Abstract
Background
Calcium phosphate mediated transfection has been used for delivering DNA into mammalian cells in excess of 30 years due to its most low cost for introducing recombinant DNA into culture cells. However, multiple factors affecting the transfect efficiency are commonly recognized meanwhile for years, the low transfection efficiency of this approach on higher differentiated and non-tumor cells such as CHO and C2C12 limits its application on research.
Results
In this paper, we systematically evaluated the possible factors affecting the transfection rate of this approach. Two categories, calcium phosphate–DNA co-precipitation and on-cell treatments were set for optimization of plasmid DNA transfection into CHO and C2C12 cell-lines. Throughout experimentation of these categories such as buffer system, transfection media and time, glycerol shocking and so on, we optimized the best procedure to obtain the highest efficiency ultimately.
During calcium phosphate DNA-precipitation, the transfection buffer is critical condition optimized with HBS at pH 7.10 (P = 0.013 compared to HEPES in CHO). In the transfection step, FBS is a necessary component in transfection DMEM for high efficiency (P = 0.0005 compared to DMEM alone), and high concentration of co-precipitated particles applied to cultured cells in combination with intermittent vortexing is also crucial to preserve the efficiency. For 6-well culture plates, 800 µl of co-precipitated particles (11.25 µg/mL of cDNA) in 1 well is the optimal (P = 0.007 compared to 200 µl). For the highest transfection efficiency, the most important condition is glycerol in shock treatment (P = 0.002 compared to no shock treatment in CHO, and P = 0.008 compared to no shock treatment in C2C12) after a 6 h incubation (P = 0.004 compared to 16 h in CHO, and P = 0.039 compared to 16 h in C2C12) on cultured cells.
Conclusions
Calcium phosphate mediated transfection is the most low-cost approach to introduce recombinant DNA into culture cells. However, the utility of this procedure is limited in highly-differentiated cells. Here we describe the specific HBS-buffered saline, PH, glycerol shock, vortex strength, transfection medium, and particle concentrations conditions necessary to optimize this transfection method in highly differentiated cells.
Abbreviations: CHO, Chinese hamster ovary cells; C2C12, mouse myoblast cells; PEG, polyethylene glycol; FBS, fetal bovine serum; Pen-Strep, penicillinstreptomycin; IntDen, integrated density
Keywords: Calcium phosphate transfection, Co-precipitation, Transfection efficiency
1. Introduction
Genetically modified organisms, including transgenic plants, animals and cultured cell lines, have gradually becoming the main experimental models in current food science, agriculture and biology research fields (Liu et al., 2016a, Liu et al., 2016b, Zhou et al., 2016). Delivery of DNA and RNA into cultured cells is a fundamental molecular biology transgenic technique. To explore the function of a gene of interest, transient transfection is normally employed to over-express or knock down gene expression from cells in a controlled setting. To conduct these seminal experiments, recombinant DNA need to be introduced into cell without damaging cell viability. There are currently three main techniques to accomplish this mission. First is lipid-mediated liposome delivery, such as lipofectamine series products manufactured by Invitrogen. This approach displays high efficiency for transient transfection in most cultured cells, but is limited by high cost and is not suitable for large quantity delivery (Junquera and Aicart, 2014, Kaestner et al., 2015, Xiong et al., 2011). The second approach is electroporation, which has the highest efficiency method of the three but also causes the highest death rate of cells (Kalli et al., 2014, Li et al., 2013, Nakamura and Funahashi, 2013). The third method of the transient transfection, and focus of this report, is calcium phosphate mediated transient transfection. In this approach, calcium phosphate forms an insoluble precipitate with DNA, this Ca-DNA complex then attaches to the cell surface where it is transported into cells by endocytosis. Due to its reasonable efficiency and low cost, this technique has been used for delivering DNA into mammalian cells for over 30 years (Dudek et al., 2001, Kwon and Firestein, 2013, Sambrook and Russell, 2001, Sun et al., 2013). However, this approach has largely been neglected in favor of the first two methods due to the myriad of factors affecting transfection efficiency in the hands of experimenters and previous belief that this method does not work well in highly differentiated cells (Inokuchi et al., 2009, Mohammad et al., 2008). In this paper, we systematically documented the optimal conditions for the most effective calcium phosphate mediated transfection in highly-differentiated cell lines, Chinese hamster ovary cells (CHO) and mouse myoblast cells (C2C12).
