Abstract
Dichlorodiphenyltrichloroethane (DDT) reportedly causes extensively acute or chronic effects to human health. Exercise can generate positive stress. We evaluated the effect of aerobic exercise on DDT degradation and oxidative stress.
Main methods: Male Wistar rats were randomly assigned into control (C), DDT without exercise training (D), and DDT plus exercise training (DE) groups. The rats were treated as follows: DDT exposure to D and DE groups at the first 2 weeks; aerobic exercise treatment only to the DE group from the 1st day until the rats are killed. DDT levels in excrements, muscle, liver, serum, and hearts were analyzed. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) levels were determined. Aerobic exercise accelerated the degradation of DDT primarily to DDE due to better oxygen availability and aerobic condition and promoted the degradation of DDT. Cumulative oxidative damage of DDT and exercise led to significant decrease of SOD level. Exercise resulted in consistent increase in SOD activity. Aerobic exercise enhanced activities of CAT and GSH-Px and promoted MDA scavenging. Results suggested that exercise can accelerate adaptive responses to oxidative stress and activate antioxidant enzymes activities. Exercise can also facilitate the reduction of DDT-induced oxidative damage and promoted DDT degradation. This study strongly implicated the positive effect of exercise training on DDT-induced liver oxidative stress.
Keywords: Aerobic exercise, DDT degradation, Oxidative stress, Rats
1. Introduction
Dichlorodiphenyltrichloroethane (DDT), one of the typical persistent organic pollutants, was used worldwide in agricultural pest control and vector-borne disease control. Although the use of DDT has been limited internationally, its presence in unchanged or modified forms is still observed in the environment (water, sediment, and soil) (Dai et al., 2014, Turgut et al., 2013, Tang et al., 2014, Veljanoska-sarafiloska et al., 2013) and can even be detected in fodder plants (Yehouenou et al., 2013) in the bodies of animals (Deribe et al., 2013) and humans (Pérez-Maldonado et al., 2013, Teeyapant et al., 2014). This finding is attributed to its persistence, bioaccumulation, toxicity, and physical–chemical capacity for long-range transport (Aneck-Hahn et al., 2007, Sadasivaiah et al., 2007, Skinner et al., 2013).
Exposure to DDT causes a wide range of acute and chronic effects on human health, including increased risk of breast cancers (Tang et al., 2014), diabetes (Everett and Thompson, 2015), endocrine disruptor chemicals (Zhuang et al., 2012), and liver disease (Robinson et al., 2014). Moreover, ancestral exposure to DDT can promote obesity and associated disease transgenerationally (Skinner et al., 2013). Therefore, how to efficiently eliminate or reduce the adverse effects of DDT has been drawing much scientific and public attention. Over the past two decades, several physicochemical and biological remediation methods have been studied. Environmental transformation is the major process in the degradation pathway and can be subdivided into abiotic and biotic transformation. Research mainly focuses on the biotic transformation, which could be carried out by a single microbial species or a combination of several microbial species (Morrison et al., 2000). Partial degradation of DDT can be conducted, but aging, sequestration, and formation of toxic metabolites result in difficulties in bioremediation of DDT-contaminated soils (Sudharshan et al., 2012).
Knowledge on DDT degradation in the human body, an important factor affecting human health, is limited. Latest reports on human toxicity showed that DDT intake still occurs even in countries that banned its use decades ago (Tang et al., 2014), due to its long half-life in the environment, which can disturb our food supplies. High lipid solubility of DDT enables it to accumulate in the lipophilic component of the plasma in the body (Jaga and Dharmani, 2003). The half-life of this insecticide in serum is approximately 10 years (Turusov et al., 2002). Animal studies showed that exposure to DDT is linked to toxicity and endocrine disruption. However, few studies have been conducted to speed up the degradation or reduce the residual of DDT or its metabolites existing in our body.
Oxidative stress is one mechanism for toxicity of pesticides resulting in cell death (necrosis and apoptosis) and metabolic changes (Karami-Mohajeri and Abdollahi, 2011, Li et al., 2015). The hepatic antioxidant system is activated to prevent oxidative stress. This system includes the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). These enzymes work in a coordinated fashion to scavenge reactive oxygen species (ROS) and resist redox disturbances in cells. Balance between ROS production and its detoxification by these antioxidant enzymes is essential to prevent oxidative damage. Physical exercises can effectively upregulate antioxidant enzyme expression and activity (Bloomer, 2008, Lima et al., 2015, Wang et al., 2016). However, little research has focused on the effect of exercise training on DDT-induced oxidative stress concerning human health. Moderate aerobic exercise is an efficient strategy for public health promotion. Therefore, the purpose of the present study was to examine the effect of aerobic exercise on DTT degradation process and attenuation oxidative damage.
