Abstract
This prospective cohort study aimed at identifying association between uric acid (UA) and peripheral arterial stiffness. A prospective cohort longitudinal study was performed according to an average of 4.8 years’ follow-up. The demographic data, anthropometric parameters, peripheral arterial stiffness (carotid-radial pulse-wave velocity, cr-PWV) and biomarker variables including UA were examined at both baseline and follow-up. Pearson’s correlations were used to identify the associations between UA and peripheral arterial stiffness. Further logistic regressions were employed to determine the associations between UA and arterial stiffness. At the end of follow-up, 1447 subjects were included in the analyses. At baseline, cr-PWV (r = 0.200, p < 0.001) was closely associated with UA. Furthermore, the follow-up cr-PWV (r = 0.145, p < 0.001) was also strongly correlated to baseline UA in Pearson’s correlation analysis. Multiple regressions also indicated the association between follow-up cr-PWV (β = 0.493, p = 0.013) and baseline UA level. Logistic regressions revealed that higher baseline UA level was an independent predictor of arterial stiffness severity assessed by cr-PWV at follow-up cross-section. Peripheral arterial stiffness is closely associated with higher baseline UA level. Furthermore, a higher baseline UA level is an independent risk factor and predictor for peripheral arterial stiffness.
Keywords: Peripheral arterial stiffness; Uric acid, risk factor; Community-based; Follow-up
Abbreviations: BMI, body mass index; Cr, creatinine; cr-PWV, carotid-radial PWV; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; OR, odds ratio; PWV, pulse-wave velocity; SBP, systolic blood pressure; UA, uric acid
1. Introduction
Hyperuricemia, a common clinical situation, has been demonstrated as an independent risk factor for cardiovascular diseases including arterial stiffening, atherosclerosis and hypertension (Chu et al., 2000, Iwashima et al., 2006, Katsiki et al., 2015). The abnormality of uric acid (UA) has also been indicated to be associated with regional arterial stiffness in patients with chronic kidney disease and diabetes mellitus (DM) (Iwashima et al., 2006, Alderman, 2007, Santos, 2012, Zhao et al., 2013). The relationship between normal serum UA and arterial stiffness has also been well documented previously (Lin et al., 2012, Shin et al., 2012). Regarding the mechanism underlying arterial stiffness in which UA participates in, it is involved in thickening vessel wall (intima-media) via proliferation and differentiation of smooth muscle cells as well as dysfunction of endothelial cells (Bian et al., 2012, Elsurer and Afsar, 2014, Ishizaka et al., 2007, Zhang et al., 2014).
It is also demonstrated that arterial stiffness is a risk factor for or pre-pathophysiological processes of various cardiovascular and cerebra-vascular diseases (Sun, 2015). Presently, the pulse wave velocity (PWV) has been used as a reproducible and valid non-invasive gold standard indicator in the assessments of arterial stiffness (Covic and Siriopol, 2015, Laurent and Boutouyrie, 2007, Liu, 2013). Nevertheless, PWVs from different arteries usually represent stiffness in distinct regions in vasculature system (Jadhav and Kadam, 2005, Weber et al., 2015), such as carotid-radial PWV (cr-PWV) which is applied to assess stiffness in arterioles (Hughes et al., 2004).
Inconsistent results on associations between serum UA level and arterial stiffness have been reported before (Elsurer and Afsar, 2014, Mallamaci et al., 2015, Zhao et al., 2013). However, majority of them were performed primarily based on a basic disease such as DM, hypertension and chronic kidney disease (Elsurer and Afsar, 2014, Zhang et al., 2014) among various ethnic groups (Ishizaka et al., 2007, Liang et al., 2012, Lim et al., 2010, Kristina and Gooyers, 2016). However, there were few follow-up studies that have been performed to identify the roles of baseline level of UA in peripheral arterial stiffness. Thus, we postulated that the higher UA level may also play a critical role in increasing peripheral arterial stiffness and performed this follow-up observational study aiming at identifying the associations between UA level and peripheral stiffness evaluated by cr-PWV, to provide novel index for stratification and risk management of arterial stiffness.
2. Material and methods
2.1. Participants and procedures
A total of 1680 health check participants were recruited between September 2007 and January 2009 from Pingguoyuan area, the Shijingshan district in this community-based follow-up cohort study according to the inclusion and exclusion criteria. The exclusion criteria were listed as following: endocrine and metabolic diseases (except DM), infection, neoplastic or severe liver or renal diseases. The inclusion criteria were as follows: residents who received a routine health examination in the community.
2.2. Follow-up and outcome assessment
Our study was reviewed and approved by the ethics committee at People’s Liberation Army General Hospital. The study was thoroughly explained to all of the subjects who agreed to participate, and all of the subjects signed informed consent forms before their examinations.
