Abstract
The family of Wuschel-related Homeobox (WOX) genes is a class of transcription factors involved in the early stages of embryogenesis and organ development in plants. Some of these genes have shown different transcription levels in embryogenic tissues and mature organs in two different cultivars of Vitis vinifera: ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS). Therefore, we investigated the genetic basis responsible for these differences by cloning and sequencing in both the cultivars the promoter regions (~2000 bp) proximal to the transcription start site of five VvWOX genes. We then introduced these promoters into Arabidopsis thaliana for expression pattern characterisation using the GUS reporter gene. In the transgenic Arabidopsis, two promoters isolated from CS (pVvWOX13C_CS and pVvWOX6_CS) induced increased expression compared to the sequence isolated in CH, confirming the data obtained in grapevine tissues. These results were corroborated by transient expression assays using the agroinfiltration approach in grapevine somatic embryos. Truncated versions of pVvWOX13C demonstrated that few nucleotide differences between the sequences isolated from CH and CS are pivotal for the transcriptional regulation of VvWOX13C. Analysis of promoters using heterologous and homologous systems appear to be effective for exploring gene modulation linked with intervarietal sequence variation in grapevine.
The regulation of gene transcription in eukaryotes is a complex mechanism that requires precise interactions among several proteins and DNA sequences. Promoters are cis-regulatory elements composed of non-coding DNA containing binding sites for transcription factors (TFs), which activate and sustain the transcription of genes1. The spatial and temporal regulation of the gene transcription is an important system influencing the plant development and the response to environmental stimuli such as light, biotic and abiotic stresses. Furthermore, the mutations affecting the gene regulation were considered important sources of evolutionary change and could be one of the most important cause of morphological divergence between organisms1,2. Indeed, the mutations in protein-coding regions could have more pleiotropic effects, because generally they affect all tissues in which the protein is active, whereas the change of a tissue-specific regulatory element in the promoter should affect only the cells interested by the specific expression change1,2. In this context, the study of promoters becomes particularly important for deepening the genetic basis of phenotypic variants in species such as grapevine (Vitis vinifera L.), characterized by thousands of different cultivars highly heterozygous3. In the last years, there has been a new impetus to study promoters in grapevine4,5, which arises from the applications of new “Sustainable Biotechnology”, i.e. cis-genesis and genome editing6,7, for which it is very important to know and access many regulatory sequences.
The WUSCHEL (WUS)-related Homeobox (WOX) gene family is a class of homeodomain transcription factors involved in plant development by regulation of cell division and differentiation8. These genes were first characterised in Arabidopsis8, and in the following years their function has been analysed in several species9,10,11,12. The WOX family consists of 15 members in the Arabidopsis genome8. The first identified WUS is required for the maintenance of stem cells in the shoot apical meristem13. The other members are involved in many different phases of plant development: WOX5 performs a function similar to that of WUS in the root meristem14,15 and WOX4 regulates the vascular cellular division16. WOX6 is required for ovule development17, and WOX2, WOX8 and WOX9 are important cell fate regulators of early pre-embryos8,18. In addition, these transcription factors generally show a high functional redundancy19, and some remain partially uncharacterised due to a lack of clear phenotypes in loss-of-function mutants.
In grapevine, 12 VvWOX genes were previously identified and their expression levels were analysed in somatic embryogenic tissues20. Somatic embryogenesis is the regenerative process most used in grapevine for genetic transformation21. However, it is negatively affected by many factors, such as genotype: indeed cultivars of V. vinifera vary widely in their potential in forming embryogenic tissues, and some are particularly recalcitrant22. VvWOX genes are important regulators of somatic embryogenesis in grapevine20 and, interestingly, their regulation during the early phase of the regenerative process differs between the two cultivars ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS), showing respectively high and low aptitude to embryogenesis. VvWOX9 is the main WOX gene expressed during the somatic embryogenesis process, and the low aptitude for embryogenesis observed in CS could be partially correlated with low expression levels of this gene20. In addition, other VvWOX genes have showed significant expression differences between CH and CS in different tissues. In particular, VvWOX3, VvWOX4, VvWOX6, VvWOX13A and VvWOX13C are expressed at high levels in undifferentiated calli of CS; VvWOX1 and VvWOX2 are expressed at high levels in embryogenic calli of CH; and VvWOX6 is expressed at a high level in the anthers of CS20.
In this work, five VvWOX genes showing the higher expression differences between CH and CS and belonging to the different evolutionary lineages previously reported: modern (VvWOX1, VvWOX4 and VvWOX6) intermediate (VvWOX9) and ancient clade (VvWOX13C)20 were chosen for further analyses. The transcriptional characterisation of these genes was extended to several grapevine organs, and promoter regions from CH and CS, proximal to transcription start sites, were cloned for the production of promoter::GUS fusion constructs. In transgenic Arabidopsis and in agroinfiltrated somatic embryos of grapevine, some point mutations associated with transcription factor binding sites (TFBSs) resulted responsible for a higher level of transcription induced by the promoters of VvWOX13C and VvWOX6 isolated from CS (pVvWOX13C_CS and pVvWOX6_CS).
Results and Discussion
Expression of VvWOX genes in different grapevine organs
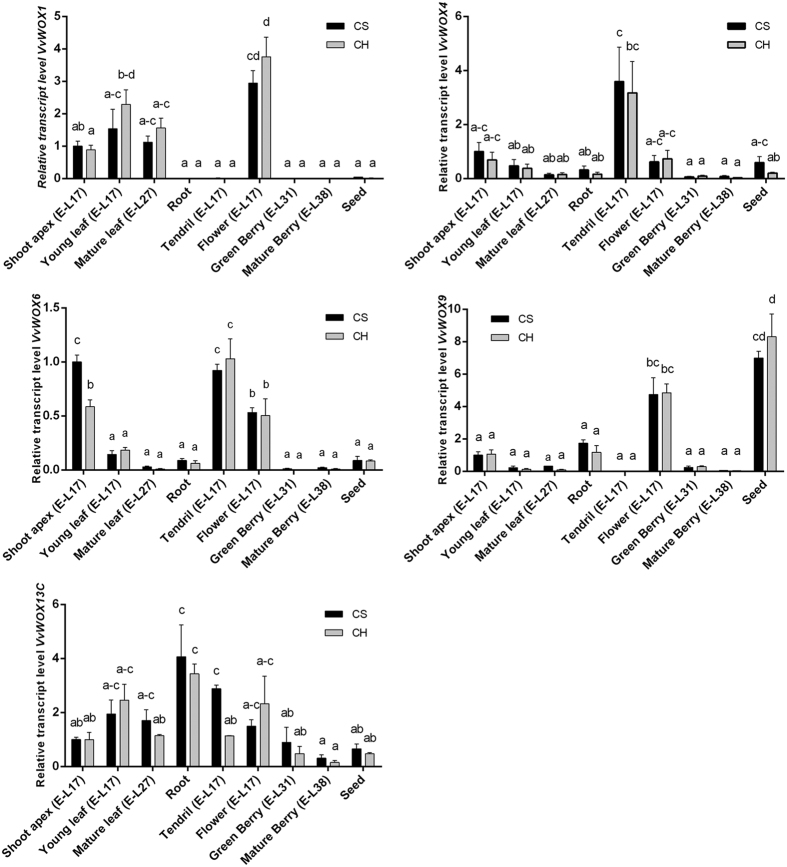
The analysis of transcript levels of five grapevine genes (VvWOX1, VvWOX4, VvWOX6, VvWOX9 and VvWOX13C), showing different regulation in embryogenic tissues of CH and CS20, was extended to different organs at different developmental stages in plants of CH and CS cultivated in field conditions.
