Abstract
Background and Aims:
Antifibrinolytics may help bleeding in orthopaedic surgeries. The present study was undertaken to compare two dose regimens of tranexamic acid (TA) on perioperative blood loss in patients undergoing hip surgeries.
Methods:
In a prospective, randomised, controlled study, 59 patients scheduled for hip surgery were divided into Group C: receiving normal saline (n – 20), Group B: receiving single dose of TA (10 mg/kg) (n – 21), and Group I: receiving a bolus (10 mg/kg) plus infusion (1 mg/kg/h) of TA up to 4 h postoperatively (n – 18). Blood loss, haemoglobin and allogeneic blood transfusions were compared between the groups. For parametric data, P was calculated by ANOVA. Intergroup comparison was done by post hoc analysis with Bonferroni test. P < 0.05 was considered significant.
Results:
The intra-operative blood loss was lower in the patients who received TA (525 ± 150, 456 ± 156 and 400 ± 133 ml in Group C, B and I respectively; P = 0.05). The 6th hourly drain collection in Group I was lower than Group B and C (41 ± 18, 46 ± 14 and 31 ± 14 ml in Group C, B, and I respectively; P = 0.018). The blood loss at 24 h was less in groups receiving TA (146 ± 32, 120 ± 76, 107 ± 37 ml for Group C, B and I, respectively; P = 0.02). The requirement of blood transfusions was lower in Group I.
Conclusions:
A bolus of tranexamic acid followed by infusion is more useful than a single dose in decreasing perioperative blood loss in patients undergoing hip surgeries. It reduces allogenic blood transfusion without increasing risk of thromboembolic events.
Key words: Blood loss, hip surgeries, tranexamic acid
INTRODUCTION
Antifibrinolytics such as aprotinin, tranexamic acid (TA) and epsilon-aminocaproic acid (EACA) have been found to consistently reduce bleeding in orthopaedic surgeries.[1,2,3] TA, a more selective inhibitor of fibrinolysis, has been reported to reduce blood loss, and the rate of blood transfusions at a low added expense in patients undergoing hip and knee replacement surgeries. However, the dosage and method of administration remains conflicting. Studies have included a single dose of 10, 15 or 20 mg/kg, single bolus followed by an infusion till the end of surgery or two bolus injections 3 h apart with varying results on blood loss.[4,5,6,7] It was reported earlier that an intravenous (IV) dose of 10 mg/kg TA will produce therapeutic blood concentration of 5–10 mg/L for 3 h, whereas a dose of 20 mg/kg will maintain the same for 8 h.[8,9,10] While the former may be insufficient to reduce bleeding, the latter was speculated to be associated with increased hazard for thrombosis. The early post-operative period is linked with increased fibrinolysis due to enhanced release of tissue plasminogen activator. This is offset by initiation of the antifibrinolytic activity which peaks only at around 24 h postoperatively. Thus, a window period of few hours with increased potential for bleeding exists in the immediate post-operative period. Whether this can be overcome by continued infusion of TA is not known as most of the studies used either one or two bolus without infusion or the infusion, if used, was discontinued at the end of surgery. The present study is based on the rationale that a bolus followed by an infusion postoperatively maintains therapeutic blood levels of TA longer than a single IV dose. This may inhibit fibrinolysis more efficiently, cause less bleeding but without the added risk of thrombosis consequent to doses more than 15 mg/kg. We, therefore, compared the effect of a single IV dose of TA with a bolus followed by infusion of TA up to 4 h postoperatively on surgical bleeding and requirement of blood transfusions in patients undergoing hip surgery.