2. Materials and methods
2.1. Cloning and sub-cloning
Four different plasmids were used in this study, EGFP-N1, EGFP-pIRES, EGFP-pIRES-mCnB, and EGFP-pIRES-mCnA. EGFP-N1 is a commercial product from Clontech. mCnB and mCnA genes were amplified from total RNA as previously described (Wang et al., 2008, Yoshiga et al., 2002), and cloned into the EGFP-pIRES plasmid with 5′ Nhe Ⅰ and 3′ Sac Ⅰ restriction sites, which were later used to confirm successful insertion.
Recombined plasmid DNAs were transfected into HB101 competent Escherichia coli. Individual colonies were transferred into Kanamycin (20 µg/ml) LB medium and incubated over night at 37 °C with vigorous shaking (250 rpm on a rotary shaker) until the bacteria reached late log phase. Plasmid DNAs were prepared using alkaline lysis with SDS were purified with polyethylene glycol (PEG, 40% PEG6000, 30 mM MgCl2) and were recovered with deionized distilled H2O or 1 × TE buffer (pH 7.6).
2.2. Cell culture
Cells for transfection were cultured at 37 °C in a humidified incubator with an atmosphere of 5% CO2, in DMEM high glucose medium (pH 7.4, Gibco) supplemented with 10% inactivated fetal bovine serum (10% FBS, Invitrogen), 100 U/ml penicillin and 100 µg/ml streptomycin (1% Pen-Strep, Invitrogen). Chinese hamster ovary (CHO) cells and mouse myoblast (C2C12) cells were two lines used in the study. Cells were transfected with expression plasmids at 50–50% confluence then sub-cultivated at 80%–90% of cell confluence. The calcium phosphate-DNA co-precipitate transient transfection method was used.
2.3. Calcium phosphate mediated transient transfection of CHO and C2C12 cell-lines
In this study, we optimized the procedure of calcium phosphate mediated transient transfection approach using CHO and C2C12 cell-lines (Supplemental Fig. 1 and detailed in Table 1) in 6-wells plates (NUNC). The traditional steps to Calcium phosphate mediated transient transfection are briefly described below:
Table 1.
Two categories optimized for establishment of best conditions are listed as experiment 1–11.
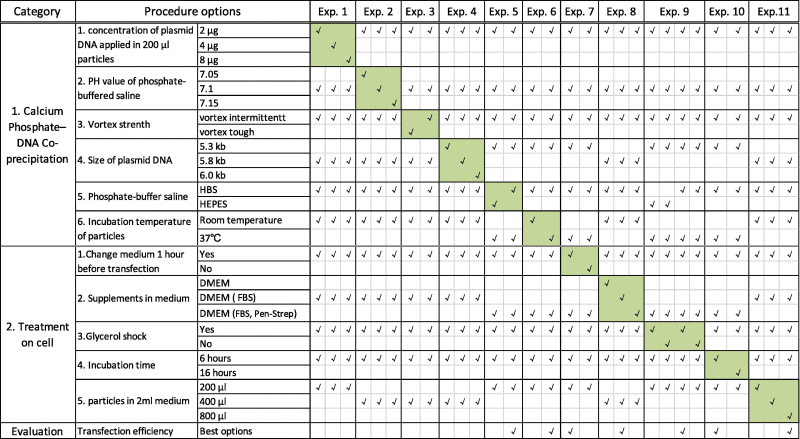 |
Notes: The green areas in table indicate the variable options and optimized options are presented in last row.
2.4. Preparation of calcium phosphate-DNA particle or complex within co-precipitation
The followings solutions were prepared for transfection experiments including CaCl2 (2.5 M); 15% (v/v) glycerol in 1 × HBS-buffered saline; 2 × HEPES-buffered saline (140 mM NaCl, 1.5 mM Na2HPO4, 50 mM HEPES, pH 7.05); and 2x HBS-buffered saline (280 mM NaCl, 1.5 mM Na2HPO4, 50 mM HEPES, 10 mM KCl, 12 mM Dextrose, pH 7.05, 7.10 or 7.15). 10 µl of 2.5 M CaCl2 and 90 µl of plasmid DNA (25 µg/ml) were mixed and added to 100 µl of HBS or HEPES buffered saline (at ratio of 1:1). Mixture was allowed to incubate at room temperature for one mixture. This total 200 µl of calcium phosphate-DNA particles complex was used for transient transfection as described below.
