Abstract
Background:
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women, affecting 5–10% of reproductive-aged women. The dermatologic manifestations of hyperandrogenism, chiefly hirsutism, acne vulgaris, androgenic alopecia, and acanthosis nigricans, are among the cardinal manifestations of PCOS.
Aim:
To study the incidence and prevalence of various cutaneous manifestations in patients with PCOS and to correlate these skin manifestations with hormonal changes.
Settings and Design:
This study was conducted at a dermatology centre over a period of 1 year from November 2012 to 2013.
Materials and Methods:
The present study included 100 women diagnosed to have PCOS. Hormonal analysis as well as radiological assessment was done in all the cases. Cutaneous manifestations were ascertained and inferences were drawn.
Statistical Analysis:
Statistical analysis was carried out by the Chi-square test and independent samples t-test. Statistical significance was determined at a level of P < 0.05.
Results:
In our study, the prevalence of hirsutism, acne, female pattern hair loss, acanthosis nigricans, seborrhea, striae and acrochordons was 78%, 48%, 31%, 30%, 29%, 13%, and 9%, respectively.
Conclusion:
Dermatologic manifestations of PCOS play a significant role in making the diagnosis and constitute a substantial portion of the symptoms experienced by women with this syndrome.
Keywords: Acanthosis nigricans, acne, androgenic alopecia, hirsutism, hyperandrogenism, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) (Stein–Leventhal syndrome) is a common hyperandrogenic disorder in women of the childbearing age group. It is a multisystem metabolic disorder, which has a major impact on the quality of life as well as fertility.[1,2] A revised definition of PCOS was proposed in 2003 at an international joint consensus meeting of the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine.[3] The group recommended that a PCOS diagnosis could be achieved if two of the following three criteria are present:
Oligo-ovulation (fewer than 8 menses per 12-month period) and/or anovulation;
Clinical hyperandrogenism and/or biochemical signs of hyperandrogenism;
Polycystic ovaries (≥12 follicles in each ovary measuring 2–9 mm in diameter and/or increased ovarian volume >10 ml) by ultrasonography.
PCOS affects women of childbearing age with no predilection for race, however, the signs and symptoms may differ in various ethnic groups. Prevalence varies from 4% to 10%, depending on the diagnostic criteria used.[4] According to the Rotterdam criteria, the prevalence is five times higher than that defined by the criteria of the National Institutes of Health (NIH).[5] The common features of PCOS are irregular or anovulatory cycles with signs of hyperandrogenism such as acne, seborrhea, hirsutism, alopecia, frank virilization, and with polycystic ovaries on pelvic sonography. Recently, PCOS has been associated with obesity, insulin-resistance (IR), and a risk of developing Type 2 diabetes mellitus (T2DM).[6] The metabolic and reproductive abnormalities predispose women to develop infertility and endometrial cancer, necessitating early diagnosis and appropriate treatment.[7]
Because there is paucity of data regarding the cutaneous changes associated with PCOS, the present study was designed to determine the incidence and prevalence of dermatological manifestations as well as their correlation with hormonal changes.
Materials and Methods
This cross-sectional clinical study was carried out at a dermatology centre over a period of 1 year from November 2012 to October 2013. One hundred consecutively diagnosed cases of PCOS, aged between 20 and 40 years, were enrolled in the present study after obtaining an informed consent. The diagnosis of PCOS was made according to the consensus criteria adopted in May 2003 in Rotterdam.[8]
A standard questionnaire including demographic variables and reproductive and gynaecological history, with emphasis on regularity of menstrual cycle, hyperandrogenic symptoms, family history of irregular menstrual cycles, and treatment history was elicited in each case during face-to-face interviews. Oligomenorrhea was defined as fewer than 9 menstrual periods per year or a cycle duration of at least 45 days. Amenorrhea was defined as absence of menstruation for 3 consecutive months during the previous year.
A detailed medical history was elicited in each case, with particular emphasis on various cutaneous complaints including their duration, evolution and progression. An informed consent was taken from each patient, after which a general physical examination, systemic examination, breast and pelvic examination, as well as a detailed dermatological examination was carried out; the relevant details were recorded and tabulated. The patients were screened for signs of hyperandrogenism such as acne vulgaris, hirsutism, seborrhea, acanthosis nigricans, acrochordons, hair loss due to androgenic alopecia, and striae distensae.