METHODS
This was a prospective, randomised, double-blinded study conducted in the Department of Anaesthesiology and Intensive care in collaboration with Department of Orthopaedics at a tertiary care centre. After obtaining clearance from the Hospital Institutional Ethics Committee and informed written consent, 59 patients belonging to either gender, American Society of Anesthesiologists Grade 1 and 2, aged between 20 and 70 years and undergoing surgery for hip fractures were included in the study. Hip fractures included fracture neck of femur, intertrochanteric and subtrochanteric fractures of femur and fractures of acetabulum. Surgeries included dynamic hip screw fixation, intramedullary nailing, plating, hemiarthroplasty, fixation of acetabulum and total hip replacement. Patients with a history of severe ischaemic heart disease, pulmonary embolism, deep vein thrombosis (DVT), hepatic or renal failure or allergy to TA were excluded from the study. Routine investigations included haemoglobin (Hb), packed cell volume, platelets, prothrombin time, activated partial thromboplastin time, serum creatinine and blood urea nitrogen. Before surgery, oral anticoagulants, antiplatelet agents such as aspirin and clopidogrel and non-steroidal anti-inflammatory medications were stopped. Patients were randomised using computer-generated random numbering into three groups, Group C: Patients received a bolus of normal saline (NS) followed by infusion of NS (n-20), Group B: Patients received single dose of TA (10 mg/kg) 10 min before incision followed by infusion of NS (n – 21) and Group I: Patients received a bolus of TA (10 mg/kg) 10 min before incision followed by continuous infusion of TA at 1 mg/kg/h for up to 4 h postoperatively (n – 18). The randomisation was accessed by the technician involved in the preparation of dose regimens before starting the case from the onsite computer. The persons involved in intra-operative (IO) blood loss estimation, those involved in post-operative drain calculations and statistical analysis were not the same and were unaware of the group allocation. After establishing IV access, all patients received 100 mL/h of Ringer's lactate. Simultaneously, the patients were given either a bolus of TA (10 mg/kg) mixed in 100 mL of NS or the same volume of placebo (NS) depending on the group allocated. All patients received continuous infusions till 4 h in the post-operative period at the rate of 3 mL/h. The patients in Group I received TA 1 mg/kg/h diluted in 3 mL of 0.9% saline (the concentration of the solution varied depending on the weight of the patient). Groups C and B patients received 3 mL of 0.9% saline without addition of TA. All the patients received central neuraxial blockade, and the mean blood pressure was maintained intraoperatively within 20% of the baseline.
The primary outcome was the IO blood loss. IO blood loss was measured by including volume collected in suction bottles and counting the number of blood-soaked sponges. One fully soaked sponge was considered to be equal to 50 mL of blood loss. Any blood loss was treated with adequate fluid replacement. Patients who lost up to 10% of blood volume received volume replacement with crystalloids in the ratio of 1:2. Those with the loss of 10%–20% of blood volume were given colloids, and loss of >20% of blood volume was replaced with packed red blood cells in 1:1 ratio. The decision whether to transfuse or not was done by the attending anaesthesiologists based on parameters such as rapidity and amount of blood loss, Hb concentration <8 g/dl, patient age, cardiovascular status and symptoms (if any). Post-operative blood loss was assessed by measurement of blood collected in the suction drains at 6, 24 and at 48 h (at each measurement point, the blood collected was measured and then discarded) postoperatively. Hb and haematocrit values were recorded at 6 h after surgery, on the morning of post-operative day 1 and 2. Patients were monitored clinically for evidence of DVT twice daily. They were assessed for calf swelling, tenderness and oedema of the leg till discharge (usually on 7th post-operative day after suture removal). All the patients were started on DVT prophylaxis with enoxaparin 40 mg subcutaneous injection daily from the 1st post-operative day.
Statistical analysis was done by SAS software version 9.3 (SAS Institute Inc, US). The sample size calculation was done based on an earlier study (10). To detect a mean difference of 225 mL in the intraoperative blood loss between the control and study groups with a standard deviation (SD) of 200 mL, a power of 90% and a type-one error probability of 5%, a minimum of 18 patients in each group were needed. Mean, SD, median and interquartile ranges are given as definitive statistics. Data for the three treatment groups were analysed by ANOVA for variables following normal distribution. For variables following non-parametric distribution, P value was calculated by Kruskal–Wallis test. Intergroup comparison was done by post hoc analysis with Bonferroni test. P < 0.05 was considered statistically significant.
