Abstract
Parkinson’s disease (PD) patients have difficulty in performing a dual-task. It has been suggested that the cerebellum is important in dual-tasking. We used functional MRI to investigate the role of the cerebellum in performing a dual motor and cognitive task in PD patients. We have examined whether there are any areas additionally activated for dual-task performance, and compared the neural activity and functional connectivity pattern in the cerebellum between PD patients and healthy controls. We found that the right cerebellar vermis and left lobule V of cerebellar anterior lobe were additionally activated for dual-task performance in healthy controls and for motor task in PD patients. We didn’t find any cerebellar regions additionally activated while performing dual-task in PD patients. In addition, the right cerebellar vermis had enhanced connectivity with motor and cognitive associated networks in PD patients. PD patients have limited cerebellar resources that are already utilized for single tasks and, for dual tasks, cannot augment as necessary in order to integrate motor and cognitive networks.
Parkinson’s disease (PD) patients commonly have difficulties in performing two tasks simultaneously1,2. This problem can be observed in performing two motor tasks, two cognitive tasks or combined cognitive and motor tasks at the same time3,4. It has been shown that dual-task performance is significantly associated to motor problems in PD, like bradykinesia5, falling6, and freezing of gait7. However, the neural reasons contributing to this problem remain unclear, and have been suggested relating to limited attentional resources3, defective central executive function and less automaticity8,9.
By now, only a small number of neuroimaging studies have investigated the neural correlates underlying dual-tasking in PD8,10,11. When performing dual-tasks combining a sequential finger movement and a counting task, the precuneus was additionally activated compared with the single component tasks in PD and healthy controls. PD patients had enhanced activity in the cerebellum, premotor cortex (PMC), parietal cortex, precuneus and prefrontal cortex compared to the controls8. A few recent studies have investigated dual-tasking related functional or structural connectivity in PD patients with freezing of gait. One showed that worse dual-task performance was associated with decreased functional connectivity within the striatum and between the caudate and superior temporal lobe, and with increased connectivity between the dorsal putamen and precuneus11. In another study, dual-task interference was correlated with asymmetry of pedunculopontine nucleus structural connectivity in PD patients10.
In a recent study, using a dual-task paradigm with a simple motor task and a cognitive (counting) task, we found that the cerebellar vermis and lobule V of the cerebellar anterior lobe are additionally activated for dual-task in healthy subjects, and these cerebellar regions had functional connectivity with extensive motor- and cognitive-related regions12. It is likely that the cerebellum is involved in integrating motor and cognitive networks in order to perform dual motor- cognitive tasks properly. The role of the cerebellum in PD has been long overlooked. However, in recent years, it has been recognized that there are reciprocal anatomical connections between the cerebellum and basal ganglia13. Moreover, increasing evidence has suggested that the cerebellum has PD-related structural and functional modulations and may contribute substantially to the clinical symptoms of PD14. Therefore, in the current study, we used functional MRI (fMRI) to investigate the neural activity and network connectivity in the cerebellum during execution of dual-task in PD patients. We hypothesized that there is no cerebellar region additionally activated, and the connectivity between the cerebellum and motor and cognitive associated networks is changed while performing dual-task in PD patients. The dysfunction of the cerebellum might be a reason contributing to the difficulty in dual-tasking in PD.
Results
Task performance
During fMRI scanning, both groups had no errors in performing the single and dual-tasks. The data were normally distributed. As the accuracy was 100% in performing all single and dual-tasks, there was no difference of performance between the dual-task and the single tasks within each group, and no difference of performance of the dual-task between the two groups (repeated-measures ANOVA, p = 1). Interference scores were calculated by taking the difference between the accuracy for the single task and dual-task, and then dividing this difference by the accuracy in the single task for each subjects. As there was no difference of accuracy between the single and dual-tasks, the interference scores were 0.
