Abstract
Degenerative disc disease (DDD) is associated with spinal pain often leading to long-term disability. However, the non-chondrodystrophic canine intervertebral disc is protected from the development of DDD, ostensibly due to its retention of notochordal cells (NC) in the nucleus pulposus (NP). In this study, we hypothesized that secretome analysis of the NC-rich NP will lead to the identification of key proteins that delay the onset of DDD. Using mass-spectrometry, we identified 303 proteins including components of TGFβ- and Wnt-signaling, anti-angiogeneic factors and proteins that inhibit axonal ingrowth in the bioactive fractions of serum free, notochordal cell derived conditioned medium (NCCM). Ingenuity Pathway Analysis revealed TGFβ1 and CTGF as major hubs in protein interaction networks. In vitro treatment with TGFβ1 and CTGF promoted the synthesis of healthy extra-cellular matrix proteins, increased cell proliferation and reduced cell death in human degenerative disc NP cells. A single intra-discal injection of recombinant TGFβ1 and CTGF proteins in a pre-clinical rat-tail disc injury model restored the NC and stem cell rich NP. In conclusion, we demonstrate the potential of TGFβ1 and CTGF to mitigate the progression of disc degeneration and the potential use of these molecules in a molecular therapy to treat the degenerative disc.
Degenerative disc disease (DDD) is a predominant contributor (~40%) to the genesis of spinal pain and is a major cause of disability worldwide, imposing enormous socio-economic burden and clinical costs to society1. The 2010 Global Burden of Disease Study estimated that low back pain is among the top 10 diseases and injuries that account for the highest number of disability-adjusted life years worldwide. It is estimated that approximately 25% of the population in United States of America suffers from lower back and neck pain at costs greater than $100 billion annually2. Current surgical treatments including fusion and disc replacement do not attempt to slow the degenerative process or promote repair3. In fact, a meta-analysis suggested that lumbar fusion might result in a higher prevalence of adjacent segment degeneration or disease than motion-preserving procedures4. Thus, there exists a need for the identification of novel, effective therapeutic agents that can be clinically translated as minimally invasive regenerative therapies for disc repair. Several approaches including gene therapy, growth factors, stem cells and tissue engineering have been proposed for the treatment of disc degeneration5,6,7,8,9,10. Growth factors including insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2) and morphogens such as bone morphogenetic protein-2 (BMP-2), osteogenic protein-1(OP-1) and growth differentiation factor-5 (GDF-5) have been investigated for their potential to stimulate extracellular matrix (ECM) synthesis in intervertebral disc (IVD) cells6. However, the use of BMP-2 for the treatment of DDD in humans is limited by its osteo-inductive characteristics11. Similarly, the injection of rhBMP-7/OP-1 resulted in exogenous bone formation with no evidence of disc regeneration in the beagle IVD - NP12. Moreover, administration of anti-inflammatory agents including interleukin 1 receptor antagonist (IL-1Ra) and synthetic inhibitors targeting tumor necrosis factor α (TNFα) or nuclear factor kappa B (NFκB) have shown limited efficacy in stimulating the anabolic response13,14. Therefore, the identification of novel biologically active agents that can restore the homeostatic microenvironment in a degenerative disc would revolutionize the treatment of DDD.
The intervertebral disc (IVD) is composed of a central nucleus pulposus (NP) surrounded by the concentric annulus fibrosus and attached to the adjacent vertebrae by thin cartilaginous end plates15,16. The NP plays an important role in maintaining the biomechanical properties of the spine such that in youth the healthy NP is rich in large, vacuolated notochordal cells (NCs) and possesses a proteoglycan rich ECM. However, NCs observed during childhood are gradually replaced by small chondrocyte-like cells (CLCs) by early adolescence in humans, a NP cellular phenotype often associated with disc degeneration15,16. The degenerative disc NP is a catabolic phenotype representing a failure of homeostatic regulation of the microenvironment with the increased expression of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) leading to progressive cell death, loss of proteoglycan content and development of an inferior, fibrocartilaginous extra-cellular matrix (ECM). This affects IVD structural integrity, compromising its biomechanical properties often leading to pain, neurological compromise and disability15,16,17,18,19. With respect to the development of DDD, a temporal relationship has been suggested between the loss of NCs and the progression of DDD15,16,17.
Unlike humans, non-chondrodystrophic (NCD) canines, porcine and rodents preserve large, vacuolated NCs within their NPs and are relatively resistant to DDD, until much later in their lives19. In our previous studies, we reported that conditioned medium collected from alginate cultures of notochordal cells (NCs) derived from NCD-canines reduced cell death and stimulated collagen 2 synthesis in bovine NP cells20,21. Similarly, other studies have demonstrated increased proteoglycan synthesis in NP cells treated with notochordal cell derived conditioned medium (NCCM) in vitro22. A recent study also demonstrated the anti-angiogeneic effects of NCCM in vitro23. These studies provide compelling evidence that NCs secrete proteins, which may confer anabolic characteristics, maintain a homeostatic microenvironment in NP and resists DDD. We hypothesized that secretome analysis of NC-rich NPs will give us insight into the protein networks regulating the development of DDD and may be exploited for regenerating the degenerative discs in humans. In this study, we identified the proteins in serum free NCCM using mass spectrometry and, improved and characterized a pre-clinical rodent disc - injury model of DDD. We used Ingenuity Pathway Analysis (IPA) to identify protein networks that might be of significance in the homeostatic regulation of the healthy disc NP. Next we evaluated the regenerative and anti-inflammatory potential of connective tissue growth factor (CTGF) and transforming growth factor beta 1 (TGFβ1), identified as major hub proteins in network analysis, using in vitro assays and an in vivo pre-clinical rodent disc - injury model of DDD.
Results
Evaluation of a pre-clinical rat-tail disc injury model of DDD
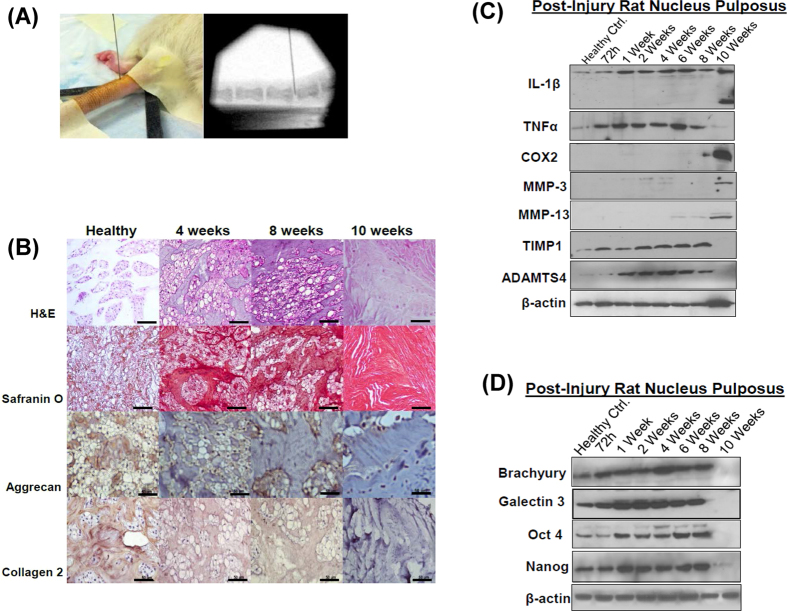
We characterized a pre-clinical rodent model of DDD to establish a platform for the evaluation of therapeutic agents. Using fluoroscopic image guidance, we performed needle puncture injuries using a 27-gauge needle in the caudal (tail) discs of 12 week-old, healthy Wistar rats (n = 21, 4 discs per animal). Alterations in the composition of the ECM and the cellular phenotype were determined in a time dependent manner (72 hrs–10 weeks, Fig. 1A–D). Histological analysis revealed a gradual loss of NCs, increased Safranin-O staining intensity and reduced expression of aggrecan and collagen 2 by the end of 10 weeks post injury (p.i.) in comparison to uninjured, healthy control IVD-NP obtained from age matched healthy controls (Fig. 1B, Supplementary Fig. 1A). Of note, needle puncture injury increased the expression of the pro-inflammatory cytokines, TNFα within 72 hrs and interleukin-1β (IL-1β) as early as 1 week p.i. (Fig. 1C, Supplementary Fig. 1B). However, the active form of IL-1β (~17 kDa) was not observed until between 8–10 weeks, coincident with an increased expression of the inflammatory mediator, cyclooxygenase 2 (COX2) and the ECM degrading enzymes, matrix metalloproteinases-3 (MMP-3) and MMP-13 as compared to no treatment controls (NTCs, i.e. tissue lysates obtained from uninjured IVD-NPs, Fig. 1C, Supplementary Fig. 1B). Interestingly, the loss of tissue inhibitor of metalloproteinases 1 (TIMP1), a natural inhibitor of MMPs was also observed at the end of 10 weeks p.i. coincident with the onset of increased levels of the MMPs (Fig. 1C, Supplementary Fig. 1B). We also observed increased expression of A disintegrin-like and metalloprotease with thrombospondin type 1 motif 4 (ADAMTS4), one of the major enzymes responsible for aggrecan degradation (Fig. 1C, Supplementary Fig. 1B). In addition to ECM changes, we also determined the expression of notochordal and stem cell markers in healthy and injured IVD-NP lysates. Our western blotting results demonstrated the loss of the NC markers (brachyury and galectin 3) and stem cell markers (Oct4 and Nanog) in the injured disc NPs, 10 weeks p.i. (Fig. 1D, Supplementary Fig. 1C). These findings clearly demonstrated a shift in the NP milieu from a healthy, homeostatically regulated environment to a pro-inflammatory, catabolic state with loss of both NCs and stem cells, similar to the human degenerative disc NP. Comparative analysis of the histological characteristics of human degenerative disc NPs with bovine, NCD - canine and Wistar rat IVDs is described in Supplementary Data (Supplementary Fig. 2A). Negative control tissue sections (rat healthy/mongrel IVD – NP) wherein the primary antibody was replaced by rabbit or goat isotype IgG controls revealed no immunostaining thereby demonstrating the specificity of the antibodies (Supplementary Fig. 2B).