2.5. Calcium phosphate mediated transient transfection
CHO and C2C12 Cells at exponential growth phase were harvested and re-plated at a density of 1–4 × 105 cells/cm2 20–24 h before transfection. Cell media was replaced 1 h before transfection. 200 µl of the calcium phosphate-DNA particles was added to the well drop wise to ensure even addition. The cells were incubated at 37 °C for 6 h before transfection media was replaced with fresh medium. Cells were then culture under standard conditions for 1–3 days for further study.
In Table 1 Experiment 9, the glycerol shock applied to CHO and C2C12 cells was conducted by replacing the medium with 1000 µl of 15% glycerol solution directly after transfection and incubating these cells for 1–2 min at 37 °C. Following PBS wash, the glycerol solution was replaced with fresh medium and cells were incubated for an additional 1–3 days. Transfection efficiency was evaluated under inverted fluorescence microscope 48 h after transfection.
2.6. Microscopy and statistics
Inverted fluorescence microscopy (Carl Zeiss Microscopy GmbH) and manufacturer software ZEN was used to document fluorescent signals of successful transfection. Images were analyzed using NIH Image J software for transfection efficiency. Numbers of fluorescent cells targeted with plasmid DNA and total cells (the fluorescent cells plus non-fluorescent cells) were counted, and the integrated density (IntDen) and area of fluorescent cells were obtained with the Image J software. SPSS was used to generate figures and statistical analysis on the fluorescent cell number / total cell number ratio.
3. Result and discussion
Two categories, calcium phosphate–DNA co-precipitation and on-cell treatments were used to optimize plasmid DNA transfection into CHO and C2C12 cell-lines (Table 1). These two cells lines were selected for their low transfection rates using the calcium phosphate approach.
3.1. Varying preparation of calcium phosphate–DNA co-precipitation
Six variables were selected for optimization in preparation of calcium phosphate-DNA complex (Table 1): (1) concentration of plasmid DNA used for preparation of the calcium phosphate-DNA particle (2 µg/200 µl, 4 µg/200 µl and 8 µg/200 µl); (2) PH of solutions used for complex preparation (pH 7.05, pH 7.10 and pH 7.15); (3) calcium phosphate-DNA particle size (varied by strength of vortex used); (4) Size of plasmid used DNA for transfection; (5) buffer solution types (HBS-buffered and HEPES-buffered saline); and (6) Incubation temperature during calcium phosphate–DNA complex preparation.
