Abstract
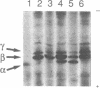
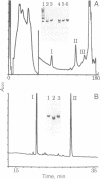
Resting human platelets contain approximately 0.3 mM unpolymerized actin. When freshly drawn and washed platelets are treated with saponin, 85-90% of the unpolymerized actin diffuses out. Analysis by polyacrylamide gel electrophoresis under nondenaturing conditions shows that the bulk of this unpolymerized actin migrates with a higher mobility than does pure G-actin, profilactin, or actin-gelsolin complex. When muscle G-actin is added to fresh or boiled saponin extract, the added muscle actin is shifted to the high-mobility form. The saponin extract contains an acidic peptide having a molecular mass in the range of 5 kDa, which has been purified to homogeneity by reverse-phase HPLC. This peptide also shifts muscle actin to the high-mobility form. Addition of either boiled saponin extract or the purified peptide to muscle G-actin also strongly and stoichiometrically inhibits salt-induced polymerization, as assayed by falling-ball viscometry and by sedimentation. We conclude that this peptide binds to the bulk of the unpolymerized actin in platelets and prevents it from polymerizing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Markey F., Lindberg U., Vandekerckhove J. The primary structure of human platelet profilin: reinvestigation of the calf spleen profilin sequence. FEBS Lett. 1988 Feb 8;228(1):17–21. doi: 10.1016/0014-5793(88)80575-1. [DOI] [PubMed] [Google Scholar]
- Bamburg J. R., Harris H. E., Weeds A. G. Partial purification and characterization of an actin depolymerizing factor from brain. FEBS Lett. 1980 Nov 17;121(1):178–182. doi: 10.1016/0014-5793(80)81292-0. [DOI] [PubMed] [Google Scholar]
- Bray D., Thomas C. Unpolymerized actin in fibroblasts and brain. J Mol Biol. 1976 Aug 25;105(4):527–544. doi: 10.1016/0022-2836(76)90233-3. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Markey F., Blikstad I., Persson T., Lindberg U. Reorganization of actin in platelets stimulated by thrombin as measured by the DNase I inhibition assay. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6376–6380. doi: 10.1073/pnas.76.12.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios C. G., Cooke R. Fluorescence energy transfer between probes on actin and probes on myosin. Biochim Biophys Acta. 1984 Jul 31;788(2):193–205. doi: 10.1016/0167-4838(84)90262-0. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Polymerization and organization of actin filaments within platelets. Semin Hematol. 1983 Oct;20(4):243–260. [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Boyer J. L., Korn E. D. Comparative biochemistry of non-muscle actins. J Biol Chem. 1977 Nov 25;252(22):8300–8309. [PubMed] [Google Scholar]
- Harris H. E., Weeds A. G. Platelet actin: sub-cellular distribution and association with profilin. FEBS Lett. 1978 Jun 1;90(1):84–88. doi: 10.1016/0014-5793(78)80303-2. [DOI] [PubMed] [Google Scholar]
- Kurth M. C., Bryan J. Platelet activation induces the formation of a stable gelsolin-actin complex from monomeric gelsolin. J Biol Chem. 1984 Jun 25;259(12):7473–7479. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambooy P. K., Korn E. D. Purification and characterization of actobindin, a new actin monomer-binding protein from Acanthamoeba castellanii. J Biol Chem. 1986 Dec 25;261(36):17150–17155. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J. L. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton. 1988;10(3):349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- Lind S. E., Janmey P. A., Chaponnier C., Herbert T. J., Stossel T. P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987 Aug;105(2):833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S. D., Pollard T. D. Viscometric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J Cell Biol. 1980 May;85(2):414–428. doi: 10.1083/jcb.85.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey F., Lindberg U., Eriksson L. Human platelets contain profilin, a potential regulator of actin polymerisability. FEBS Lett. 1978 Apr 1;88(1):75–79. doi: 10.1016/0014-5793(78)80610-3. [DOI] [PubMed] [Google Scholar]
- Mc Leod J. F., Kowalski M. A., Haddad J. G., Jr Interactions among serum vitamin D binding protein, monomeric actin, profilin, and profilactin. J Biol Chem. 1989 Jan 15;264(2):1260–1267. [PubMed] [Google Scholar]
- Miron T., Wilchek M., Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988 Dec 15;178(2):543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- Philp N. J., Nachmias V. T. Components of the cytoskeleton in the retinal pigmented epithelium of the chick. J Cell Biol. 1985 Aug;101(2):358–362. doi: 10.1083/jcb.101.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Rosenberg S., Stracher A., Lucas R. C. Isolation and characterization of actin and actin-binding protein from human platelets. J Cell Biol. 1981 Oct;91(1):201–211. doi: 10.1083/jcb.91.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D. An electrophoretic procedure for detecting proteins that bind actin monomers. Anal Biochem. 1989 Apr;178(1):32–37. doi: 10.1016/0003-2697(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Schering B., Bärmann M., Chhatwal G. S., Geipel U., Aktories K. ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin. Eur J Biochem. 1988 Jan 15;171(1-2):225–229. doi: 10.1111/j.1432-1033.1988.tb13780.x. [DOI] [PubMed] [Google Scholar]
- Schröer E., Wegner A. Purification and characterization of a protein from chicken gizzard, which inhibits actin polymerization. Eur J Biochem. 1985 Dec 16;153(3):515–520. doi: 10.1111/j.1432-1033.1985.tb09332.x. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Isolation of an inhibitor of actin polymerization from human polymorphonuclear leukocytes. J Biol Chem. 1981 Mar 25;256(6):3030–3036. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tilney L. G., Hatano S., Ishikawa H., Mooseker M. S. The polymerization of actin: its role in the generation of the acrosomal process of certain echinoderm sperm. J Cell Biol. 1973 Oct;59(1):109–126. doi: 10.1083/jcb.59.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G. The polymerization of actin. II. How nonfilamentous actin becomes nonrandomly distributed in sperm: evidence for the association of this actin with membranes. J Cell Biol. 1976 Apr;69(1):51–72. doi: 10.1083/jcb.69.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Bryan J. Isolation of calcium-dependent platelet proteins that interact with actin. Cell. 1981 Sep;25(3):637–649. doi: 10.1016/0092-8674(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Stark F., Nachmias V. T. Comparison of the effects of phorbol 12-myristate 13-acetate and prostaglandin E1 on calcium regulation in human platelets. Biochem J. 1988 Jan 15;249(2):487–493. doi: 10.1042/bj2490487. [DOI] [PMC free article] [PubMed] [Google Scholar]