The participants were followed up for cardiovascular diseases mortality, all-cause mortality, and the development of DM from the initial screening to September 30, 2013. After a median of 4.8 years’ follow-up for 1680 subjects, 181 participants were lost for follow-up and excluded from analysis. Therefore, 1499 subjects (follow-up rate 89.2%) finished the follow-up and fifty-two of which were excluded because of death. In the final data analysis, 1447 participants were included.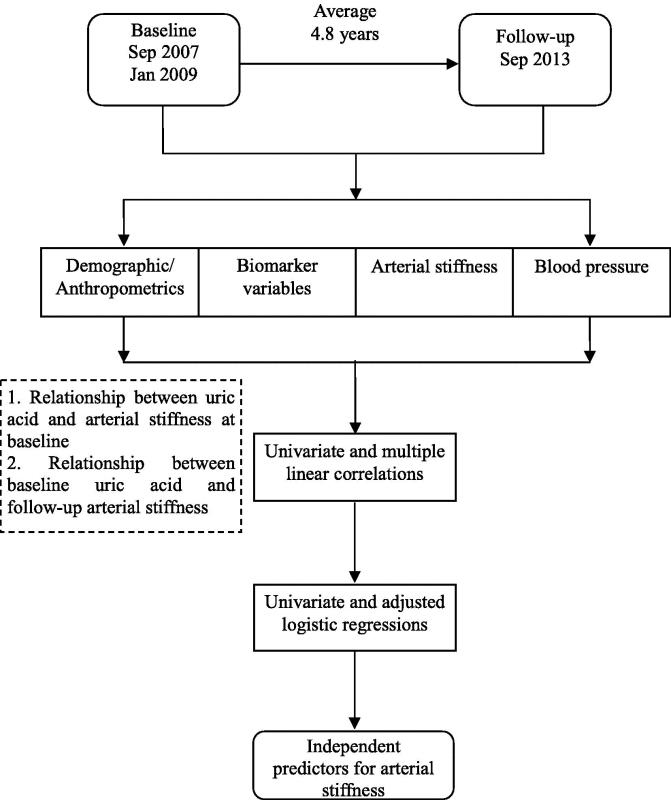
2.3. Clinical data collection
The lifestyle factors, prevalent diseases, demographic information, anthropometrics, family history and medication use were recorded using a standardized self-reported questionnaire in our following-up study. Smoking status and alcohol use was categorized as current, former, or never drinking/smoking, respectively. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were also examined on the right arm in a sitting position after a rest of five minutes.
2.4. Biomarker variable determination
Between 8 am and 10 am after an overnight fast (at least 12 h), the venous blood samples were obtained from all participants. Plasma aliquots were obtained and stored at −80 °C for further study. Concentrations of plasma UA, total cholesterol (TC), triglyceride (TG), fasting blood glucose (FBG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) concentrations were measured from venous blood samples using commercially available ELISA kits by Roche enzymatic assays (Roche Diagnostics GmbH, Mannheim, Germany). Concentration of plasma creatinine (Cr) was also measured by enzymatic assay (Roche Diagnostics GmbH) on a Hitachi 7600 autoanalyzer (Hitachi, Tokyo, Japan).
The biochemical variables were measured from the blood specimens in the Departments of Clinical Laboratory, Chinese PLA General Hospital, following the criteria of the World Health Organization Lipid Reference Laboratories.
2.5. Assessments of arterial stiffness
Caffeine, smoking and alcohol were avoided before the assessment for at least 12 h. Arterial stiffness was assessed by automatic cr-PWV measurements using a Complior SP device (Createch Industrie, France) after a 5–10 min rest in supine position in the morning, in a quiet environment, and at a stable temperature. PWV along the artery was measured with two strain-gauge transducers in the noninvasive procedure using the TY-306 Fukuda pressure-sensitive transducer (Fukuda Denshi Co, Japan) that is fixed transcutaneously over the course of a pair of arteries separated by a known distance on the carotid and radial arteries (both on the right side). Two transducers were used: one positioned at the base of the neck over the common carotid artery and the other over the radial artery. Cr-PWV were obtained simultaneously. The measurement was repeated over 10 different cardiac cycles. PWV was calculated from the measurement of the pulse transit time and the distance traveled by the pulse between the two recording sites: PWV (m/s) = distance (m)/transit time (s) (O'Rourke et al., 2002).
2.6. Definition of variables
Smoking status was defined as smoking 1 or more cigarettes per day for at least 1 year. Body mass index (BMI) was defined as weight (kilograms) divided by square of height (meters). DM was defined as a fasting glucose ⩾7.0 mmol/L, glucose ⩾11.1 mmol/L at two hours after an oral 75 g glucose challenge, or both, or use of antihyperglycemic medication. Alcohol users were defined as drinking once a week (white spirit or beer or red wine).
2.7. Statistical analysis
Normally distributed baseline continuous variables are expressed as mean ± standard deviation (SD) and analyzed with Student’s t-tests while the baseline dichotomous variables are presented as numbers (percentages) and compared using chi-square test. Non-normally distributed variables such as UA levels and other biomarkers were normalized by natural logarithm transformation as necessary.
A Pearson’s regression analysis, a stepwise multivariate linear regression analysis and a multicollinearity analysis were performed to evaluate the associations between UA (natural logarithm transformed) level and arterial stiffness (cr-PWV) and other parameters at both the baseline and the end of the 4.8-year follow-up. Plasma UA levels at baseline were categorized as Quartile 1 (⩽238.95 mmol/L, n = 370), Quartile 2 (239–284.60 mmol/L, n = 352), Quartile 3 (284.61–341.85 mmol/L, n = 372), and Quartile 4 (⩾341.90 mmol/L, n = 353).
