Abstract
To study the influence of stachydrine hydrochloride on the inflammatory cytokines and tissue morphology of the re-perfusion model of mice with repetitive cerebral ischemia and probe into the protection mechanism of stachydrine hydrochloride for cerebral ischemia reperfusion impairment. Build a repetitive cerebral ischemia reperfusion model by first blocking the common carotid artery on both sides for 10 min, then resuming perfusion for 10 min and then blocking the common carotid artery on both sides again for 10 min. Before the operation, all the mice in the Nimodipine group, and the big, medium and small stachydrine hydrochloride dose groups were given corresponding gastric perfusion, the mice in the sham operation group and the modeled groups were at the same time given 0.5% sodium carboxymethyl cellulose for gastric perfusion of the same volume. The medicine was fed daily for 7 consecutive days. The model was built 1 h after the last feed and the perfusion continued for 24 h after the operation. Then the death rate of the mice was calculated. The mouse brains were taken out to test the ICAM-1 level and the TNF-α level, and the serum was taken out to test the NSE level and the MPO level. The tissue morphology changes were also observed. All the repetitive cerebral ischemia reperfusion models were successfully duplicated. The stachydrine hydrochloride in all the dose groups significantly reduced the death rates of big and small mice, reduced the level of ICAM-1 and the level of TNF-α in the brain tissues and the NSE level and the MPO level in the serum, significantly alleviating the pathological impairment in the hippocampus. Stachydrine hydrochloride can significantly reduce the death rate of mice, improve the pathological changes in the hippocampus, inhibit inflammatory reactions after ischemia, thus reducing the re-perfusion impairment after cerebral ischemia.
Keywords: Stachydrine hydrochloride, Cerebral ischemia reperfusion
1. Introduction
Stachydrine hydrochloride is an important part of the pharmacological basis for alkaloids in the Leonurus japonicus Houtt - motherwort. The motherwort tastes spicy and slightly bittersweet. The property slightly, it can be used for clearing heat and detoxification. Stachydrine hydrochloride is also called Proline Betaine or N-Dimethyl proline and is the simplest pyrrole alkaloid. Its basis structure is l-stachydrine and its molecular weight M = 143.18 and the pH = 7. The Chinese Pharmacopoeia 2015 has already designated the concentration of stachydrine hydrochloride as the standard for testing dry motherwort – the concentration of stachydrine hydrochloride must not be below 0.50%. The pyrrole rings in their chemical structure have very good medical effects. Modern pharmacological studies have shown that the alkaloids in motherwort have extensive pharmacological activities in treating cardiovascular and cerebral diseases and resisting inflammations. Cerebral ischemia is a common disease among old people and the pathological mechanism of impairment caused by cerebral ischemia is very complex, which includes many factors, such as the emergence of inflammatory media, exhaustion of energy (Liu, 2016), the emergence of radicals, and the activation of apoptosis pathway. Early studies have found that motherwort, having the capacity to clear heat, detoxify, activate blood and remove blood stasis, can be used in alleviating the impairment caused by cerebral ischemia. “Activate blood and remove blood stasis” is commonly used in clinical treatment of impairment caused by cerebral ischemia and the effect is significant. “Clear heat and detoxify” is a new viewpoint in alleviating such impairment put forward by traditional Chinese medicine researchers.
2. Materials and methods
2.1. Drugs and reagents
Stachydrine hydrochloride, was provided by the Chemical Lab of Henan University of Traditional Chinese Medicine, concentration >9%, batch No: 20091212; Nimodipine pills, by Yabao Pharmaceutical Group Co., Ltd, batch No: 130150; penicillin sodium for injection use, by Huabei Pharmaceuticals Co., Ltd, batch No. C1206807; CMC, by Hengxing Chemical Reagent Production Co., Ltd of Tianjin, batch No.: 20120418; ICAM-1 ELISA Testing Reagent, R&D Company, batch No. 20130901A; TNF-α ELISA testing reagent box, R&D Company, batch No. 20130901A; NSE ELISA testing reagent box, R&D Company, batch No. 20130901A; MPO testing box, Nanjing Jiancheng Bio-engineering Institute, batch No. 20130914. Ultraviolet–visible spectrophotometer, provided by Shanghai Tianmei Scientific Instrument Co., Ltd, type: UV 1000; reader, BIO-RAD Company (US), type: BIORAD-68 (Table 1, Table 2).