RESULTS
The baseline demographic data of the three groups are shown in Table 1. All the three groups were comparable with regard to their age, male: female ratio, weight, ASA status, type of hip surgery and their baseline Hb levels. Details of IO blood loss and collection in the suction drains at 6, 24 and 48 h are given in Table 2. The blood loss was lower in the patients who received TA (the mean IO blood loss was 525 mL in the Group C, 456 mL in the Group B and 400 mL in the Group I), but the difference did not reach statistical significance (P = 0.05). In the post-operative period, the 6th hourly drain collection showed a significant difference between the three groups (P = 0.018). Post hoc analysis showed that blood loss was significantly lower between the Group I and other groups (with Group C [P = 0.001] and Group B [P = 0.047]). The 24 hourly drain also showed a significant difference between the three groups (P = 0.02). Blood loss was reduced in both groups which received TA than in Group C {versus Group I (P = 0.009) and Group B (P = 0.03)}. Although bleeding was less in Group I than Group B at 24 h, it did not reach statistical significance (P = 0.95). The blood loss on 2nd post-operative day did not show any difference (P = 0.06) between the three groups.
Table 1.
Demographic data of the study groups
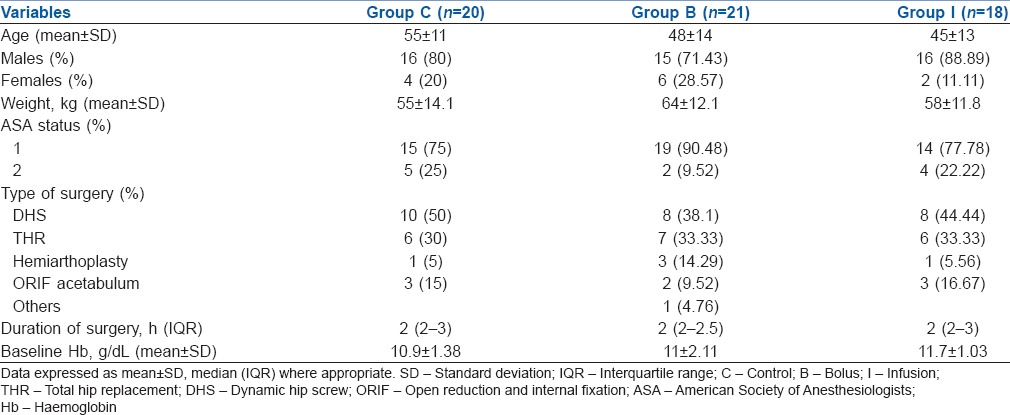
Table 2.
Details of intra-operative blood loss and collection in the suction drains at 6, 24 and 48 h
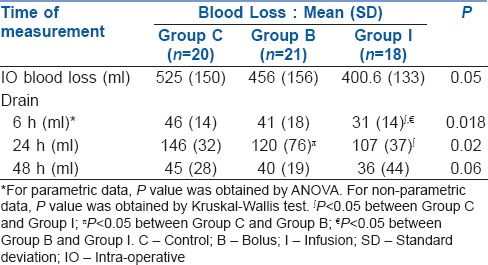
Hb done at 6 h, on 1st and 2nd post-operative day showed a significant difference between the three groups (P = 0.01) [Table 3]. Post hoc test showed that Hb at 6 h was better maintained with infusion as compared to Group C (P = 0.004). There was no difference in the 6 h Hb between control and bolus (P = 0.22) or bolus and infusion (P = 0.07). On post-operative day 1 and 2, post hoc test showed significantly lower Hb in the controls in comparison to the groups receiving TA (Group I [P < 0.01] and Group B [P < 0.01]). However, no difference in the Hb, either on 1st or 2nd day, was found between Group B versus Group I (P = 0.72).
Table 3.
Comparison of haemoglobin and packed cell volume between groups: Mean (SD)
A total of 22 transfusions were given in the post-operative period. Fourteen patients in Group C, six patients in Group B and two patients in Group I received transfusion of packed red blood cells. The number of transfusions was significantly less in the Group I compared to Group B and Group C. There was no clinically detected DVT or thromboembolic episodes in any of the groups.