The presence of comedones, erythematous papules and pustules, and nodules and cysts on the face, neck, upper chest, upper back, and upper arms were classified as acne. Pigmented, raised, warty, velvety skin patches on the intramammary folds, nape of neck, and antecubital fossae were classified as acanthosis nigricans. Androgenic alopecia was evaluated according to Ludwig's classification. Hirsutism was assessed using the modified Ferriman-Gallwey (F–G) score, in which a score >8 indicates hirsutism. The presence of greasy or oily and shiny skin on the nasolabial folds, the forehead, or behind the ear and hair was defined as seborrhea or oily skin. Women were given a questionnaire in which they were asked if their skin felt greasy, shiny, or oily on nasolabial folds, forehead, or behind their ear, and they were asked to chose an answer from two choices (yes or no).
Height and weight were recorded by standard methods while the patients were lightly clothed and barefooted. Waist circumference was measured in centimeters at the level of the umbilicus, without clothing and in standing position. Hip circumference was also measured in centimeters at the level of greater trochanters. Waist-to-hip (W/H) ratios and body mass index (BMI; kg/m2) were calculated. W/H ratio greater than 0.85 indicated android fat distribution.
After an overnight fasting, venous blood samples were obtained on the second day of spontaneous menstruation for assessment of baseline levels of follicle stimulating hormone (FSH; normal range: 2.5–10.2 IU/L), luteinizing hormone (LH; normal range: 1.9–12.5 IU/L), testosterone (normal range: 14–76 ng/dl), dehydroepiandrosterone sulfate (DHEAS; normal range: 61.2–493.6 µg/dl), and prolactin (PRL; normal range: 2.8–29.2 IU/L) using enzyme immunoassay (EIA).
In addition to hormonal analysis, fasting glucose, fasting insulin, serum lipids, and serum TSH were also measured in these patients. Insulin resistance (IR) was determined by calculating homeostasis model assessment (HOMA) score as fasting plasma glucose (mg/dL) × fasting plasma insulin (mU/mL)/405.
For the diagnosis of PCO morphology, all women underwent a transvaginal ultrasonography during the early follicular phase. The presence of PCO was diagnosed by the presence of 12 or more follicles measuring 2–9 mm in diameter in each ovary and/or increased ovarian volume (>10 cm3).
Exclusion criteria were patients with known thyroid disorder, hypothalamic, pituitary, or adrenal disease; aged less than 20 years; and taking hormonal medications or drugs such as atorvastatin calcium, simvastatin calcium, metformin hydrochloride, or glipizide during the 2 months preceding the study.
Statistical analysis of the data was performed by appropriate statistical methods using the Statistical Package for Social Sciences (SPSS Version 17). Statistical significance of the results was determined by the Chi-square test and independent samples t-test. Statistical significance was determined at a level of P < 0.05.
Results
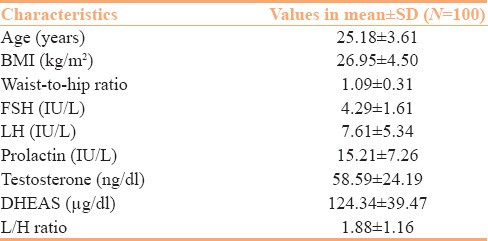
The present study comprised 100 female patients diagnosed to have PCOD. The anthropometric characteristics of the study population including age, BMI, and W/H ratio are given in Table 1.
Table 1.
Anthropometric and hormonal characteristics of women in the study group
Mean age of menarche in our patients was 13.8 ± 0.9 years. Menstrual disturbances were seen in 65 females, whereas 35 females had a normal menstrual pattern. The most common menstrual disturbance noticed was oligomenorrhea, seen in 57 females, followed by amenorrhea, seen in 8 females.
As per BMI, the prevalence of obesity and overweight in our study group was 27% and 53%, respectively, whereas 20% of our patients in the study group had a normal weight.
Among 100 study patients, 15 patients were married whereas 85 were unmarried. Among the married group of 15 patients, 11 (73.3%) had normal obstetric history, whereas 4 (26.7%) patients had an issue of primary infertility.
Mean ± SD values of LSH, FSH, prolactin, testosterone, DHEAS, as well as LH/FSH ratio in our study patients are shown in Table 1.