Table 1.
The influence on the death rate, serum NSE and serum MPO levels of the modeled mice ( ± s).
| Group | Dose (mg/kg) | Number of animals modeling |
Death rate (%) | NSE (ng/ml) | MPO (U/L) | |
|---|---|---|---|---|---|---|
| Before | Post | |||||
| Sham-operation group | – | 16 | 16 | 0 | 4.401 ± 0.587** | 66.808 ± 24.759** |
| Model group | – | 16 | 10 | 37.5 | 5.401 ± 0.546 | 118.964 ± 38.892 |
| Nimodipine group | 30 | 16 | 12 | 25 | 4.848 ± 0.539* | 86.806 ± 27.390* |
| Big-dose group | 60 | 16 | 13 | 18.75 | 4.892 ± 0.410* | 79.679 ± 23.303** |
| Medium-dose group | 30 | 16 | 11 | 31.25 | 4.929 ± 0.493* | 82.838 ± 26.744** |
| Small-dose group | 15 | 16 | 11 | 31.25 | 5.046 ± 0.476 | 85.493 ± 33.322* |
Note: Compared with the modeled group, **P < 0.01, *P < 0.05.
Table 2.
Influence on the ICAM-1 and TNF-α level of the cerebral tissue of the mice ( ± s).
| Group | n | Dose (mg/kg) | ICAM-1 (ng/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|
| Sham-operation group | 16 | – | 63.239 ± 14.605** | 85.539 ± 10.305** |
| Model group | 10 | – | 81.204 ± 14.328 | 104.431 ± 14.311 |
| Nimodipine group | 12 | 30 | 69.048 ± 9.816** | 91.275 ± 8.210** |
| Big-dose group | 13 | 60 | 69.826 ± 9.783* | 90.965 ± 11.694** |
| Medium-dose group | 11 | 30 | 71.861 ± 10.636* | 93.316 ± 9.824* |
| Small-dose group | 11 | 15 | 72.259 ± 9.162 | 94.973 ± 8.421* |
Note: Compared with the modeled group, **P < 0.01, *P < 0.05.
2.2. Animals
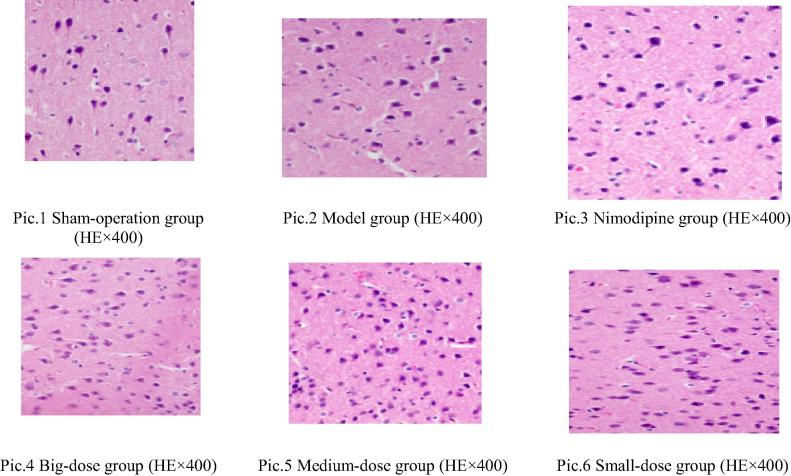
KM mice, SPF level, male, 96 in number, weighted 25–30 g, were provided by the Experiment Animal Center of Henan Province, license No: 41003100000258; lab license No.: SYXK (Henan) 2010-001 (Fig. 1).
Figure 1.
The pathological photos of the cerebral cortex of modeled mice.