Further analysis by a stepwise multivariate logistic regression analysis was performed to identify the risk of UA (baseline) on the follow-up arterial stiffness (cr-PWV ⩾8.76 m/s vs. cr-PWV <8.76 m/s) (Lin et al., 2012). Regression models were adjusted for age and sex as the independent variable (Model 1) and additionally adjusted for alcohol use (g/day), smoking, DM, LDL-C, TG, SBP, TC, DBP, HDL-C and Cr as the independent variables (Model 2).
All analyses were performed using SPSS 19.0 for Windows (SPSS, Chicago, IL, USA). P values <0.05 were considered as statistically significant. Statisticians from the People’s Liberation Army General Hospital were consulted for all of the statistical methods and results.
3. Results
3.1. Baseline clinical characteristics
The mean age of the subjects was 61.40 ± 11.4 years and 59.98% were women. The baseline clinical characteristics were categorized into four groups by quartiles of UA. The cr-PWV were significantly higher in Quartiles 2, 3 and 4 than in Quartile 1 at baseline (all p < 0.05, Table 1).
Table 1.
Characteristics of the subjects categorized by Uric acid levels at baseline.
| Variable | Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|---|
| ⩽238.95 | 239–284.60 | 284.61–341.85 | ⩾341.90 | ||
| No. of subjects | 1447 | 370 | 352 | 372 | 353 |
| Age (years) | 61.40 ± 11.4 | 57.13 ± 10.87 | 61.64 ± 11.59* | 61.68 ± 9.69* | 66.16 ± 8.73* |
| Women [n (%)] | 868(59.98) | 310(83.78) | 252(71.59)* | 223(59.94) * | 83(23.51) * |
| Current smoking [n (%)] | 380(26.26) | 81(21.89) | 55(15.62) ** | 96(25.80) | 148(42.92) * |
| Current alcohol use [n (%)] | 274(18.93) | 63(17.02) | 49(13.92) | 75(20.16) | 87(24.64) ** |
| BMI(kg/m2) | 25.41 ± 3.32 | 24.72 ± 3.52 | 26.41 ± 3.31* | 25.68 ± 2.93* | 25.37 ± 3.43* |
| TG (mmol/l) | 1.90 ± 1.24 | 1.53 ± 0.93 | 2.23 ± 1.58* | 1.87 ± 1.17* | 1.88 ± 1.07 |
| TC (mmol/l) | 5.03 ± 0.93 | 4.88 ± 0.93 | 4.94 ± 0.96 | 5.06 ± 0.88** | 4.91 ± 0.99 |
| HDL-C (mmol/l) | 1.38 ± 0.36 | 1.51 ± 0.42 | 1.25 ± 0.33* | 1.34 ± 0.31* | 1.36 ± 0.41 |
| LDL-C (mmol/l) | 2.91 ± 0.71 | 2.83 ± 0.69 | 2.89 ± 0.74 | 3.02 ± 0.69* | 2.89 ± 0.72 |
| SBP (mmHg) | 128.74 ± 17.71 | 125.93 ± 17.32 | 133.17 ± 18.86* | 129.58 ± 16.34** | 136.79 ± 19.65* |
| DBP (mmHg) | 76.92 ± 10.23 | 75.37 ± 9.93 | 78.80 ± 10.51* | 76.89 ± 9.98 | 76.93 ± 11.34 |
| FBG (mmol/L) | 5.39 ± 1.65 | 5.69 ± 2.13 | 5.38 ± 1.29 | 5.18 ± 1.09* | 5.73 ± 1.79** |
| Cr (μmoI/L) | 66.14 ± 18.16 | 57.24 ± 13.22 | 76.57 ± 16.69* | 67.49 ± 14.94* | 71.16 ± 19.70* |
| cr-PWV (m/s) | 9.38 ± 1.47 | 9.11 ± 1.41 | 9.71 ± 1.57* | 9.40 ± 1.44** | 9.72 ± 1.61* |
Notes: Characteristics are reported as percentages for categorical variables and means (±SD) or medians (with interquartile range) for continuous variables. The study participants were divided into four groups based on the baseline levels of the quartile of Uric acid (⩽238.95, 239–284.60, 284.61–341.85, ⩾341.90 mmol/L). Categorical variables are presented as counts (percentages). The values outside the parentheses are the number of subjects, and the values inside the parentheses are prevalence. The quartile 1 level of Uric acid was used as the reference and quartiles 2, 3, 4 evaluated vs. quartile 1.
<0.05 vs. Quartile 1,
<0.01 vs. Quartile 1.
3.2. Associations between peripheral arterial stiffness and UA level at baseline
At the baseline, peripheral artery stiffness index, cr-PWV was strongly related to UA level (r = 0.200, p < 0.001) in the Pearson’s analysis. In addition, cr-PWV was also closely correlated with DBP, SBP, Cr and TG in univariate linear analysis, while it negatively associated with HDL-C (Table 2).
Table 2.