Cutaneous manifestations secondary to a hyperandrogenic state in these patients are depicted in Figure 1. The prevalence of hirsutism, acne, female pattern hair loss, acanthosis nigricans, seborrhea, striae, and acrochordons was found to be 78%, 48%, 31%, 30%, 29%, 13%, and 9%, respectively.
Figure 1.
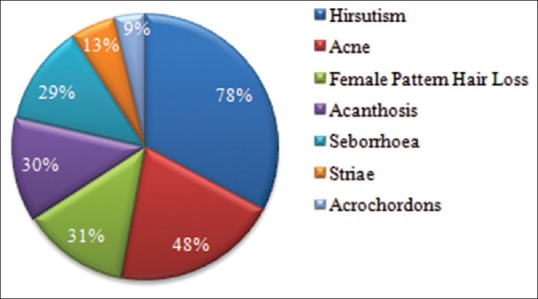
Prevalence of cutaneous manifestations in the study group of PCOS patients
Figure 2 shows the increased levels of hormones in the patients of PCOS. As depicted in the figure, the most common hormonal abnormality encountered in our patients was raised testosterone levels (28%). The results of logistic regression analysis for the cutaneous features hirsutism and seborrhea as dependent variables are presented in Table 2.
Figure 2.
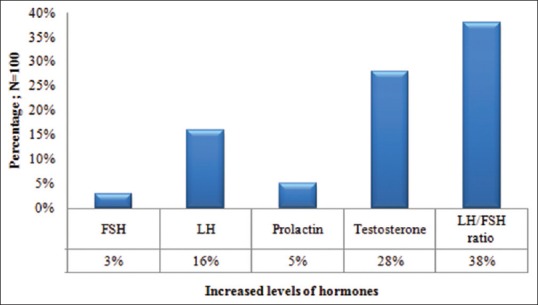
Increased hormonal levels in the study group of PCOS patients
Table 2.
Logistic regression analysis with hirsutism and seborrhea as dependent variables
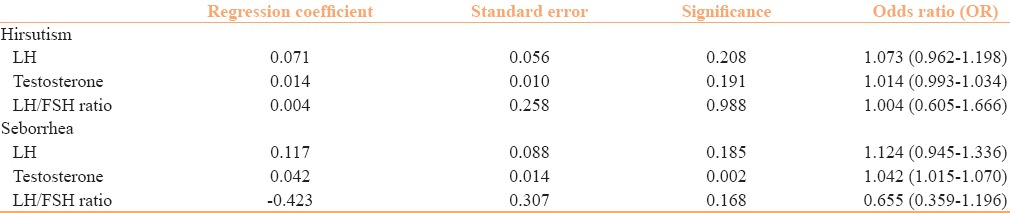
There was a statistically significant association between age and hirsutism. Patients with hirsutism were found to be younger (mean age: 24.72 ± 3.39 years) compared to non-hirsute women (mean age: 26.82 ± 3.96 years), with a P value of 0.015. No statistically significant association was seen between age and other cutaneous features.
Discussion
In 1935, Stein and Leventhal reported a series of 7 women who presented with oligo/amenorrhoea, hirsutism, obesity, infertility, and bilateral polycystic ovaries (Stein–Leventhal syndrome).[9] They concluded that there was a primary ovarian defect, and the disorder was termed polycystic ovarian disease (PCOD). In present times, PCOD has been associated with various metabolic disorders, and is now known as polycystic ovarian syndrome (PCOS).