2. Material and methods
2.1. Animals and treatments
Animal Care and Use Committee of Shandong Sport University approved the experimental protocol, which was in compliance with the Guidelines for Care and Use of Laboratory Animals in the research prescribed by the Ministry of Science and Technology of China (2006). The principles of laboratory animal care (NIH publication No. 86-23, revised 1985) were followed.
Ninety male Wistar rats [mean (±SD) bodyweight of 156.4 (±5.6) g], provided by Shandong University laboratory animal center (China), were used in these experiments. All animals were maintained (2 rats per cage) in the same environment, temperature (23 ± 2 °C), humidity (50 ± 20%), illumination (12 h light/dark cycle), and with free access to standard pellet diet and water. DDTs (78.21% p,p′-DDT, 19.85% o,p′-DDT, 1.86% p,p′-DDD, and 0.08% p,p′-DDE) were purchased from National Research Center for Certified Reference Materials, China. After 1 week of acclimatization to the testing environment, the rats were randomly divided into three groups (n = 18/group): control (C), DTT without exercise training (D), and DTT plus exercise training (DE). The groups were treated for 6 weeks as follows: no treatment to C group; DDT exposure, dose of 40 mg/kg DTT per day, to D and DE groups for the first 2 weeks; aerobic exercise treatment using a treadmill at 18 m/min for 30 min per day, only to DE group. Excrement was collected throughout the experiment.
2.2. Sample preparation
In each group, the animals were sacrificed by cervical dislocation 15, 30, and 45 days later. Blood was drained from the neck and collected into 5 ml test tubes, centrifuged at 2938g at 4 °C for 10 min. Supernatant serum was immediately analyzed. The gastrocnemius muscle, liver, and heart were quickly removed, snap-frozen in liquid nitrogen, and stored at −80 °C for further analyses.
2.3. DDTs analyses
Levels of dichlorodiphenyl-tricloroethanes (DDTs) in excrements, muscles, livers, serum, and hearts from rats were analyzed as described by Johnson et al. (2012). The samples were analyzed for the following compounds: p,p′-DDT, p,p′-DDE, p,p′-DDD, o,p′-DDT, and total DDT (DDT).
2.4. Determination of SOD, CAT, GSH-Px, and MDA (malondialdehyde)
Liver homogenates were used to determine SOD, CAT, GSH-Px, and MDA levels. Commercial SOD, CAT, GSH-Px, and MDA assay kits (Nanjing Jiancheng Bioengineering Institute, China) were used in accordance with the manufacturer’s instructions.
2.5. Statistical analysis
Data of SOD, CAT, GSH-Px, and MDA levels are presented as mean ± SE, and the others are presented as mean ± SD. One-way analysis of variation with Tukey’s post hoc tests was used to compare the means between multiple groups. Analyses were performed using IBM SPSS Statistic V20.0 software, with P < 0.05 considered significant and P < 0.01 considered greatly significant.
3. Results
3.1. Effects of aerobic exercise on DDTs biodegradation
3.1.1. Levels of ∑DDT in different organs
As shown in Table 1, ∑DDT distributes similarly among tissues in different groups. In C group, the total DDT level on wet weight basis in the tissues always followed this order: liver > heart > muscle > excretion > serum. In D and DE groups, the DDT distributions between tissues were not the same as the C group. However, the liver, in general, contained the highest ∑DDT with two exceptions: D group, 4 weeks; and DE group, 6 weeks.
Table 1.
Level of ∑DDT (ng/g, wet wt; mean ± SD) in the organs of Wistar rats exposed to DDTs after 2, 4, and 6 weeks, respectively.