VvWOX1 was expressed in a specific way in leaves, shoot apexes and in flowers, while no expression was detected in other organs, except for a faint signal in seeds (Fig. 1). This expression pattern confirms the previous observations in grapevine20 and in other plants. Indeed, in Arabidopsis, WOX1 was initially identified in the initiating vascular primordium of the cotyledons during the heart and torpedo stages of the embryos8. Since then, it has been demonstrated that WOX1, in association with WOX3/PRESSED FLOWER, is expressed in a middle domain downstream of adaxial/abaxial polarity establishment in the leaf primordia and promotes leaf blade outgrowth and margin development23. This close association between WOX1 and leaf morphology was also observed in Populus tomentosa24 and Pisum sativum25, in which this gene is also involved in flower development, such as in Medicago truncatula26.
Figure 1. Relative expression level of VvWOX1, VvWOX4, VvWOX6, VvWOX9 and VvWOX13C in different organs of ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS) as determined by qRT-PCR.
Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3).
The expression of VvWOX6 was concentrated in young tissue as the shoot apexes, in flowers and tendrils, while in mature organs such as fully developed leaves and berries the expression decreased significantly (Fig. 1). VvWOX9 was expressed at high levels in seeds and flowers, at lower levels in the root and shoot apexes, and was almost absent in other organs (Fig. 1). The expression in shoot apexes substantially reflects the activity of WOX9 as regulator of WUS expression in the shoot apical meristem27.
VvWOX4 was expressed in almost all grapevine organs analysed, particularly in tendrils (Fig. 1). This gene is a key regulator of auxin-dependent cambium stimulation in the main stem of Arabidopsis16,28 and regulates vascular cellular division29. The high expression of VvWOX4 in tendrils observed in grapevine could be related to their high level of vascularisation (similar to that of stems). Finally, VvWOX13C, which belongs to the WOX13 subfamily and is involved in replum development, lateral root formation, and vegetative to floral growth transition30,31, was expressed in all grapevine organs analysed (Fig. 1).
Interestingly, for all genes considered, expression in berries was absent or very limited, suggesting little involvement of these genes in the berry development. In addition, the expression of VvWOX1, VvWOX4 and VvWOX9 changed considerably between flowers and tendrils, different structures generated from a common primordium: Vitis tendrils are modified reproductive organs adapted to climb32. These high transcriptional differences, especially for VvWOX1, might suggest a direct involvement of these transcription factors in the regulation of the transition of lateral meristems to flowers or tendrils in grapevine.
The differences between CH and CS in the level of expression of VvWOX1, VvWOX4, VvWOX6, VvWOX9 and VvWOX13C observed during the embryogenetic process20 were only partially confirmed in the grapevine organs. In CS the high expression of VvWOX6 and VvWOX13C observed in shoot apexes and tendrils respectively (Fig. 1), confirmed the expression levels in CS previously reported20. Conversely, no significant difference between the two cultivars was observed for VvWOX1, VvWOX4, and VvWOX9 (Fig. 1). The reasons for these discrepancies may be multiple. For example, some differences between the two cultivars can only be highlighted in embryogenic tissues. Moreover, in the in vitro culture, the presence of high concentrations of plant growth regulators (PGRs)20 may be responsible for different transcriptional modulation between CH and CS, which is not always detectable in plants cultivated in filed conditions.
Sequencing and computational analyses of promoters isolated from VvWOX genes
In order to deepen the understanding of the molecular bases of gene modulation observed in CH and CS, the promoter sequences associated to five VvWOX genes were analysed. Using the grapevine genome sequence PN4002433, regions (~2000 bp) proximal to the transcription start site of each gene were cloned and sequenced. The sequences of promoters (pVvWOX) deposited in GenBank under accession numbers from KY492279 to KY492288 were aligned in order to identify nucleotide differences between the sequences isolated from CH and CS (Supplementary Fig. S1). For each gene, the percentage identity between the promoters isolated from the two cultivars ranged from 96.7% for pVvWOX4_CH vs pVvWOX4_CS to 99.8% for pVvWOX1_CH vs pVvWOX1_CS. The differences in some cases were limited to a few point mutations as in pVvWOX1_CH vs pVvWOX1_CS, while in other promoters a combination of several point mutations and small deletions (pVvWOX6_CH vs pVvWOX6_CS and pVvWOX13C_CH vs pVvWOX13C_CS) were observed. Interestingly, pVvWOX4_CS was characterised by a long deletion of 60 bp at the position -703 to transcription start site (Supplementary Fig. S1).
In a phylogenetic analysis, the promoter sequences were divided in two major groups in which pVvWOX1, pVvWOX4 and pVvWOX6 constituted a separate clade from pVvWOX9 and pVvWOX13C (Fig. 2). The relationship among these promoters is typical of a subdivision into evolutionary lineages previously reported for the WOX proteins in grapevine and other species: a modern clade (WUS, WOX1, 2, 3, 4, 5, 6, and 7) separated from an intermediate clade (WOX8, 9, 11, and 12) and an ancient clade (WOX10, 13, and 14)8,20,34. These results showed that the evolutionary relations among different WOX proteins can also be conserved in promoter sequences, suggesting a parallel evolution between coding sequences and gene regulation35.
Figure 2. The Neighbor-Joining tree of the promoters pVvWOX1, pVvWOX4, pVvWOX6, pVvWOX9 and pVvWOX13C isolated from ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS).
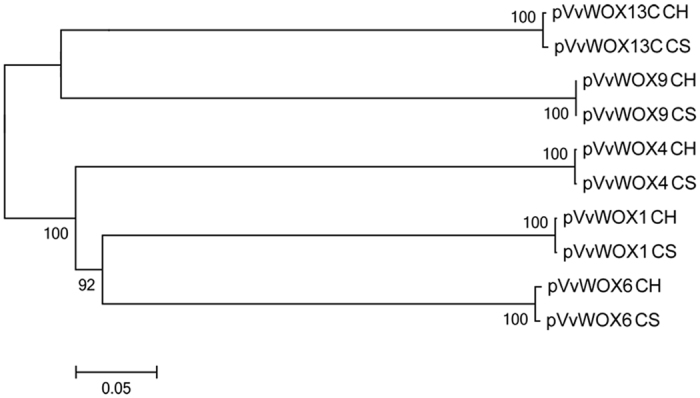
The significance of each node was tested using 1000 bootstrap replicates.