FMRI results
Brain activity
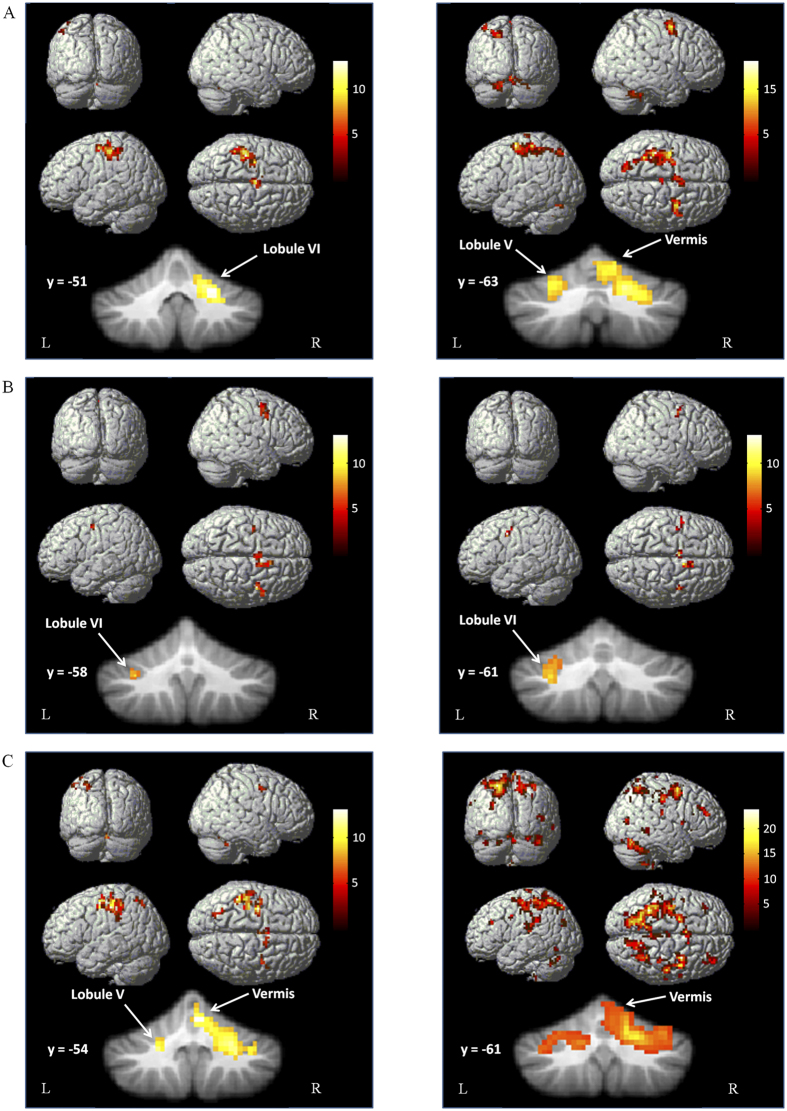
Performance of the single tapping task was associated with activation in the left primary sensorimotor cortex (M1), caudal supplementary motor area (SMA-proper), and left premotor cortex (PMC) in both groups (one sample t-test, P < 0.05, FWE corrected). Bilateral cerebellum was activated in PD patients, while only the right lobule VI of cerebellum was activated in controls (Fig. 1A). The counting task activated the rostral SMA (pre-SMA), bilateral PMC, and left lobule VI of cerebellum in both PD and control subjects (Fig. 1B). During performance of the dual-task, both groups had activations in the left M1, pre-SMA, SMA-proper, bilateral PMC, bilateral parietal cortex, precuneus, and bilateral cerebellum, while PD patients additionally activated bilateral prefrontal cortex (Fig. 1C).
Figure 1. Brain activity in PD patients and controls.
Brain regions activated during performing the right hand finger tapping task (A), counting task (B) and dual-task (C) in the healthy control group (left column), and in PD patients (right column). One sample t-test, P < 0.05, FWE corrected. T-value bars are shown on the right.
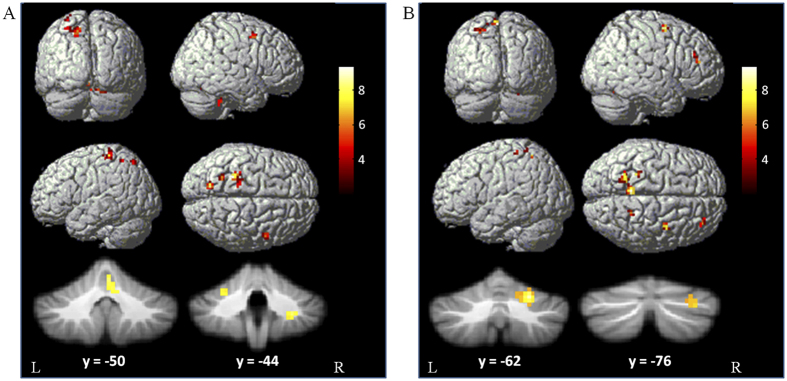
Compared with the single component tasks, dual-task performance additionally activated the RVM, LCV, and precuneus in controls; in contrast, the precuneus was the only additionally activated area in PD patients (one-way ANOVA, P < 0.05, FWE corrected; Fig. 2, Table 1).
Figure 2. Brain regions additionally activated in the dual-task.
