Abstract
Our purpose was to discuss the biological function of Hpa gene and its regulatory network in invasion and metastasis of colon cancer. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes database were used to perform functional annotation and pathway analysis on Hpa gene. Gene Ontology analysis results showed that Hpa plays a significant role in cellular component, molecular function and biological process; and combined with Kyoto Encyclopedia of Genes and Genomes database, regulatory network of angiogenesis of colon cancer was drawn out. Through analysis of regulatory network linked to angiogenesis in invasion and metastasis of colon cancer, the study lays foundation for further prevention, diagnosis and treatment of colon cancer.
Keywords: Invasion and metastasis of colon cancer, Hpa gene, Angiogenesis, GO analysis, Network pathway
1. Introduction
Colon cancer, a common malignant digestive tract tumor, has a high incidence ranking second on the list of digestive tract tumor, greatly threatening human life and health. With the improvement of living standard, people’s diet structure becomes more complicated, and the incidence of colon cancer increases year by year; besides, it tends to attack younger people (Blazeby et al., 2010). In recent years, China has made great progress in treating colon cancer, and in addition to conventional surgical treatment, various protocols have been updated. The postoperative survival rate, however, is still low, and the main reason is that the invasion and metastasis of the tumor lead to recurrence, which finally results in death of patients (Del Pulgar et al., 2007). Since invasion and metastasis of tumor is a complex process with multistage, multi-gene involvement and multi-factor accumulation, it is significant for treatment and prognosis of patients with colon cancer to study the potential molecular mechanism linked to invasion and metastasis of colon cancer.
Heparanase (Hpa), a kind of glucuronic acid enzyme, is commonly seen and over expressed in lots of malignant tumors, and more and more studies indicated that it plays a significant role in invasion and metastasis of tumor (Jiang et al., 2009). Hpa can degrade basement membrane and heparan sulfate proteoglycan (HSPG) on extracellular matrix, resulting in destruction of the basement membrane and extracellular matrix and opening channel for the invasion and metastasis of tumor; most importantly, it promotes angiogenesis of tumor with invasion and metastasis, thereby accelerating growth of tumor cells (Vaday and Lider, 2000). To date, there are few reports on analysis of regulatory network correlated to Hpa in colon cancer. Therefore, this study aims to study molecular regulation gene related to colon cancer from the perspective of bioinformatics, and to systematically and comprehensively analyze the regulatory network of Hpa linked to angiogenesis in process of invasion and metastasis of colon cancer, thus providing significant theoretical foundation for improvement of therapy and prognosis of patients with colon cancer.
2. Material and methods
2.1. Gene Ontology (GO) analysis
Online website Gene Ontology Consortium (http://geneontology.org/) was employed to conduct GO analysis for Hpa gene. GO analysis began with entering the homepage of Gene Ontology Consortium followed by typing in screening conditions, “Hpa” and “Homo sapiens” in order, and then the primary GO functional annotation for Hpa gene correlated to colon cancer cells was performed.
2.2. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
The online website KEGG PATHWAY Database (http://www.kegg.jp/kegg/pathway.html) was adopted to analyze network pathway linked to Hpa gene in colon cancer. KEGG analysis began with entering the homepage of KEGG PATHWAY Database before typing in screening conditions, “hsa” for organism, and “Hpa” for keywords, and then regulatory network of the gene in angiogenesis of patients with colon cancer was searched.
3. Results
3.1. GO analysis of Hpa gene
Gene Ontology, mainly used in studying specific functions of gene, covers three domains: molecular function, cellular component and biological process. GO analysis results of Hpa gene are shown in Table 1. As shown, Hpa possesses all the three functions, among which molecular function involves heparan sulfate proteoglycan binding, heparanase activity, beta-glucuronidase activity, and so on; cellular component involves proteinaceous extracellular matrix, nucleus, nucleoplasm, and so on; biological process involves positive regulation of vascular endothelial growth factor production, positive regulation of osteoblast proliferation, cell proliferation, glycosaminoglycan catabolic process, and so on.
Table 1.