Since even few mutations in regulatory sequences can modify the regulation of a gene1, the TFBSs present in the promoter sequences and their corresponding TFs were analysed by the software PlantPAN 2.036. In pVvWOX1 and pVvWOX9 the small differences retrieved in the nucleotide sequences isolated from CH and CS (Supplementary Fig. S1) imply few changes in the potential TFBSs predicted using a bioinformatics approach (Supplementary Table S1). Conversely, in pVvWOX4_CS, the deletion of 60 bp (Supplementary Fig. S1) determined the loss of several TFBSs in comparison to pVvWOX4_CH, and point mutations reported in pVvWOX6_CH vs pVvWOX6_CS and pVvWOX13C_CH vs pVvWOX13C_CS produced several variations in the predicted TFBSs (Supplementary Fig. S2, Supplementary Table S1). Interestingly, in pVvWOX13C_CS a TATA-box was identified at position -393 not present in pVvWOX13C_CH, due to a single nucleotide mutation at position -387 (Supplementary Fig. S2, Supplementary Table S1). Several TFs linked to the regulation of development (Homeodomain, Growth-regulating factor-GRF, Squamosa promoter binding protein-SBP, Alpha-amylase, WOX), response to hormones (AUX/IAA, ARF, AP2-ERF, CAMTA, EIN3) and response to stresses (Dehydrin, LEA5, NAC, WRKY) were identified (Supplementary Table S2). In addition, pVvWOX4, pVvWOX9 and pVvWOX13C contained some TFBSs putatively recognised by WOX TFs (Supplementary Table S2), suggesting a strong interaction between the components of this gene family and a possible self-regulation of these TFs. A high number of sequences recognised by B3 domain-containing TFs, involved in embryo and flower developments37, and sequences recognised by TFIID (TATA-box binding protein), which contribute to the expression of most RNA polymerase II-transcribed genes38, was present in pVvWOX6_CS and pVvWOX13C_CS (Supplementary Table S2).
Overall analyses suggested that the promoters of VvWOX4, VvWOX6 and VvWOX13C showed more variations between CH and CS in comparison to the promoters of VvWOX1 and VvWOX9. Therefore, subsequent analyses have been limited to pVvWOX4, pVvWOX6 and pVvWOX13C.
Characterisation of promoters isolated from VvWOX genes in Arabidopsis thaliana
The sequences of pVvWOX4, pVvWOX6 and pVvWOX13C isolated from CH and CS were cloned in the expression vector pMDC16439 for the production of promoter::GUS fusion constructs (Supplementary Fig. S3). The constructs were inserted in Arabidopsis and transcriptional and histochemical analyses in different organs were carried out. Cauliflower mosaic virus 35S promoter inserted in pMDC164 (p35S::GUS) and the empty vector (p0::GUS) were used for Arabidopsis transformation as positive and negative controls, respectively, (Supplementary Fig. S4).
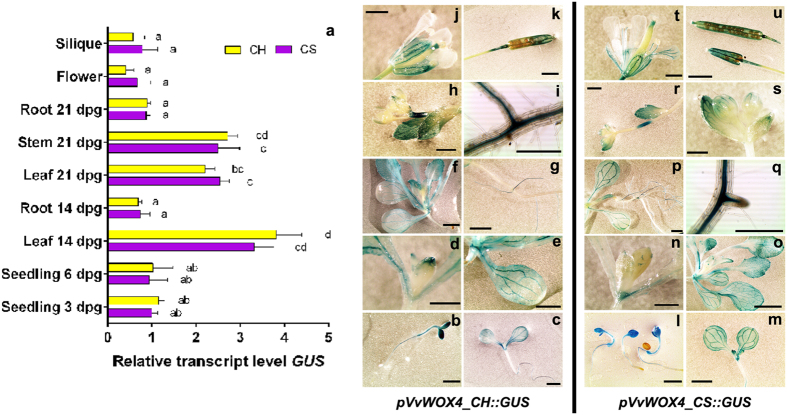
The histochemical assay for determining GUS activity in Arabidopsis plantlets at 3 days post germination (3 dpg) showed a very intense blue staining induced by both the constructs pVvWOX4_CH::GUS and pVvWOX4_CS::GUS in cotyledons and in all the other organs including roots (Fig. 3b,c,l). At 6 dpg the GUS activity started during leaf development at the apex (Fig. 3d,n,m), and increased to higher levels in adult leaves, where intense blue staining was observed in veins at 14 and 21 dpg (Fig. 3e,f,o). During development, the primary roots showed a discontinuous blue staining at 14 and 21 dpg, with an irregular activity of GUS in old tissues, while expression remained high in younger secondary roots (Fig. 3g,i,p,q). The young flower organs appeared less coloured, as reported for the young leaves, while during flower development the sepals, the filaments of anthers, and the stigma showed a high GUS activity (Fig. 3h,j,r,s,t). The siliques appeared intensely stained and no blue signal was detected in the external teguments of the seeds (Fig. 3k,u). The quantification by qRT-PCR of GUS expression confirmed that the mature leaves at 14 and 21 dpg, and the inflorescence stems are the organs with highest expression (Fig. 3a). Interestingly, the distribution of GUS activity in Arabidopsis organs driven by pVvWOX4 appears correlated to the biological functions of WOX4, linked to cambium differentiation in the stem16,28, suggesting that Arabidopsis is an adequate system for the characterisation of promoters of VvWOX genes.
Figure 3. Characterisation of pVvWOX4 isolated from ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS) in Arabidopsis thaliana.
(a) Relative expression level of GUS under control of pVvWOX4_CH and pVvWOX4_CS in transgenic Arabidopsis organs collected at different developmental stages. Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3). (b–u) Histochemical assay for GUS expression in transgenic Arabidopsis organs. Data shown are representative of three independent transgenic lines. Size bar = 2 mm.
There was no significant difference between the expression levels of the constructs pVvWOX4_CH::GUS and pVvWOX4_CS::GUS in all organs and in all developing stages of transgenic plants (Fig. 3). Consequently, the deletion of 60 bp in the pVvWOX4_CS and the differences in the putative TFBSs (Supplementary Fig. S2) do not seem to influence the transcription of GUS in Arabidopsis.
In order to verify whether pVvWOX4_CH::GUS and pVvWOX4_CS::GUS are influenced by PGRs, plantlets at 6 dpg were transferred to a medium containing 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-Benzyladenine (BA) at the same concentrations used to induce embryogenesis in grapevine20. After 8 days of culture, GUS expression was stable in leaves independently of conditions (with or without PGRs) and constructs, while in roots cultured on PGRs the expression increased significantly for both the constructs (Supplementary Fig. S5a). Interestingly, the pVvWOX4 contains some regulatory regions influenced by PGRs, while even in presence of PGRs no expression difference between pVvWOX4_CH::GUS and pVvWOX4_CS::GUS was observed.