Univariate and stepwise multiple linear regression analyses at baseline.
| Pearson correlation |
Multiple linear correlation |
||||
|---|---|---|---|---|---|
| r | P | β | 95%CI | P | |
| cr-PWV | |||||
| UA# | 0.200 | <0.001** | 0.284 | −0.061–0.629 | 0.107 |
| Age | −0.027 | 0.337 | 0.049 | 0.037–0.062 | <0.001** |
| SBP | 0.178 | <0.001** | 0.003 | 0.003–0.009 | 0.271 |
| DBP | 0.241 | <0.001** | 0.020 | 0.010–0.031 | <0.001** |
| LDL-C | 0.002 | 0.942 | 0.003 | −0.234–0.241 | 0.980 |
| HDL-C | −0.091 | 0.001** | 0.207 | −0.091–0.505 | 0.173 |
| Cr | 0.201 | <0.001** | 0.058 | 0.048–0.068 | <0.001** |
| TC | 0.028 | 0.321 | 0.022 | −0.175–0.220 | 0.824 |
| TG | 0.102 | <0.001** | 0.201 | 0.010–0.393 | 0.039* |
Natural logarithm transformed.
<0.05;
<0.01.
However, in multivariate analysis, cr-PWV was uncorrelated to UA level (β = 0.284, p = 0.107) which may be adjusted by other variates such as age, DBP, Cr and TG in multivariate linear analysis (Table 2).
3.3. Follow-up cr-PWV was tightly associated with baseline UA level
Pearson’s correlation analyses were employed to identify the association between follow-up peripheral arterial stiffness and baseline UA. As shown in Table 3, both univariate and multivariate linear regression analyses revealed statistically significant association between cr-PWV and UA (r = 0.145, p < 0.001 and β = 0.493, p = 0.013). Furthermore, the significant associations between follow-up cr-PWV and baseline parameters such as age, blood pressure, HDL-C (r = −0.061, p = 0.028), Cr (r = 0.132, p < 0.001) and TG (r = 0.085, p = 0.002) were also present in univariate analysis.
Table 3.
Univariate and multiple linear regression analysis of baseline parameters and follow-up cr-PWV.
| Pearson correlation |
Multiple linear correlation |
||||
|---|---|---|---|---|---|
| r | P | β | 95%CI | P | |
| cr-PWV | |||||
| UA# | 0.145 | <0.001** | 0.493 | 0.106–0.880 | 0.013* |
| Age | 0.163 | <0.001** | 0.000 | −0.014–0.014 | 0.968 |
| SBP | 0.108 | <0.001** | 0.005 | −0.002–0.012 | 0.136 |
| DBP | 0.205 | <0.001** | 0.016 | 0.004–0.027 | 0.009** |
| LDL-C | 0.028 | 0.314 | 0.083 | −0.183–0.349 | 0.541 |
| HDL-C | −0.061 | 0.028 | 0.390 | 0.056–0.724 | 0.022* |
| Cr | 0.132 | <0.001** | 0.031 | 0.020–0.042 | <0.001** |
| TC | −0.036 | 0.202 | −0.197 | −0.419–0.024 | 0.081 |
| TG | 0.085 | 0.002** | 0.312 | 0.097–0.527 | 0.004** |
Natural logarithm transformed.
<0.05;
<0.01.
3.4. Logistic regressions for UA and arterial stiffness
Logistic regression using univariate UA for peripheral artery stiffness measured as cr-PWV indicated only Quartile 4 were risk for peripheral artery stiffening (OR: 1.260; 95%CI: 1.000–1.588; p = 0.050). In the Model 2 for cr-PWV, Quartile 3 UA range revealed significantly risk for peripheral artery stiffening (OR: 1.781; 95%CI: 1.156–2.744; p = 0.009) (Table 4). Furthermore, the UA level in the Quartile 4 showed significant risk for peripheral artery stiffening (OR: 1.259; 95%CI: 0.942–1.681; p = 0.019).
Table 4.
Logistic regression analysis for baseline levels of uric acid and follow-up cf-PWV.
| Quartile 2 vs. Quartile 1 239–284.60 vs ⩽238.95 |
Quartile 3 vs. Quartile 1 284.61–341.85 vs ⩽238.95 |
Quartile 4 vs. Quartile 1 ⩾341.9 vs. ⩽238.95 |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| cr-PWV | ||||||
| Unadjusted | 0.645(0.264–1.579) | 0.337 | 1.390(0.980–1.970) | 0.065 | 1.260(1.000–1.588) | 0.050* |
| Model 1 | 0.534(0.207–1.379) | 0.195 | 1.515(1.058–2.171) | 0.023* | 1.322(1.045–1.672) | 0.020* |
| Model 2 | 0.453(0.005–1.241) | 0.123 | 1.781(1.156–2.744) | 0.009** | 1.259(0.942–1.681) | 0.019* |
Notes: Data were presented as ORs (per SD increase in uric acid level) and corresponding 95% CIs. In the logistic regression model, cr-PWV (PWV ⩾ 8.76m/s vs. PWV < 8.76 m/s) were treated as the dependent variable.
Model 1: adjusted for age, gender; Model 2: adjusted for age, gender, DM, current smoking, MetS, DBP, SBP and levels of TC, TG, HDL-C, LDL-C and Cr.
: p < 0.05.
3.5. Subgroups analyses in MetS and non-MetS
We performed the subgroup analyses to identify the affection of MetS on arterial stiffness. However, cr-PWV showed no correlation with UA in the MetS subgroup (all p > 0.05). Interestingly, in the non-MetS group, UA was associated with cr-PWV in both linear analyses (r = 0.190, p < 0.001 and β = 0.561, p = 0.017). Association’s analyses for subgroups are displayed in Table 5.
Table 5.