Figure 1. Needle puncture injury in rat-tail disc leads to the development of fibrocartilaginous matrix and loss of notochordal (NC) and stem cells in nucleus pulposus (NP).
(A) Representative fluoroscopic image-guided needle puncture injury in rat-tail disc NP. (B) Histological analysis demonstrating representative hematoxylin and eosin (H&E) and Safranin O staining images showing development of a fibrocartilaginous matrix over a period of 10 weeks p.i. in rat NP. Both H&E and Safranin O staining was done in duplicates on IVD sections obtained from all the animals in each group. Immunohistochemistry showing the loss of the ECM proteins, aggrecan and collagen 2 in time dependent manner (healthy to 10 weeks p.i., Scale bar 50μ, n = 2 per group). (C) Representative Western blot panels showing alterations in the expression of pro-inflammatory cytokines (IL-1β and TNFα), inflammation mediator, Cox2 and ECM proteins (MMP-3, MMP-13, TIMP1 and ADAMTS4) in a time dependent manner in post-injury rat NP tissue lysates. (D) Representative Western blot panels showing loss of NC markers (brachyury, galectin 3) and stem cell markers (Oct4, Nanog) in NPs obtained from rat-tail injured discs over a period of 10 weeks. β-actin was used a loading control in Western blots. All Western blots were run under the same experimental conditions as described in Materials and Methods.
Intra-discal injection of NCCM promotes regeneration of the degenerative disc nucleus pulpous in a pre-clinical rodent model of DDD
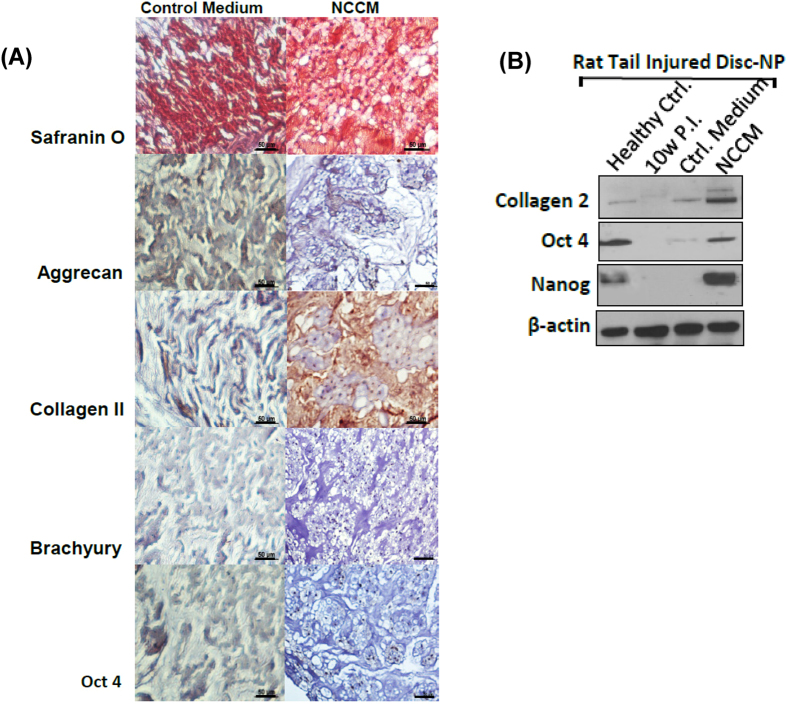
We injected concentrated, serum free NCCM or control medium (~8 μL/disc) into the injured (4-week p.i.) rat-tail disc NPs (n = 6 animals/group, 4 discs per animal) using a specially designed, 32-gauge needle with a 35 degree bevel under fluoroscopic guidance. Ten weeks p.i., histological analysis revealed NC-rich NPs with moderate Safranin-O staining in NCCM injected rat-tail injured discs (Fig. 2A). In contrast, rat-tail injured discs injected with control medium showed low cellularity and displayed a fibrocartilaginous matrix, with intense Safranin-O staining (Fig. 2A). Immunohistochemistry and western blotting revealed that discs injected with NCCM displayed restoration of aggrecan, collagen 2, brachyury, Oct4 and Nanog in contrast to the sham/control medium injected discs (Fig. 2A,B, Supplementary Fig. 2C). However, protein extraction using RIPA lysis buffer is a potential limitation for estimation of Collagen2 expression in tissues using Western blotting. Nonetheless, these results validated our hypothesis and demonstrated that soluble factors within NCCM have therapeutic potential for a degenerative IVD - NP.
Figure 2. NCCM confers anabolic and anti-catabolic characteristics to degenerating NP in rat-tail injured IVD - NP.
(A) Representative Safranin O staining sections showing fibro-cartilaginous matrix and small NP cells in rat tail injured disc NPs injected with control medium as compared to the injured disc NPs injected with serum free NCCM showing proteoglycan-rich ECM with large notochordal cells (n = 12 tissue sections/group). Representative panel showing immunohistochemistry of aggrecan, collagen 2, brachyury and Oct4 in paraffin embedded sections of rat-tail injured discs treated with protein free Hybridoma medium used as control or NCCM (Scale bar 50μ). Immunohistochemistry for all the proteins was performed in duplicates in at least 3 different IVD sections obtained from different animals from each group used in this study. (B) Representative Western blot panels showing expression of collagen 2 and the stem cell markers Oct4 and Nanog in tissue lysates obtained from healthy controls, rat-tail injured disc NPs left untreated, injected with control medium or NCCM.
Identification of soluble factors in NCCM using mass spectrometry
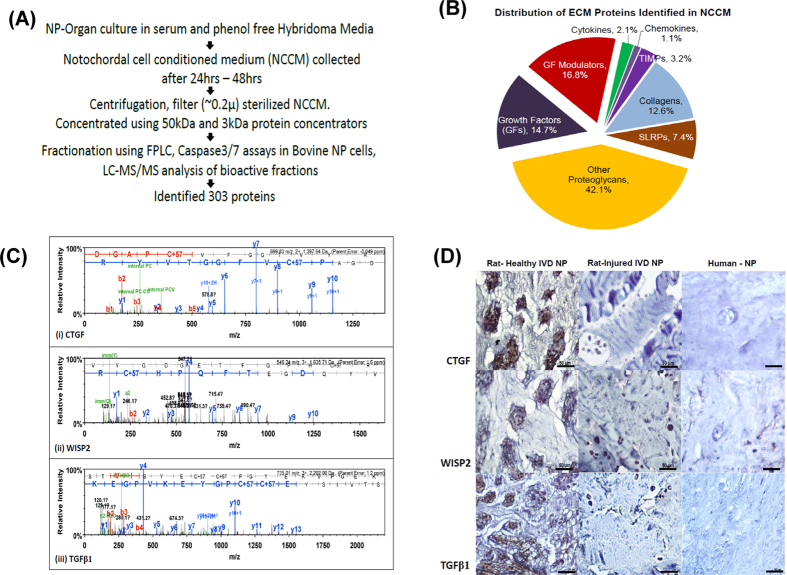
Serum-free NCCM was concentrated sequentially using 50 kDa and 3 kDa filters, followed by fractionation using size exclusion chromatography (Fig. 3A). Of the 30 fractions collected, only five of these fractions (50PF4, 50PF5, 50PF6, 3PF4 and 3PF5) suppressed etoposide - induced caspase-3/7 activity in bovine NP cells (Supplementary Fig. 3A–C). Using mass-spectrometry analysis, we identified 303 non-redundant proteins in these bioactive fractions of NCCM, of which 31% proteins have been reported within the ECM (Fig. 3B, Table 1, Supplementary Table S1). We identified several growth factors including transforming growth factor beta 1 (TGFβ1), connective tissue growth factor (CTGF), Wnt-induced soluble protein 2 (WISP2), insulin-like growth factor binding protein 7, angiopoietin-like 7 and their modulators, including chordin, sclerostin and cartilage intermediate layer protein (CILP, Fig. 3C, Table 1). For a complete list of proteins see Supplementary Table S1. Immunohistochemical analysis demonstrated moderate to strong immunostaining of CTGF, Wnt–inducible soluble protein 2 (WISP2) and TGFβ1 in NC cells and ECM in healthy rat-tail disc NPs. However, no detectable expression of CTGF, WISP2 or TGFβ1 was observed in the ECM of degenerative needle puncture injured rat-tail or human disc NPs (Fig. 3D). These findings suggested an association between the loss of CTGF, WISP2 and TGFβ1 with the development of DDD.
