the pathogenesis of schizophrenia (Norton et al., 2006; Silberberg et al., 2006; Nicodemus et al., 2006; Hahn et al., 2006; Law et al., 2007) and there is evidence of an interaction between a specific variant in this gene, rs4673628, and the NRG1 Icelandic haplotype (Norton et al., 2006). The same variant within ErbB4 has also been shown to influence splice-variant expression, being associated with an abundance of JM-a variant isoforms in subjects affected by schizophrenia. These variant isoforms contain a metalloprotease cleavable extracellular domain rendering them susceptible to processing by the metalloprotease/presenilin-dependent γ-secretase proteolytic pathway further suggesting that this polymorphism may have important functional consequences (Silberberg et al., 2006; Law et al., 2007). The NRG1-ErbB4 pathway is involved in synaptic plasticity (Mei and Xiong, 2008), and in the development of thalamo-cortical axon finding (Lopez-Bendito et al., 2006). Reduced NRG1-ErbB4 signalling may lead to deficits in pyramidal and GABAergic neuronal migration, neurite outgrowth, axon projection and myelination, processes of likely relevance to both schizophrenia and bipolar disorder (Maier et al., 2006; Mei and Xiong, 2008). Although, to our knowledge, no studies have investigated the genetic involvement of ErbB4 in the pathogenesis of bipolar disorder, disrupted NRG1-ErbB4 signalling may be a mechanism shared by both disorders with common effects on brain structure. The heritability of brain structure along with replicated imaging abnormalities in both affected patients and their unaffected relatives also suggest that the brain structure may be sensitive to these genetic effects and have utility as a disease endophenotype.
In a previous study in healthy subjects, we found that a risk-associated variation in NRG1 affects the integrity of the anterior limb of the internal capsule (ALIC; McIntosh et al., 2008), an area reported to be involved in the pathogenesis of schizophrenia and bipolar disorder (Zhou et al., 2003; McIntosh et al., 2005b; Sussmann et al., 2009). Attention, motivation, and memory are amongst the many cognitive processes understood to involve the ALIC. Memory together with working memory has also been proposed as an endophenotypic marker for the functional psychoses (Savitz et al., 2005; Glahn et al., 2006a; Glahn et al., 2006b; Glahn et al., 2007), with evidence that abnormalities of memory appear to be related to an increased liability to both bipolar disorder and schizophrenia (Glahn et al., 2004; McIntosh et al., 2005a; Balanza-Martinez et al., 2008). Moreover, Nicodemus et al.(2006) found that the rs4673628 T(A) allele (within a haplotype) was negatively associated with both verbal memory factor score and digit span factor scores in healthy controls. Disconnectivity in the internal capsule may therefore affect specific cognitive processes and contribute to genetic vulnerability to schizophrenia and bipolar disorder (Wobrock et al., 2008).
In the current study we sought to determine whether the rs4673628 SNP at ErbB4, previously associated with schizophrenia development by interaction with the NRG1 Icelandic risk haplotype (Norton et al., 2006), affects brain white matter integrity. We specifically considered the ALIC, a region in which we have previously shown NRG1 associated reductions in integrity. We also tested for associations between ErbB4 variants and memory, whose dysfunction has been proposed as an endophenotypic marker for schizophrenia and bipolar disorder, and for associations between white matter structure and memory function.
2. Methods
2.1. Participants
Thirty-six people with no known personal or close family history of either schizophrenia or bipolar disorder were recruited from previous studies (McIntosh et al., 2008; Sussmann et al., 2009). Controls were identified from the personal contacts and non-genetic relatives of the patients involved in previous studies, some of whom were recruited from the local community, and from the social networks of control subjects who had already participated.
The diagnostic status of subjects was confirmed using data obtained at face-to-face interview. Subjects were screened using the SCID or the Present State Examination, and an extensive enquiry was made about the lifetime presence of psychiatric symptoms. Individuals with a current or past history of previous treatment with antidepressants or antipsychotics were also excluded. All clinical assessments were conducted by a trained psychiatrist.
Exclusion criteria included a history of head injury, neurological disorder, illicit drug dependence, learning disability, pregnancy or other standard MRI exclusions. Additional demographic and historical information was collected at the interview on all subjects with a semi-structured questionnaire. All eligible subjects were interviewed with the Positive and Negative Syndrome Scale (Kay et al., 1987), Hamilton Depression Rating Scale (Hamilton, 1960), and the Young Mania Rating Scale (Young et al., 1978).