GO analysis results of Hpa gene.
| Gene | Name | Ontology | Accession |
|---|---|---|---|
| Hpa | Heparan sulfate proteoglycan binding | Molecular_function | GO:0043395 |
| Beta-glucuronidase activity | Molecular_function | GO:0004566 | |
| Protein binding | Molecular_function | GO:0005515 | |
| Heparanase activity | Molecular_function | GO:0030305 | |
| Syndecan binding | Molecular_function | GO:0045545 | |
| Nucleoplasm | Cellular_component | GO:0005654 | |
| Proteinaceous extracellular matrix | Cellular_component | GO:0005578 | |
| Nucleus | Cellular_component | GO:0005634 | |
| Intracellular membrane-bounded organelle | Cellular_component | GO:0043231 | |
| Positive regulation of vascular endothelial growth factor production | Biological_process | GO:0010575 | |
| Positive regulation of osteoblast proliferation | Biological_process | GO:0033690 | |
| Positive regulation of protein kinase B signaling | Biological_process | GO:0051897 | |
| Angiogenesis involved in wound healing | Biological_process | GO:0060055 | |
| Positive regulation of cell proliferation | Biological_process | GO:0008284 | |
| Glycosaminoglycan catabolic process | Biological_process | GO:0006027 |
3.2. KEGG analysis of Hpa gene
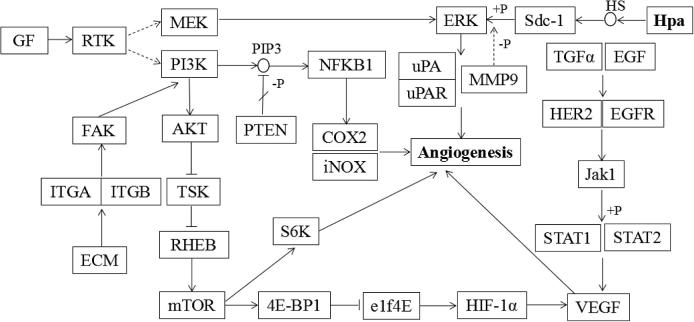
According to the KEGG analysis of Hpa gene based on KEGG PATHWAY database, Hpa participated in development of angiogenesis in colon cancer, and regulatory network of the angiogenesis which involved Hpa was established, as shown in Fig. 1.
Figure 1.
Regulatory network on development of angiogenesis in colon cancer.
4. Discussion
Invasion and metastasis is not only the essential biological characteristic of malignant tumor cells but also a primary reason for the death of patients with tumor. Hpa was firstly found correlated to metastasis of tumor in 1980s. Later, more and more studies indicated that it plays a positive-regulation role in invasion and metastasis of tumor. Friedmann et al. (2000) systematically analyzed the expression of Hpa in human colon cancer, which finally found Hpa had a high expression in the process of colon cancer cells metastasizing to lung, liver and lymph node. Besides, Hpa in tumor tissues of colon cancer had higher expression level and activity as compared to normal colon tissues. Monoclonal antibody was adopted to track the activities of Hpa in colonic epithelial adenoma carcinoma sequence, and results indicated that the expression, processing and location of Hpa in development of colon cancer were closely related to colonic adenoma carcinoma sequence (Doviner et al., 2006). Study by El-Assal et al. (2001) on patients with liver cancer found that mRNA expression of Hpa was significantly correlated to tumor size and invasion of tumor, and they further found that Hpa promoted the growth, invasion and angiogenesis of tumor. In addition, results of study on breast cancer also indicated that Hpa’s correlation to tumor size and metastasis of sentinel lymph node of breast cancer. Koliopanos et al. (2001) conducted over-expression transfection of Hpa in pancreatic carcinoma cells cultured in vitro and results indicated that over expression of Hpa enhanced invasion capability of pancreatic carcinoma cells, thereby promoting the potential of tumor metastasis; besides, the expression of Hpa was negatively correlated to survival rate of patients with pancreatic carcinoma. Research by Goldshmidt et al. (2002) proved the key role that Hpa’s expression and secretion outside the cell played in promoting tumor angiogenesis and metastasis. Elevation of Hpa gene induces tumor angiogenesis and tissue angiogenesis (Elkin et al., 2001). In gastric cancer, study also showed that with the inhibition of Hpa’s expression by siRNA, invasion capability of gastric cancer decreased as well (Zhang et al., 2007). In this study, GO analysis found that Hpa had not only molecular function (including heparan sulfate proteoglycan binding, heparanase activity, etc) but also biological process (including positive regulation of vascular endothelial growth factor production, etc), which is in line with previous studies and intuitively illustrates that Hpa is involved in biological process including tumor metastasis and angiogenesis. Since all above studies showed that Hpa had positive correlation to invasion and metastasis of tumor, researchers have been seeking for an Hpa inhibitor for tumor treatment. For the time being, study of the inhibitor mainly focuses on sulfated polysaccharides or negative ion polymer structurally similar to polysaccharide, among which PI-88 is now the only Hpa inhibitor that enters phase III clinical trials.