Figure 3. Analysis of NCCM.
(A) Schematic representation of the methodology for identification of proteins in NCCM using mass - spectrometry. (B) Pie-chart showing distribution of ECM proteins identified in NCCM. (C) Peptide signature peaks for CTGF, WISP2 and TGFβ1 observed in mass – spectrometry analysis of NCCM. (D) Immunohistochemistry showing expression of CTGF, WISP-2 and TGFβ1 in paraffin-embedded sections of rat NP (healthy and injured discs) and human degenerative disc NP (Scale bar 50μ).
Table 1. Representative list of proteins identified in NCCM and their functions.
| S. No. | Accession Number | Protein Name-FASTA | Function/Cell Signaling | References |
|---|---|---|---|---|
| 1 | F1PW10_CANFA (+1) | Transforming Growth Factor Beta-1 | Involved in development of notochord, Stimulates ECM synthesis | 24,26 |
| 2 | J9NTX8_CANFA | Connective Tissue Growth Factor | Involved in development of notochord, Stimulates ECM synthesis, Regulates TGFβ signaling | 27, 28, 29, 30, 31 |
| 3 | E2RJ75_CANFA | WNT1 Inducible Signaling Pathway Protein 2(CCN5) | Negatively regulates TGFβ signaling | 32 |
| 4 | F1PM26_CANFA | CD109 | Negatively regulates TGFβ signaling | 34, 35, 36 |
| 5 | F6V790_CANFA | CILP | Regulates TGFβ signaling | 37 |
| 6 | PGS2_CANFA | Decorin | Negatively regulates TGFβ signaling | 38 |
| 7 | E2RJE0_CANFA [2] | Cartilage Oligomeric Matrix Protein1 | Regulates TGFβ signaling | 39 |
| 8 | J9P9E4_CANFA | sclerostin (BMP antagonist) | Negatively regulates TGFβ signaling | 40 |
| 9 | E2RBE4_CANFA (+1) | Chordin | Negatively regulates TGFβ signaling | 40 |
| 10 | F1PY69_CANFA (+1) | Follistatin-Like Protein 1 | Regulates TGFβ signaling | 51 |
| 11 | F6XL96_CANFA | Von Willebrand Factor A Domain-Containing Protein 1 | Promotes matrix mineralization through TGFβ signaling | 52 |
| 12 | E2R612_CANFA | EGF-Containing Fibulin-Like Extracellular Matrix Protein | Regulates cell proliferation and apoptosis | 53 |
| 13 | E2RNR0_CANFA | Osteoglycin | Involved in muscle and bone linkage. Activates Samd3/4 independent of TGFβ. | 54 |
| 14 | E2R8H9_CANFA (+1) | pleiotrophin, Neurite Growth-Promoting Factor 11 | Involved in Angiogenesis and neurite growth | 55 |
| 15 | F1P7Y6_CANFA | nidogen-2 | Involved in ECM assembly, cell invasion, involved in Angiogenesis | 56 |
| 16 | F1PQV4_CANFA | Olfactomedin-Like 3 | Binds to BMP4 enhances the canonical SMAD1/5/8 signaling pathway, involved in Angiogenesis | 57 |
| 17 | F1PN55_CANFA | Frizzled-Related Protein 1 | Regulates Wnt signalling | 41 |
| 18 | E2R2G1_CANFA | Angiopoietin-Like 7 | Involved in Angiogenesis | 45 |
| 19 | E2R0R3_CANFA | Semaphorin | Inhibits angiogenesis and nerve ingrowth in IVD | 46,47 |
| 20 | F1Q3G2_CANFA | Slit Homolog 3 (Drosophila) | Involved in Angiogenesis | 58 |
| 21 | E2RMA3_CANFA | Secreted Protein, Acidic, Cysteine-Rich (Osteonectin) | Involved in ECM assembly, invasion and proliferation | 59 |
| 22 | E2R161_CANFA (+1) | Secreted Phosphoprotein 1 (Osteopontin) | Involved in ECM composition, invasion and migration | 59 |
Network Analysis of Proteins Identified in Secretome Analysis
Using Ingenuity Pathway Analysis (IPA) software, we analyzed the networks inherent to the healthy, notochordal cell rich NP. Among the major signaling pathways, TGFβ, Wnt/β-catenin, axonal guidance, nitric oxide synthase (eNOS), leucocyte extravasation signaling and inflammation associated matrix degradation emerged as significant pathways (p < 0.05, Supplementary Fig. 4A–E). In a hierarchical model, TGFβ1 emerged as the top protein hub interacting or regulating the expression of several other proteins including CTGF, platelet-derived growth factor (PDGF), secreted protein acidic and rich in cysteine (SPARC), MMPs and collagens identified in this study (Fig. 4). In another model, CTGF appeared as a central hub protein interacting with BMPs, TGFβ1, Smads, WISP2, several ECM proteins (aggrecan, collagens, decorin, HAPLN1, fibulin1) and kinases (Supplementary Fig. 4B).
Figure 4. Ingenuity Pathway Analysis (IPA).
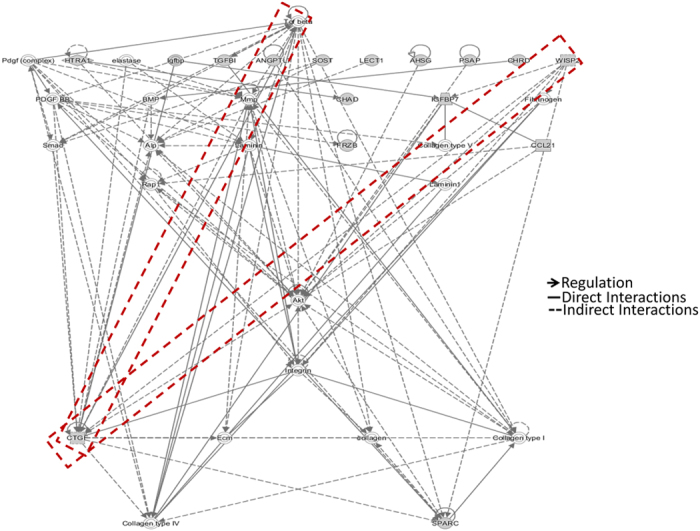
IPA was performed using 94 ECM proteins identified in our secretome analysis to determine the significant protein networks. Hierarchical model showing TGFβ1 as a major hub protein, interacting with or regulating expression of several other proteins in the network. Bold arrows show direct regulation, bold lines show direct interactions while dotted lines represent indirect interactions among proteins shown in the network.