Subgroups analyses by linear regressions in baseline parameters and follow-up arterial stiffness.
| MetS |
Non-MetS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation |
Multiple linear correlation |
Pearson correlation |
Multiple linear correlation |
|||||||
| r | P | β | 95%CI | P | r | P | β | 95%CI | P | |
| cr-PWV | ||||||||||
| UA# | 0.059 | 0.274 | 0.271 | −0.394–0.935 | 0.424 | 0.190 | <0.001** | 0.561 | 0.099–1.024 | 0.017* |
| Age | −0.215 | <0.001** | 0.002 | −0.025–0.029 | 0.869 | −0.150 | <0.001** | 0.002 | −0.014–0.018 | 0.772 |
| SBP | 0.112 | 0.025* | 0.007 | −0.003–0.017 | 0.170 | 0.110 | 0.001** | 0.007 | −0.002–0.016 | 0.133 |
| DBP | 0.238 | <0.001** | 0.016 | −0.002–0.034 | 0.074 | 0.208 | <0.001** | 0.015 | 0.000–0.030 | 0.045 |
| LDL-C | −0.060 | 0.237 | 0.478 | 0.009–0.947 | 0.046* | −0.067 | 0.040* | −0.095 | −0.421–0.232 | 0.570 |
| HDL-C | 0.027 | 0.591 | 1.160 | 0.418–1.901 | 0.002** | −0.049 | 0.128 | 0.227 | −0.147–0.600 | 0.235 |
| Cr | 0.057 | 0.261 | 0.029 | 0.009–0.049 | 0.005** | 0.157 | <0.001** | 0.032 | 0.019–0.046 | <0.001** |
| TC | −0.058 | 0.249 | −0.590 | −1.005 to −0.174 | 0.006** | −0.027 | 0.409 | −0.071 | −0.336–0.195 | 0.601 |
| TG | 0.055 | 0.277 | 0.581 | 0.134–1.027 | 0.011* | 0.114 | <0.001** | 0.369 | 0.108–0.629 | <0.001* |
Natural logarithm transformed.
<0.05;
<0.01.
Regarding the prediction roles of UA in MetS group, logistic regressions revealed no predictive values for arterial stiffening. Importantly, in the non-MetS subgroup, UA (Quartile 4 vs. Quartile 1) was an independent risk factor or predictor for arterial stiffening reflected by cr-PWV in Model 2 which was adjusted by age, gender, hypertension, DM, current smoking, DBP, SBP and levels of serum TC, TG, HDL-C, LDL-C and Cr (Table 6).
Table 6.
Logistic regressions for cr-PWV in the subgroups.
| MetS | Non-MetS | |||
|---|---|---|---|---|
| Quartile 4 vs. Quartile 1 |
Quartile 4 vs. Quartile 1 |
|||
| OR (95%CI) | p | OR (95%CI) | p | |
| cr-PWV | ||||
| Unadjusted | 1.441(0.637–3.259) | 0.380 | 0.909(0.633–1.305) | 0.604 |
| Model1 | 1.370(0.491–3.828) | 0.548 | 1.117(0.775–1.653) | 0.581 |
| Model2 | 1.252(0.469–3.345) | 0.654 | 1.034(0.661–1.619) | 0.043 |
In the logistic regression model, cr-PWV (PWV ⩾ 8.76 m/s vs. PWV < 8.76 m/s) were treated as the dependent variable.
Model 1: adjusted for age, gender; Model 2: adjusted for age, gender, DM, current smoking, DBP, SBP and levels of TC, TG, HDL-C, LDL-C and Cr.
*: <0.05; **: <0.01.
4. Discussion
In the current 4.8 years of follow-up study, we found that UA level was associated with cr-PWV. However, only Quartile 3 and 4 levels of baseline UA were independent predictors for follow-up peripheral arterial stiffness. Furthermore, UA predicts arterial stiffness in a MetS independent way.
4.1. UA and its distribution in the health check up population
UA as the end-product of purine nucleotide metabolism participates in many pathophysiological processes in vascular diseases (Sabio et al., 2010). Over the past decades, hyperuricemia has been indicated to be a risk factor for cardiovascular and cerebrovascular diseases such as atherosclerosis and hypertension (Katsiki et al., 2015, Sabio et al., 2010). However, the role of UA in its normal physiological range, such as upper level, was remained elusive.
In our present study, we did observe that the higher UA levels were accompanied with higher cr-PWV, indicating that higher range of UA may contribute to peripheral arterial stiffening. This result was partly in consistent with previous studies (Hsu et al., 2013). Furthermore, individuals with higher levels (such as Quartiles 3 and 4) of UA account for almost half of the health check up population. Thus, the higher normal UA levels should be given proper attention with critical importance. Additionally, our observations that both SBP and DBP were lower in the lowest normal UA level interval (Quartile 1), combining with the results on arterial stiffness mentioned-above may provide clue for further stratifications and managements of cardiovascular diseases risk.
4.2. Peripheral stiffness were associated with UA levels
It has been demonstrated that arterial stiffness occurs in both aortic and peripheral arteries, and the latter one is prior to the former one (Lee and Oh, 2010, Sun, 2015). There are also a number of studies that have indicated that UA was related to arterial stiffening that assessed by brachial-ankle PWV or heart-femoral PWV (Ishizaka et al., 2007, Lim et al., 2010). In our current study, we evaluated peripheral arterial stiffness using cr-PWV. We also found that cr-PWV was associated with baseline UA which is partly similar to the previous results (Liang et al., 2012).