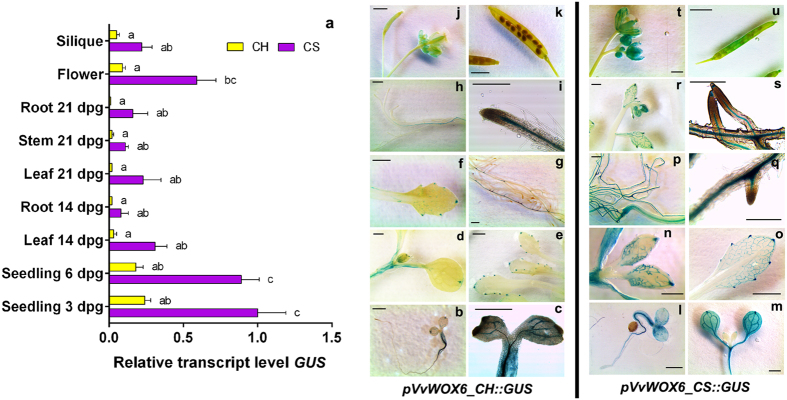
In Arabidopsis plantlets at 3 dpg, several differences in GUS activity between pVvWOX6_CH::GUS and pVvWOX6_CS::GUS were detected (Fig. 4). Seedlings transformed with pVvWOX6_CH::GUS showed an intense staining in hypocotyl and root, decreasing toward the root tip. In cotyledons blue signals were limited to the veins and to some cells at the end of the cotyledon (Fig. 4b,c). In contrast, seedlings under the control of the promoter isolated from CS showed an intense blue staining in all parts of the plantlets, particularly on the cotyledons, in the hypocotyl and in the vascular system along the root (Fig. 4l). In both constructs, the activity of GUS at 6 dpg was stable in cotyledons and roots, while the young emerging leaves were less stained, with some signal localised in the veins and at the apical extremity (Fig. 4d,m,n). At 14 and 21 dpg in the plants transformed with pVvWOX6_CH::GUS, the blue staining was limited to a few groups of cells on the edges of leaves and in roots, which gradually lost the GUS activity in the older tissues (Fig. 4e–i). Conversely, plants transformed with pVvWOX6_CS::GUS at 14 and 21 dpg, showed a general more intense blue staining. In addition, in leaves the midribs were not stained, but the secondary veins appeared blue (Fig. 4o–s). In flowers, sepals and styles were intensely stained in pVvWOX6_CS::GUS, with activity also detectable in the siliques (Fig. 4t,u). In pVvWOX6_CH::GUS transgenic plants, the activity was lower in flowers (Fig. 4j) and not detectable in siliques (Fig. 4k). The qRT-PCR analyses confirmed a higher expression associated with pVvWOX6_CS::GUS; in all organs, the promoter isolated from CS induced a GUS expression at least five times higher than that induced by pVvWOX6_CH (Fig. 4a). These results suggest that the mutations identified in these promoters (Supplementary Figs S1 and S2) are involved in the different transcriptional regulation of VvWOX6 in the two grapevine cultivars20 (Fig. 1). In particular, the mutations around the position -478, associated with changes in several TFBSs, could be potentially good candidates to explain this different gene regulation (Supplementary Fig. S2, Supplementary Table S1). In addition, as observed for pVvWOX4, the promoters of VvWOX6 in transgenic Arabidopsis induced a GUS expression in agreement with the expression reported in grapevine. In particular, the expression of both constructs in Arabidopsis was localised in young organs, seedlings at 3 and 6 dpg, and in flowers (Fig. 4A), as well as high expression was detected in shoot apexes and in flowers of grapevine (Fig. 1).
Figure 4. Characterisation of pVvWOX6 isolated from ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS) in Arabidopsis thaliana.
(a) Relative expression level of GUS under control of pVvWOX6_CH and pVvWOX6_CS in transgenic Arabidopsis organs collected at different developmental stages. Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3). (b–u) Histochemical assay for GUS expression in transgenic Arabidopsis organs. Data shown are representative of three independent transgenic lines. Size bar = 2 mm.
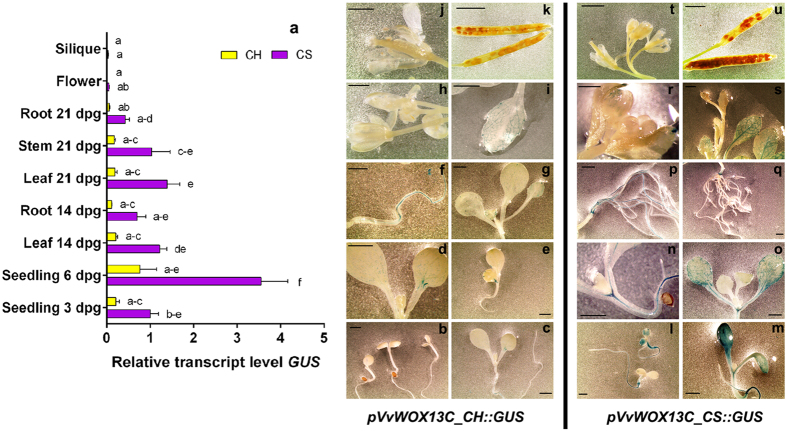
In Fig. 5 is reported the evolution of the expression of the GUS gene under the control of the promoters pVvWOX13C_CH and pVvWOX13C_CS in Arabidopsis. In seedlings at 3 dpg the first differences between the two constructs were observed; the promoter isolated from CS induced GUS activity in all parts of the plantlets, particularly in hypocotyls and roots (Fig. 5l), while the activity of pVvWOX13C_CH::GUS was barely detectable by histochemical assay (Fig. 5b). These differences were also conserved in the following developmental phases: at 6 dpg for pVvWOX13C_CH::GUS blue staining was localised in few cells in cotyledons and roots with no signal in young leaves (Fig. 5c–e), while for pVvWOX13C_CS::GUS the blue signals were much more extended in cotyledons, hypocotyls and roots (Fig. 5m–o). In mature organs at 14 and 21 dpg, GUS activity decreased for both constructs (Fig. 5f–i, p–s); in plants transformed with pVvWOX13C_CH::GUS, no activity was detected in flowers and siliques (Fig. 5h,j,k), and for pVvWOX13C_CS::GUS, only weak signals were detected in young sepals (Fig. 5r,t,u). These expression levels were confirmed by qRT-PCR, with higher expression induced by pVvWOX13C_CS::GUS in young organs, particularly at 6 dpg (Fig. 5a). In addition, also in the presence of PGRs, pVvWOX13C_CS induced more GUS expression than pVvWOX13C_CH in the leaves and roots of Arabidopsis (Supplementary Fig. S5b). As reported above for VvWOX6, the point mutations identified in these promoters (Supplementary Figs S1 and S2) were likely responsible for the different transcriptional regulation of VvWOX13C in CH and CS20 (Fig. 1).
Figure 5. Characterisation of pVvWOX13C isolated from ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS) in Arabidopsis thaliana.
(a) Relative expression level of GUS under control of pVvWOX13C_CH and pVvWOX13C_CS in transgenic Arabidopsis organs collected at different developmental stages. Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3). (b–u) Histochemical assay for GUS expression in transgenic Arabidopsis organs. Data shown are representative of three independent transgenic lines. Size bar = 2 mm.