Evaluation of the anabolic effects of CTGF, WISP2 and TGFβ1 on NP cells
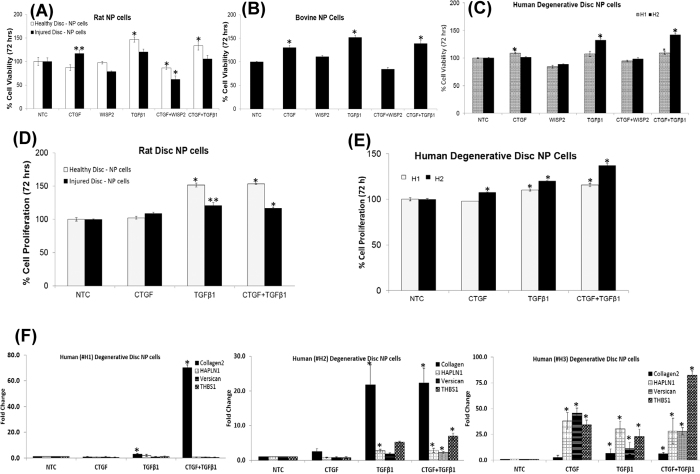
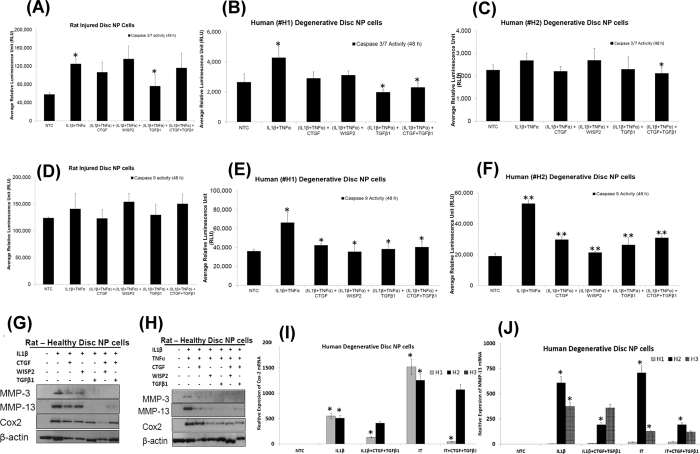
We treated rat-tail disc NP cells with recombinant human (rh) CTGF, WISP2 or TGFβ1 proteins in order to evaluate their effect upon cell viability in a dose and time dependent manner (5 ng/mL–100 ng/mL, 24 hr–96 hrs, Supplementary Fig. 5A–D). Treatment with CTGF (100 ng/ml) or TGFβ1 (10 ng/ml) alone or in combination increased the viability of NP cells derived from healthy rat-tail discs and degenerative discs (rat-tail injured discs, bovine and human) in 48 hrs–72 hrs (Fig. 5A–C, Supplementary Fig. 6A–C). Treatment with a combination of CTGF (100 ng/ml) and TGFβ1 (10 ng/ml) increased cell viability by ≥35% within 72 hrs in human degenerated disc NP cells (Fig. 5C, Supplementary Fig. 6C). However, treatment with WISP2 showed no significant differences in cell viability of NP cells derived from rat, bovine and human discs (Fig. 5A–C). This was further confirmed with cell proliferation assays using bromodeoxyuridine (BrdU) incorporation. We observed a significant increase in DNA synthesis in rat-tail (healthy/injured) and human degenerated disc NP cells upon treatment with TGFβ1 (10 ng/ml) alone or in combination with CTGF (100 ng/ml) within 72 hrs (Fig. 5D,E). We also observed increased mRNA levels of important ECM proteins including collagen type 2, hyaluronan and proteoglycan link protein 1 (HAPLN1), versican and thrombospondin1 (THBS1) in human degenerative disc NP cells upon treatment with a combination of CTGF and TGFβ1 (Fig. 5F). Western blotting verified an increase in collagen 2 expression on treatment with a combination of CTGF and TGFβ1 within 24 hrs (Supplementary Fig. 7A and B), supporting their anabolic roles on human degenerated disc NP cells.
Figure 5. Anabolic effects of CTGF, WISP2 and TGFβ1 in an in vitro model of DDD.
Effect of CTGF, WISP2 and TGFβ1 treatment alone or in combination on cell viability (72 hrs) as determined using MTT assays in NP cells obtained from (A) rat tail (healthy/injured) discs **p = 0.049, *p ≤ 0.02), (B) bovine degenerative disc NPs, *p < 0.01 and (C) human (H1, H2) degenerative disc NPs (*p < 0.005). Each bar represents mean ± S.D. of 3 independent experiments done in triplicates (n = 9). Cell proliferation assays (72 hrs) in (D) rat NP cells (healthy/injured discs), *p < 0.001, **p < 0.01 and (E) human (H1, H2) degenerative disc NPs treated with CTGF and TGFβ1 alone or in combination as determined using colorimetric anti-BrdU – ELISA, *p < 0.001. The p-values were determined using paired Student’s t-test, for treatment with CTGF, WISP2 or TGFβ1 alone or in combination with respect to no treatment control (NTC). (F) Histograms showing alterations in expression of collagen 2, HAPLN1, versican and thrombospondin1 (Thbs1) on treatment of human degenerative disc NP cells (H1, H2, H3) with CTGF and TGFβ1 as revealed by real time PCR analysis. Each bar in the histogram represents the mean ± S.D. of 3 independent experiments done in duplicates (n = 6, *p < 0.001).
Evaluation of anti-catabolic effects of CTGF, WISP2 and TGFβ1 on NP cells
Human and rat NP cells were treated with pro-inflammatory cytokines, IL-1β (10 ng/ml) alone or a combination of IL-1β (10 ng/ml) and TNFα (50 ng/ml) in the presence of CTGF (100 ng/ml), WISP2 (100 ng/ml) and TGFβ1 (10 ng/ml) alone or in combination for 24–48 hrs. Treatment with IL-1β alone or a combination of IL-1β and TNFα induced apoptosis as revealed by increased caspase-3/7 activity in NP cells (Fig. 6A–C). However, a significant decrease in inflammation–induced caspase-3/7 activity was observed in degenerative disc NP cells (rat/human) in the presence of TGFβ1 alone or combined with CTGF (Fig. 6A–C). A significant reduction in IL-1β and TNFα induced caspase-9 activity was also observed in the presence of CTGF, WISP2 or TGFβ1 in human degenerative disc NP cells (Fig. 6E,F). Treatment with CTGF, WISP2 or TGFβ1 reduced expression of MMP-3, MMP-13 and Cox2 proteins in rat NP cells treated with IL-1β and TNFα (Fig. 6G,H, Supplementary Fig. 8, A and B). Treatment of human degenerative NP cells with the pro-inflammatory cytokines, IL-1β and TNFα, induced expression of Cox2 (H1 and H2) and MMP13 in all 3 cases (H1, H2 and H3) as compared to their respective no treatment controls (NTC, Fig. 6I,J). However, the addition of CTGF and TGFβ1 reduced Cox2 and MMP-13 mRNA levels induced by IL-1β and TNFα in human degenerative discs NP cells demonstrating their anti-catabolic effects (Fig. 6I,J).
Figure 6. Anti-catabolic effects of CTGF, WISP2 and TGFβ1 in an in vitro model of DDD.
Histograms showing caspase 3/7 activity in NP cells derived from (A) rat injured IVD (*p = 0.008), (B,C) human degenerative disc NP treated with pro-inflammatory cytokines, IL-1β and TNFα alone or in presence of CTGF, WISP2 and TGFβ1 (*p < 0.05). Histograms showing caspase 9 activity in NP cells (D) rat injured IVD, (E,F) human degenerative disc NP cells treated with pro-inflammatory cytokines, IL-1β and TNFα alone or in presence of CTGF, WISP2 and TGFβ1(*p < 0.05, **p < 0.005). Each bar in the caspase assays is showing mean ± S.D. of 2 independent experiments done in quadruplets (n = 8). The p-values were determined using paired Student’s t-test. For the combination of IL-1β and TNFα are with respect to no treatment control (NTC), while p-values for the groups containing growth factors (CTGF, WISP2 or TGFβ1) are with respect to the group containing combination of IL-1β and TNFα only. Representative Western blot panels showing expression of MMP-3, MMP-13 and Cox2 in rat healthy IVD NP cells treated with (G) IL-1β alone, (H) IL-1β and TNFα in combination, and in the presence of CTGF, WISP2 or TGFβ1. All Western blots were run under the same experimental conditions as described in Materials and Methods. Histograms showing decreased expression of (I) Cox2, (J) MMP-13 mRNA levels in human degenerative disc NP cells treated with IL-1β and TNFα in presence of CTGF and TGFβ1 in comparison to IL-1β and TNFα only treatments. Each bar in the histogram represents mean ± S.D. of 3 independent experiments done in duplicates (n = 6, *p < 0.001).
Intra-discal injection of CTGF and TGFβ1 mediates progression of degenerative disc disease in a pre-clinical rodent model
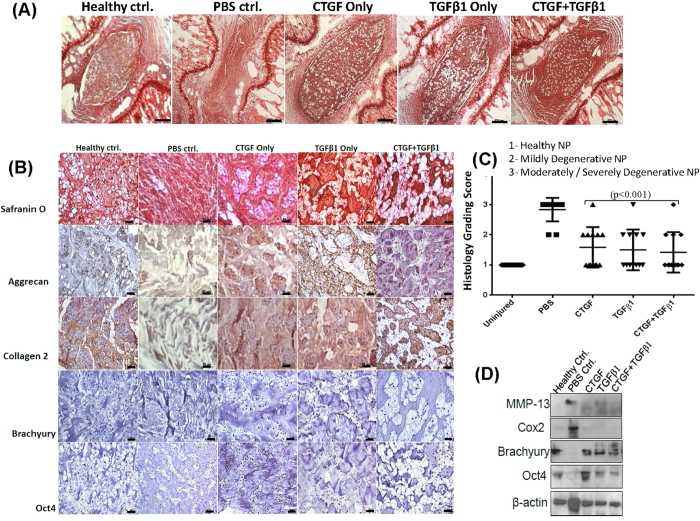
To test the potential of CTGF and TGFβ1 to mediate progression of DDD in a pre-clinical rodent model, we performed image guided rat-tail disc injuries (n = 24) in two independent experiments. Four weeks following injury, the animals were randomized into four groups (n = 6 animals/group) and an intra-discal injection (~8 μL) of CTGF (100 ng/mL i.e. 0.80 ng/disc), TGFβ1 (10 ng/mL, i.e. 0.08 ng/disc), a combination of CTGF (100 ng/mL) and TGFβ1 (10 ng/mL) or phosphate buffered saline (PBS, 1X, pH = 7.2) as a vehicle control was administered in each injured disc NPs (4 discs per animal). Histological analysis of discs injected with PBS (1X, pH = 7.2) showed a fibrocartilaginous, acellular matrix with intense Safranin-O staining within the NP (Fig. 7A–C). However, injured rat-tail discs treated with CTGF, TGFβ1 alone or in combination, demonstrated a healthy disc, rich in NCs, 10 weeks p.i. (Fig. 7A–C). Immunohistochemical analysis confirmed the restoration of a healthy NP on treatment with rh CTGF or TGFβ1, demonstrating strong expression of aggrecan, collagen 2, Brachyury and Oct4 as compared to injured disc NPs injected with PBS (1X, pH = 7.2) used as sham controls (Fig. 7B). Western blotting validated the restoration of Brachyury and Oct4 expression in rat-tail injured disc NPs following treatment with rh-CTGF and TGFβ1 proteins (Fig. 7D, Supplementary Fig. 9A). Further, treatment with CTGF and TGFβ1 also suppressed levels of the ECM degrading enzyme, MMP-13 and inflammation mediator, cyclooxygenase-2 (Cox2) in injured disc NPs (Fig. 7C, Supplementary Fig. 9A).