Of these variables, the buffer system played a key role on the efficiency of calcium phosphate mediated transfection. HBS-buffered saline delivered better transfection efficiency than HEPES-buffered saline when used in conjunction with glycerol shock, a variable discussed in detail below. The HBS-buffered saline delivered over 100% more transfected cells than HEPES-buffered saline in both CHO and C2C12 cells (Fig. 1, Table 1 Experiment 5). However, glycerol shock is necessary discussed for this improved performance, as discussed in varying treatments on culture cells.
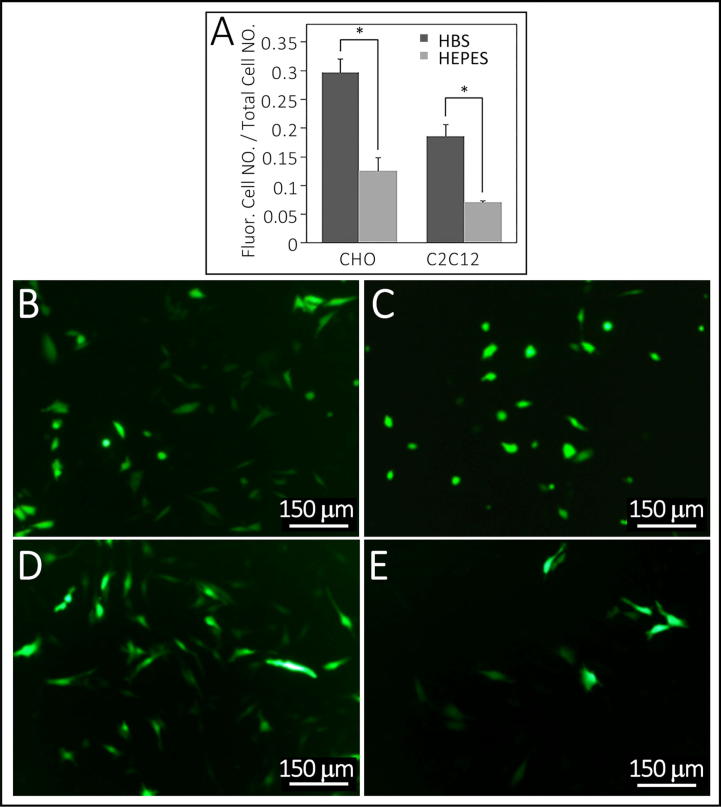
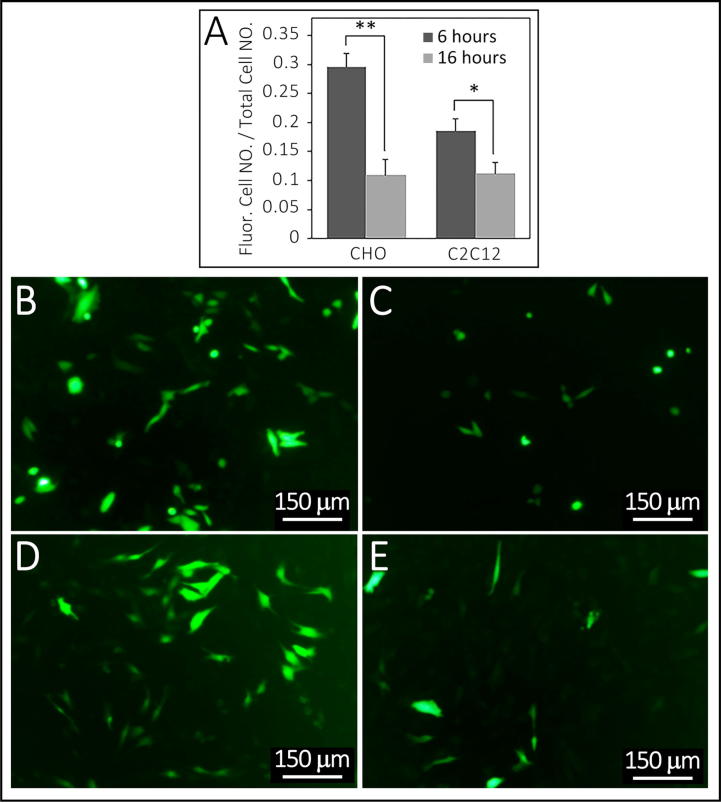
Fig. 1.
Comparison of transfection efficiency between HBS- and HEPES-buffer saline for preparation of particles. Summarized in Table 1, Experiment 5. The efficiency of calcium phosphate–DNA particles preparation with HBS-buffer saline is greater than HEPES-buffer saline in both CHO and C2C12 cell-lines, respectively, F = 0.909 and 0.028, P = 0.013 and 0.025. Corresponding fluorescence images B and C are of CHO cell transfections using particles prepared with HBS and HEPES. Images D and E are of C2C12 cell transfections using particles prepared with HBS and HEPES. *indicates p-value < 0.05. Bars in B-E are 150 µm.
Despite the accepted convention that buffering system pH is critical on transfection [10], our experiments indicated that a pH in the range of 7.05 and 7.10 using the HBS-buffering system is sufficient for optimal transfection. Both pH 7.05 and pH 7.10 induced higher efficiency in the transfection for CHO cells, but a higher pH of 7.15 resulted in lower efficiency (Supplemental Fig. 2, Table 1 Experiment 2). Transfection efficiency was also unaffected plasmid DNA size within the range of 5.3 kb to 6.0 kb (Supplemental Fig. 4, Table 1 Experiment 4). However, transfection efficiency was dependent on vortex strength, indicating that particle size is an important factor. Higher strength vortex generally induced lower efficiency (Supplemental Fig. 3, Table 1 Experiment 3).
Surprisingly, increasing plasmid DNA concentration in the calcium phosphate-DNA particle improves transfection efficiency. In experiment 1, 8 µg plasmids DNA per 200 µl particles displayed the highest efficiency transfection for CHO cells (Supplemental Fig. 5, Table 1 Experiment 1). Incubating temperature during calcium phosphate–DNA particles formation was also important with particles prepared at 37 °C demonstrating higher rate of transfection than those prepared at room temperature (Supplemental Fig. 6, Table 1 Experiment 6).