In accordance with many others’ reports, the current study showed that UA was correlated with peripheral stiffening at baseline (Shin et al., 2012, Bian et al., 2012); Homma et al., 2015). These results suggested that the subclinical arterial stiffening may exist in normal healthy persons. Particularly, the association between baseline UA and follow up peripheral arterial stiffness suggests that UA may be used to predict the incident of arterial stiffness which will be discussed in the next section.
Up to date, though various studies have focused on the association between UA and arterial stiffness (Lin et al., 2012, Shin et al., 2012, Hsu et al., 2013, Bae et al., 2013, Cicero et al., 2014), the mechanisms underlying the pathogenesis that UA participates in arterial stiffening have not been clearly uncovered. However, several basic investigations demonstrated prominent roles of UA in arterial stiffness (Iwashima et al., 2006, Bobik and Grassi, 2012, Kanellis et al., 2003, Kang et al., 2005, Luft, 2012).
First of all, UA has been indicated to thick vascular wall via promoting proliferation and differentiation of smooth muscle cells. The biological behaviors of smooth muscle cells mentioned above were triggered by the activated renin-angiotensin system and reactive oxygen species (Bobik and Grassi, 2012, Jae et al., 2012, Jain et al., 2014, Park and Lakatta, 2012). Those results subsequently lead to the reduction in elasticity of artery wall promoting the arterial stiffness. Secondly, numerous studies also conclude that the essential roles of UA in arterial stiffness prior to hypertension process may be attributed to the inflammatory activation (increased levels of C-reactive protein and other pro-inflammatory factors) (Kang et al., 2005). Thirdly, UA has also implicated in endothelial cell dysfunction which plays crucial roles in arterial stiffening (Bellien et al., 2010). It is elucidated that UA participates in arterial stiffness maybe also via the nitric oxide pathway dysfunction, oxidative stress, insulin resistance which result endothelial cell dysfunction (Bellien et al., 2010). Endothelial cell dysfunction further increases proliferation and migration of smooth muscle cells and the rearrangement of artery wall components. Those changes decrease the complaisance and stiffen arteries functionally and structurally. Lastly, it has also been addressed that UA injures arteries chronically through up-regulating the expression of platelet-derived growth factor and monocyte chemotactic protein-1 (Kanellis et al., 2003). In a word, though UA participates in arterial stiffness primarily through the four mechanisms mentioned above, other potential mechanisms still warrant further study.
4.3. Higher baseline UA level was an independent predictor for peripheral arterial stiffness
Further analyses by logistic regressions indicated that higher baseline UA level was an independent risk factor and predictor for central stiffness and peripheral stiffness. In both adjusted models, higher UA levels exhibited risk for arterial stiffness measured by cr-PWV indicating that higher UA level was an independent predictor for peripheral arterial stiffening. In addition, baseline UA level in Quartiles 3 and 4 were still an independent predictor for peripheral arterial stiffening (cr-PWV).
Though others’ cross sectional studies have indicated the risk for arterial stiffness ubiquitously (Lin et al., 2012, Bae et al., 2013), the predictive value of UA for arterial stiffness has not been confirmed in a long time longitudinal study. Thus, we performed this follow up survey and demonstrated that higher normal UA levels were risk factors and predictors for arterial stiffness. Particularly, the UA level ⩾341.90 mmol/L was an independent risk factor and predictor for central artery stiffening (vs. ⩽238.95 mmol/L). Our observations confirm the association between UA and arterial stiffness ((Lin et al., 2012, Cicero et al., 2014) and its predictive value for arterial stiffness which may facilitate us to prevent arterial stiffening.
4.4. UA predicts arterial stiffness in a MetS independent pattern
The association between UA and MetS is debatable (Santos, 2012, Lim et al., 2010, Sun et al., 2013), though a vast amount of previous studies have indicated that UA was closely related to part components of MetS. Furthermore, no positive relationship between UA and MetS independent of other variables was addressed, though it has elucidated that prevalence of MetS increases with UA level positively and hyperuricemia was an independent predictor of MetS incident (Ishizaka et al., 2007). Thus, we performed the subgroup analysis to identify whether the predictive value of UA for peripheral arterial stiffness depended on MetS.
Partly in accordance with the previous studies (Lim et al., 2010, Sun et al., 2013), in MetS subgroup no significant association has been indicated between baseline UA and follow up peripheral arterial stiffness, while, in non-MetS group, UA was significantly associated with cr-PWV in both univariate and multiple linear regressions.
Although UA was partly associated with MetS in the cross-sectional studies (Sun et al., 2013), the association between peripheral arterial stiffness and UA was conducted in the MetS independent pattern. The underlying mechanisms for the interesting observation may be caused by other confounding atherogenic risk factors such as hyperglycemia and hypertension which consists of MetS. These results further indicated the predictive value of plasma UA for peripheral arterial stiffness in healthy populations with great power. Thus, in the non-MetS subjects with higher baseline UA level should be given more attention on the risk for arterial stiffness than in the MetS ones.