Figure 7. Evaluation of the therapeutic potential of CTGF and TGFβ1 in a pre-clinical in vivo rodent disc injury model of DDD.
(A) Representative Safranin-O staining photomicrographs representing uninjured, healthy IVD and injured rat IVDs injected with phosphate - buffered saline (PBS, used as a control), CTGF, TGFβ1 or a combination of CTGF and TGFβ1 (Scale bar 500μ, n = 12 tissue sections/group). (B) Representative Safranin-O and immunohistochemical staining of ECM proteins, aggrecan and collagen 2 in paraffin embedded sections of rat tail injured IVD - NPs treated with phosphate - buffered saline (PBS, used as a control), CTGF, TGFβ1 or a combination of CTGF and TGFβ1 (Scale bar 50μ). Immunohistochemistry for all the proteins was performed in duplicates in atleast 3 different IVD sections obtained from different animals from each group. (C) Scatter plots showing histological grading scores (mean ± S.D.) of rat healthy tail disc-NPs and injured tail disc-NPs following treatment with PBS, CTGF, TGFβ1 and a combination of CTGF and TGFβ1 (**p < 0.001). The p-values were determined for treatment with CTGF, TGFβ1 alone or in combination with respect to PBS. (D) Representative Western blot panels showing decreased expression of MMP-13 and Cox2, and restoration of the NC marker, brachyury and stem cell marker, Oct4 in NP tissue lysates (pooled) obtained from rat tail injured discs treated with CTGF, TGFβ1 or a combination of CTGF and TGFβ1.
Discussion
Degenerative disc disease (DDD) is associated with the progressive loss of notochordal cells (NCs) and the development of an inferior NP matrix compromising the structural integrity and biomechanical properties of the spine15,16,17,18,19. In this study, we characterized a pre-clinical rodent disc injury model that mimics progressive disc degeneration, similar to that observed in humans. Our in vitro and pre-clinical rodent model emphasized the role of inflammation in deterioration of the NP - ECM and cell death during progressive disc degeneration. Further, we have identified the key factors secreted by NCD-canine notochordal cells responsible for maintaining a healthy, hydrophilic, proteoglycan rich IVD NP that resists DDD. Our secretome analysis of the NC-rich nucleus pulposus revealed components of TGFβ- and Wnt-signaling, anti-angiogeneic factors (semaphorins, nidogen), several proteogylcans including aggrecan, small leucine rich proteoglycans (SLRPs) and collagens in serum free NCCM. Both TGFβ1 and CTGF emerged as the major protein hubs regulating the expression of several ECM proteins and interacting with other growth factors in protein network analysis. These protein networks are important for maintenance of a healthy disc NP and might be involved in resisting the development of DDD.
Both TGFβ1 and CTGF play a critical role in the development of the IVD and cartilage in the embryonic stage as well as in post-natal development of the spine24,25. TGFβ1 belongs to the transforming growth factor beta (TGFβ) superfamily of proteins known to play an important role in cartilage and bone development26. CTGF is an important constituent of the intervertebral disc microenvironment and interacts with several growth factors and matrix proteins including integrins and heparan sulfate proteoglycans27,28,29,30,31. CTGF and WISP2 (CCN5) belong to the connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) - family of matricellular proteins that possess an amino-terminal secretory peptide followed by four conserved domains with sequence homologies to insulin-like growth factor-binding proteins, von Willebrand factor C (VWC) domain, thrombospondin type 1 repeat (TSR) and a carboxy-terminal (CT) domain that contains a cysteine-knot motif27. Interestingly, the von Willebrand factor C (VWC) domain present in CTGF mediates physical interactions with members of the TGFβ-superfamily, including bone morphogenetic proteins (BMPs) and other TGFβ ligands27,28,29,30,31. In contrast, CCN5 negatively regulates TGFβ signaling by suppressing the expression of TGFβRII. Knocking down expression of CCN5 in breast cancer cells (MCF-7) resulted in deregulation of several components of the TGFβ - signaling pathway including its downstream effector SMAD signaling proteins32.
Our data showed the loss of TGFβ1, CTGF and WISP2 expression in degenerative disc NPs implicating their association with the loss of NCs. Notably, treatment with a combination of TGFβ1 and CTGF restored a healthy, cellular NP rich in NC and stem cells as compared to sham control discs in our pre-clinical models. In addition, our results demonstrated that treatment with TGFβ1 and CTGF increased cell viability, DNA synthesis and expression of healthy ECM genes in NP cells derived from degenerative human discs. The mitigation of inflammation induced caspase-3/7 activity, Cox2, MMP-3 and MMP-13 expression levels by recombinant TGFβ1 and CTGF demonstrated their synergistic action. CTGF has been shown to suppress IL-1β - induced mRNA levels of MMP-3, ADAMTS5, syndecan-4 and prolyl hydroxylase 3 by binding to cell surface integrin receptors αvβ3 and α5β1 in healthy NP cells33. These finding clearly suggest the potential overlap in the cell signaling activated downstream of TGFβ1 and CTGF regulating their anti-inflammatory and anti-catabolic activities in NP cells. However, our study is limited to studying the changes in the cellular and biochemical composition of NP in response to inflammation in vitro and disc injury in vivo but lacks determination of changes in disc height, if any. Further investigation needs to done in animal models to determine if such treatments have the capacity to retain or gain increased disc height in addition to biochemical changes in response to these treatments.
Among other proteins, we identified CD109, COMP, CILP, decorin, chordin and sclerostin proteins that are known activators or modulators of TGFβ-Smad dependent signaling. Soluble CD109 can bind to all 3 isoforms of TGFβ-1/2/3, thus interfering with the activation of TGFβ dependent signaling34. In fact, membrane bound CD109 acts as a co-receptor for TGFβ1 and results in internalization and degradation of TGFβ receptors via binding to Smad735,36. Further, CILP inhibits transcriptional activation of matrix genes in NP cells by binding to TGFβ1 and inhibiting the downstream phosphorylation of Smads, thereby promoting disc degeneration37. In addition, small leucine rich proteoglycans (SLRPs) like decorin sequester multiple growth factors including TGFβ1 and directly antagonizes the function of EGFR and IGF-IR38. In contrast, COMP activates TGF-β dependent transcriptional activity39. Ishida et al.39, proposed that COMP may serve as an “instructive matrix” component in vivo slowing the diffusion of the growth factors and promoting their biological activity. Similarly, sclerostin binds to BMP-6 and BMP-7 while chordin binds to BMP-2 and BMP-4 preventing their interaction with BMP receptors40. Taken together, these studies suggest the presence of a delicate balance in TGFβ- signaling in NC - rich NPs that is well regulated and responsible for maintaining a healthy, proteoglycan rich NP.
In addition, sclerostin and soluble frizzled-related protein 1 (sFRP1) identified in NCCM are known antagonists of Wnt-signaling pathway. The secreted frizzled-related proteins (sFRP) bind to Wnt ligands, whereas the sclerostin binds to components of the Wnt receptor41. We also identified LRP1, LRP2 and VLDLR, which act as receptors for activation of Wnt-signaling. Hiyama et al.42, reported that the activation of Wnt signaling upregulates the pro-inflammatory cytokine TNF-α leading to disc degeneration. Thus blocking Wnt signaling might protect nucleus pulposus cells against degeneration43. Further, Iwata et al.44, demonstrated that Wnt/β-catenin signaling could enhance Runx2 expression in NPs resulting in IVD calcification. Thus, cross talk between TGFβ- and Wnt-signaling pathways might play an important role in the development and progression of DDD. However, these findings require further investigation in order to understand their impact on the development and progression of DDD.