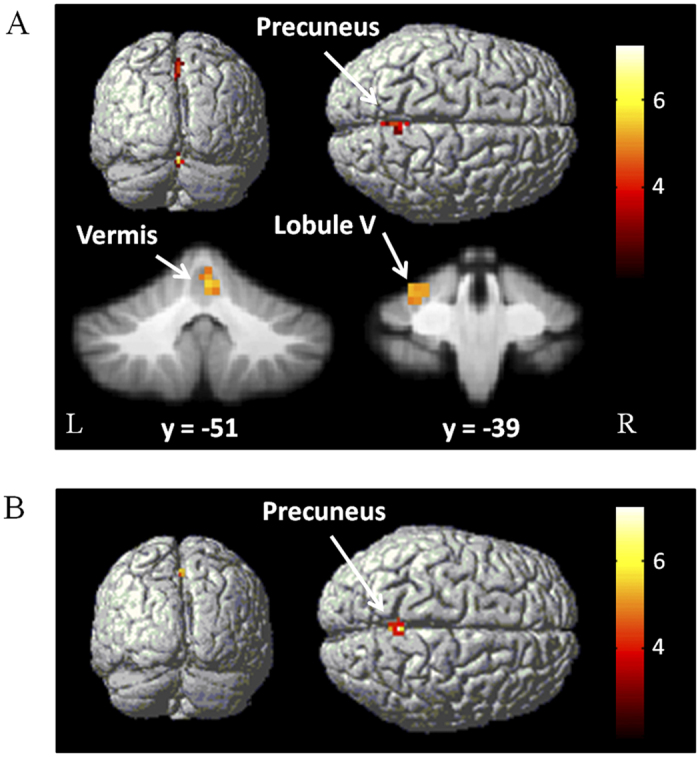
Brain areas more activated in the dual-task compared with single component tasks in controls (A), and in PD patients (B). One-way ANOVA, P < 0.05, FWE corrected. T-value bars are shown on the right.
Table 1. Brain regions additionally activated in dual-task.
| Group | Brain region | Brodmann area | MNI coordinates | t value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controls | R Precuneus | 7 | 3 | −57 | 51 | 5.84 | 594 |
| L Cerebellum, Anterior Lobe, Culmen, Lobule V | −27 | −39 | −23 | 6.17 | 899 | ||
| R Cerebellum, Vermis | 3 | −51 | −15 | 6.51 | 972 | ||
| PD patients | Precuneus | 7 | 0 | −51 | 51 | 5.72 | 675 |
Brain regions additionally activated during performance of dual-task compared with tapping and counting tasks in each group. Abbreviations: L, left; and R, right.
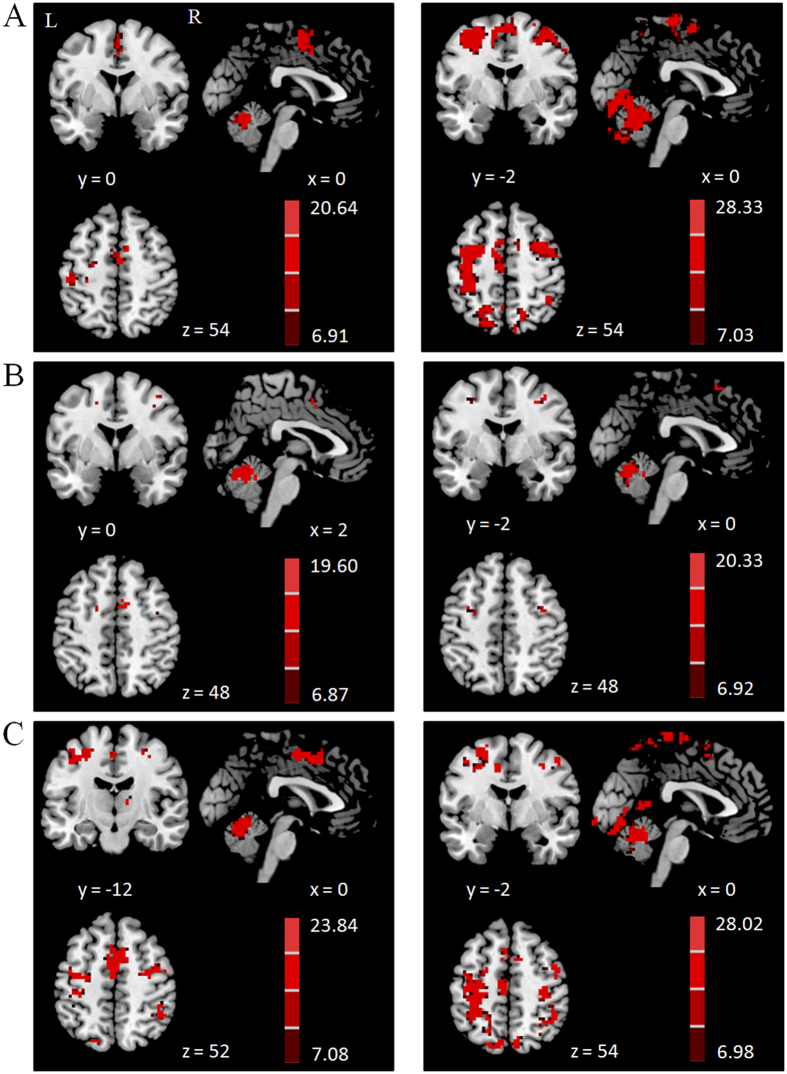
Compared with the controls, PD patients had more activation in the left M1, right PMC, left parietal cortex, and bilateral cerebellum during performance of the tapping task, and had more activation in the right prefrontal cortex, right PMC, bilateral parietal cortex, bilateral precuneus, and right cerebellum while performing the dual-task (two sample t-test, p < 0.05, FWE corrected; Fig. 3, Table 2). There was no difference between the groups in performing the counting task. We did not detect more activity in controls than in patients in any region while performing the tapping, counting, or dual-task (two sample t-test, P < 0.05, FWE corrected).