4.5. Limitations
The participants in this study were recruited from two districts in Beijing instead of the random sampling from all over the country; the results may not represent the Chinese individuals from other areas. The unavoidable limitation is that a total of 181 subjects (10.7%) were lost to follow-up which may induce bias to the conclusions.
5. Conclusions
Given a higher baseline level of UA in normal range, UA is closely related with peripheral arterial stiffness and it is an independent predictor for peripheral arterial stiffness suggesting that the plasma UA level may be used for stratification and management of risk factors for arterial stiffness. Furthermore, baseline UA predicts peripheral arterial stiffness in a MetS independent pattern.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors' contributions
Ping Ye and Xiao–Han Ding participated the design of this research. Xiao–Han Ding and Xiaona Wang also have drafted the manuscript and performed the statistical analyses. Ping Ye and Xiao–Han Ding have reviewed and revised this manuscript critically for important intellectual content. Xiaona Wang, Ruihua Cao, Xu Yang, Wenkai Xiao, Yun Zhang, Yongyi Bai and Hongmei Wu finished Clinical data collection and the measurements of BP, HR and the biomarker. Xu Yang and Wenkai Xiao carried out the arterial stiffness measurements.
Acknowledgements
This research is supported by the grant from the National Nature Science Foundation of China (81270941), the Key National Basic Research Program of China (2012CB517503, 2013CB530804) to Dr. Ye Ping. We are also grateful to all study participants for their participation in the study. We thank colleagues at the Department of Laboratory Medicine, the PLA General Hospital for help with biochemical measurements.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alderman M.H. Podagra, uric acid, and cardiovascular disease. Circulation. 2007;116:880–883. doi: 10.1161/CIRCULATIONAHA.107.728600. [DOI] [PubMed] [Google Scholar]
- Bae J.S., Shin D.H., Park P.S., Choi B.Y., Kim M.K., Shin M.H., Lee Y.H., Chun B.Y., Kim S.K. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: the Korean Multi-Rural Communities Cohort study. Atherosclerosis. 2013;231:145–151. doi: 10.1016/j.atherosclerosis.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Bellien J., Favre J., Iacob M., Gao J., Thuillez C., Richard V., Joannides R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- Bian S., Guo H., Ye P., Luo L., Wu H., Xiao W. Serum uric Acid level and diverse impacts on regional arterial stiffness and wave reflection. Iran. J. Public Health. 2012;41:33–41. [PMC free article] [PubMed] [Google Scholar]
- Bobik A., Grassi G. Low-grade inflammation and arterial stiffness in the elderly. J. Hypertens. 2012;30:679–681. doi: 10.1097/HJH.0b013e328351b1fd. [DOI] [PubMed] [Google Scholar]
- Chu N.F., Wang D.J., Liou S.H., Shieh S.M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur. J. Epidemiol. 2000;16:13–17. doi: 10.1023/a:1007654507054. [DOI] [PubMed] [Google Scholar]
- Cicero A.F., Salvi P., D'Addato S., Rosticci M., Borghi C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J. Hypertens. 2014;32:57–64. doi: 10.1097/HJH.0b013e328365b916. [DOI] [PubMed] [Google Scholar]
- Covic A., Siriopol D. Pulse wave velocity ratio: the new “gold standard” for measuring arterial stiffness. Hypertension. 2015;65:289–290. doi: 10.1161/HYPERTENSIONAHA.114.04678. [DOI] [PubMed] [Google Scholar]
- Elsurer R., Afsar B. Serum uric acid and arterial stiffness in hypertensive chronic kidney disease patients: sex-specific variations. Blood Press. Monit. 2014;19:271–279. doi: 10.1097/MBP.0000000000000056. [DOI] [PubMed] [Google Scholar]
- Homma S., Kato K., Hayashi J., Yamamoto M. Negative associations between arterial stiffness parameter evaluated by cardio-ankle vascular index and serum low-density lipoprotein cholesterol concentration in early-stage atherosclerosis. Angiology. 2015;66:143–149. doi: 10.1177/0003319713516853. [DOI] [PubMed] [Google Scholar]
- Hsu P.F., Chuang S.Y., Cheng H.M., Sung S.H., Ting C.T., Lakatta E.G., Yin F.C., Chou P., Chen C.H. Associations of serum uric acid levels with arterial wave reflections and central systolic blood pressure. Int. J. Cardiol. 2013;168:2057–2063. doi: 10.1016/j.ijcard.2013.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S.M., Dixon L.J., McVeigh G.E. Arterial stiffness and pulse wave velocity: problems with terminology. Circulation. 2004;109 doi: 10.1161/01.CI.0000111121.03758.12. e3; author reply e3. [DOI] [PubMed] [Google Scholar]
- Ishizaka N., Ishizaka Y., Toda E., Hashimoto H., Nagai R., Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192:131–137. doi: 10.1016/j.atherosclerosis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Iwashima Y., Horio T., Kamide K., Rakugi H., Ogihara T., Kawano Y. Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension. 2006;47:195–202. doi: 10.1161/01.HYP.0000200033.14574.14. [DOI] [PubMed] [Google Scholar]
- Jadhav U.M., Kadam N.N. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005;57:226–232. [PubMed] [Google Scholar]