Another class of soluble factors identified in NCCM included proteins that prevent the ingrowth of blood vessels and neurites into the NP, a hallmark of DDD. Angiopoeitin like 7 (ANGPTL7) has been proposed as an anti-angiogeneic protein abundantly expressed in keratocytes and plays a major role in maintaining corneal avascularity and transparency45. Semaphorin 3E identified in NCCM belongs to class 3 semaphorin family of proteins, known as potent inhibitors of both pathological nerve innervation and vascular proliferation46,47. Another member of the family, Semaphorin 3A is highly expressed by healthy disc cells, primarily localized to the outer annulus fibrosus, but decreases significantly in the degenerative disc nucleus pulposus46,47. Further, low expression of semaphorin 3A showed a significant correlation with overexpression of endothelial cell marker, CD31 and PGP9.5, marker for neurons47. However, the role of ANGPTL7 and Semaphorin 3E in disc degeneration is currently unknown and requires further investigations.
Conclusions
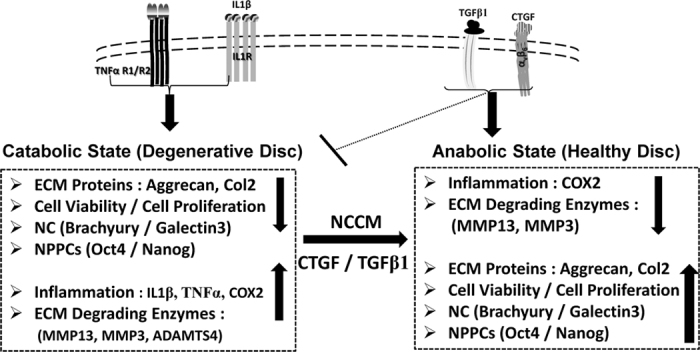
In conclusion, we have identified the soluble proteins present within NCCM that have the ability to maintain a healthy, proteoglycan rich nucleus pulpous and delay DDD. We have further identified the proteins networks important for homeostatic regulation of the healthy IVD - NP. Our findings suggest that the loss of TGFβ1, CTGF and WISP2 is associated with the progression of DDD. Both TGFβ1 and CTGF represent pivotal protein hubs involved in cellular signaling and ECM regulation of the healthy IVD NP. We further demonstrated the potential utility of a combination of TGFβ1 and CTGF as novel, minimally invasive molecular therapeutic agents for the biological treatment of degenerative disc disease (Fig. 8).
Figure 8. Proposed model demonstrating the mechanism of progressive disc degeneration in presence of pro-inflammatory cytokines (IL-1β and TNFα) and effect of intervention by novel, potential therapeutic agents (CTGF/TGFβ1) for regeneration of the IVD - NP.
Materials and Methods
Generation of serum free notochordal cell derived conditioned medium (NCCM)
Notochordal cell derived conditioned medium (NCCM) was collected from notcohordal cell- rich nucleus pulpous (NP) obtained from IVDs of non-chondrodystrophic (NCD) canines as described earlier20,21. All animals (n = 12) were obtained in collaboration with a licensed animal facility and all experimental protocols were approved by the Animal Care Committee, Research Animal care policy and Ethics Approval Board of University Health Networks, Toronto, Ontario, Canada. All experimental procedures were performed in accordance with the guidelines approved by Animal Care Committee and Ethics Approval Board of University Health Networks, Toronto, Ontario, Canada. All non-chondrodystrophic canines were 8 to 14 months of age and had failed at adoption or were to be euthanized for other purposes. Deep sedation was achieved using a combination of Acepromazine (10 mg/mL, Atravet-Aerst Pharmaceuticals St. Laurent, Quebec, Canada) mixed with Xylazine 100 mg/mL (Xylomax-Bimeda-NHC Animal Health, Broomhill Road, Tallaght, Dublin, Ireland) at a combined dose of 1 mL/15 Kg body weight. Once deep sedation had occurred, euthanasia was accomplished using intravenous sodium pentobarbital (CDMV) (St. Hyacinthe, Quebec, Canada at a dose of 30 mL/kg body weight. Within 2 hrs of euthanization, the lumbar spines were removed and nuclei pulposi were isolated under aseptic conditions20,21. Nuclei pulposi were washed with phosphate buffered saline (PBS, pH = 7.2) and three NPs were placed within tissue culture inserts with 40μm-filters in serum free CD Hybridoma media (protein and phenol red free, Cat No #11279-023, Life Technologies, USA) containing 100 units penicillin/streptomycin in 6 well plates under hypoxic conditions (3.5% O2, and 5% CO2, NuAire incubators) at 37 °C. The conditioned medium referred hereafter as NCCM was collected after 24 hrs – 48 hrs, centrifuged at 8000 rpm for 30 minutes, filtered through 0.2μm syringe-tip filters and stored in −80 °C until further use.
Identification of bioactive fractions of NCCM
Notochordal cell condition medium (NCCM) was concentrated sequentially using 50 kDa and 3 kDa spin-ultrafiltration protein concentrators (EMD Millipore, MA, USA) following manufacturer’s instructions. The respective concentrated NCCM samples were further fractionated by size - exclusion on a Superose 12 HR 10/30 fast protein liquid chromatography (FPLC) column (Pharmacia) in running buffer (10 mM sodium phosphate, 150 mM NaCl, 1 mM EDTA, pH = 7.4). Thirty fractions (~1 ml) were collected, measured for protein concentration by absorbance at 280 nm and stored at −80 °C until further use. Consecutive protein-containing fractions were pooled pairwise and evaluated for their effect on etoposide (cytotoxic drug) induced caspase-3/7 activity in bovine tail disc NP cells as described below. Bioactive fractions were defined as protein fractions showing a decrease in caspase-3/7 activity in bovine NP cells on treatment with etoposide. These bioactive fractions were later analyzed for identification of proteins using mass-spectrometry.
Mass spectrometry analysis
The LC-MS/MS analysis was performed at the SPARC BioCentre, The Hospital for Sick Children, Toronto, Canada. Briefly, the bioactive fractions were reduced with dithiothreitol (DTT), the free cysteine residues alkylated with iodoactetamide and digested overnight with modified bovine trypsin (Promega, Madison, USA). The tryptic peptides were desalted and loaded onto a 50 cm × 75 μm ID column containing RSLC 2 μm C18 packing material (EASY-Spray, Thermo-Fisher, Odense, Denmark) with an integrated emitter. The peptides were eluted into a Q-Exactive hybrid mass spectrometer (Thermo-Fisher, San Jose, CA) using an Easy-Spray nLC 1000 chromatography system (Thermo-Fisher, Odense Denmark) with a 90-minute gradient from 0% to 35% acetonitrile in 0.1% formic acid. The mass spectrometer was operated in a data dependent mode with 1 MS followed by 10 MS/MS spectra. The MS was acquired with a resolution of 70,000 FWHM, a target of 1 × 106 ions and a maximum scan time of 120 ms. The MS/MS scans were acquired with a resolution of 17,500 FWHM, a target of 1 × 106 ions and a maximum scan time of 120 ms using relative collision energy of 27%. A dynamic exclusion time of 15 seconds was used for the MS/MS scans. The raw data files were acquired with XCalibur 2.2 (Thermo-Fisher Scientific) and processed with the Sequest search engine (Thermo-Fisher Scientific) using the UniProt canine database August 12, 2014 version with 28,460 entries and with X!-Tandem (Beavis Informatics, Winnipeg, Canada). The processed data was imported into Scaffold 3.2 (Proteome Software, Portland, OR). Peptides were considered to be identified if the Scaffold score exceeded the 0.1% false discovery rate (FDR) as determined by searching against the reversed UniProt canine database.
Development and characterization of a pre-clinical rodent injury model of DDD
We used 12-week old female Wistar rats (n = 21, Charles River Laboratories Inc., MA, USA) to develop a pre-clinical rodent tail disc injury model of DDD as follows. Experimental protocols were approved by the Animal Care Committee and Research Ethics Approval Board of University Health Networks, Toronto, Ontario, Canada. All experimental procedures were performed in accordance with the guidelines approved by Animal Care Committee and Ethics Approval Board of University Health Networks, Toronto, Ontario, Canada. Anesthesia was achieved using isofluorane (5 L/min plus 1 L/min O2) and maintained at 3 L/min. Once deeply anaesthetized, the animal was affixed on a stereotactic procedure apparatus (Model 900, Kopf Instruments, CA, USA) with nose cone inhalation. For disc injury (4 caudal discs per animal), we used a 26-gauge (G) needle (Hamilton Company, USA) mounted on a Hamilton syringe. The needle was advanced completely through the selected tail IVD to penetrate the full thickness inclusive of the annulus fibrosus on both sides of the disc. Confirmation of needle placement was made using fluoroscopy and maintained in position for 2 minutes, withdrawn halfway to the center of the NP and left there for 1 minute and then slowly withdrawn completely over a 1-minute period. The animals were then removed from the stereotactic apparatus and allowed to recover in a warmed cage. At the end of study period i.e. 72 hrs - 10 weeks, animals were humanely euthanized using CO2 asphyxiation and each vertebral lumbar/caudal motion segment was dissected aseptically. Of the four injured IVDs, atleast one IVD per animal was fixed in formalin as a representative for histological analysis and rest of the injured IVD - nucleus pulposi were harvested and lysed in RIPA buffer (50 mM Tris, pH = 7.4, 150 mM NaCl, 1% NP-40 and protease inhibitor cocktail) for western blotting.