All participants provided signed informed consent for their participation. The study methods followed local and national guidelines on the ethical conduct of research, and the study protocol and consent procedures were approved by the Lothian Regional Ethics Committee and by the Royal Edinburgh Hospital Management Board.
2.2. Genotype analysis
Genomic DNA was extracted from venous blood samples using standard protocols. Single nucleotide polymorphisms were genotyped by the Wellcome Trust Clinical Research Facility, Edinburgh, with TaqMan assay-by-design assays. Genotyping was performed for SNP rs4673628 from the ErbB4 gene complex. This particular variant was chosen because of its previous statistical association with schizophrenia and its interaction with the 5′ NRG1 haplotype (Norton et al., 2006; Nicodemus et al., 2006; Law et al., 2007). However, the variant is also understood to affect splice-variant expression providing supportive biological evidence that the rs4673628 variant is associated with altered gene function (Law et al., 2007).
2.3. Diffusion tensor imaging (DTI) acquisition and analysis
All participants were scanned on a 1.5 T GE MRI scanner (GE Medical Systems, Milwaukee, Wisconsin) equipped with a self-shielding gradient set (22 mT/m maximum gradient strength) and manufacturer-supplied ‘birdcage’ quadrature head coil. The DTI examination employed single-shot pulsed gradient spin-echo echo planar imaging (EPI) and consisted of 3 T2-weighted (b = 0s/mm2) and sets of diffusion-weighted (b = 1000 s/mm2) EPI volumes acquired with diffusion gradients applied sequentially along 51 non-collinear directions arranged uniformly in space. Forty-eight contiguous axial slice locations were imaged with the following acquisition parameters: FOV = 220 × 220 mm, imaging matrix = 96 × 96 (zero filled to 128 × 128), slice thickness = 2.8 mm, TR = 17 s and TE = 93.4 ms.
All DICOM format magnitude images were transferred to a Sun Blade 2000 workstation (Sun Microsystems, Mountain View, CA, USA) and converted into ANALYZE 3D (Mayo Foundation, Rochester, MN, USA) file format for further processing. Using the FLIRT toolbox (www.fmrib.ox.ac.uk/fsl), a three dimensional computational image alignment program (Jenkinson and Smith, 2001), bulk patient motion and eddy current induced distortions were removed from the DTI data by registering the diffusion-weighted to the first T2-weighted EPI volume. The apparent diffusion tensor of water (D) was calculated in each voxel from the signal intensities in the component EP images (Basser et al., 1994). Maps of fractional anisotropy (FA) for every subject were generated on a voxel-by-voxel basis from the sorted eigenvalues of D and converted into Analyze format, resulting in a series of skull-stripped FA and T2-weighted volumes.
2.4. Processing of FA data
A study-specific template was constructed to minimise the amount of warping required in bringing the volumes into a common analysis space. This was achieved by co-registering each subject's T2-weighted volume with the Montreal Neurological Institute (MNI) skull-stripped T2-weighted template using only linear transformations to account for large differences in brain size and position, i.e. 9-point affine (3 translations, 3 rotations and 3 shears). All volumes were then normalised to this target using a reduced number of nonlinear warps, described by a set of 4 × 5 × 4 basis functions, to avoid distortion. These were then averaged andthe study-specific template was formed by smoothing the mean T2-weighted volume at 8 mm full-width at half maximum (FWHM). The T2-weighted volume from each subject were then normalised to the study-specific template using the standard SPM default parameters, including 7 × 9 × 7 basis functions to describe the non-linear components. Normalisation of the FA volumes was achieved by applying the same transformation parameters (Jones et al., 2005). Both normalised T2-weighted and FA volumes were smoothed at 12 mm FWHM to make statistical tests more sensitive to structures of approximately the same extent, to ensure normality of the data and to compensate for any inaccuracy in the spatial normalisation (Ashburner and Friston, 2000). Model residuals were subsequently checked to ensure that they approximated to a normal distribution.
2.5. VBM analyses of FA data
Statistical analyses were conducted on all subjects. Smoothed FA volumes were compared between genotype groups at rs4673628 using T-contrasts. In each analysis, the uncorrected threshold was set at T = 3.00 and results were considered significant when the corrected voxel-wise p-value was less than 0.05, corrected for the family-wise error rate (PFWE).