Although PCOS is a heterogeneous disorder without an easily identified single etiology, the key pathophysiologic components appear to include androgen excess, abnormal gonadotropin dynamics, and IR. Excess androgen production in the ovary impairs follicle maturation, leading to follicular atresia and decreased reproductive function. In addition, the resultant hyperandrogenemia may produce clinical hyperandrogenism. Whether due to an underlying primary hypothalamic defect in the gonadotropin releasing hormone (GnRH) pulse generator or a secondary effect of low levels of progesterone resulting from oligo- or anovulation,[10] an increased pulse frequency of hypothalamic GnRH is thought to produce elevated levels of luteinizing hormone (LH) found in women with PCOS.[11] This increase in LH secretion relative to FSH stimulates production of androstenedione by ovarian theca cells. Insulin also plays a central role in PCOS pathophysiology, acting to increase androgen levels by direct and indirect mechanisms.[12]
The patient may seek a dermatology consultation for one or more complaints of hyperandrogenism. PCOS is a multisystem disorder closely associated with obesity. The prevalence of obesity and overweight women in our study was 27% and 53%, respectively. A study conducted by Majumdar et al. revealed the prevalence rate of obesity to be 37.5%.[13] Lim et al. in a systemic review and meta-analysis concluded that women with PCOS had a greater risk of overweight, obesity, and central obesity.[14]
In our study group, menstrual disturbances were seen in 65% of the PCOS women, whereas 35% of the patients had normal menstrual cycles. Among these, oligomenorrhea was the most common menstrual disturbance, seen in 57%, followed by amenorrhea seen in 8% of the PCOS women. Ramanand et al. reported irregular cycles in 100% of their study participants, whereas infertility was present in 21%.[15]
Gowri et al. evaluated the incidence and prevalence of skin manifestations in 40 patients with PCOS. In their study, of all the cutaneous manifestations, acne was seen in the highest percentage (67.5%), followed by hirsutism (62.5%), seborrhea (52.5%), androgenetic alopecia (AGA) (30%), acanthosis nigricans (22.5%), and acrochordons (10%).
The prevalence of hirsutism [Figure 3] in our study group of PCOS women was found to be 78%. Some studies report a prevalence of hirsutism in women with PCOS in the range of 50–76%.[16,17] Saxena et al. in their study reported that the prevalence of hirsutism was 89% and 80% in obese and lean PCOS, respectively.[18] In our study, the modified F–G score was used to determine the severity of hirsutism.[19] Maximum hirsutism F–G score in our patients was 16 with a mean ± SD of 12 ± 2.44.
Figure 3.
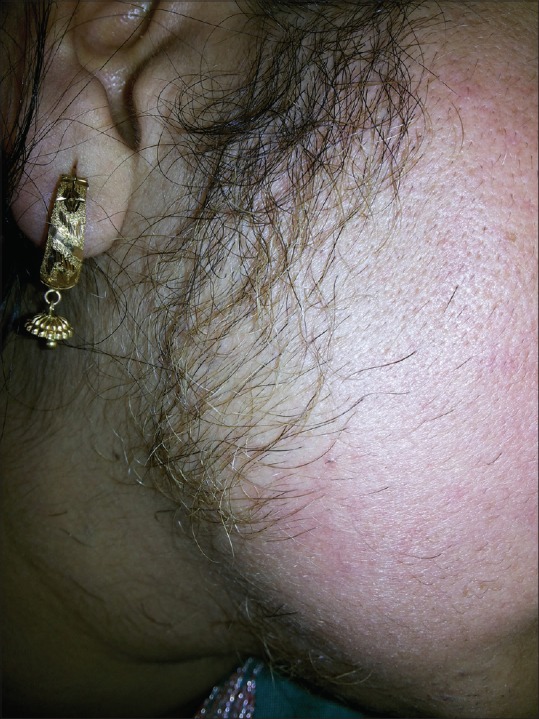
Facial hirsutism in a female with PCOS
The next common clinical manifestation of hyperandrogenism noted in our study was acne [Figure 4], which was seen in 48% of the women. Past studies have estimated the prevalence of acne in patients with PCOS at 9.8–34%.[20,21] One study among 129 women seen at a dermatology clinic for acne found that 19% met the criteria for PCOS defined by menstrual disturbances, hyperandrogenism, polycystic ovaries on ultrasound, and an elevated LH:FSH ratio.[22]
Figure 4.