DISCUSSION
Surgery for traumatic hip fractures is associated with significant blood loss. Treatment of perioperative anaemia with allogeneic blood transfusion increases the risk of infections, lung injury, renal failure, intravascular haemolysis, volume overload, immunomodulation as well as increased cost and prolonged hospital stay.[11,12,13] Different methods to reduce surgical bleeding such as IO acute normovolaemic haemodilution and hypotensive anaesthesia are time consuming, technically demanding and need logistic support. Antifibrinolytics such as EACA, aprotinin and TA have been found useful in reducing blood loss in cardiac, liver, orthopaedic and dental surgeries.[4,14,15,16,17] Apart from being more cost effective than aprotinin and more potent than EACA, TA is particularly useful in orthopaedic surgeries due to better penetration into the joint spaces. Several studies have shown that TA decreases bleeding and requirement of transfusions in hip, knee and spine surgeries, but confusion persists regarding the correct dosage and method of administration.[1,2,3] Recent literature has revealed that TA causes seizures by inhibition of gamma-aminobutyric acid receptors and alterations in cerebral blood flow and this risk is dose dependent.[18] With the possibility of seizures at higher doses and inadequate antifibrinolysis at low doses, administration of optimal dose of TA at optimal rate becomes important.
Varying doses of TA ranging from 10 to 100 mg/kg have been used. An IV dose of 10 mg/kg TA produces a therapeutic blood concentration of 5–10 mg/L for 3 h while a dose of 20 mg/kg maintains the same for 8 h. While the former may be insufficient to reduce bleeding, the latter was speculated to be associated with increased thrombosis. The present study found an insignificant decrease in IO blood loss with the administration of 10 mg/kg of TA as compared to the controls. This is similar to previous studies which also reported that use of TA decreased post-operative blood loss but not IO blood loss.[19,20] It was postulated that irrespective of the dose, this delayed response is expected as TA acts mainly on fibrinolysis and not on primary coagulation.[20]
However, a single IV dose of 10 mg/kg of TA has not consistently reduced bleeding and transfusion requirements. The short duration of action may not be adequate to suppress the fibrinolysis triggered in the early post-operative period, and as the compensatory antifibrinolytic mechanisms take up to 24 h to peak action, there is increased risk of bleeding in the immediate post-operative period. A higher initial dose or a repeat bolus dose may not be useful to reduce post-operative bleeding as uniform therapeutic levels may not be achieved. An infusion may provide constant blood levels and initiate or augment the antifibrinolytic activity earlier without the risk of thrombosis and seizures seen with higher doses. Confirming our rationale, bleeding 6 h postoperatively was significantly decreased when patients were continued on infusion than if they received a bolus of TA or placebo. However, decrease in bleeding was seen in both bolus and infusion groups at 24 h (though marginally more in Group I). This could be because TA as a single bolus (apart from infusion) was also effective in hastening the onset and amplification of antifibrinolysis as compared to placebo.
The fall in Hb also followed a similar pattern with the Group I maintaining better Hb at 6 h and the TA groups having higher Hb on day 1. The Hb on day 2 was also higher in the TA groups though no obvious bleeding was observed in the drains at 48 h. This may be because the overall perioperative blood loss was greater in the control group. Expectedly, the number of patients who required transfusion in the groups given TA was less.
There were some limitations to the present study. First, only clinical examination was relied upon for the diagnosis of DVT. Using objective imaging such as colour Doppler or venography of the lower limbs may have helped in increasing the accuracy of incidence of DVT. Second, the assessment of blood loss could have been comprehensive if internal haematomas were also measured either with an ultrasound or computed tomography scan of the surgical site. Third, an initial bolus of 15 mg/kg may have yielded better results in terms of bleeding. However, as it was the most commonly reported dose in literature, we used 10 mg/kg bolus in our study.