Small volume corrections (SVC), manually drawn on the template on the basis of previously defined boundaries (Wobrock et al., 2008), were applied bilaterally to the ALIC. FA values were then extracted at each reported voxel of maximum difference and imported into SPSS (SPSS Inc, Chicago, Ill, USA).
2.6. Memory assessment and analyses
Declarative memory was assessed with the Extended Rivermead Behavioural Memory Test (de Wallet al., 1994), an ecologically based test that includes tasks of relevance to everyday function. The Rivermead Profile score was the dependent measure used in the subsequent analyses. Verbal working memory was assessed with the Digit Span Subset of the Wechsler Adult Intelligence Scale III (WAIS 3) (The Psychological Corporation, Lutz, FL, USA; 1997), with the forward condition measuring the total number of digits recalled from digit strings of increasing length, and the backward condition requiring subjects to reorder digits held in memory.
Neuropsychological data were analyzed using analysis of variance, comparing each measure between the genotype groups. When the ANOVA showed an overall difference, further post hoc pair-wise comparisons were then computed. Since no significant differences were found in age, sex, or premorbid IQ (NART), no covariates were used in the analysis. Finally a linear regression analysis was used to examine the functional consequences of reduced FA, using each cognitive measure as the dependent variable and FA as the independent variable. All analyses considered the effect of genotype on FA or cognitive measures using the general linear model. We compared people homozygous for the A allele with those carrying the G allele.
3. Results
Genetic and MRI data were available for all 36 healthy subjects. The average age and IQ of the sample were 37.7 (12.1) years and 113.4 (6.8) respectively, and 52.8% of the subjects were male. The three genotype groups at SNP rs4673628 did not differ in age, premorbid IQ (NART) or gender (Table 1), and the frequencies did not differ from those expected under the Hardy-Weinberg equilibrium (p = 0.2816).
Table 1.
Characteristics of the 36 study subjects.
| ErbB4 genotype | ||||
|---|---|---|---|---|
| Genotype | GG | GA | AA | Significance |
| N | 10 | 14 | 12 | |
| Male N (%) | 5 (50.0) | 8 (57.1) | 6 (50.0) | P(χ2) = 0.73 |
| Age Mean (S.D.) | 34.4 (10.7) | 37.3 (11.7) | 40.9 (13.7) | F(0.90), p=0.46 |
| NART-IQ Mean (S.D.) | 113.7 (8.4) | 111.5 (5.7) | 115.4 (6.6) | F(0.91), p=0.41 |
3.1. Effects of rs4673628 on white matter integrity
Genetic variation at rs4673628 was significantly associated with FA within the ALIC. Significant differences were found in both the right (voxel level: T = 3.48, PFWE = 0.047, peak MNI coordinates = [16, 6, 8]; cluster level: kE =7, pCOR = 0.041) and left ALIC (voxel level: T = 3.98, PFWE = 0.016, peak MNI coordinates = [−20, 4, 10]; cluster level: kE = 61, pCOR = 0.011). In each case subjects bearing the G allele at rs4673628 showed lower FA relative to subjects who were AA homozygous (Fig. 1).
Fig. 1.
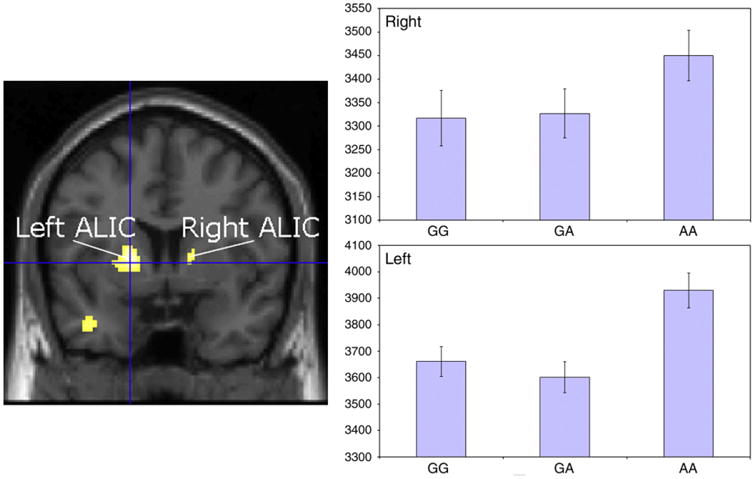
Reductions in ALIC white matter integrity by genotype. The y-axis scales on the two rightmost graphs represent the fractional anisotropy (×104) at each voxel of maximum difference. Error bars represent ± 1 S.E.