| C group | D group | DE group | ||
|---|---|---|---|---|
| 2 weeks | Liver | 1089.5 ± 245.4 | 6837.3 ± 429.4 | 14216.0 ± 2106.7 |
| Heart | 774.6 ± 223.2 | 2422.3 ± 395.2 | 8389.3 ± 483.3 | |
| Muscle | 658.4 ± 234.2 | 3088.1 ± 389.3 | 1162.6 ± 272.3 | |
| Serum | 4.2 ± 1.2 | 69.2 ± 9.3 | 21.4 ± 7.3 | |
| Excretion | 567.2 ± 123.1 | 7217.4 ± 4131.1 | 4827.6 ± 3930.7 | |
| 4 weeks | Liver | 1085.7 ± 244.5 | 3915.6 ± 414.2 | 18112.6 ± 2197.9 |
| Heart | 790.8 ± 229.4 | 9601.5 ± 388.8 | 8530 ± 581.9 | |
| Muscle | 679.5 ± 229.4 | 651.3 ± 237.3 | 285.2 ± 129.2 | |
| Serum | 4.7 ± 0.9 | 449.8 ± 54.1 | 27.4 ± 8.1 | |
| Excretion | 583.7 ± 145.2 | 4444.8 ± 2705.6 | 2389.4 ± 541.3 | |
| 6 weeks | Liver | 1094.4 ± 252.3 | 1727.8 ± 395.8 | 1183.3 ± 345.5 |
| Heart | 785.1 ± 318.4 | 1285.1 ± 378.6 | 5884.1 ± 493.4 | |
| Muscle | 667.3 ± 228.3 | 388.8 ± 226.3 | 1970.4 ± 332.5 | |
| Serum | 4.5 ± 1.1 | 26.7 ± 7.8 | 22.3 ± 6.2 | |
| Excretion | 554.4 ± 141.3 | 1921.2 ± 787.5 | 4388.4 ± 5041.5 | |
3.1.2. Biodegradation characteristic of DDTs in various tissues
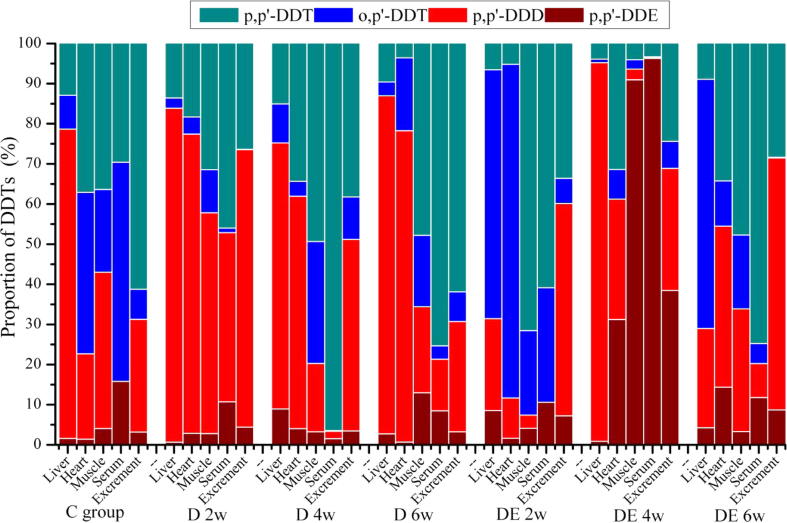
DDT can be converted to other more stable forms, including p,p′-DDD and p,p′-DDE. Similar to their mother compound, these metabolites are also hydrophobic and persistent. Fig. 1 demonstrates the proportion distributions of four DDT components (p,p′-DDT, o,p′-DDT, p,p′-DDD, and p,p′-DDE) in various organs of C, D, and DE groups, respectively. In the DE group at the 4th week, the percentage of p,p′-DDE obviously exceeded that of the other groups in the heart, muscle, serum, and excrement, respectively. However, p,p′-DDD was dominant in the D groups in the 2nd, 4th, and 6th weeks. In the liver, there was more p,p′-DDD than p,p′-DDE and less p,p′-DDE than that in the heart, muscle, serum, and excrement.
Figure 1.
The distribution proportion patterns of four DDT components in various organs of C, D, and DE groups, respectively. D 2 w = D group, 2 weeks; D 4 w = D group, 4 weeks; D 6 w = D group, 6 weeks; DE 2 w = DE group, 2 weeks; DE 4 w = DE group, 4 weeks; DE 6 w = DE group, 6 weeks.
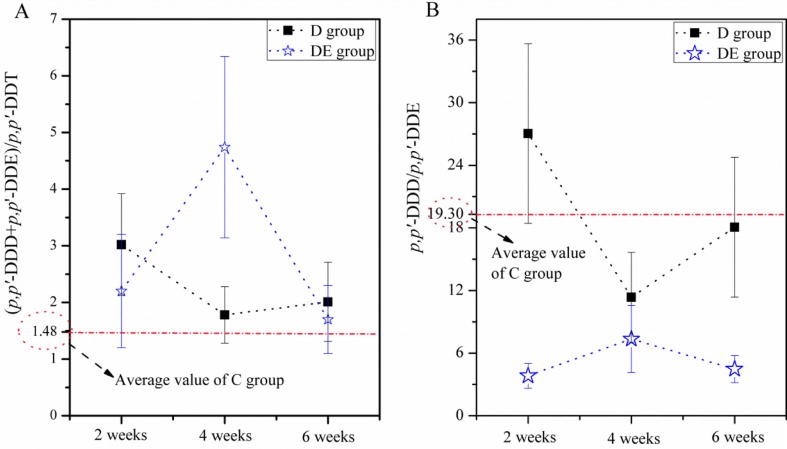
The ratios of parent DDT to its metabolites offer available messages on the identification of pollution source. Ratio of (p,p′-DDE + p,p′-DDD)/p,p′-DDT is a helpful indicator to judge DDT age and its decomposition rate. In the C group (Fig.2A), we found nearly the same ratio between 2, 4, and 6 weeks (data not shown). Therefore, to clearly illustrate the change between C group and the other two groups (i.e., D and DE groups), we took an average ratio among the three periods as reference value (1.48) and made a reference line in Fig.2A. The values in D and DE groups were all remarkably higher than in the C group, largely because rats in these two groups were exposed to additional DDTs. The highest degradation ratio was observed in the DE group after the rats were subjected to DTT plus exercise training for 4 weeks. Surprisingly, at 2 and 6 weeks, they were both lower than that in the D group. The results showed that these pollutant biotransformations were very efficaciously induced by aerobic exercise at 4 weeks.