2.3. Methods
96 healthy mice, male, weighted 25–30 g, were fed normally for 3 days, and then weighed and classified into 6 groups at random, 16 in each group. The groups were the sham operation group, the modeled group, the Nimodipine Group, the big doze stachydrine hydrochloride group, the medium dose stachydrine hydrochloride group and the small dose stachydrine hydrochloride group. Nimodipine suspension gastric perfusion (positive control drug, fed dose 30 mg/kg, equivalent to 15 times that of clinical dose, formulate the medicine concentration of 3 mg/nl with 0.5% CMC prior to use) were conducted for mice in the Nimodipine group at the concentration level of 0.1 ml/10 g. The mice in the big, medium and small dose groups were fed stachydrine hydrochloride suspension (medicine fed is respectively 60 mg/kg, 30 mg/kg and 15 mg/kg, formulate medicine mixtures with concentrations of 6 mg/ml, 3 mg/ml, and 1.5 mg/ml with 0.5% CMC). The mice in the sham operation group and in the model group were given gastric perfusion with 0.5% CMC of the same volume. The mice were fed once daily for 7 consecutive days.
From 8 p.m. on the sixth day, abrosia was conducted for the mice batch by batch. After 12 h, the mice were weighed and fed batch by batch. After 1 h, celiac anesthesia was conducted by injecting 10% chloral hydrate (0.03 ml/10 g), the neck was sterilized with alcohol, and then we used operation knives to conduct median slicing and gradually separated surrounding muscles until the common carotid artery (CCA) on both sides were seen. Then we used the bent precision forcep to peel off surrounding nerves, separated CCA and threaded it for back-up use. Each CCA was sutured with an acupuncture needle whose diameter was around 0.3 mm, and was made 90 degrees, stayed lack of blood for 10 min, and then we let loose of the acupuncture needle, resumed perfusion for 10 min, then blocked the blood for 10 min, and then resumed blood perfusion. After that, we applied penicillin powder on the wounds, and sutured the wound at the cervix. For the sham operation group, except that the CCA was not blocked, other operations were the same as those in the modeled group (Tan et al., 2002).
We gave all the mice perfusion for another 24 h, removed the eyeballs to extract blood, kept the blood still for half an hour, conducted centrifugal operation of the blood at 3500 r/min for 10 min, obtained the serum, and tested the concentration of NSE and MPO in the serum. Then we removed the cervical vertebra to execute the mice, obtained the brains quickly, vector cut half of the brain and put in 10% formalin, kept still for a week and imbedded it with paraffin, conducted HE staining and observed tissue morphological changes. For the other half of the brain, we used saline to erase the blood stains, and used the filter paper to fully absorb the saline on it, accurately measured its weight and calculated the amount of cold saline added with this weight, made 10% brain homogenate with glass homogenate machine in a big kiln at a proportion that saline (ml): brain tissue (g) = 9:1, conducted centrifugal operation of the homogenate at 3000 r/min at 4 °C, obtained the supernatant and stored it at a temperature below −20 °C, and tested the concentration of ICAM-1 and that of TNF-α in the homogenate (Fan et al., 2015) (Table 3, Table 4).
Table 3.
The influence on the pathological changes in the cerebral cortex of the modeled mice.
| Group | n | Dose (mg/kg) | − | + | ++ | +++ | P |
|---|---|---|---|---|---|---|---|
| Sham-operation group | 16 | – | 16 | 0 | 0 | 0 | ** |
| Model group | 10 | – | 0 | 0 | 2 | 8 | |
| Nimodipine group | 12 | 30 | 5 | 4 | 2 | 1 | ** |
| Big-dose group | 13 | 60 | 3 | 6 | 3 | 1 | ** |
| Medium-dose group | 11 | 30 | 3 | 4 | 3 | 1 | ** |
| Small-dose group | 11 | 15 | 2 | 5 | 2 | 2 | ** |
Note: Compared with the modeled group, **P < 0.01, *P < 0.05.
“−” the nerve cells were normal; “+” cerebral cortex nerve cells had edema, were interspersed, a small amount of neurons degenerated, the cytoplasm was lightly dyed and the structure blurred; “++” the nerve cells in the cerebral cortex had edema, chunks of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; “+++” the cerebral cortex nerve cells had edema and most neurons were necrotic.
Table 4.