3.2. Effects of rs4673628 and white matter integrity on mnemonic function
No significant differences in mnemonic function were found between groups defined on the basis of their ErbB4 genotype. However, FA within the voxel of peak difference in the ALIC was significantly and positively associated with both forward (left: r = 0.45, p = 0.01; right: r = 0.52, p = 0.003) and backward (left: r = 0.35, p = 0.051; right: r = 0.40, p = 0.028) digit span and with Rivermead Behavioural Memory Test scores (left: r = 0.36, p = 0.039; right: r = 0.37, p = 0.034) bilaterally (Supplementary Fig. 1).
4. Discussion
In the current study we show that genetic variation at rs4673628 in the ErbB4 gene is related to white matter integrity reductions in both the left and right ALIC, with subjects bearing the G allele showing reduced FA values relative to subjects homozygous for the A allele. These integrity reductions were associated with mnemonic impairments suggesting that this variation may also have deleterious effects on cognition.
Several studies have shown that ALIC white matter FA values are reduced in both schizophrenia and bipolar disorder (Zhou et al., 2003; McIntosh et al., 2005b; Sussmann et al., 2009). The brain structure is highly heritable and reductions of white matter integrity in the ALIC have recently been associated with risk-associated variation in NRG1 (McIntosh et al., 2008). ALIC integrity impairments due to NRG1 variations are also supported by the evidence of an association between NRG1 and thalamo-cortical projection development as well as possible effects on myelination (Lopez-Bendito et al., 2006).
The single risk SNP rs4673628 is an intronic variant in the NRG1 receptor ErbB4 associated with the development of schizophrenia (Law et al., 2007; Mei and Xiong, 2008), shown to interact with the 5′ NRG1 haplotype (Norton et al., 2006) and to alter splice-variant expression in several areas of the brain in schizophrenic patients (Silberberg et al., 2006; Law et al., 2007). Specifically, individuals homozygous for the G allele show a lower level of JM-a variant ErbB4 isoform expression, with higher levels previously associated with the development of schizophrenia. Although the effect of this variant on brain structure and function is not completely understood, its effects on splice-variant expression and interaction with NRG1 haplotype (HAPICE) may well underlie its risk association and, on that basis, we focused on the analysis of rs4673628 in the current study.
The current findings are similar to our previous work showing an association between the NRG1 SNP rs6994992 and ALIC white matter integrity (McIntosh et al., 2008). These convergent findings suggest that the NRG1-ErbB4 signalling pathway is important for the development of normal white matter and that disruptions in this process may have consequences for white matter integrity, cognition and ultimately risk of psychosis.
In a previous association study, Konrad et al. (2009) showed that a separate ErbB4 variant, namely rs839523, was associated with temporal white matter integrity. These studies considered ErbB4 variants in different regions of the gene and are therefore unlikely to be marking the same risk-associated variation. It is likely that either more than one functional genetic variant exists in ErbB4 or that some of the differences may yet be explained by differences in the population under study or by other methodological factors.
Whilst no differences were found in mnemonic function between the genotype groups, working and long-term memory were both positively related to white matter integrity in the internal capsule. This suggests that abnormalities within this area might be associated with some of the cognitive deficits commonly reported (Savitz et al., 2005; Glahn et al., 2006a; Glahn et al., 2006b; Glahn et al., 2007) in patients with schizophrenia or bipolar disorder. Memory function is subserved by systems of fibres some of which pass through the ALIC. It is perhaps unsurprising that disruption of these fibres has the potential to impair memory and this evidence is also in agreement with some results obtained in subjects affected by multiple sclerosis (Sepulcre et al., 2008). This possibility is supported by the findings of Nicodemus et al. (2006) who demonstrated that subjects homozygous for the T allele at rs4673628 show impairments on tasks involving both declarative and working memory.
In conclusion, although confirmatory data are needed and a larger sample size would strengthen these findings, the current study provides further evidence that the ALIC is susceptible to disruptions in the NRG1-ErbB4 signalling path and that these changes may underlie some of the cognitive deficits found in people with schizophrenia and bipolar disorder.