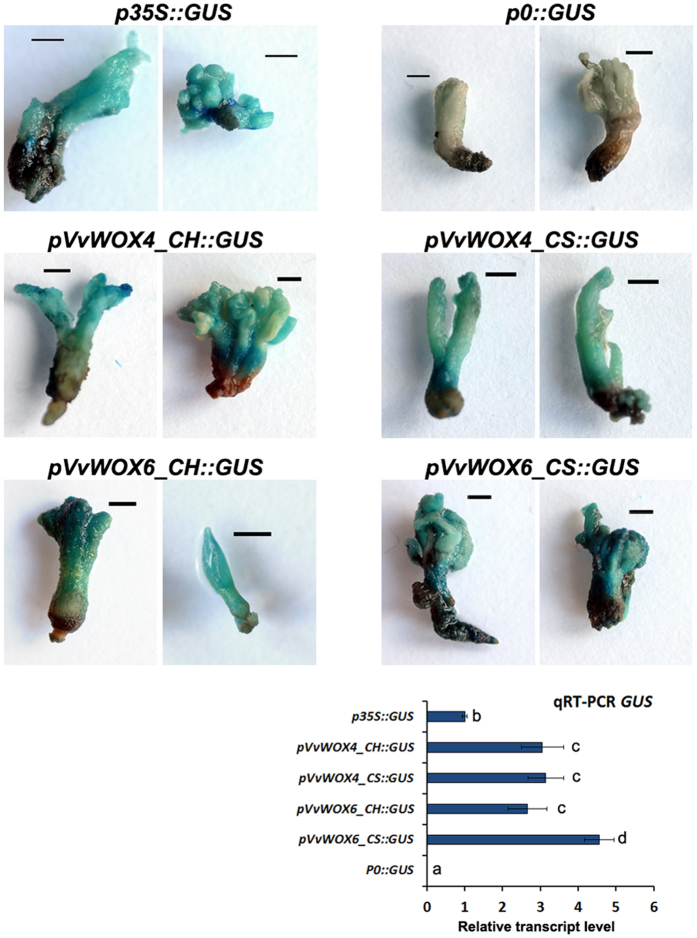
GUS expression levels induced in Arabidopsis by the six promoters isolated from VvWOX4, VvWOX6 and VvWOX13C were significantly lower than those induced by the constitutive p35S (Supplementary Fig. S6). Analysing the relative expression levels, the activity of the VvWOX promoters appeared concentrated in young plantlets, whereas in mature tissues (e.g. roots at 21 dpg) the expression was generally low, as demonstrated by the expression induced by pVvWOX6_CH resulting at least 1000 times lower than for p35S::GUS (Supplementary Fig. S6). These indications could be crucial for the application of these promoters in functional studies in grapevine, in substitution of the traditional p35S4.
Characterisation of promoters isolated from VvWOX genes in grapevine embryos
In order to extend the characterisation of VvWOX promoters in grapevine, a transient transformation of somatic embryos was used. This approach has proven effective in grapevine4,40 and it represents a good alternative to stable genetic transformation that is still a time consuming process in grapevine21.
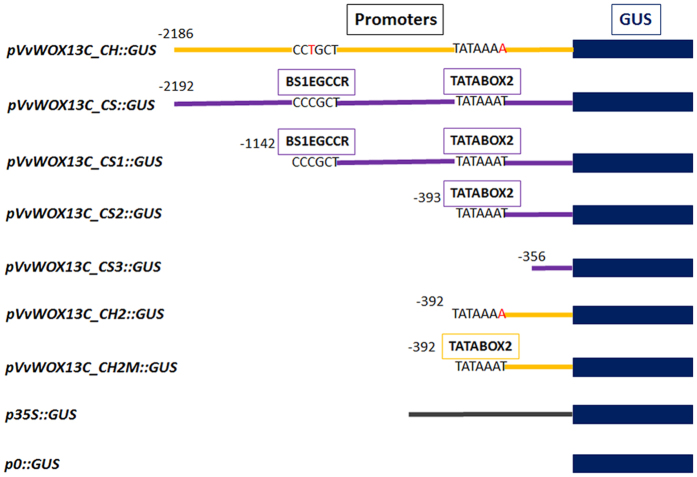
For the promoters isolated from VvWOX4 and VvWOX6, the histochemical and qRT-PCR experiments carried out on transiently transformed embryos substantially confirmed the results reported above for Arabidopsis. No differences in expression levels were observed for the pVvWOX4_CH::GUS and pVvWOX4_CS::GUS constructs, while pVvWOX6_CS::GUS induced high GUS activity in comparison to pVvWOX6_CH::GUS (Fig. 6). In addition, for the promoters of VvWOX13C the transient transformation confirmed a higher capacity of the sequence isolated from CS to activate the GUS transcription in somatic embryos (Fig. 7). In order to understand the role in the gene regulation of some point mutations associated with putatively TFBSs present only in pVvWOX13C_CS, truncated versions of pVvWOX13C_CH and pVvWOX13C_CS were analysed (Fig. 8). Three truncated versions of pVvWOX13C_CS were used to produce promoter::GUS fusion constructs: i) pVvWOX13C_CS1::GUS, containing a fragment of 1142 bp with the TFBS ‘BS1EGCCR’ (Supplementary Fig. S2) involved in vascular expression of the cinnamoyl CoA reductase gene41 and present in a single copy only in the sequence isolated from CS; ii) pVvWOX13C_CS2::GUS containing a fragment of 393 bp with a TATA-box (Supplementary Fig. S2) absent in the sequence of CH; iii) pVvWOX13C_CS3::GUS containing a fragment of 356 bp proximal to the 5′-end of the gene and with seven point mutations between CH and CS (Fig. 8, Supplementary Fig. S1). For the promoter pVvWOX13C_CH, two truncated versions were generated and used in promoter::GUS fusion constructs: i) pVvWOX13C_CH2::GUS, containing a fragment of 392 bp, homologous to pVvWOX13C_CS2, but devoid of TATA-box and with nine point mutations (Fig. 8, Supplementary Fig. S1); ii) pVvWOX13C_CH2M::GUS, containing the same fragment of pVvWOX13C_CH2 but with a mutation in a single base (the A was mutated to T at position -387) to generate a functional TATA-box (Fig. 8). The three shortened versions of pVvWOX13C_CS induced a progressive reduction of GUS expression (Fig. 7), suggesting that the deleted regions contained important TFBSs involved in gene regulation, even in the more distal regions beyond the position -1142 (Fig. 8, Supplementary Fig. S1). The construct pVvWOX13C_CS2::GUS induced a twofold GUS expression compared with its homologous pVvWOX13C_CH2::GUS cloned from CH. However, a single mutation generating a functional TATA-box (pVvWOX13C_CH2M::GUS) was enough to bring the GUS expression to the same levels detected for pVvWOX13C_CS2::GUS (Figs 7and 8). Then, of the nine point mutations present in pVvWOX13C_CH2 (Supplementary Fig. S1), only the one at position -387 associated to a TATA-box was necessary to significantly increase the expression induced by the proper sequence isolated from CH (Fig. 7).
Figure 6. Histochemical and qRT-PCR analysis of GUS activity in transiently transformed grapevine somatic embryos.
Data shown are representative of three independent experiments using the constructs pVvWOX4_CH::GUS, pVvWOX4_CS::GUS, pVvWOX6_CH::GUS and pVvWOX6_CS::GUS. p35S::GUS and p0::GUS were used respectively as positive and negative controls of agroinfiltration. Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3). Size bar = 1 mm.
Figure 7. Histochemical and qRT-PCR analysis of GUS activity in transiently transformed grapevine somatic embryos.