3.2. Varying treatments on cultured cells
Five variables were established for optimization during cell transfection. As listed in Table 1: (1) medium-change 1-h prior to transfection; (2) FBS/Pen-Strep supplementation in medium; (3) glycerol shock post transfection incubation; (4) incubation time with the transfection particle, and (5) concentration of particles for transfection incubation (200 µl, 400 µl and 800 µl of particles in 2 ml of transfection medium).
Unlike liposome transfection, FBS and Pen-Strep in the transfection media did not disturb transfection efficiency and even improved efficiency given the appropriate conditions. The highest transfection efficiency was achieved when transfection media was supplemented with FBS or FBS plus Pen-Strep in comparison to un-supplemented media. However, this efficiency was depended on glycerol shock (Fig. 2, Table 1 Experiment 8). Increasing calcium phosphate–DNA particles density also improves transfection efficiency (Fig. 3, Table 1).The addition of glycerol shock post-transfection incubation was a significant contributor to transfection efficiency (Fig. 4, Table 1 Experiment 9). Transfection particles incubation time was also an important factor. An incubation time of 6–7 h is critical for efficient transfection and should not exceed 10 h. Over incubation results in reduced efficiency due to cell damage induced by particle-over delivery (Fig. 5, Table 1 Experiment 10).
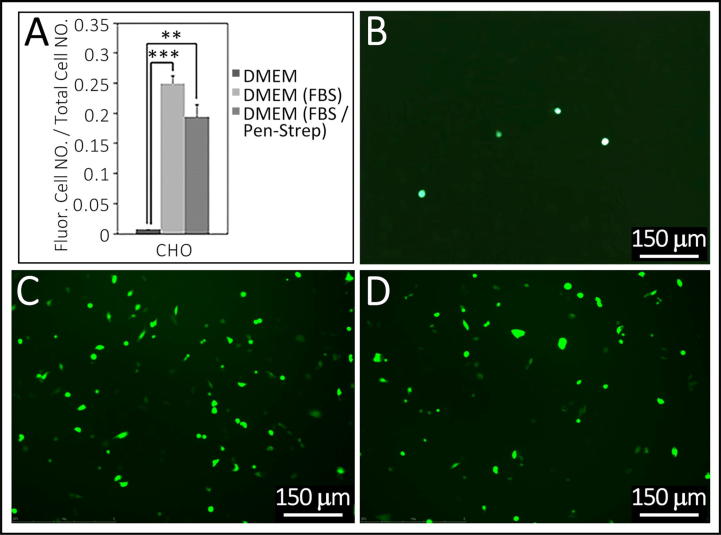
Fig. 2.
Comparison of transfection efficiency between media supplemented with FBS and Pen-Strep to un-supplemented media. Summarized in Table 1, Experiment 8. Panel A, transfection of CHO cell-lines is more efficient in both FBS-DMEM and FBS/Pen-Strep DMEM in comparison to DMEM alone, F = 0.084 and 0.060, P = 0.0005 and 0.004. Fluorescence images showed in B-D. There is no significant difference between FBS-DMEM and FBS/Pen-Strep-DMEM (F = 0.306, P = 0.056). ** indicates p-value < 0.01 and *** indicates P-value < 0.001. Bars in B–D are 150 µm.
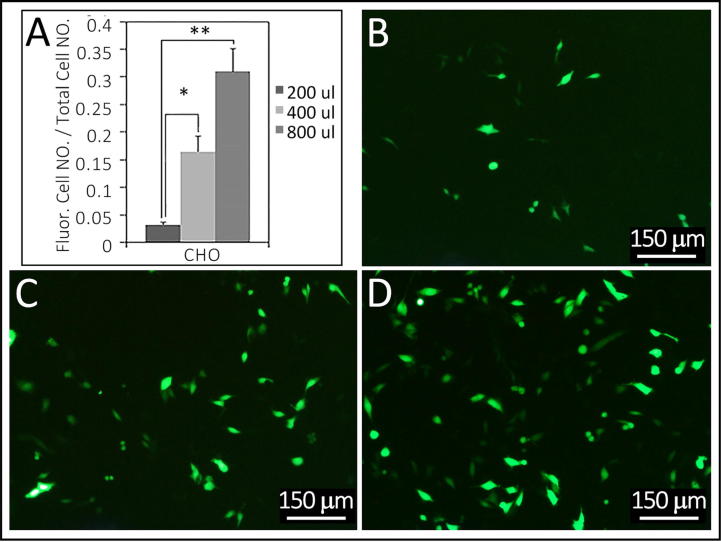
Fig. 3.