The invasion and metastasis of tumor needs two essential conditions: one is the break of natural barrier composed of extracellular matrix and basement membrane; the other is tumor angiogenesis. Angiogenesis can maintain and promote normal growth of tumor, and tumor cells can invade surrounding tissues and spread with distant metastasis until forming metastatic lesions in secondary parts; besides, the growth and metastasis of metastatic lesions also need angiogenesis (Fidler and Ellis, 1994), in which the role of angiogenesis is more significant than that in primary lesion (Tanaka et al., 2001). Apparently, angiogenesis is important for invasion and metastasis of tumor. Angiogenesis consists of four steps, which are endovascular dissolution of endothelial basement membrane and endothelial cell activation, endothelial cell migration, endothelial cell proliferation, and formation of blood vessels. The mechanism of angiogenesis is complicated, and it is regulated by various oncogenes and tumor suppressor genes. Through the online website, KEGG PATHWAY Database, this study analyzed the pathways of Hpa on angiogenesis in process of invasion and metastasis. In addition to Hpa, the pathways in this study also involve other angiogenesis-associated factor, such as hypoxia inducible factor 1 alpha (HIF-1α), phosphatase and tensin homolog deleted on chromosome ten (PTEN), cyclooxygenase-2 (COX-2), urokinase plasminogen activator (uPA), and VEGF. Hpa can directly act on vascular endothelium and promote vessel formation by budding; at the same time, it enhances the activity of other growth factors through the degradation of HS so as to speed up the formation of blood vessels (Uno et al., 2001). On the one hand, HIF-1α is a hypoxia inducible factor, which plays an important role in the promotion of tumor angiogenesis and invasion and metastasis of tumor cells. On the one hand, HIF-1α promotes the proliferation and angiogenesis of cells by elevating expression of vascular endothelial growth factor (VEGF) (Liu et al., 2015, Bakirtzi et al., 2014); on the other hand, hypoxia induced by HIF-1α reduces the activity of angiogenesis inhibiting factor so as to provide an appropriate environment for angiogenesis (Ruan et al., 2009). Similarly, uPA and urokinase plasminogen activator receptor (uPAR), through stimulating the migration of vascular smooth muscle cell (Kiyan et al., 2009), degrade extracellular matrix and microvascular basement membrane of adjacent tissues, clear a variety of obstacles for the migration of endothelial cells within a certain range, and provide suitable microenvironment for the formation of new blood vessels, thereby promoting the proliferation of tumor endothelial cells. PTEN is a tumor suppressor gene found and confirmed in 1997 (Li and Ross, 2007), and its inhibition effect on angiogenesis manifests as follows: PTEN dephosphorylates the PIP3 on cell membrane and generate PIP2, and it antagonizes formation of PIP3-mediated proangiogenic pathways (Kuramochi et al., 2016). VEGF is the first protein found to stimulate tumor angiogenesis and also now the unique growth factor that works on vascular endothelial cells only, and it is directly involved in the induction of tumor angiogenesis and also enhances vascular permeability (Hanrahan et al., 2003).
5. Conclusions
Through analysis of regulatory network linked to Hpa gene in the process of invasion and metastasis of colon cancer, this study not only facilitates deep understanding of onset and development of colon cancer but also lays theoretical foundation and provides direction for further researches on colon cancer. Furthermore, it offers evidence for prevention, diagnosis, and treatment of colon cancer.