Data shown are representative of three independent experiments using the complete and truncated constructs derived by pVvWOX13C_CH and pVvWOX13C_CS. p35S::GUS and p0::GUS were used respectively as positive and negative controls of agroinfiltration. Lowercase letters denote significant differences attested by Tukey’s HSD test (P < 0.05). Data are expressed as means ± SE (n = 3). Size bar = 1 mm.
Figure 8. Schematic representation of truncated versions of promoters derived by pVvWOX13C_CH and pVvWOX13C_CS and fused with GUS gene in the expression vector pMDC164.
Conclusion
In recent years, a substantial amount of information has been collected on grapevine genome structure and genetic variation within cultivars42,43,44. However, little is known about how these sequence variations influence gene expression and phenotype in different cultivars. The family of WOX genes is a class of transcription factors that, in V. vinifera, showed different transcription levels in two different cultivars, CH and CS20 (Fig. 1). We hypothesised that transcriptional regulation would be different for these genes. In the present work, the analyses of the regions proximal to the transcription start site of VvWOX1 and VvWOX9 did not show substantial differences in nucleotides and TFBSs between CH and CS, suggesting that other regulatory regions and/or epigenetic changes45 are likely responsible for the transcriptional differences previously reported20. Similarly, the same conclusion can be drawn for pVvWOX4_CH and pVvWOX4_CS after the analyses in Arabidopsis and grapevine somatic embryos. Although pVvWOX4_CS is characterised by a long deletion of 60 bp at about 700 bp proximal to the transcription start site of the gene, apparently this did not influence the transcription and no difference was detected in the transcriptional levels induced by pVvWOX4_CH. Recently, an increasing number of computational approaches have been developed to evaluate DNA sequences regulating the transcription of many genes46,47. However, our results confirm that a purely computational evaluation of regulatory elements involved in the gene modulation in some cases can be misleading, and a functional assessment is often preferable or necessary, especially for the evaluation of a limited number of sequences.
pVvWOX13C_CS and pVvWOX6_CS induced a significantly higher expression of GUS compared to sequences cloned from CH in both the system adopted, the stable transformation of Arabidopsis and the transient expression in grapevine. Interestingly, these differences were linked to some mutations in proximal regulatory regions. In particular, we demonstrated that a TATA-box, present only in pVvWOX13C_CS at -393 bp to the transcription start site of the gene, was pivotal for the transcriptional regulation of VvWOX13C.
The understanding of VvWOX regulation in different cultivars of grapevine, which are characterised by different potentials to form embryogenic tissues, would be useful for understanding and improving this regenerative process, fundamental to prospective large-scale applications in new “Sustainable Biotechnology”, i.e. cis-genesis and genome editing in grapevine6,48. Indeed, the characterisation of VvWOX promoters has provided new information about tissue- and time-specific promoters useful for functional studies and as an alternative to traditional constitutive promoters in cis-genetic approaches. In addition, these results also provide interesting information for genome editing in grapevine7,49, suggesting that point mutations in regulatory sequences could be determinant for increasing or decreasing gene transcription.
Methods
Plant materials
The plant material for the expression analysis of VvWOX genes in grapevine was collected in 2015 in an experimental vineyard located in the Piedmont (Northwestern Italy). Vines were trained to a vertical trellis with Guyot pruning; conventional agronomic management was regularly applied in the vineyard. Samples were collected from V. vinifera ‘Chardonnay’ (CH) and ‘Cabernet Sauvignon’ (CS), both grafted onto rootstock 1103 Paulsen (V. rupestris x V. berlandieri), from different organs and in different phenological phases according to the E-L System modified by Coombe50: shoot apexes, young leaves, tendrils and flowers (E-L17, May 2015), mature leaves (E-L27, June 2015), green berry at pea size (E-L31, July 2015), berries at harvest and seeds (E-L38, September 2015). The roots were collected from three-year-old greenhouse-grown potted plants. For each cultivar, samples from three plants were pooled to form a biological replicate and immediately frozen in liquid nitrogen (in total three independent biological replicates). Pools were stored at −80 °C until molecular analyses.
Somatic embryogenesis from immature anthers of CH and development of somatic embryos were induced following the protocols reported previously in Gambino et al.20.
Promoter isolation, sequencing and construction of transformation vectors
Promoter sequences (~2000 bp) proximal to transcription start sites of the genes VvWOX1, VvWOX4, VvWOX6, VvWOX9 and VvWOX13C20 were identified into the grapevine genome PN4002433. The DNA extracted from leaves of CH and CS using a Plant/Fungi DNA Isolation kit (Norgen Biotek Corp.) was amplified by PCR using specific primers for each promoter (Supplementary Table S3). The PCR reaction mix (50 μl) contained 100 ng of DNA, 0.3 mM dNTPs, 0.3 μM each primer, 1 mM MgSO4 and 1 unit of High Fidelity Taq polymerase (Platinum™ Pfx DNA Polymerase, Invitrogen). Cycling conditions for PCRs consisted of initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 15 s, 50 °C for 30 s, and 68 °C for 3 min. Products were analysed by electrophoresis on 1% agarose gels buffered in TBE (45 mM Tris-borate, 1 mM EDTA) and visualised by UV-light after staining with ethidium bromide. Amplified products were gel-purified by the Wizard® SV Gel and PCR Clean-Up System (Promega) and cloned into the pDONR™/Zeo plasmid (Invitrogen) following the Gateway® Technology39 to produce Entry Clones using the Gateway® BP Clonase® II enzyme (Invitrogen). Plasmid DNA was isolated by the Wizard Plus SV Minipreps DNA Purification System (Promega) following the Promega protocol, and sequenced using M13 primers (forward and reverse) and internal primers specific to each promoter (Supplementary Table S3) in order to sequence the entire regions. Products were sequenced using Big-Dye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems), following the manufacturer’s instructions. PCR products were purified using an AutoSeq G-50 Dye Terminator Removal kit (GE Healthcare) and analysed using a 3130 Genetic Analyzer capillary sequencer (Applied Biosystems).
The nucleotide sequences of pVvWOX were aligned with the Clustal MUSCLE software (http://www.ebi.ac.uk/Tools/msa/muscle/) using default settings. The phylogenetic analysis based on the Neighbor-Joining (NJ) method was carried out using MEGA7 software51. The significance of each node was tested using 1000 bootstrap replicates. The TFBSs present in the promoter sequences and their corresponding TFs were analysed by The Plant Promoter Analysis Navigator (PlantPAN 2.0; http://PlantPAN2.itps.ncku.edu.tw)36.
The six promoters pVvWOX4_CH, pVvWOX4_CS, pVvWOX6_CH, pVvWOX6_CS, pVvWOX13C_CH and pVvWOX13C_CS inserted in pDONR™/Zeo were transferred to the destination vector pMDC16439 (https://www.arabidopsis.org/servlets/TairObject?type=vector&id=501100124) for the production of promoter::GUS fusion constructs using the Gateway® LR Clonase® II enzyme (Invitrogen) (Supplementary Fig. S3). The promoters were inserted between the recombination sites attR1 and attR2 localized immediately at 5′ of gusA gene in the plasmid pMDC164, which contains the Kanamycin resistance for the bacterial selection and the Hygromycin resistance for the selection in plant. The truncated version of the promoters pVvWOX13C_CH and pVvWOX13C_CS (Fig. 8) were amplified from DNA extracted from CH and CS using specific primers (Supplementary Table S3), sequenced and inserted in pMDC164 as described above. The constitutive p35S promoter was amplified by PCR from the plasmid pGA643 (GenBank accession: AY804024), introduced in pMDC164 and used as positive control for GUS expression. All the constructs were inserted in the Agrobacterium tumefaciens strain GV3101 by the freeze-thaw method52.