Figure 2.
The ratios of (p,p′-DDE + p,p′-DDD)/p,p′-DDT (A) and p,p′-DDD/p,p′-DDE (B) in C, D, and DE groups, respectively. Results are presented as mean ± SD.
DDT is reductively dechlorinated to DDD under anaerobic conditions and to DDE under aerobic conditions. Thus, the prevailing DDD presence (high DDD/DDE ratio) indicates that the biotransformation rate of pollutants is under more anaerobic conditions. Results in Fig.2B display the ratio of p,p′-DDD to p,p′-DDE in C, D, and DE groups, respectively. As mentioned above (Fig.2A), the value of C group (19.30) was shown as a reference line. In Fig.2B, the DDD/DDE ratios were all more than 1. Compared with the C group, the ratio was significantly higher only in the 2nd week in the D group. In addition, the metabolic ratio values in the DE group were all lower than those in the D group.
3.2. Effects of aerobic exercise on oxidative stress
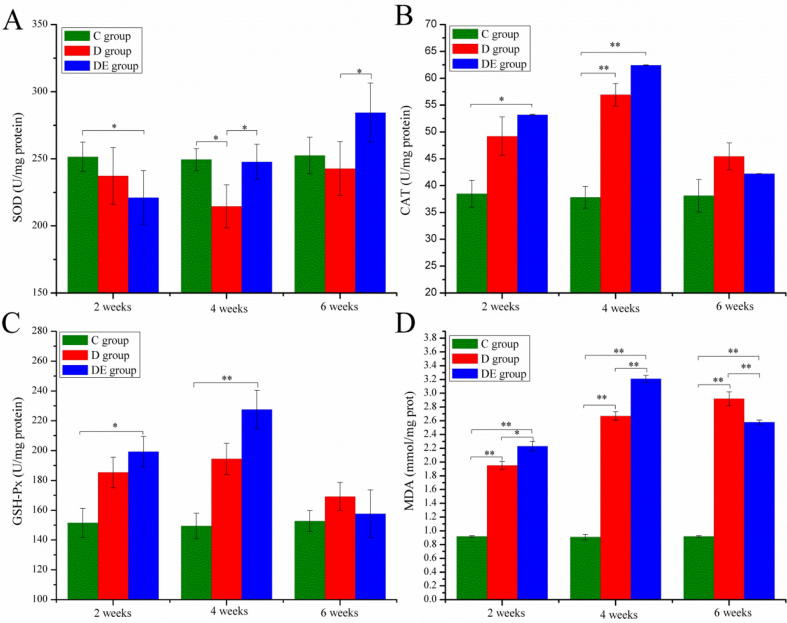
Fig. 3 illustrates the levels of SOD, CAT, GSH-Px, and MDA. In the 2nd week, the SOD activity in the DE group significantly decreased compared with that in C group (P < 0.05). No significant difference was found between D and C groups, although a downtrend was observed. Until the 4th week, D group showed marked decline compared with C and DE groups. From the 2nd week to the 6th week, SOD activity in DE group gradually increased and was significantly higher than in the D group in the 6th week (Fig.3A). From the 2nd week to the 4th week, the CAT activity in the D and DE groups continuously increased (Fig.3B). Compared with the C group, the D group showed significant difference in the 4th week, and the DE group did the same in the 2nd and 4th weeks, respectively. By contrast, after 6 weeks, CAT restored to the level of the C group. In Fig.3C, similar results to CAT activity were found in GSH-Px. However, no significant difference was found between the C and D groups in the 4th week. As shown in Fig.3D, significantly higher MDA level was detected in the D and DE groups. Compared with the D group, the DE group displayed significantly higher MDA level in the 2nd and 4th weeks and significantly lower level in the 6th week.
Figure 3.
Exercise training effects on SOD (A), CAT (B), GSH-Px (C), and MDA (D) level. Results are presented as mean ± SE. *P < 0.05, **P < 0.01.