The influence on the pathological changes in the hippocampus of the modeled mice.
| Group | n | Dose (mg/kg) | − | + | ++ | +++ | P |
|---|---|---|---|---|---|---|---|
| Sham-operation group | 16 | – | 16 | 0 | 0 | 0 | ** |
| Model group | 10 | – | 0 | 0 | 3 | 7 | |
| Nimodipine group | 12 | 30 | 4 | 3 | 3 | 2 | ** |
| Big-dose group | 13 | 60 | 3 | 4 | 5 | 1 | ** |
| Medium-dose group | 11 | 30 | 2 | 3 | 4 | 2 | ** |
| Small-dose group | 11 | 15 | 1 | 2 | 5 | 3 | * |
Note: Compared with the modeled group, **P < 0.01,*P < 0.05.
“−” the nerve cells were normal; “+” hippocampus nerve cells had edema, were interspersed, a small amount of neurons degenerated, the cytoplasm was lightly dyed and the structure blurred; “++” the nerve cells in the hippocampus had edema, chunks of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; “+++” the hippocampus nerve cells had edema and most neurons were necrotic.
2.4. Statistical analysis
Statistical analysis was conducted with SPSS 17.0. The calculated materials were represented with mean ± standard deviation, and inter-group comparison was conducted with single factor analysis of variance. For those with the same variance, the LSD method was used. For those with different variances, the Games-Howeel method was used. The level materials were tested with Radit (Zhang et al., 2012).
3. Results & discussion
3.1. The influence on the death rate, serum NSE and serum MPO levels of the modeled mic
From the table above, it can be seen that the death rate in the modeled group was the highest, and the death rates in the Nimodipine Group and the big, medium and small dose stachydrine hydrochloride groups were all lower to some extent, indicating that the medicine fed in each group could all reduce the death rates of the modeled mice, reduce cerebral tissue impairment and protect the cerebral tissue. Compared with the sham operation group, the NSE level and the MPO level in the serum both significantly increased (P < 0.05), the NSE levels in the serum in the big dose and medium dose group were obviously reduced (P < 0.05), the serum NSE level in the small dose group revealed a trend of decline (P > 0.05), the serum MPO level in the big dose and medium dose group declined remarkably (P < 0.01), and the serum MPO level in the small dose group remarkably declined, suggesting that the medicine fed had protective effects for the neurological cells of the mice at varied degrees and could reduce the NSE level and the MPO level in the serum.
3.2. Influence on the ICAM-1 and TNF-α level of the cerebral tissue of the mice
From the table above, it can be seen that compared with the sham operation group, the level of ICAM-1 and TNF-α in the cerebral tissue of the mice in the modeled group both increased significantly (P < 0.01), suggesting that the modeling was successful; compared with the modeled group, the ICAM-1 level of cerebral tissue of the mice in the Nimodipine group decreased significantly (P < 0.01), the ICAM-1 level in the cerebral tissues of the mice in the small dose group declined significantly (P > 0.05), the TNF-α level of the cerebral tissues of the mice in the Nimodipine and big dose group decreased significantly (P < 0.01), the TNF-α level in the medium and small dose group obviously decreased (P < 0.05), suggesting that the medicine fed had the ability to reduce the ICAM-1 and TNF-α levels in the brain tissues and reduce the impairment inflammations could do to cerebral tissues.
3.3. The influence on the pathological changes in the cerebral cortex of the modeled mice
The pathological observation of the cerebral cortex of the modeled mice is as follows (see Fig. 1 for the pictures): in the sham operation group, the nerve cells in the cerebral cortex were normal; in the modeled group, the nerve cells in the cerebral cortex had edema and most neurons were necrotic; in the Nimodipine group, a small amount of nerve cells had edema, a small amount of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; in the big dose stachydrine hydrochloride group, a small amount of nerve cells in the cerebral cortex had edema, were interspersed, a small amount of neurons degenerated, the cytoplasm was lightly dyed and the structure blurred; in the medium dose group, part of nerve cells in the cerebral cortex had edema, were interspersed, a small amount of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; in the small dose group, the nerve cells in the cerebral cortex had edema, chunks of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic.