Supplementary Material
Acknowledgments
We would like to thank all of the participants who took part in the study and the staff of the Scottish Funding Council Brain Imaging Research Centre, University of Edinburgh, where the MRI scanning was conducted. This study and two of the investigators (AMM and SML) were supported by the Dr. Mortimer and Theresa Sackler Foundation during the period of data collection. Dr. Hall is funded by an MRC Clinical Research Training Fellowship. Dr. McIntosh is currently supported by the Health Foundation through a Clinician Scientist Fellowship and by NARSAD through an Independent Investigator Award.
Drs. McIntosh, Hall and Professor Lawrie report having jointly received research funding from the Translational Medical Research Institute supported by Wyeth.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2010.11.001.
Financial disclosures: Drs. Zuliani, Moorhead, Bastin, Job, McKirdy, Sussmann, Brambilla and Professor Johnstone report no financial interests.
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sanchez-Moreno J, Salazar-Fraile J, Vieta E, Tabares-Seisdedos R. Neurocognitive endophe-notypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neuroscience & Biobehavioral Reviews. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The continuum of psychosis and its genetic origins. The sixty-fifth Maudsley lecture. Br J Psychiatry. 1990;156:788–797. doi: 10.1192/bjp.156.6.788. [DOI] [PubMed] [Google Scholar]
- de Wall C, Wilson BA, Baddeley AD. The Extended Rivermead Behavioural Memory Test: a measure of everyday memory performance in normal adults. Memory. 1994;2:149–166. doi: 10.1080/09658219408258942. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Barrett J, Bearden CE, Mintz J, Green MF, Serap Monkul E, Najt P, Soares JC, Velligan DI. Dissociable mechanisms for memory impairment in bipolar disorder and schizophrenia. Psychological Medicine. 2006a;36:1085–1095. doi: 10.1017/S0033291706007902. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biological Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disorders. 2006b;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disorders. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Green EK, Raybould R, Macgregor S, Gordon-Smith K, Heron J, Hyde S, Grozeva D, Hamshere M, Williams N, Owen MJ, O'Donovan MC, Jones L, Jones I, Kirov G, Craddock N. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Archives of General Psychiatry. 2005;62:642–648. doi: 10.1001/archpsyc.62.6.642. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-ErbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature Medicine. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, Lawrie SM. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nature Neuroscience. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N, Winterer G. ErbB4 genotype predicts left frontotemporal structural connectivity in human brain. Neuropsychopharmacology. 2009;34:641–650. doi: 10.1038/npp.2008.112. [DOI] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Human Molecular Genetics. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Current Opinion in Psychiatry. 2006;19:165–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. The British Journal of Psychiatry. 2005a;186:378–385. doi: 10.1192/bjp.186.5.378. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biological Psychiatry. 2005b;58:254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, Sussmann JE, Baig BJ, Bastin ME, Porteous D, Evans KL, Johnstone EC, Lawrie SM, Hall J. The effects of a neuregulin 1 variant on white matter density and integrity. Molecular Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Prata DP, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Demjaha A, Kravariti E, Toulopoulou T, Murray R, Collier DA, McGuire PK. The effects of neuregulin1 on brain function in controls and patients with schizophrenia and bipolar disorder. Neuroimage. 2008;42:817–826. doi: 10.1016/j.neuroimage.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Reviews Neuroscience. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Molecular Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor ErbB4 increases susceptibility to schizophrenia. American Journal of Medical Genetics Part B Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disorders. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Masdeu JC, Sastre-Garriga J, Goni J, Velez-de-Mendizabal N, Duque B, Pastor MA, Bejarano B, Villoslada P. Mapping the brain pathways of declarative verbal memory: evidence from white matter lesions in the living human brain. Neuroimage. 2008;42:1237–1243. doi: 10.1016/j.neuroimage.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. American Journal of Medical Genetics Part B Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. American Journal of Human Genetics. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Maniega SM, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ, Muir WJ, Blackwood DH, Evans KL. Association of Neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Molecular Psychiatry. 2007;12:94–104. doi: 10.1038/sj.mp.4001889. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, Maier W, Rietschel M, Schulze TG, Scherk H, Schild HH, Block W, Traber F, Tepest R, Honer WG, Falkai P. Reduction of the internal capsule in families affected with schizophrenia. Biological Psychiatry. 2008;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Winterer G, Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N. Association of 5′ end neuregulin-1(NRG1) gene variation with subcortical medial frontal microstructure in humans. NeuroImage. 2008;1(40):712:8. doi: 10.1016/j.neuroimage.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biological Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