Acknowledgments
The authors acknowledge the financial support from the Scientific and Technological Development Program in year 2016 in Henan Province China (Grant: 162102310228).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bakirtzi K., West G., Fiocchi C., Law I.K., Iliopoulos D., Pothoulakis C. The neurotensin-HIF-1α-VEGFα axis orchestrates hypoxia, colonic inflammation, and intestinal angiogenesis. Am. J. Pathol. 2014;184:3405–3414. doi: 10.1016/j.ajpath.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazeby J.M., Soulsby M., Winstone K., King P.M., Bulley S., Kennedy R.H. A qualitative evaluation of patients’ experiences of an enhanced recovery programme for colorectal cancer. Colorectal Dis. 2010;12(10 Online):e236–e242. doi: 10.1111/j.1463-1318.2009.02104.x. [DOI] [PubMed] [Google Scholar]
- Del Pulgar T.G., Bandrés E., Espina C. Differential expression of Rac1 identifies its target genes and its contribution to progression of colorectal cancer. Int. J. Biochem. Cell Biol. 2007;39:2289–2302. doi: 10.1016/j.biocel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Doviner V., Maly B., Kaplan V., Gingis-Velitski S., Ilan N., Vlodavsky I., Sherman Y. Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence. Mod. Pathol. 2006;19:878–888. doi: 10.1038/modpathol.3800603. [DOI] [PubMed] [Google Scholar]
- El-Assal O.N., Yamanoi A., Ono T., Kohno H., Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expression in hepatocellular carcinoma. Clin. Cancer Res. 2001;7:1299–1305. [PubMed] [Google Scholar]
- Elkin M., Ilan N., Ishai-Michaeli R., Friedmann Y., Papo O., Pecker I., Vlodavsky I. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- Fidler I.J., Ellis L.M. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Friedmann Y., Vlodavsky I., Aingorn H., Aviv A., Peretz T., Pecker I., Pappo O. Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Evidence for its role in colonic tumorigenesis. Am. J. Pathol. 2000;157:1167–1175. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmidt O., Zcharia E., Abramovitch R., Metzger S., Aingorn H., Friedmann Y., Schirrmacher V., Mitrani E., Vlodavsky I. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. PNAS. 2002;99:10031–10036. doi: 10.1073/pnas.152070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan V., Currie M.J., Gunningham S.P., Morrin H.R., Scott P.A., Robinson B.A., Fox S.B. The angiogenic switch for Vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J. Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- Jiang F., Cui D.W., Su S.Y., Yuan Y., Wang L., Lü C. The expression of HPA in nasopharyngeal carcinoma and its clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;22:21–23. [PubMed] [Google Scholar]
- Kiyan J., Haller H., Dumler I. The tyrosine phosphatase SHP-2 controls urokinase-dependent signaling and functions in human vascular smooth muscle cells. Exp. Cell Res. 2009;315:1029–1039. doi: 10.1016/j.yexcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Koliopanos A., Friess H., Kleeff J., Shi X., Liao Q., Pecker I., Vlodavsky I., Zimmermann A., Büchler M.W. Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res. 2001;61:4655–4659. [PubMed] [Google Scholar]
- Kuramochi H., Nakamura A., Nakajima G., Kaneko Y., Araida T., Yamamoto M., Hayashi K. PTEN mRNA expression is less pronounced in left- than right-sided colon cancer: a retrospective observational study. BMC Cancer. 2016;13:366. doi: 10.1186/s12885-016-2400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ross A.H. Why is PTEN an important tumor suppressor? J. Cell. Biochem. 2007;102:1368–1372. doi: 10.1002/jcb.21593. [DOI] [PubMed] [Google Scholar]
- Liu M., Du K., Fu Z., Zhang S., Wu X. Hypoxia-inducible factor 1-alphaup-regulates the expression of phospholipase D2 in colon cancer cells under hypoxic conditions. Med. Oncol. 2015;32:394. doi: 10.1007/s12032-014-0394-9. [DOI] [PubMed] [Google Scholar]
- Ruan K., Song G., Ouyang G. Role of hypoxia in the hallmarks of human cancer. J. Cell Biochem. 2009;107:1053–1062. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Konno H., Baba S., Kanai T., Matsumoto K., Matsuda I., Ohba K., Ohta M., Kamiya K., Nakamura S. Prevention of hepatic and peritoneal metastases by the angiogenesis inhibitor FR-118487 after removal of growing tumor in mice. Jpn. J. Cancer Res. 2001;92:88–94. doi: 10.1111/j.1349-7006.2001.tb01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno F., Fujiwara T., Takata Y., Ohtani S., Katsuda K., Takaoka M., Ohkawa T., Naomoto Y., Nakajima M., Tanaka N. Anti sense-mediated suppression of human heparanase gene expression inhibits pleural dissemination of human cancer cells. Cancer Res. 2001;61:7855–7860. [PubMed] [Google Scholar]
- Vaday G.G., Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Z.N., Zhang X., Xu H.M., Jiang L., Luo Y., Xing L.L., Xu M.D., Li J. Inhibitory effect of siRNA on heparanase expression and invasion ability of gastric cancer cells: an in vitro experiment. Zhonghua Yi Xue Za Zhi. 2007;87:1717–1720. [PubMed] [Google Scholar]