- Jae S.Y., Heffernan K.S., Yoon E.S., Park S.H., Choi Y.H., Fernhall B., Park J.B. Pulsatile stress, inflammation and change in arterial stiffness. J. Atheroscler. Thromb. 2012;19:1035–1042. doi: 10.5551/jat.13516. [DOI] [PubMed] [Google Scholar]
- Jain S., Khera R., Corrales-Medina V.F., Townsend R.R., Chirinos J.A. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237:381–390. doi: 10.1016/j.atherosclerosis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Kanellis J., Watanabe S., Li J.H., Kang D.H., Li P., Nakagawa T., Wamsley A., Sheikh-Hamad D., Lan H.Y., Feng L., Johnson R.J. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- Kang D.H., Park S.K., Lee I.K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- Katsiki N., Doumas M., Athyros V.G., Karagiannis A. Hyperuricemia as a risk factor for cardiovascular disease. Expert Rev. Cardiovasc. Therapy. 2015;13:19–20. doi: 10.1586/14779072.2015.987129. [DOI] [PubMed] [Google Scholar]
- Kristina G.M., Gooyers C.E. Explorations into the design of mobile police and warning information application system under 4G background. J. Mech. Eng. Res. Dev. 2016;39(1):204–210. [Google Scholar]
- Laurent S., Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206. doi: 10.1161/HYPERTENSIONAHA.106.076166. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Oh B.H. Aging and arterial stiffness. Circ. J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- Liang J., Li Y., Zhou N., Teng F., Zhao J., Zou C., Qi L. Synergistic effects of serum uric acid and cardiometabolic risk factors on early stage atherosclerosis: the cardiometabolic risk in Chinese study. PLoS One. 2012;7:e51101. doi: 10.1371/journal.pone.0051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.H., Kim Y.K., Kim Y.S., Na S.H., Rhee M.Y., Lee M.M. Relationship between serum uric acid levels, metabolic syndrome, and arterial stiffness in korean. Korean Circ. J. 2010;40:314–320. doi: 10.4070/kcj.2010.40.7.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Lai X., Chen G., Xu Y., Huang B., Wu Y., Chen Z., Yao L., Lin F., Qiao Y., Zhu S., Huang H., Wen J. Association among serum uric acid, cardiovascular risk, and arterial stiffness: a cross-sectional study in She ethnic minority group of Fujian Province in China. J. Endocrinol. Invest. 2012;35:290–297. doi: 10.3275/7765. [DOI] [PubMed] [Google Scholar]
- Liu Z. Evaluation on urban developing level by matter-element extension model. J. Appl. Sci. 2013;13(21):4702–4707. [Google Scholar]
- Luft F.C. Molecular mechanisms of arterial stiffness: new insights. J. Am. Soc. Hypertens. 2012;6:436–438. doi: 10.1016/j.jash.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Mallamaci F., Testa A., Leonardis D., Tripepi R., Pisano A., Spoto B., Sanguedolce M.C., Parlongo R.M., Tripepi G., Zoccali C. A genetic marker of uric Acid level, carotid atherosclerosis, and arterial stiffness: a family-based study. Am. J. Kidney Dis. 2015;65:294–302. doi: 10.1053/j.ajkd.2014.07.021. [DOI] [PubMed] [Google Scholar]
- O'Rourke M.F., Staessen J.A., Vlachopoulos C., Duprez D., Plante G.E. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- Park S., Lakatta E.G. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med. J. 2012;53:258–261. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio J.M., Vargas-Hitos J.A., Mediavilla J.D., Navarrete-Navarrete N., Zamora-Posadas M., Perez-Vicente S., Hidalgo-Tenorio C., Diaz-Chamorro A., Jaimez L., Jimenez-Alonso J. Correlation of asymptomatic hyperuricaemia and serum uric acid levels with arterial stiffness in women with systemic lupus erythematosus without clinically evident atherosclerotic cardiovascular disease. Lupus. 2010;19:591–598. doi: 10.1177/0961203309355301. [DOI] [PubMed] [Google Scholar]
- Santos R.D. Elevated uric acid, the metabolic syndrome and cardiovascular disease: cause, consequence, or just a not so innocent bystander? Endocrine. 2012;41:350–352. doi: 10.1007/s12020-012-9657-4. [DOI] [PubMed] [Google Scholar]
- Shin J.Y., Lee H.R., Shim J.Y. Significance of high-normal serum uric acid level as a risk factor for arterial stiffness in healthy Korean men. Vasc. Med. (London, England) 2012;17:37–43. doi: 10.1177/1358863X11434197. [DOI] [PubMed] [Google Scholar]
- Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Zhang Y., Tian J.L., Wang H. Relationship between uric acid and arterial stiffness in the elderly with metabolic syndrome components. Chin. Med. J. 2013;126:3097–3102. [PubMed] [Google Scholar]
- Weber T., Wassertheurer S., Hametner B., Parragh S., Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J. Hypertens. 2015 doi: 10.1097/HJH.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xiang G., Xiang L., Sun H. Serum uric acid is associated with arterial stiffness in men with newly diagnosed type 2 diabetes mellitus. J. Endocrinol. Invest. 2014;3:441–447. doi: 10.1007/s40618-013-0034-9. [DOI] [PubMed] [Google Scholar]
- Zhao G., Huang L., Song M., Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosism. 2013;231:61–68. doi: 10.1016/j.atherosclerosis.2013.08.023. [DOI] [PubMed] [Google Scholar]