4. Discussion
In addition to having health-promoting effects, exercise can induce oxidative stress as well as DDT (Shi et al., 2007). Oxidative stress is characterized by an imbalance between ROS and antioxidants defenses. When present in a high enough concentration, ROS can affect cellular components, especially lipids, which initiate a chain reaction, i.e., lipid peroxidation (Urso and Clarkson, 2003, Yoon and Park, 2014). DDT’s persistence and bioaccumulation are positively correlated with lipid content. Meanwhile, exercise reportedly generates positive stress. ROS produced during exercise sessions can be harmful to unprepared tissues but may also activate adaptive responses to oxidative stress inducing antioxidant defense systems by upregulation of responsible gene expression (Bouzid et al., 2014, Radak et al., 2008). Therefore, the aim of this study was to investigate the impact of exercise training on DDT degradation pattern and DDT-induced oxidative stress.
We investigated the effects of aerobic exercise on DDT distribution in rats. The samples were taken from the liver, heart, blood, muscle, and excrement. The heart and blood were included as sample materials due to their circulatory functions. Muscle was chosen because it participates in a dominant portion of exercise. Excrement was selected because its DDT content directly reflects the reduction of DDT metabolites. The liver is an organ associated with detoxification and tends to accumulate toxic substance. ∑DDT distributed similarly among tissues in different groups. The liver contained the highest ∑DDT (Table 1). Results suggested that the liver is the dominant tissue for organochloride accumulation due to its affinity to their lipid component; their concentrations in tissues were positively correlated with lipid content (Van Wyk et al., 2001, Wafo et al., 2005).p,p′-DDE is the primary metabolite and is generated by dehydrochlorination of p,p′-DDT. In addition, p,p′-DDT can be reductively dechlorinated to p,p′-DDD under reducing conditions (Sudharshan et al., 2012, Gao et al., 2011). Dehydrochlorination is the dominant reaction that facilitates the degradation of DDT primarily to DDE under aerobic conditions, whereas more rapid dechlorination results primarily in the formation of DDD under anaerobic conditions (Manz et al., 2001, Maliszewska-Kordybach et al., 2014). Aerobic exercise is widely accepted to involve more oxygen circulation throughout the body. In our research, p,p′-DDE is the dominant metabolite in the DE group, especially in the heart, muscle, serum, and excrement at the 4th week, and p,p′-DDD is dominant in the D groups. A higher proportion of p,p′-DDD is found in the liver, and a lower proportion of p,p′-DDE is found in the heart, muscle, serum, and excrement. These results may attribute to better oxygen availability in the heart, muscle, and serum, which possess circulatory functions, and during aerobic exercise, which involves more oxygen circulation.
Fig. 2 illustrates the degradation rate of DDT. The ratio of (p,p′-DDE + p,p′-DDD)/p,p′-DDT is a helpful indicator to judge p,p′-DDT age, i.e., old or new DDT input or exposure, and is often used to reveal DDT decomposition rate. In Fig.2A, the level of (p,p′-DDE + p,p′-DDD)/p,p′-DDT in D and DE groups was remarkably higher than that of control group, largely because the rats were exposed to additional DDTs. In the 2nd week, the ratio of DE group was lower than that of the D group. Nonetheless, it came to a culmination at the 4th week, gradually decreased thereafter, and was lower than D group again. In our research, we found a positive correlation between degradation ratio and oxidative stress, which will be discussed below. The lower degradation rate may be due to the cumulative stress from exercise, and DDT depressed the capability of DDT decomposition in rats. Afterward, exercise speeded up adaptive responses to oxidative stress or activated relevant enzymes and then promoted degradation of DDT in the body. Therefore, the highest value was observed in the 4th week. Therefore, the biotransformation rate of these pollutants was efficaciously induced by aerobic exercise.
Lower proportions of p,p′-DDD to p,p′-DDE suggest that DDT endures more aerobic transform in organisms. As shown in Fig.2B, the DDD/DDE ratios were all more than 1, indicating relatively anaerobic conditions during the DDT degradation process. Compared with the C group, the ratio was significantly higher only in the 2nd week in the D group, suggesting higher anaerobic conditions in this period. Notably, metabolic ratio values in the DE group were all lower than in the D group. Lower proportions of p,p′-DDD to p,p′-DDE suggested that DDT endured more aerobic transformation in exercising rats (DE group) than sedentary rats (C and D groups). Thus, aerobic exercise induced a significant increase in the degradation of DDT to DDE levels compared with the sedentary animals throughout the experimental period.