Transfection efficiency in relation to particle densities on CHO cells. Summarized in Table 1, Experiment 11. Panel A, transfection efficiency improves as particle density increases in CHO cell-lines. 800 µl-particles are significantly more efficient than 200 µl, F = 0.117, P = 0.007, and 400 µl-particles are significantly more efficient than 200 µl, F = 0.107, P = 0.024. There is no significant difference between 400 µl- and 800 µl-particles (F = 0.579, P = 0.076). Fluorescence images B–D are of transfected CHO cells with 200 µl (B), 400 µl (C) and 800 µl (D) of particles in 2 ml medium. * indicates p-value < 0.05 and ** indicates p-value < 0.01. Bars in B–D are 150 µm.
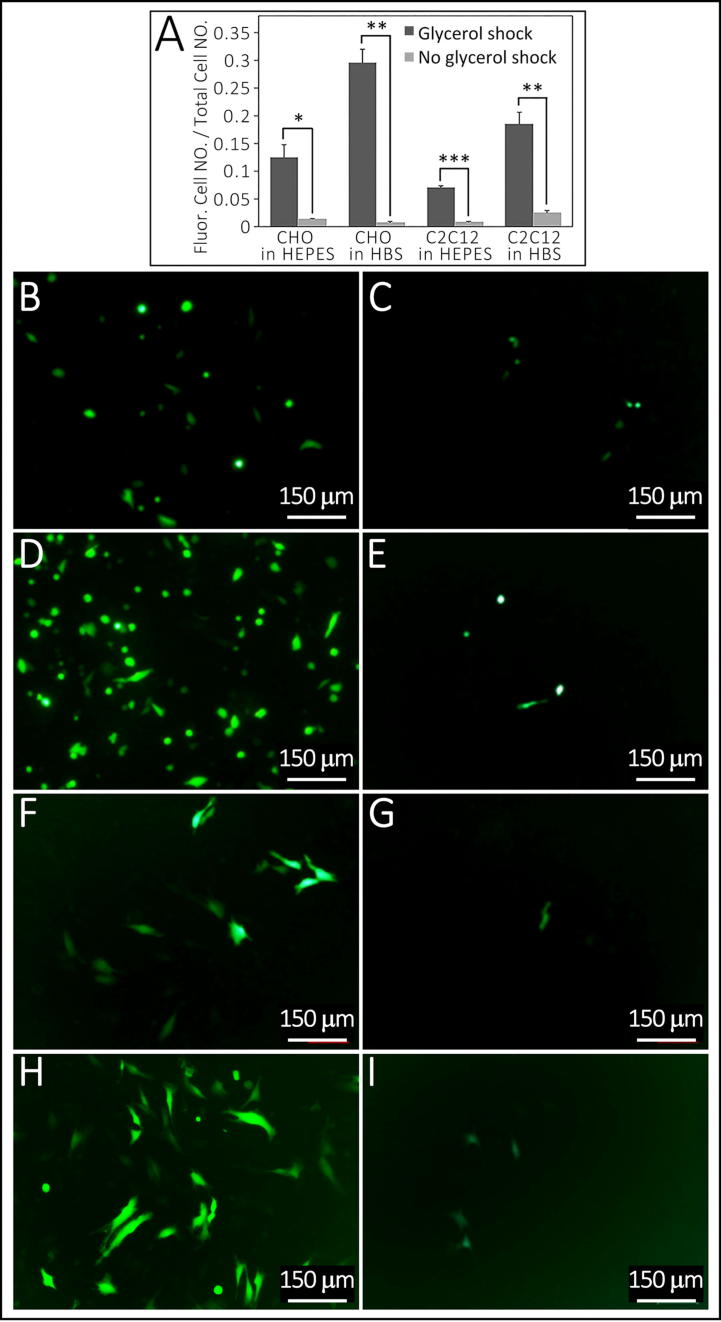
Fig. 4.