Arabidopsis transformation
The promoter::GUS fusion constructs (Supplementary Fig. S3) were used for the transformation of Arabidopsis thaliana, ecotype Columbia (Col-0), using the ‘floral dip’ method53. Wild type seeds were distributed on the surface of the moistened potting soil, pots were placed at 4 °C for 48 h and then transferred to a growth room with a 16 h photoperiod at 24 °C. After 4 to 6 weeks, when inflorescences were composed of many unopened floral buds, plants were dipped upside down in the Agrobacterium inoculum obtained resuspending the bacterial cells immediately before use in a liquid infiltration medium [0.5X Murashige & Skoog (MS) basal salt mixture54, 5% sucrose, pH 5.7 and 0.005% Silwet L-77]. The plants were transferred to the growth room until the harvest of seeds. For the screening of transformants, seeds sterilised with sodium hypochlorite (1.5% available chlorine) were distributed in Petri dishes on a selection medium containing 1X MS, 3.0% sucrose, 0.6% agar, pH 5.7, and 30 mg/L of Hygromycin, added after sterilisation at 121 °C for 10 min. Resistant seedlings that survived to selection were transferred to pots and to the growth room for the production of T2 seeds. In the following generations, the seeds sowed in vitro under selective conditions were used to determine the ratio of resistant to sensitive seedlings and to calculate the number of segregating T-DNA loci. For each construct, three independent transgenic lines, containing a single insertion in homozygosis, were analysed. qRT-PCR and GUS histochemical assays were carried out on T4 seedlings cultured in vitro and collected at 3, 6, 14 and 21 days post germination (dpg). Flowers and siliques were collected from the same plants after the transfer to pots and growth room.
The effects of PGRs on VvWOX promoters were studied on Arabidopsis seedlings at 6 dpg, transferring the plantlets onto the same selection medium described above with the addition of 4.5 μM 2,4-D and 8.9 μM BA20. After 8 days of culture, control seedlings and seedlings subjected to PGRs were collected and analysed by qRT-PCR.
Transient expression assays in grapevine embryos
Somatic embryos of CH were subjected to agroinfiltration following the protocol of Xu et al.55 using the same promoter::GUS fusion constructs inserted into Arabidopsis (Supplementary Fig. S3) and the truncated version of pVvWOX13C (Fig. 8). After an overnight culture of Agrobacterium, the bacteria were collected by centrifugation, resuspended in an infiltration medium (10 mM MES pH 5.6, 10 mM MgCl2, 2% (w/v) sucrose and 150 μM of acetosyringone) and incubated at 28 °C for 3 h to reach a final concentration of 0.6 OD600. The embryos were placed in the bacterial solution in a desiccator and subjected to vacuum (0.07 MPa) for 1 h, during which the vacuum was quickly released and restored several times to facilitate the entry of the bacterial suspension into the embryo tissues. The infiltrated embryos were then washed in sterile water, placed over a wet Whatman paper in a Petri dish and incubated in a growth chamber at 25 °C for three days before qRT-PCR and GUS histochemical assays. For each construct, three independent experiments, used as independent biological replicates, were carried out.
qRT-PCR analysis
Total RNA was extracted from grapevine tissues, agroinfiltrated somatic embryos and plantlets of Arabidopsis using the Spectrum™ Plant Total RNA extraction kit (Sigma Aldrich). Total RNA quantity was checked using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) and treated with DNase I (Invitrogen) in accordance with the manufacturer’s instructions. For each biological replicate, first-strand cDNA was synthesised from a starting quantity of 1 μg of total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer’s instructions. Target-specific primers (Supplementary Table S3) were designed using Primer Express® software (v3.0, Applied Biosystems). Reactions were carried out using Power SYBR® Green PCR Master Mix (Applied Biosystems) as reported in Gambino et al.20. Three technical replicates were run for each biological replicate, and the expression of transcripts was quantified after normalisation to two housekeeping genes: ubiquitin (VvUBI) and actin (VvACT) for grapevine tissues20, AtSAND and AtTIP41 for Arabidopsis plantlets56.
The data were subjected to statistical analysis by one-way analyses of variance (ANOVA) with treatment as the main factor using the SPSS 23.0 statistical software package (SPSS Inc., Cary, NC, USA). Tukey’s HSD test was applied when ANOVA showed significant differences (P < 0.05). The standard error (SE) of all means were calculated.
GUS histochemical assays
The histochemical staining of Arabidopsis transgenic plantlets and grapevine agroinfiltrated embryos was carried out following the protocol of Jefferson57. Plant tissues immersed in GUS staining solution [50 mM NaH2PO4 pH 7.0, 5 mM K4Fe(CN)6·3H2O, 5 mM K3Fe(CN)6, 0.1% Triton X-100 and 1 mM of 5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid (X-Gluc, Sigma-Aldrich)] were subjected to vacuum (0.07 MPa) for 5 min and then incubated for 24 h at 37 °C. For Arabidopsis plantlets, chlorophyll was removed from the leaves by successive washes with 70% ethanol at room temperature. Imaging was performed using an optical microscope with a 10X objective (Nikon Eclipse 55i, Tokyo, Japan).
Additional Information
How to cite this article: Boccacci, P. et al. Cultivar-specific gene modulation in Vitis vinifera: analysis of the promoters regulating the expression of WOX transcription factors. Sci. Rep. 7, 45670; doi: 10.1038/srep45670 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
Irene Perrone was financed by the Italian Ministry of University and Research, FIR project RBFR13GHC5: “The Epigenomic Plasticity of Grapevine in Genotype per Environment Interactions”.
Footnotes
The authors declare no competing financial interests.
Author Contributions G.G. and P.B. designed the study. P.B., A.M., C.P.M., W.C., I.P., I.G. and G.G. carried out data acquisition and analysis. G.G. wrote the paper. All authors reviewed the manuscript.