Exercise improves the antioxidant defense in many organs and pathologies. Lima et al. (2015) reported that exercise training attenuates the diabetes-induced alterations on antioxidant enzymes by improving the hepatic ROS scavenging and redox status. Peeri et al. (2013) demonstrated that regular aerobic exercise protects against nitric oxide deficiency-induced kidney damage by modifying HSP70, up-regulating SOD activity, and depleting TBARS. To further investigate the liver antioxidant enzyme response to aerobic exercise in DDT-induced oxidative stress, we analyzed the levels of total SOD, CAT, GSH-Px, and MDA. In this study, a combination of exercise and DDT treatment resulted in a significant decrease of SOD level compared with the control group and sedentary with DDT group (Fig.3A). This apparent reduction emerged most likely not only because of DDT induction but also because of the transitory production of ROS in the liver induced by aerobic exercise. Alternatively, it may be due to the fact that the body cannot timely activate adaptive responses to oxidative stress by upregulating antioxidant defenses. Exercise induced consistent increases in hepatic SOD activity for up to 6 weeks. However, when exposed to DDT only, SOD activity initially came to low ebb in the 4th week and gradually increased thereafter. These results suggested that exercise training could effectively benefit DDT-induced hepatic oxidative stress through SOD regulation.
Similar activity changes were found for CAT and GSH-Px levels (Fig.3B and C). In exercising rats, compared with the control group, significantly higher CAT and GSH-Px activity were observed from the 2nd week to the 4th week and then restored to the level of the C group. The increase in the CAT activity could be due to a higher production of the endogenous H2O2. In the 6th week, the H2O2 level restored to the normal level. This may be due to the fact that H2O2 was scavenged by cumulative effect from CAT and GSH-Px. We also found an increase of these two antioxidant enzymes in DDT-only rats compared with that in the controls, especially CAT level in the 4th week showed significant increase. CAT is often one of the earliest antioxidant enzymes to be induced (Cheung et al., 2001, Richardson et al., 2008). A similar result was observed in our research, i.e., CAT was induced in the 2nd week and was induced earlier than SOD. A positive correlation between hepatic CAT and GSH-Px was observed, probably because of their analogical functions, catalyzing transformation of hydroperoxide to molecular water. Together with SOD test, the above results suggested that exercise may boost activity and expression of antioxidant enzymes (SOD, CAT, and GSH-Px).
MDA, a biomarker to measure oxidative stress level, is formed during decomposition of polyunsaturated fatty acids induced by free radical attacks. A significant increase of MDA level was measured in the D and DE groups. Increases of MDA levels in both groups may result from the ROS induced by DDT or/and exercise. Compared with the D group, the DE group displayed significantly higher MDA level. This increase was observed to reach a peak in the 4th week and decreased thereafter. However, a consistent increase was observed in the D group. Thus, in the 6th week, significantly lower level of MDA was observed in the DE group compared with the D group, which may be a reflection of favorable effects of aerobic exercises on antioxidant defense. This finding agrees with the results of the SOD, CAT, and GSH-Px experiments.
5. Conclusions
Consistent with other studies, the present study shows that the liver, the detoxification organ in the animal body, is the dominant tissue for DDT metabolite accumulation, even after aerobic exercise. Aerobic exercise can accelerate degradation of DDT primarily to DDE due to better oxygen availability and aerobic condition. For the same reason, more DDE was generated in the heart, muscle, and serum than in the liver. Interestingly, at first, a combination of exercise and DDT inhibited DDT biotransformation most likely due to accumulating oxidative damage of DDT and exercise. Afterward, exercise speeded up adaptive responses to oxidative stress or activated relevant enzymes and then promoted DDT degradation in the body. Aerobic exercise also improved the antioxidant defense in DDT-induced oxidative stress. In accordance with DDT degradation, at first, cumulative oxidative damage of DDT and exercise led to significant decrease of SOD level. Exercise induced consistent increases in hepatic SOD activity by effectively upregulating SOD activity. Activities of CAT and GSH-Px were also enhanced by aerobic exercise. In addition, aerobic exercise can promote MDA scavenging, which is a final product of peroxidation. Together, results suggested that aerobic exercise may boost activity and expression of antioxidant enzymes and facilitate reduction of DDT-induced oxidative damage. This finding may provide new insight into the function of aerobic exercise in DDT degradation pattern and DDT-induced oxidative stress.