Influence of glycerol treatment on CHO and C2C12 cells transfection efficiency. Summarized in Table 1, Experiment 9. Panel A, glycerol shock generated significantly more efficient transfection when used with HEPES buffer saline and HBS buffer saline in both CHO and C2C12 cell lines (F = 0.003, P = 0.044 with HEPES in CHO, F = 0.138, P = 0.002 with HBS in CHO, F = 0.082, P = 0.00003 with HEPES in C2C12, and F = 0.092, P = 0.008 with HBS in C2C12). Panels B–E show CHO cells, comparison of transfection efficiency between glycerol shock (B) and no shock (C) in HEPES buffer saline and comparison of transfection efficiency between glycerol shock (D) and no shock (E) in HBS buffer saline. Panels F-I show C2C12 cells, comparison of transfection efficiency between glycerol shock (F) and no shock (G) in HEPES buffer saline and comparison of transfection efficiency between glycerol shock (H) and no shock (I) in HBS buffer saline. * indicates p-value < 0.05, ** indicates p-value < 0.01, and *** indicates p value < 0.001. Bars in B–I are 150 µm.
Fig. 5.
Comparison of incubation time effect on transfection efficiency of in CHO and C2C12 cells. Summarized in Table 1, Experiment 10. Panel A, the efficiency with 6 h incubation in both CHO and C2C12 cell-lines is significantly more effective than 16 h incubation, F = 0.841 and 0.981, P = 0.004 and 0.039, separately. Panel B and C show fluorescence images of CHO cells. Panel D and E show fluorescence images of C12C12 cells. * indicates p-value < 0.05 and ** indicates p-value < 0.01. Bars in B–E are 150 µm.
4. Conclusion
Calcium phosphate mediated transfection is the most low-cost approach to introducing recombinant DNA into culture cells. It is commonly used to transfect recombined DNA into cells for packaging of virus-carrying gene of interest (Shunaeva et al., 2015, Yaswen et al., 1993). However, the low transfection efficiency of this approach on higher differentiated cells such as CHO and C2C12 limits its research application. Our study systematically evaluated possible factors affecting successful transfection rate and optimized transfection conditions for the highest efficiency (Table 1).
When calcium phosphate–DNA co-precipitations or particles are prepared, an accurate pH value of HBS-buffered saline is critical to guarantee appropriate formation of the calcium–DNA complex. HBS-buffered saline is preferred over HEPES-buffered saline and transfection efficiency is greatly improved when particle assembly is carried out at 37 °C. And a glycerol shock post incubation with calcium phosphate–DNA particles is vital to efficient transfection. Although FBS- and FBS/Pen-Strep free medium is required for liposome transfection (Cui et al., 2012, Felgner et al., 1987), in calcium phosphate mediated transfection, FBS is necessary for high efficiency on higher differentiated cells such as CHO and C2C12. Furthermore, since fresh media just prior to transfection had no significant effect on efficiency (Supplemental Fig. 7, Table 1 Experiment 7), removing this step will conserve time and further lower the cost of our optimized procedure for the calcium phosphate mediated transfection.
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
XXH conceived of the study. GL, WLY and YRH developed protocols and collected all data. GL, WLY and YRH analysed the data. MMX, ZX, FR and GGK provided direction and advisement. XXH, GL, WLY and YRH prepared the manuscript and XXH, MMX, MN, WI and JM edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31371256, 31571273), the National Department of Education Central Universities Research Fund (Grant No. GK20130100), the Foreign Distinguished Scientist Program (Grant No. MS2014SXSF038), US Maryland Stem Cell Research Fund (2009MSCRFE008300), Qinba Mountain Developing Center (Grant No. CIC-QBRSD) and the Outstanding Doctoral Thesis fund (Grant Nos. X2014YB02, X2015YB05).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.sjbs.2017.01.034.
Appendix A. Supplementary data
References
- Cui, S., Zhang, S., Chen, H., Wang, B., Zhao, Y., Zhi, D., 2012. The mechanism of lipofectamine 2000 mediated transmembrane gene delivery engineering 04, 172–175 http://dx.doi.org/10.4236/eng.2012.410B045.