References
- Wittkopp P. J. & Kalay G. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 13, 59–69 (2012). [DOI] [PubMed] [Google Scholar]
- Carroll S. B. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36 (2008). [DOI] [PubMed] [Google Scholar]
- Myles S. et al. Genetic structure and domestication history of the grape. Proc. Natl Acad. Sci. USA 108, 3530–3535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. T., Kim K. H., Jasinski J. R., Creech M. R. & Gray D. J. Large-scale characterization of promoters from grapevine (Vitis spp.) using quantitative anthocyanin and GUS assay systems. Plant Sci. 196, 132–142 (2012). [DOI] [PubMed] [Google Scholar]
- Toth Z. et al. Expression of a grapevine NAC transcription factor gene is induced in response to Powdery Mildew colonization in Salicylic Acid-independent manner. Sci. Rep. 6, 30825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchiswamy C. N., Sargent D. J., Velasco R., Maffei M. E. & Malnoy M. Looking forward to genetically edited fruit crops. Trends Biotechnol. 33, 62–64 (2015). [DOI] [PubMed] [Google Scholar]
- Ren C. et al. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 6, 32289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker A. et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131, 657–668 (2004). [DOI] [PubMed] [Google Scholar]
- Nardmann J., Zimmermann R., Durantini D., Kranz E. & Werr W. WOX gene phylogeny in Poaceae: A comparative approach addressing leaf and embryo development. Mol. Biol. Evol. 24, 2474–2484 (2007). [DOI] [PubMed] [Google Scholar]
- Jain M., Tyagi A. K. & Khurana J. P. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J. 275, 2845–2861 (2008). [DOI] [PubMed] [Google Scholar]
- Hedman H., Zhu T. Q., von Arnold S. & Sohlberg J. J. Analysis of the WUSCHEL-RELATED HOMEOBOX gene family in the conifer Picea abies reveals extensive conservation as well as dynamic patterns. BMC Plant Biol. 13, 89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. et al. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc. Natl. Acad. Sci. USA 110, 366–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K. F. et al. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815 (1998). [DOI] [PubMed] [Google Scholar]
- Sarkar A. K. et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814 (2007). [DOI] [PubMed] [Google Scholar]
- Forzani C. et al. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr. Biol. 24, 1939–1944 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suer S., Agusti J., Sanchez P., Schwarz M. & Greb T. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. Plant Cell 23, 3247–3259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. O., Zheng Z.-, Oppenheimer D. G. & Hauser B. A. The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development 132, 841–849 (2005). [DOI] [PubMed] [Google Scholar]
- Breuninger H., Rikirsch E., Hermann M., Ueda M. & Laux T. Differential expression of WOX genes mediates apical–basal axis formation in the Arabidopsis embryo. Dev. Cell 14, 867–876 (2008). [DOI] [PubMed] [Google Scholar]
- Dolzblasz A. et al. Stem cell regulation by Arabidopsis WOX Genes. Mol. Plant. 9, 1028–1039 (2016). [DOI] [PubMed] [Google Scholar]
- Gambino G. et al. Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J. Exp. Bot. 62, 1089–1101 (2011). [DOI] [PubMed] [Google Scholar]
- Gambino G. & Gribaudo I. Genetic transformation of fruit trees: current status and remaining challenges. Transgenic Res. 21, 1163–1181 (2012). [DOI] [PubMed] [Google Scholar]
- Martinelli L. & Gribaudo I. Strategies for effective somatic embryogenesis in grapevine (Vitis spp.): an appraisal. In Grapevine Molecular Physiology & Biotechnology Second edition(ed Roubelakis-Angelakis K.) 461–494 (Springer, 2009). [Google Scholar]
- Nakata M. et al. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 24, 519–535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. et al. WUSCHEL-related Homeobox genes in Populus tomentosa: diversified expression patterns and a functional similarity in adventitious root formation. BMC Genomics 15, 296–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L.-L. et al. LATHYROIDES, encoding a WUSCHEL-related Homeobox1 transcription factor, controls organ lateral growth, and regulates tendril and dorsal petal identities in garden pea (Pisum sativum L.). Mol. Plant 5, 1333–1345 (2012). [DOI] [PubMed] [Google Scholar]
- Niu L. F. et al. LOOSE FLOWER, a WUSCHEL-like Homeobox gene, is required for lateral fusion of floral organs in Medicago truncatula. Plant J. 81, 480–492 (2015). [DOI] [PubMed] [Google Scholar]
- Wu X., Dabi T. & Weigel D. Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr. Biol. 15, 436–440 (2005). [DOI] [PubMed] [Google Scholar]
- Hirakawa Y., Kondo Y. & Fukuda H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22, 2618–2629 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells J. P., Provost C. M., Mishra L. & Turner S. R. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140, 2224–2234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveaux Y. et al. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 8, 291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera-Branchat M., Ripoll J. J., Yanofsky M. F. & Pelaz S. The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J. 37, 37–49 (2012). [DOI] [PubMed] [Google Scholar]
- Calonje M., Cubas P., Martínez-Zapater J. M. & Carmona M. J. Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol. 135, 1491–1501 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon O. et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463–468 (2007). [DOI] [PubMed] [Google Scholar]
- Vandenbussche M. et al. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 21, 2269–2283 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiño J. M. et al. Evolution of DNA-binding sites of a floral master regulatory transcription factor. Mol. Biol. Evol. 33, 185–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.-N. et al. PlantPAN 2.0: an update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 44(D1), D1154–D1160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirauch M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158, 1431–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury S., Bernecky C. & Cramer P. Structural basis of transcription initiation by RNA polymerase II. Nat. Rev. Mol. Cell. Biol. 16, 129–143 (2015). [DOI] [PubMed] [Google Scholar]
- Curtis M. D. & Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelly N. S., Valat L. & Walter B. & Maillot, P. Transient expression assays in grapevine: a step towards genetic improvement. Plant Biotechnol. J. 12, 1231–1245 (2014). [DOI] [PubMed] [Google Scholar]
- Lacombe E., Van Doorsselaere J., Boerjan W., Boudet A. M. & Grima-Pettenati J. Characterization of cis-elements required for vascular expression of the Cinnamoyl CoA reductase gene and for protein-DNA complex formation. Plant J. 23, 663–676 (2000). [DOI] [PubMed] [Google Scholar]
- Da Silva C. et al. The high polyphenol content of grapevine cultivar Tannat berries is conferred primarily by genes that are not shared with the reference genome. Plant Cell 25, 4777–4788 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Genova A. et al. Whole genome comparison between table and wine grapes reveals a comprehensive catalog of structural variants. BMC Plant Biol. 14, 7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M. F. et al. Inter-varietal structural variation in grapevine genomes. Plant J. 88, 648–661 (2016) [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mega E. et al. Role of transcriptional regulation in the evolution of plant phenotype: a dynamic systems approach. Dev. Dyn. 244, 1074–1095 (2015). [DOI] [PubMed] [Google Scholar]
- Meng H. & Wang Y. Cis-acting regulatory elements: from random screening to quantitative design. Quant. Biol. 3, 107–114 (2015). [Google Scholar]
- Koryachko A. et al. Computational approaches to identify regulators of plant stress response using high-throughput gene expression data. Curr. Plant Biol. 3–4, 20–29 (2015). [Google Scholar]
- Belhaj K., Chaparro-Garcia A., Kamoun S., Patron N. J. & Nekrasov V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 32, 76–84 (2015). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Identification of genomic sites for CRISPR/Cas9-based genome editing in the Vitis vinifera genome. BMC Plant Biol. 16, 96 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe B. G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1, 100–110 (1995). [Google Scholar]
- Kumar S., Stecher G. & Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen R. & Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 16, 9877 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Murashige T. & Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497(1962). [Google Scholar]
- Xu W., Yu Y., Ding J., Hua Z. & Wang Y. Characterization of a novel stilbene synthase promoter involved in pathogen- and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta 231, 475–487 (2010). [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M. K. & Scheible W. R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5, 387–405 (1987). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.