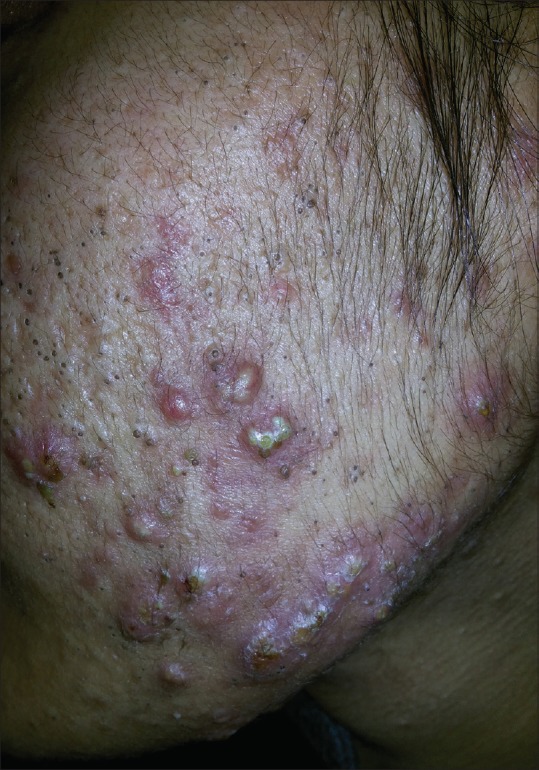
Severe pustular acne in a young female with PCOS
In the present study, the prevalence of female pattern hair loss [Figure 5] in PCOS women was found to be 31%. Only a few studies have specifically examined pattern of hair loss in women with PCOS. In a cohort of 950 women referred for clinical hyperandrogenism, of whom 72% were diagnosed with PCOS, alopecia ranging from Ludwig pattern type I (mild) to type III (severe) was found in 3.2%.[23]
Figure 5.
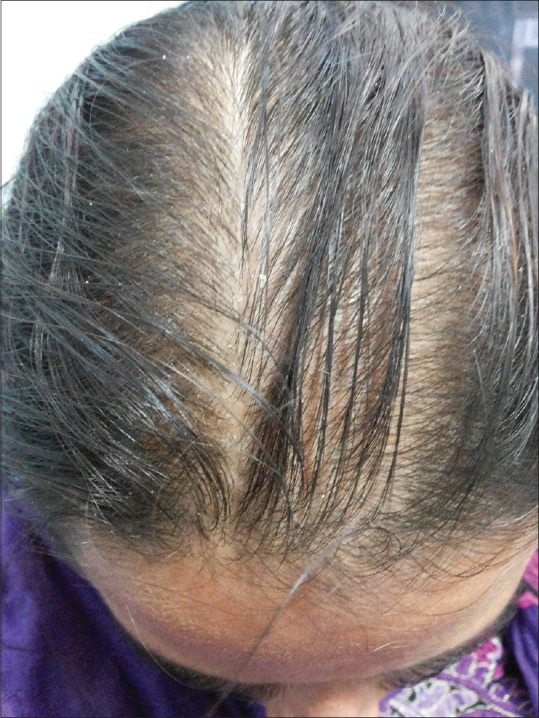
Female pattern hair loss
Seborrhea has also been described as a cutaneous feature in PCOS. However, its prevalence in PCOS is not exactly known.[24] The prevalence of seborrhea was found to be 29% in the present study. In addition to androgen hormones, the patient's genetic predisposition, and climate and emotional factors are other important factors affecting seborrhea. For these reasons, clinical, serological, and radiological examinations are important for the diagnosis of seborrhea related to PCOS. We evaluated seborrhea in a parametric manner. It may be suggested that hormonal and metabolic factors related with seborrhea in PCOS should be assessed using a Sebumeter for a metric measurement.
Acanthosis nigricans is characterized by velvety, brown, thickened plaques with accentuated skin markings. Although acanthosis nigricans is most commonly observed in settings such as obesity, PCOS, and diabetes; it can also be associated with multiple genetic variants, reactions to medications such as nicotinic acid,[25] and malignancy.[26] In our study, the prevalence of acanthosis nigricans [Figure 6] in PCOS women was seen to be 30%, and the most common site was nape of the neck, followed by axillae and antecubital fossae. In other studies, the prevalence of acanthosis nigricans in PCOS women was found to be 22.5% to 44.16%.[15,27] Straie distensae [Figure 7] and acrochordons [Figure 8] were other cutaneous manifestations seen in 13% and 9% of the PCOS women, respectively. However, further epidemiologic studies need to be conducted to establish the prevalence of acanthosis nigricans in patients with PCOS.
Figure 6.