- Dudek, H., Ghosh, A., Greenberg, M.E., 2001. Calcium phosphate transfection of DNA into neurons in primary culture. Curr. Protoc. Neurosci. Chapter 3: Unit 3 11 http://dx.doi.org/10.1002/0471142301.ns0311s03. [DOI] [PubMed]
- Felgner P.L. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi J., Narula N., Yee D.S., Skarecky D.W., Lau A., Ornstein D.K., Tyson D.R. Annexin A2 positively contributes to the malignant phenotype and secretion of IL-6 in DU145 prostate cancer cells. Int. J. Cancer. 2009;124:68–74. doi: 10.1002/ijc.23928. [DOI] [PubMed] [Google Scholar]
- Junquera E., Aicart E. Cationic lipids as transfecting agents of DNA in gene therapy. Curr. Top. Med. Chem. 2014;14:649–663. doi: 10.2174/1568026614666140118203128. [DOI] [PubMed] [Google Scholar]
- Kaestner L., Scholz A., Lipp P. Conceptual and technical aspects of transfection and gene delivery. Bioorg. Med. Chem. Lett. 2015;25:1171–1176. doi: 10.1016/j.bmcl.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kalli C., Teoh W.C., Leen E. Introduction of genes via sonoporation and electroporation. Adv. Exp. Med. Biol. 2014;818:231–254. doi: 10.1007/978-1-4471-6458-6_12. [DOI] [PubMed] [Google Scholar]
- Kwon M., Firestein B.L. DNA transfection: calcium phosphate method. Methods Mol. Biol. 2013;1018:107–110. doi: 10.1007/978-1-62703-444-9_10. [DOI] [PubMed] [Google Scholar]
- Li L., Allen C., Shivakumar R., Peshwa M.V. Large volume flow electroporation of mRNA: clinical scale process. Methods Mol. Biol. 2013;969:127–138. doi: 10.1007/978-1-62703-260-5_9. [DOI] [PubMed] [Google Scholar]
- Liu S.M. Transgenic plants expressing the AaIT/GNA fusion protein show increased resistance and toxicity to both chewing and sucking pests. Insect Sci. 2016;23:265–276. doi: 10.1111/1744-7917.12203. [DOI] [PubMed] [Google Scholar]
- Liu X. N-3 polyunsaturated fatty acids attenuates triglyceride and inflammatory factors level in hfat-1 transgenic pigs. Lipids Health Dis. 2016;15:89. doi: 10.1186/s12944-016-0259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad H.S. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int. J. Oncol. 2008;33:1157–1163. [PubMed] [Google Scholar]
- Nakamura H., Funahashi J. Electroporation: past, present and future. Dev. Growth Differ. 2013;55:15–19. doi: 10.1111/dgd.12012. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Russell, D.W., 2001. Introducing cloned genes into cultured mammalian cells. In: Argentine, J. (Ed.), Molecular Cloning a Laboratory Manual, vol 3, 3rd ed., Cold Spring Harbor Laboratory Press, New York, pp. 16.17–16.54.
- Shunaeva, A., et al., 2015. Improvement of HIV-1 and HTLV-1 replication-dependent vectors via optimization of reporter gene reconstitution and modification with intronic shRNA. J. Virol. http://dx.doi.org/10.1128/jvi.01940-15. [DOI] [PMC free article] [PubMed]
- Sun, M., Bernard, L.P., Dibona, V.L., Wu, Q., Zhang, H., 2013. Calcium phosphate transfection of primary hippocampal neurons. J. Vis. Exp. e50808. http://dx.doi.org/10.3791/50808. [DOI] [PMC free article] [PubMed]
- Wang Y.L., Wang Y., Tong L., Wei Q. Overexpression of calcineurin B subunit (CnB) enhances the oncogenic potential of HEK293 cells. Cancer Sci. 2008;99:1100–1108. doi: 10.1111/j.1349-7006.2008.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F., Mi Z., Gu N. Cationic liposomes as gene delivery system: transfection efficiency and new application. Pharmazie. 2011;66:158–164. [PubMed] [Google Scholar]
- Yaswen L.R., Stephens E.B., Davenport L.C., Hutt-Fletcher L.M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology. 1993;195:387–396. doi: 10.1006/viro.1993.1388. [DOI] [PubMed] [Google Scholar]
- Yoshiga T., Yokoyama N., Imai N., Ohnishi A., Moto K., Matsumoto S. CDNA cloning of calcineurin heterosubunits from the pheromone gland of the silkmoth, Bombyx mori. Insect Biochem. Mol. Biol. 2002;32:477–486. doi: 10.1016/s0965-1748(01)00125-4. [DOI] [PubMed] [Google Scholar]
- Zhou, X., et al., 2016. Liver specific NG37 over-expression leads to diet-dependent fatty liver disease accompanied by cardiac dysfunction. Genes Nutr. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.