Acknowledgments
This work was supported by the Key Program of General Administration of Sport of China (2014B059), Natural Science Foundation of Shandong Province (ZR2013CM034), Science and Technology Program of Educational Commission of Shandong Province (J12LE04), Shandong Provincial Education Association for International Exchanges (2014), and the International Cooperation Program for Excellent Lecturers of 2013 by Shandong Provincial Education Department, P.R. China.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aneck-Hahn N.H., Schulenburg G.W., Bornman M.S., Farias P., De Jager C. Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J. Androl. 2007;28:423–434. doi: 10.2164/jandrol.106.001701. [DOI] [PubMed] [Google Scholar]
- Bloomer R.J. Effect of exercise on oxidative stress biomarkers. Adv. Clin. Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- Bouzid M.A., Hammouda O., Matran R., Robin S., Fabre C. Low intensity aerobic exercise and oxidative stress markers in older adults. J. Aging Phys. Act. 2014;22:536–542. doi: 10.1123/japa.2013-0037. [DOI] [PubMed] [Google Scholar]
- Cheung C.C., Zheng G.J., Li A.M., Richardson B.J., Lam P.K. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat Toxicol. 2001;52:189–203. doi: 10.1016/s0166-445x(00)00145-4. [DOI] [PubMed] [Google Scholar]
- Dai G.H., Liu X.H., Liang G., Gong W.W. Evaluating the exchange of DDTs between sediment and water in a major lake in North China. Environ. Sci. Pollut. Res. Int. 2014;21:4516–4526. doi: 10.1007/s11356-013-2400-8. [DOI] [PubMed] [Google Scholar]
- Deribe E., Rosseland B.O., Borgstrøm R., Salbu B., Gebremariam Z., Dadebo E. Biomagnification of DDT and its metabolites in four fish species of a tropical lake. Ecotoxicol. Environ. Saf. 2013;95:10–18. doi: 10.1016/j.ecoenv.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Everett C.J., Thompson O.M. Association of DDT and heptachlor epoxide in human blood with diabetic nephropathy. Rev. Environ. Health. 2015 doi: 10.1515/reveh-2015-0003. [Online 30 Mar 2015] [DOI] [PubMed] [Google Scholar]
- Gao B., Liu W.B., Jia L.Y., Xu L., Xie J. Isolation and characterization of an Alcaligenes sp. strain DG-5 capable of degrading DDTs under aerobic conditions. J. Environ. Sci. Health B. 2011;46:257–263. doi: 10.1080/03601234.2011.540534. [DOI] [PubMed] [Google Scholar]
- Jaga K., Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int. J. Occup. Med. Environ. Health. 2003;16:7–20. [PubMed] [Google Scholar]
- Johnson N.A., Ho A., Cline J.M., Hugh C.L., Foster W.G., Davis V.L. Accelerated mammary tumor onset in a HER2/Neu mouse model exposed to DDT metabolites locally delivered to the mammary gland. Environ. Health Perspect. 2012;120:1170–1176. doi: 10.1289/ehp.1104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami-Mohajeri S., Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum. Exp. Toxicol. 2011;30:1119–1140. doi: 10.1177/0960327110388959. [DOI] [PubMed] [Google Scholar]
- Li D.L., Peng W.X., Ge S.B., Li S.S., Mo B., Ohkoshi M. Groups characteristics of bioactivator extractives in three poplar woods. Wood Res. 2015;60(5):755–762. [Google Scholar]
- Lima T.I., Monteiro I.C., Valença S., Leal-Cardoso J.H., Fortunato R.S., Carvalho D.P. Effect of exercise training on liver antioxidant enzymes in STZ-diabetic rats. Life Sci. 2015;128:64–71. doi: 10.1016/j.lfs.2015.01.031. [DOI] [PubMed] [Google Scholar]
- Maliszewska-Kordybach B., Smreczak B., Klimkowicz-Pawlas A. Evaluation of the status of contamination of arable soils in Poland with DDT and HCH Residues; National and Regional Scales. Pol. J. Environ. Stud. 2014;23:139–148. [Google Scholar]
- Manz M., Wenzel K.D., Dietze U., Schüürmann G. Persistent organic pollutants in agricultural soils of central Germany. Sci. Total Environ. 2001;277:187–198. doi: 10.1016/s0048-9697(00)00877-9. [DOI] [PubMed] [Google Scholar]
- Morrison D.E., Robertson B.K., Alexander M. Bioavailability to earthworms of aged DDT, DDE, DDD, and dieldrin in soil. Environ. Sci. Technol. 2000;34:709–713. [Google Scholar]
- Peeri M., Habibian M., Azarbayjani M.A., Hedayapt M. Protective effect of aerobic exercise against L-NAME-induced kidney damage in rats. Arh. Hig. Rada Toksikoi. 2013;64:229–235. doi: 10.2478/10004-1254-64-2013-2260. [DOI] [PubMed] [Google Scholar]
- Pérez-Maldonado I.N., Trejo-Acevedo A., Pruneda-Alvarez L.G., Gaspar-Ramirez O., Ruvalcaba-Aranda S., Perez-Vazquez F.J. DDT, DDE, and 1-hydroxypyrene levels in children (in blood and urine samples) from Chiapas and Oaxaca, Mexico. Environ. Monit. Assess. 2013;185:9287–9293. doi: 10.1007/s10661-013-3251-y. [DOI] [PubMed] [Google Scholar]