After Ridit test, compared with the sham operation group, the modeled group had more statistical significance (P < 0.01), suggesting that prominent pathological changes took place in the cerebral cortex of the mice and that the modeling was successful; compared with the modeled group, the Nimodipine and the big, medium and small dose groups all had prominent statistical significance (P < 0.01), suggesting that the medicine fed in each group could significantly reduce the pathological damages of the cerebral cortex and protect the cerebral tissues.
3.4. The influence on the pathological changes in the hippocampus of the modeled mice
The pathological observations of the hippocampus of the cerebral tissues of the modeled mice are as follows: in the sham operation group, the hippocampus nerve cells were normal; in the modeled group, the hippocampus never cells had edema, most neurons were necrotic; in the Nimodipine group, a small amount of nerve cells had edema, a small amount of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; in the big dose group, a small amount of nerve cells had edema, were interspersed and a small amount of neurons degenerated, the cytoplasm was lightly dyed and the structure blurred; in the medium dose group, part of the nerve cells in the hippocampus had edema, were interspersed, a small amount of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic; in the small dose group, the nerve cells in the hippocampus had edema, chunks of neurons degenerated, the cytoplasm was lightly dyed, the structure blurred and certain neurons were necrotic.
After Ridit test, compared with the sham operation group, the modeled group had prominent statistical significance (P < 0.01), suggesting that prominent pathological changes took place in the hippocampus of the mice and that the modeling was successful; compared with the modeled group, the Nimodipine and the big, the medium and the small dose groups all had prominent statistical significance (P < 0.01); the small dose group had obvious statistical significance, suggesting that the medicine fed in each group could reduce the pathological damages of the cerebral cortex at varied degrees and protect cerebral tissues.
4. Conclusion
Ischemia took place twice in the modeling process. In the second ischemia, maybe because of acute loss of blood, the mice were dead. Most deaths took place during the 10 min of the second ischemia and the 10 h after ischemia. The reasons might be that it was difficult for individual mice to form collateral circulation of cerebral veins, the blood flow in the brain declined dramatically, or that the cerebral tissues had a poor resistance against lack of oxygen, causing the respiratory center to be inhibited, or that the blood pipes were pierced open, the nerves were broken in the operation, or that the physical conditions of the mice were weak. Therefore, it can be seen that since the factors that might cause death of the mice were many, death rates could only be used as an auxiliary indicator for (Fan, 2015, Zhou, 2014).
After ischemia took place, through inducing IL-1β and other cytokines to release themselves, TNF-α could exacerbate inflammatory reactions, damage the endothelial cells in the capillary, damage blood brain barrier, exacerbate cerebral edema, add to the formation and development of artery thrombus, and cause many other mechanism to have effect, leading to cerebral ischemia reperfusion damages; it could also increase the ICAM-1 gene express by inducing the express of NF-κB to increase, intensify the syntheses of ICAM-1, and merge with corresponding recipients in the leukocyte, causing the leukocyte to be attached to endothelia cells, then emigrate and enter the cerebral substance outside the blood pipes and release inflammatory media and cytokines. Meanwhile, these inflammatory media and cytokines further promoted the gene express of ICAM-1 and caused a vicious cycle, thus exacerbating the damages. The major leukocytes that invaded the brain during an ischemia were neutrophil cells and single-core cells and MPO was a unique enzyme for them both. The activity of MPO reflected the immersed level of the two leukocytes in cerebral tissues. In normal cerebral tissues, NSE only existed in neuron cytoplasm. After ischemia continued for 24 h, NSE would be released from cerebral neurons that were damaged due to ischemia and enter blood circulation by crossing blood brain barriers (Ma and Yang, 2006). The testing of the variance of NSE levels in the serum could reflect the impairment degree of cerebral neurons. The study result suggested that compared with the sham operation group, the TNF-α, ICAM-1, and MPO levels all obviously increased. Stachydrine hydrochloride could reduce the levels of TNF-α, ICAM-1, and MPO and this suggested that stachydrine hydrochloride could inhibit the attachment of leukocyte to endothelia cells and its emigration, thus reducing the release of inflammatory media and cytokines (Wang and Wang, 2012).