Evaluation of NCCM and soluble factors as therapeutic agents in pre-clinical rodent model of DDD
We used a pre-clinical rat-tail disc injury model of DDD for evaluating the therapeutic potential of NCCM and soluble factors identified. For the 1st set of experiments involving NCCM, animals (n = 6/group) were randomized four weeks post-injury and an intra-discal injection (~8 μL) of either concentrated NCCM (2.2 μg/μl) or the control medium was given under general anesthesia using fluoroscopic image guidance in 4 injured discs per animal as described earlier. For the 2nd set of experiments involving growth factors including CTGF and TGFβ1, animals (n = 6/group) were randomized four weeks post-injury and an intra-discal injection (~8 μL) of either phosphate buffered saline (PBS, 1X, pH = 7.2), CTGF (100 ng/mL), TGFβ1 (10 ng/mL) or a combination of CTGF (100 ng/mL) and TGFβ1 (10 ng/mL) was given under general anesthesia using fluoroscopic image guidance in 4 injured discs per animal as described above. Further, a group of six animals were left uninjured that served as age matched healthy controls in each set of experiments. At the end of experiments (i.e. six weeks later), at least one IVD per animal was fixed in formalin as a representative for histological analysis. The remainder of the NPs harvested from rat caudal IVDs were pooled together according to their respective groups (healthy/treated) for lysis in RIPA lysis buffer for western blotting. Each of these experiments was repeated independently to ensure reproducibility.
Histology and immunohistochemistry (IHC)
For formalin fixation, at least one IVD per animal (healthy/injured following treatment with NCCM, PBS, CTGF or TGFβ1 alone or in combination) was harvested for paraffin embedding. The tissues were fixed in 10% formalin followed by decalcification and paraffin embedding. Hemaetoxylin and eosin (H&E) and Safranin-O staining was performed in duplicates on paraffin-embedded sections (5 μm) to assess general morphology and proteoglycan content21. Histological grading of IVD-NP (injury followed by treatment) was carried out on the basis of NP-cellularity in H&E sections as described earlier48. Following H&E and Safranin O staining, serial tissue sections were deparaffinized in xylene, hydrated in gradient alcohol followed by antigen retrieval in Tris-EDTA buffer (pH = 9.0) for IHC. The sections were incubated with hydrogen peroxide (0.3% v/v) in methanol for 15 minutes, followed by blocking with 10% serum. Thereafter, the slides were incubated with either rabbit/goat polyclonal or mouse monoclonal primary antibodies overnight (O/N) at 4 °C. Protein expression was detected using respective secondary antibodies (rabbit/goat/mouse) from Vectastain ABC kit and diaminobenzidine (DAB) as a chromogen. In negative controls, the primary antibody was replaced by isotype-matched IgG. Immunohistochemistry was done in at least 3 different IVD sections per group. The bright field sections were evaluated by light microscopic examination using a ScanScope XT, Aperio Whole Slide Scanner and Nikon bright field microscope.
Ingenuity Pathway Analysis (IPA)
Ingenuity Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com) was used to identify the canonical pathways, major protein hubs and the associated networks involved in the maintenance of a healthy disc NP48. Of the 303 proteins identified in the bio-active fractions of NCCM, only 94 ECM proteins were used for network analysis. The protein accession numbers were used to determine IPA abbreviated names. These notations were then used to navigate the databases available online. The eligible proteins served as nodes for generating the canonical and novel proteins interaction networks. All networks were scored [−log (p-value)] based on the number of network eligible molecules contained in these networks. The higher the score, the lower was the probability of finding the observed number of network eligible molecules by random chance. The canonical pathways were scored based on their p-values, calculated using the right-tailed Fisher Exact Test; a p-value ≤ 0.05 indicates a statistically significant, non-random association.
Cell culture, Western blotting and quantitative real time PCR (qPCR)
Healthy rat lumbar/caudal spine IVDs, injured tail discs and bovine caudal IVD NPs were removed aseptically. Bovine caudal disc NPs were obtained from six, 3-year old steers. Human degenerative disc nucleus pulposus tissues were obtained from patients (n = 3) undergoing discectomy or fusion surgery at Toronto Western Hospital, University Health Network (UHN), Toronto. For human degenerative disc NP samples used in this study, informed consent was obtained from all the patients prior to surgery. All human tissue samples were collected in accordance to the guidelines approved by the Research Ethics Board, Toronto Western Hospital, UHN, Toronto. The nucleus pulposus (NP) was separated and enzymatically digested according to our established methods20,21. The next day, the cells were filtered with a 70 μm cell strainer and cultured within a hypoxic incubator (NuAire, MN, USA) in 3.5% O2, 5% CO2, in Advanced Dulbecco’s modified Eagle’s medium (ADMEM) supplemented with 8% fetal bovine serum (FBS), penicillin and streptomycin (100 U/mL) until passage (P2) as described earlier20,21. The NP cells obtained from rat IVDs were pooled together for treatment with respective agents as follows. The cells were either cultured in serum free DMEM (no treatment controls) or treated with IL-1β (10 ng/mL), TNFα (50 ng/mL), CTGF (10–100 ng/mL), WISP2 (10–100 ng/mL) or TGFβ1 (5–20 ng/mL) for various time points (24 hrs–72 hrs) under hypoxic conditions. The effect of pro-inflammatory cytokines (IL-1β and TNFα) and soluble factors (CTGF/WISP2/TGFβ1) on expression of ECM genes and proteins in NP cells were determined using qPCR and Western blotting respectively (See Supplementary Data for details). For Western blotting, protein estimation was performed using Bradford assay (BioRad, CA). Briefly, equal amounts of whole cell or tissue lysates prepared using RIPA lysis buffer were subjected to Western blotting as described earlier49. Total lysates (30 μg) were resolved on 10% sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE) under reducing conditions and then proteins were electro-transferred onto polyvinyledendifluoride (PVDF) membranes (BioRad, CA). After blocking with 5% non-fat powdered milk in Tris-buffered saline (TBS, 0.1 M, pH = 7.4), blots were incubated with rabbit/goat polyclonal or mouse monoclonal primary antibodies at 4 °C overnight (See Supplementary Data for antibody details). Membranes were washed three times with Tween (0.1%)-Tris-buffer saline (TTBS) and then incubated for 2 hrs at room temperature (RT) with the respective HRP-conjugated anti-IgG secondary antibodies (BioRad, CA), diluted as per the manufacturers suggestions in 2% non-fat milk in TBS (pH = 7.2, 1X). Blots were washed three times with TTBS for 15 minutes and protein bands were detected by the enhanced chemiluminescence method (BioRad, CA) on Kodak Hyperfilm50. β-actin was used as a loading control for each experiment. All Western blots were run under the same experimental conditions and repeated at least 3 times to ensure reproducibility. All Western blots were quantified using densitometry values calculated using Image J software (available online). For calculation of fold changes representing expression levels of protein under each treatment conditions, densitometry values of each band for the protein of interest was normalized with β-actin used as a loading control in each experiment50.
Cell viability, cell proliferation and apoptosis assays
Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, USA) as described earlier50. We used a BrdU - ELISA (colorimetric) assay (Cat# ab126556, Abcam) to determine the effect of treatment with CTGF (100 ng/mL) or TGFβ1 (10 ng/mL) on human and rat NP cells (healthy/injured) following the manufacturer’s instructions. Apoptosis in NP cells (rat and human) was determined using Caspase-3/7 and Caspase-9 specific Lumi-Glo assays (Promega, Madison). See Supplementary Data for details.
Statistical analysis
All data for cell viability, proliferation, apoptosis (casase-3/7, caspase-9) assays and qPCR are expressed as means ± SD. Significant differences in test and no treatment controls (NTC) were determined using the paired Student’s t-test. Statistical analysis was performed using the Graphpad Prism. Data analysis for qPCR was carried out using calculation of ΔΔCt values, fold changes and p-values using Student’s t-test. P-value < 0.05 was defined as statistically significant for all tests. Ingenuity Pathway Analysis (IPA) software calculated p-values for canonical pathways based on the Fisher Exact Test; a p-value < 0.05 indicates a statistically significant, non-random association.
Additional Information
How to cite this article: Matta, A. et al. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 7, 45623; doi: 10.1038/srep45623 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We are thankful to Drs. Steven Lewis, Mohammad Shamji and Michael Fehlings for providing the human surgical disc samples used in this study and our summer student, Stanley Zhou, BSc for his technical assistance in some of the preliminary experiments. We also gratefully acknowledge Dr. Robert Inman for manuscript review and editorial suggestions. We are grateful to The Skoll Family Trust for financial assistance with the production of this work.
Footnotes
The authors declare no competing financial interests.