Several investigators have expressed concern that TA causes a hypercoagulable state and there is a trend in increased risk of vascular events.[7,21] However, the present study was not designed to measure long-term outcome measures such as myocardial infarction, cerebrovascular events and mortality in the 1st year after surgery. Two large meta-analyses have not found any increased risk of thromboembolic episodes with systemic TA.[1,22] The effects of TA are more pronounced in operative wounds than in peripheral venous blood because generation of tissue plasminogen activator occurs in wounds, and this may be the reason why systemic TA may be safe. However, further prospective studies addressing the issue of hypercoagulability with TA with specific emphasis on long-term vascular events are needed before the use of TA can be routinely recommended.
CONCLUSIONS
Tranexamic acid is effective in reducing perioperative blood loss when given as either bolus or bolus followed by infusion. Continuation of an infusion postoperatively is more useful than a single dose in decreasing blood loss in the first 6 h after surgery. Tranexamic acid significantly reduces the need for allogenic blood transfusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: A meta-analysis. Anaesth Intensive Care. 2003;31:529–37. doi: 10.1177/0310057X0303100507. [DOI] [PubMed] [Google Scholar]
- 2.Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am. 2008;90:2399–407. doi: 10.2106/JBJS.G.01179. [DOI] [PubMed] [Google Scholar]
- 3.Huang F, Wu D, Ma G, Yin Z, Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: A meta-analysis. J Surg Res. 2014;186:318–27. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: A randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–9. [PubMed] [Google Scholar]
- 5.Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemelä HM, Mäntylä SK, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–44. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: A randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72:442–8. doi: 10.1080/000164701753532754. [DOI] [PubMed] [Google Scholar]
- 7.Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 8.Jansen AJ, Andreica S, Claeys M, D'Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 9.Sano M, Hakusi H, Kojima C, Akimoto T. Absorption and excretion of tranexamic acid following intravenous, intramuscular and oral administrations in healthy volunteers. Jpn J Clin Pharmacol Therapeutics. 1976;7:375–81. [Google Scholar]
- 10.Benoni G, Bjorkman S, Fredin H. Application of pharmacokinetic data from healthy volunteers for the prediction of plasma concentrations of the tranexamic acid in surgical patients. Clin Drug Investig. 1995;10:280–7. [Google Scholar]
- 11.Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96:272–8. doi: 10.2106/JBJS.L.01268. [DOI] [PubMed] [Google Scholar]
- 12.Vamvakas EC, Blajchman MA. Transfusion-related mortality: The ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 13.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 14.Massicotte L, Denault AY, Beaulieu D, Thibeault L, Hevesi Z, Roy A. Aprotinin versus tranexamic acid during liver transplantation: Impact on blood product requirements and survival. Transplantation. 2011;91:1273–8. doi: 10.1097/TP.0b013e31821ab9f8. [DOI] [PubMed] [Google Scholar]
- 15.Dhir A. Antifibrinolytics in cardiac surgery. Ann Card Anaesth. 2013;16:117–25. doi: 10.4103/0971-9784.109749. [DOI] [PubMed] [Google Scholar]
- 16.Coetzee MJ. The use of topical crushed tranexamic acid tablets to control bleeding after dental surgery and from skin ulcers in haemophilia. Haemophilia. 2007;13:443–4. doi: 10.1111/j.1365-2516.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 17.Rannikko A, Pétas A, Taari K. Tranexamic acid in control of primary hemorrhage during transurethral prostatectomy. Urology. 2004;64:955–8. doi: 10.1016/j.urology.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: Causes and treatment. Ann Neurol. 2016;79:18–26. doi: 10.1002/ana.24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91:776–83. doi: 10.1302/0301-620X.91B6.22393. [DOI] [PubMed] [Google Scholar]
- 20.Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107:397–401. doi: 10.1080/00015458.2007.11680081. [DOI] [PubMed] [Google Scholar]
- 21.Reid RW, Zimmerman AA, Laussen PC, Mayer JE, Gorlin JB, Burrows FA. The efficacy of tranexamic acid versus placebo in decreasing blood loss in pediatric patients undergoing repeat cardiac surgery. Anesth Analg. 1997;84:990–6. doi: 10.1097/00000539-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty: A meta-analysis. J Arthroplasty. 2006;21:869–73. doi: 10.1016/j.arth.2005.09.009. [DOI] [PubMed] [Google Scholar]