After observing the pathological tissue slices, we found that the cerebral nerve cells were damaged after ischemia. After ischemia, the metabolism of the cerebral nerve cells became disordered, causing nerve cell damages or apoptosis. In this experiment, we adopted the HE staining method to observe the damages of the nerve cells. The major area we observed was the hippocampus, which was vulnerable to damages caused by ischemia reperfusion. The cerebral tissue slice could directly reflect the volume of cerebral cells and the changes near the cytoplasm, nucleus, and blood pipes. The experiment results suggested that compared with the sham operation group, significant pathological changes took place in the cerebral tissues of the modeled animals, which were mainly the edema and degeneration of the nerve cells in hippocampus. Stachydrine hydrochloride could reduce edema in the cerebral nerve cells and this suggested that stachydrine hydrochloride could soothe the pathological changes in the hippocampus caused by ischemia reperfusion and could be useful in treating ischemia. In terms of treatment effect, the bigger the dose of stachydrine hydrochloride, the better. The experiment result proved that stachydrine hydrochloride could protect nerve cells from inflammatory impairment and the effect of big dose stachydrine hydrochloride was the best (Tan, 2013, Miao and Rui, 2016).
Stachydrine hydrochloride, the pharmacological active component of motherwort, a traditional Chinese herb that can clear heat, detoxify, activate blood and remove blood stasis, could reduce ischemia reperfusion impairment from many aspects (Yoo, 2012, Miao and Cheng, 2015). This finding has laid a laboratory foundation for developing medicines that can prevent and treat ischemia diseases in a secure and effective way.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (Grant No. 81173474), Outstanding scientific and technological innovation team of Henan province (Grant No. TCJ2014-391) and Zhengzhou science and technology innovation team (Grant No. 131PCXTD612).
Footnotes
Peer review under responsibility of King Saud University.
References
- Fan M.Y. Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurol. Sci. 2015;36(2):315–321. doi: 10.1007/s10072-014-1943-x. [DOI] [PubMed] [Google Scholar]
- Fan M.Y. Lithium chloride administration prevents spatial learning and memory impairment in repeated cerebral ischemia-reperfusion mice by depressing apoptosis and increasing BDNF expression in hippocampus. Behav. Brain Res. 2015;291:399–406. doi: 10.1016/j.bbr.2015.05.047. [DOI] [PubMed] [Google Scholar]
- Liu Z.J. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci. Ther. 2016;22(1):43–52. doi: 10.1111/cns.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.H., Yang J.R. Protective effect of stachydrine hydrochloride on myocardial ischemia reperfusion injury of rats. Chin. J. Exp. Tradit. Med. Formulas. 2006;05:40–42. [Google Scholar]
- Miao M.S., Cheng B.L. Effect of curcumin on diabetic rat model of cerebral ischemia. Pak. J. Pharm. Sci. 2015;28(1 Suppl.):401–405. [PubMed] [Google Scholar]
- Miao M.S., Rui Q. Radix Ilicis Pubescentis total flavonoids combined with mobilization of bone marrow stem cells to protect against cerebral ischemia/reperfusion injury. Neural Regener. Res. 2016;11(2):278–284. doi: 10.4103/1673-5374.177736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z.J. Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke. 2013;44(12):3490–3497. doi: 10.1161/STROKEAHA.113.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.B., Gong Q.Y., Yao M.H. Effects of Ginkgo biloba extract on inflammation following focal ischemic brain injury in rats. Chin. New Drugs Clin. Rem. 2002;27(21):385–389. [Google Scholar]
- Wang F., Wang C. Anti-inflammatory activity of stachydrine hydrochloride. China Pharm. 2012;3:212–214. [Google Scholar]
- Yoo D.Y. Chronic effects of pyridoxine in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neurochem. Res. 2012;37(5):1011–1018. doi: 10.1007/s11064-011-0696-7. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang X.L., Miao M.S. Effect of total ilex pubescens flavone on cerebral ischemic mouse model. Tradit. Chin. Drugs Res. Clin. Pharm. 2012;23(4):409–412. [Google Scholar]
- Zhou Y. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine. 2014;21(7):998–1003. doi: 10.1016/j.phymed.2013.12.005. [DOI] [PubMed] [Google Scholar]