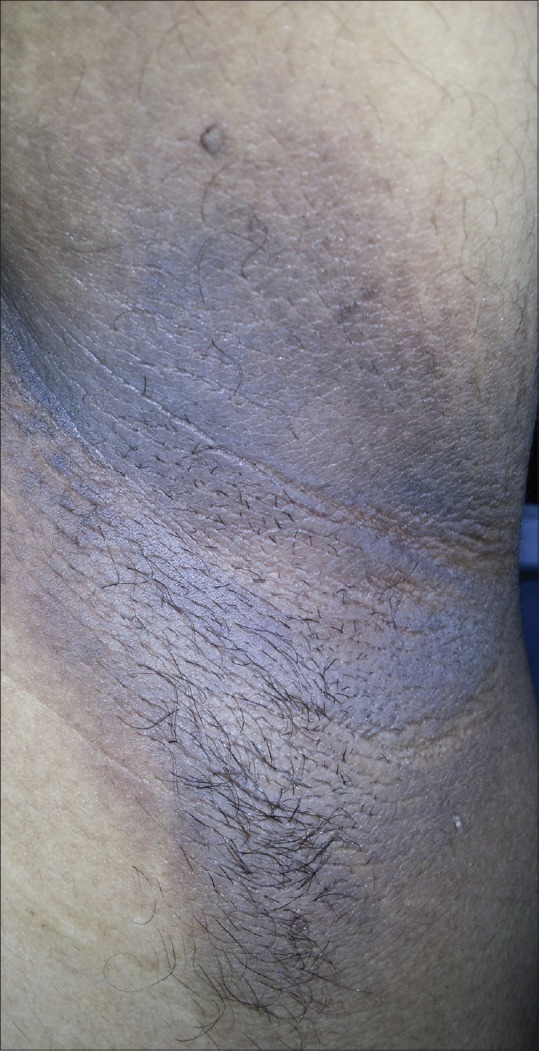
Acanthosis nigricans in the axillary region
Figure 7.
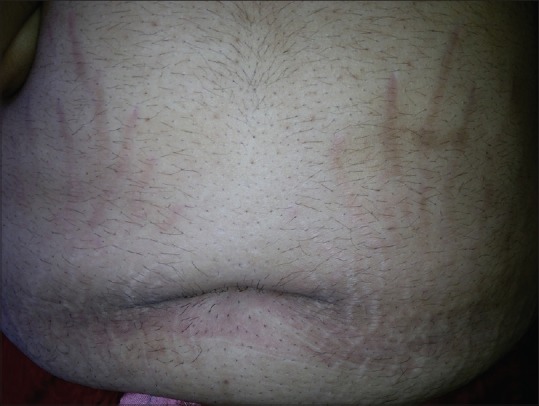
Striae distensae in a female with PCOS
Figure 8.
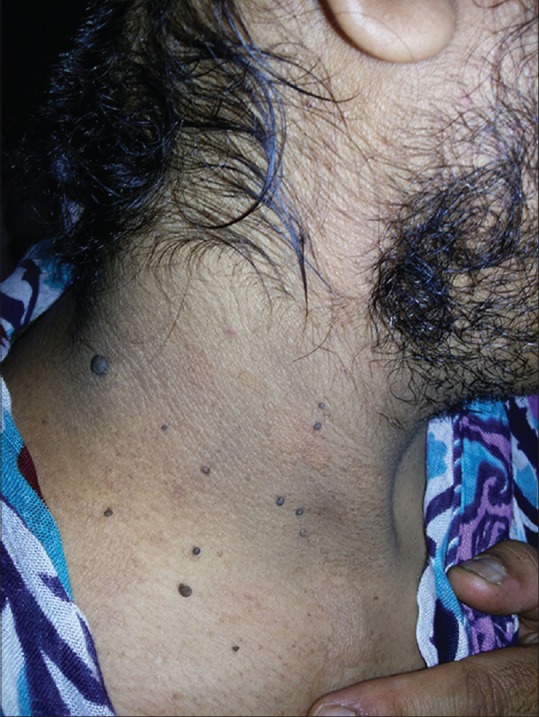
Acrochordons, acanthosis, and facial hirsutism in a female with PCOS
Conclusion
PCOS is one of the most common endocrine disorders in women of reproductive age and can be associated with multiple long-term health risks and substantial psychological impact. The dermatologic manifestations of PCOS play a significant role in diagnosis and constitute a substantial portion of the symptoms experienced by women with this syndrome. Patients must be counseled regarding the long duration of treatment that includes lifestyle modifications along with systemic treatment. Success in the effective management of women with PCOS is through a synchronized effort between the dermatologist, endocrinologist, gynecologist, nutritionist, and physical trainer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Frank S, Gharani N, McCathy M. Candidates genes in Polycystic Ovary Syndrome. Hum Reprod. 2001;7:405–0. doi: 10.1093/humupd/7.4.405. [DOI] [PubMed] [Google Scholar]
- 2.Elsenbruch S, Hahn S, Kowalsky D, Offner AH, Schedlowski M, Mann K, et al. Quality of life, psychosocial wellbeing, and sexual satisfaction in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003;88:5801–7. doi: 10.1210/jc.2003-030562. [DOI] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi MO, Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193–205. doi: 10.1016/j.beem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Buccola JM, Reynolds EE. Polycystic ovary syndrome: A review for primary providers. Prim Care. 2003;30:697–710. doi: 10.1016/s0095-4543(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Oh JY, Sung YA, Chung H, Cho WY. The prevalence and risk factors for glucose intolerance in young Korean women with polycystic ovary syndrome. Endocrine. 2009;36:326–32. doi: 10.1007/s12020-009-9226-7. [DOI] [PubMed] [Google Scholar]
- 7.Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Australian ovarian cancer study group and australian national endometrial cancer study group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control. 2010;21:2303–8. doi: 10.1007/s10552-010-9658-7. [DOI] [PubMed] [Google Scholar]
- 8.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 10.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–7. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: Implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–26. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- 12.Stubbs SA, Webber LJ, Stark J, Rice S, Margara R, Lavery S, et al. Role of Insulin-like growth factors in initiation of follicle growth in normal and polycystic human ovaries. J Clin Endocrinol Metab. 2013;98:3298–305. doi: 10.1210/jc.2013-1378. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar A, Singh TA. Comparison of clinical features and health manifestations in lean vs obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12–7. doi: 10.4103/0974-1208.51336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovarian syndrome: A systemic review and meta-analysis. Hum Reprod Update. 2012;18:618–37. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 15.Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17:138. doi: 10.4103/2230-8210.107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: Clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–23. doi: 10.1016/j.fertnstert.2005.01.096. [DOI] [PubMed] [Google Scholar]
- 17.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 18.Saxena P, Prakash A, Nigam A, Mishra A. Polycystic ovarian syndrome: Is obesity a sine qua non? A clinical, hormonal and metabolic assessment in relation to body mass index. Indian J Endocr Metb. 2012;16:996–9. doi: 10.4103/2230-8210.103011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coskun A, Ercan O, Arikan DC, Ozer A, Kilinc M, Kiran G, et al. Modified Ferriman-Gallwey hirsutism score and androgen levels in Turkish women. Eur J Obstet Gynecol Reprod Biol. 2011;154:167–71. doi: 10.1016/j.ejogrb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–8. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 21.Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ. The Polycystic Ovary Syndrome health-related quality of life Questionnaire (PCOSQ): A validation. Hum Reprod. 2004;19:371–7. doi: 10.1093/humrep/deh048. [DOI] [PubMed] [Google Scholar]
- 22.Borgia F, Cannavo S, Guarneri F, Cannavò SP, Vaccaro M, Guarneri B. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201–4. doi: 10.1080/00015550410023248. [DOI] [PubMed] [Google Scholar]
- 23.Carmina E, Rosato F, Janni A, Rizzo M, Longo RA. Extensive clinical experience: Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91:2–6. doi: 10.1210/jc.2005-1457. [DOI] [PubMed] [Google Scholar]
- 24.Lee AT, Zane LT. Dermatologic manifestations of polycystic ovary syndrome. Am J Clin Dermatol. 2007;8:201–19. doi: 10.2165/00128071-200708040-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hartman R, Defelice T, Tzu J, Meehan S, Sanchez M. Acanthosis nigricans in the setting of niacin therapy. Dermatol Online J. 2011;17:11. [PubMed] [Google Scholar]
- 26.Singh GK, Sen D, Mulajker DS, Suresh MS. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722–5. doi: 10.4103/0019-5154.91837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gowri BV, Chandravathi PL, Sindhu PS, Naidu KS. Correlation of skin changes with hormonal changes in polycystic ovarian syndrome: A cross-sectional study clinical study. Indian J Dermatol. 2015;60:419. doi: 10.4103/0019-5154.160505. [DOI] [PMC free article] [PubMed] [Google Scholar]