- Radak Z., Chung H.Y., Koltai E., Taylor A.W., Goto S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Richardson B.J., Mak E., De Luca-Abbott S.B., Martin M., McClellan K., Lam P.K. Antioxidant responses to polycyclic aromatic hydrocarbons and organochlorine pesticides in green-lipped mussels (Perna viridis): do mussels ‘‘integrate”biomarker responses? Mar. Pollut. Bull. 2008;57:503–514. doi: 10.1016/j.marpolbul.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Robinson O., Want E., Coen M., Kennedy R., Van den Bosch C., Gebrehawaria Y. Hirmi Valley liver disease: a disease associated with exposure to pyrrolizidine alkaloids and DDT. J. Hepatol. 2014;60:96–102. doi: 10.1016/j.jhep.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Sadasivaiah S., Tozan Y., Breman J.G. Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control? Am. J. Trop. Med. Hyg. 2007;77:249–263. [PubMed] [Google Scholar]
- Shi M., Wang X., Yamanaka T., Ogita F., Nakatani K., Takeuchi T. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ. Health Prev. Med. 2007;12:202–208. doi: 10.1265/ehpm.12.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.K., Manikkam M., Tracey R., Guerrero-Bosagna C., Haque M., Nilsson E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013 doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharshan S., Naidu R., Mallavarapu M., Bolan N. DDT remediation in contaminated soils: a review of recent studies. Biodegradation. 2012;23:851–863. doi: 10.1007/s10532-012-9575-4. [DOI] [PubMed] [Google Scholar]
- Tang M., Zhao M., Zhou S., Chen K., Zhang C., Liu W. Assessing the underlying breast cancer risk of Chinese females contributed by dietary intake of residual DDT from agricultural soils. Environ. Int. 2014;73:208–215. doi: 10.1016/j.envint.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Teeyapant P., Sikaphan S., Parnmen S. Bioaccumulation of DDT residues in human serum: an historical use of DDT Indoor residual spraying in malaria endemic regions of Thailand. Environ. Asia. 2014;7:1–6. [Google Scholar]
- Turgut C., Cutright T.J., Mermer S., Atatanir L., Turgut N., Usluy M. The source of DDT and its metabolites contamination in Turkish agricultural soils. Environ. Monit. Assess. 2013;185:1087–1093. doi: 10.1007/s10661-012-2616-y. [DOI] [PubMed] [Google Scholar]
- Turusov V., Rakitsky V., Toamtis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ. Health Perspect. 2002;110:125–128. doi: 10.1289/ehp.02110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso M.L., Clarkson P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Van Wyk E., Bouwman H., Van der Bank H., Verdoorn G.H., Hofmann D. Persistent organochlorine pesticides detected in blood and tissue samples of vultures from different localities in South Africa. Comp. Biochem. Physiol. C. 2001;129:243–264. doi: 10.1016/s1532-0456(01)90201-7. [DOI] [PubMed] [Google Scholar]
- Veljanoska-sarafiloska E.M., Jordanoski M., Stafilov T. Presence of DDT metabolites in water, sediment and fish muscle tissue from Lake Prespa, Republic of Macedonia. J. Environ. Sci. Health B. 2013;48:548–558. doi: 10.1080/03601234.2013.774879. [DOI] [PubMed] [Google Scholar]
- Wafo E., Sarrazin L., Diana C., Dhermain F., Schembri T., Lagadec V. Accumulation and distribution of organochlorines (PCBs and DDTs) in various organs of Stenella coeruleoalba and a Tursiops truncatus from Mediterranean littoral environment (France) Sci. Total Environ. 2005;348:115–127. doi: 10.1016/j.scitotenv.2004.12.078. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang Y.Q., Silva J. Mechanism of pavement compaction detection technology to improve the quality of pavement construction. J. Mech. Eng. Res. Dev. 2016;39(1):112–118. [Google Scholar]
- Yehouenou A., Pazou E., Azehoun J.P., Aléodjrodo P.E., Van Straalen N.M., Van Hattum B., Van Gestel C.A. Health risks associated with pesticide residues in sediments, fish, and plants from the Ouémé Valley in the Republic of Bénin. Arch. Environ. Contam. Toxicol. 2013;65:260–265. doi: 10.1007/s00244-013-9895-3. [DOI] [PubMed] [Google Scholar]
- Yoon G.A., Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014;8:618–624. doi: 10.4162/nrp.2014.8.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S.L., Zhang J., Wen Y.Z., Zhang C.L., Liu W.P. Distinct mechanisms of endocrine disruption of DDT-related pesticides toward estrogen receptor α and estrogen-related receptor γ. Environ. Toxicol. Chem. 2012;31:2597–2605. doi: 10.1002/etc.1986. [DOI] [PubMed] [Google Scholar]