Author Contributions A.M. and W.M.E. designed experiments, wrote and edited manuscript. A.M., M.Z.K. and W.M.E. performed experiments and analyzed data. D.E.I. provided reagents and infrastructure for size exclusion chromatography and edited the manuscript. W.M.E. provided all the reagents, financial support and infrastructure for performing the experiments.
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman B. R., Belmont P. J. Jr. & Schoenfeld A. J. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J 12, 63–70 (2012). [DOI] [PubMed] [Google Scholar]
- Phillips F. M., Slosar P. J., Youssef J. A., Andersson G. & Papatheofanis F. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976) 38, E409–22 (2013). [DOI] [PubMed] [Google Scholar]
- Bydon M. et al. Lumbar fusion versus non-operative management for treatment of discogenic low back pain: a systematic review and meta-analysis of randomized controlled trials. J. Spinal Disord Tech. 27, 297–304 (2014). [DOI] [PubMed] [Google Scholar]
- Nishida K. et al. Gene therapy approach for disc degeneration and associated spinal disorders. Eur. Spine J 17, 459–66 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J 17, 441–51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Efficacy of intervertebral disc regeneration with stem cells - a systematic review and meta-analysis of animal controlled trials. Gene. 564, 1–8 (2015). [DOI] [PubMed] [Google Scholar]
- Sakai D. & Andersson G. B. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat. Rev. Rheumatol. 11, 243–56 (2015). [DOI] [PubMed] [Google Scholar]
- Kandel R., Roberts S. & Urban J. P. Tissue engineering and the intervertebral disc: the challenges. Eur. Spine J. 17, 480–91 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A. J., Bradford D. S. & Lotz J. C. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976). 29, 156–63 (2004). [DOI] [PubMed] [Google Scholar]
- Malham G. M. et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J. Neurosurg. Spine 21, 851–60 (2014). [DOI] [PubMed] [Google Scholar]
- Willems N. et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 17, 137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupille P., Mulleman D. & Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis. Res. Ther. 9, 110 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair S. M. et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 36, 1190–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud M. V. & Shapiro I. M. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit. Rev. Eukaryot.Gene Expr. 21, 29–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D., et al. Discs degenerate before facets. Spine. 15, 111–3, (1990). [DOI] [PubMed] [Google Scholar]
- Aguiar D. J., Johnson S. L. & Oegema T. R. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Experimental Cell. Research 246, 129–137 (1999). [DOI] [PubMed] [Google Scholar]
- Risbud M. V. & Shapiro I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10, 44–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders L. A. et al. Intervertebral disc degeneration in the dog. Part 2: chondrodystrophic and non-chondrodystrophic breeds. Vet. J 195, 292–9 (2013). [DOI] [PubMed] [Google Scholar]
- Erwin W. M., Islam D., Inman R. D., Fehlings M. G. & Tsui F. W. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res. Ther. 13, R215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin W. M., Ashman K., O’Donnel P. & Inman R. D. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 54, 3859–67 (2006). [DOI] [PubMed] [Google Scholar]
- Bach F. C. et al. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur. Cell Mater. 30, 132–47 (2015). [DOI] [PubMed] [Google Scholar]
- Cornejo M. C., Cho S. K., Giannarelli C., Iatridis J. C. & Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage 23, 487–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H. et al. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 585, 1209–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedore J. et al. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 65, 2634–44 (2013). [DOI] [PubMed] [Google Scholar]
- Li X. & Cao X. BMP signaling and skeletogenesis. Ann. N. Y. Acad. Sci. 1068, 26–40 (2006). [DOI] [PubMed] [Google Scholar]
- Leask A. & Abraham D. J. All in the CCN family: essential matricellular signaling modulators merge from the bunker. J. Cell Sci. 119, 4803–10 (2006). [DOI] [PubMed] [Google Scholar]
- Erwin W. M. The Notochord, Notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J. Cell Commun. Signal 2, 59–65 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T. et al. CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J. Cell Commun. Signal 1, 45–58 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu J. G., Ketpura N. I., Reversade B. & De Robertis E. M. Connective tissue growth factor (CTGF) modulates cell signalling by BMP and TGFbeta. Nat. Cell Biol. 4, 599–604 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C. M. et al. Regulation of CCN2/connective tissue growth factor expression in the nucleus pulposus of the intervertebral disc: role of Smad and activator protein 1 signaling. Arthritis. Rheum. 62, 1983–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M. et al. CCN5, a novel transcriptional repressor of the transforming growth factor β signaling pathway. Mol. Cell Biol. 31, 1459–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C. M. et al. CCN2 suppresses catabolic effects of interleukin-1β through α5β1 and αVβ3 integrins in nucleus pulposus cells: implications in intervertebral disc degeneration. J. Biol. Chem. 289, 7374–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. D. et al. Soluble CD109 binds TGF-β and antagonizes TGF-β signaling and responses. Biochem. J pii: BJ20141488 (2015). [DOI] [PubMed] [Google Scholar]
- Bizet A. A. et al. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim. Biophys. Acta. 1813, 742–53 (2011). [DOI] [PubMed] [Google Scholar]
- Bizet A. A., Tran-Khanh N., Saksena A., Liu K., Buschmann M. D. & Philip A. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J. Cell Biochem. 113, 238–46 (2012). [DOI] [PubMed] [Google Scholar]
- Seki S. et al. Cartilage intermediate layer protein promotes lumbar disc degeneration. Biochem. Biophys. Res. Commun. 446, 876–81 (2014). [DOI] [PubMed] [Google Scholar]
- Neill T., Schaefer L. & Iozzo R. V. Decorin: a guardian from the matrix. Am. J. Pathol. 181, 380–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K. et al. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone. 55, 23–35 (2013). [DOI] [PubMed] [Google Scholar]
- Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 16, 309–17 (2005). [DOI] [PubMed] [Google Scholar]
- Mii Y. & Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 53, 911–23 (2011). [DOI] [PubMed] [Google Scholar]
- Hiyama A., Yokoyama K., Nukaga T., Sakai D. & Mochida J. A complex interaction between Wnt signaling and TNF-α in nucleus pulposus cells. Arthritis Res. Ther. 15, R189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama A. et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 62, 3036–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M. et al. Enhancement of Runx2 expression is potentially linked to β-catenin accumulation in canine intervertebral disc degeneration. J. Cell Physiol. 230, 180–90 (2015). [DOI] [PubMed] [Google Scholar]
- Toyono T. et al. Angiopoietin-like 7 is an anti-angiogenic protein required to prevent vascularization of the cornea. PLoS One. 10, e0116838 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P., Lv H., Zhang L., Zhang L. & Tang P. Semaphorin 3A: A Potential Target for Low Back Pain. Front. Aging Neurosci. 26, 7:216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binch A. L. et al. Class 3 semaphorins expression and association with innervation and angiogenesis within the degenerate human intervertebral disc. Oncotarget. 6, 18338–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B. et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine (Phila Pa 1976) 33, 1925–34 (2008). [DOI] [PubMed] [Google Scholar]
- Matta A., Masui O., Siu K. W. & Ralhan R. Identification of 14-3-3zeta associated protein networks in oral cancer. Proteomics. 16, 1079–89 (2016). [DOI] [PubMed] [Google Scholar]
- Matta A., DeSouza L. V., Ralhan R. & Siu K. W. Small interfering RNA targeting 14-3-3ζ increases efficacy of chemotherapeutic agents in head and neck cancer cells. Mol. Cancer Ther. 9, 2676–88 (2010). [DOI] [PubMed] [Google Scholar]
- Chaly Y. et al. Follistatin-like protein 1 regulates chondrocyte proliferation and chondrogenic differentiation of mesenchymal stem cells. Ann. Rheum. Dis. 74, 1467–73 (2015). [DOI] [PubMed] [Google Scholar]
- Ohyama Y. et al. Modulation of matrix mineralization by Vwc2-like protein and its novel splicing isoforms. Biochem. Biophys. Res. Commun. 418, 12–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaj P. et al. EFEMP1 binds the EGF receptor and activates MAPK and Akt pathways in pancreatic carcinoma cells. Biol. Chem. 390, 1293–302 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka K. et al. Role of osteoglycin in the linkage between muscle and bone. J. Biol. Chem. 287, 11616–28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidlinger-Wilke C. et al. Mechanical stimulation alters pleiotrophin and aggrecan expression by human intervertebral disc cells and influences their capacity to stimulate endothelial migration. Spine (Phila Pa 1976). 34, 663–9 (2009). [DOI] [PubMed] [Google Scholar]
- Köhling R. et al. Nidogen and nidogen-associated basement membrane proteins and neuronal plasticity. Neurodegener. Dis. 3, 56–61 (2006). [DOI] [PubMed] [Google Scholar]
- Miljkovic-Licina M. et al. Targeting olfactomedin-like 3 inhibits tumor growth by impairing angiogenesis and pericyte coverage. Mol. Cancer Ther. 11, 2588–99 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L. J. et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci. 99, 510–7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan S. P. et al. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue Eng. Part A. 20, 264–74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.