Figure 3. Brain areas more activated in PD patients.
Brain areas more activated in PD patients than in controls during performing tapping task (A), and dual-task (B). Two-sample t-test, P < 0.05, FWE corrected. T-value bars are shown on the right.
Table 2. Brain regions more activated in PD patients compared to controls.
| Task | Brain region | Brodmann area | MNI coordinates |
t value | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Tapping | L M1 | 4 | −30 | −33 | 63 | 8.01 | 918 |
| R PMC | 6 | 48 | 2 | 51 | 7.29 | 378 | |
| L Superior Parietal Lobule | 7 | −18 | −69 | 51 | 7.67 | 621 | |
| L Inferior Parietal Lobule | 40 | −27 | −54 | 57 | 7.88 | 351 | |
| L Cerebellum, Anterior Lobe, Culmen, lobule V | −26 | −45 | −27 | 7.31 | 486 | ||
| R Cerebellum, Anterior Lobe, Vermis | 3 | −50 | −16 | 8.07 | 891 | ||
| R Cerebellum, Posterior Lobe, Declive, lobule VI | 23 | −65 | −25 | 7.83 | 810 | ||
| R Cerebellum, Posterior Lobe, Tonsil | 28 | −44 | −42 | 7.92 | 513 | ||
| Dual-task | R Precuneus | 5 | −6 | −45 | 66 | 7.89 | 1215 |
| R Paracentral Lobule | 5 | 18 | −45 | 57 | 6.10 | 432 | |
| R PMC | 6 | 30 | −10 | 54 | 6.64 | 1404 | |
| R Prefrontal Cortex | 9 | 33 | 25 | 39 | 6.05 | 864 | |
| L Postcentral Gyrus | 40 | −23 | −42 | 50 | 8.69 | 1944 | |
| R Cerebellum, Posterior Lobe, Declive, lobule VI | 22 | −62 | −24 | 8.11 | 1161 | ||
| R Cerebellum, Posterior Lobe, Uvula | 32 | −76 | −32 | 6.02 | 594 | ||
Abbreviations: L, left; R, right; M1, primary motor cortex; PMC, premotor cortex.
Brain deactivation
During performance of dual-task, there was deactivation in the precuneus, left inferior parietal lobule and left middle temporal gyrus in healthy controls. In PD patients, there was deactivation in the precuneus, left inferior parietal lobule and left middle frontal gyrus (Supplementary Fig. 1; one sample t-test, P < 0.05, FWE corrected). There was no significant between-group difference of deactivation while performing these tasks.
Network connectivity
Connectivity in the RVM
In healthy controls, the RVM functionally connected with the bilateral cerebellum, left M1, left PMC, and SMA-proper while performing the finger tapping task, and had connectivity with the bilateral cerebellum, pre-SMA, and bilateral PMC when performing the counting task. During performance of the dual-task, the RVM connected with the bilateral cerebellum, left M1, bilateral PMC, pre-SMA, SMA-proper, bilateral parietal cortex, and right thalamus (Fig. 4, Supplementary Table 3).
Figure 4. Brain regions connected with the right vermis of the cerebellum (RVM).
Brain regions functionally connected with the RVM during performing the right hand finger tapping task (A), counting task (B) and dual-task (C) in the healthy control group (left column), and in PD patients (right column). One sample t-test, P < 0.05, FWE corrected. T-value bars are shown on the right.
In PD patients, the RVM functionally connected with the bilateral cerebellum, left M1, bilateral PMC, pre-SMA, SMA-proper, right prefrontal cortex and bilateral parietal cortex while performing the finger tapping task, and had connectivity with the bilateral cerebellum, pre-SMA, and bilateral PMC when performing the counting task. During performance of the dual-task, the RVM connected with bilateral cerebellum, bilateral M1, bilateral PMC, pre-SMA, SMA-proper, bilateral temporal lobe, bilateral parietal cortex, and left thalamus (Fig. 4, Supplementary Table 2).
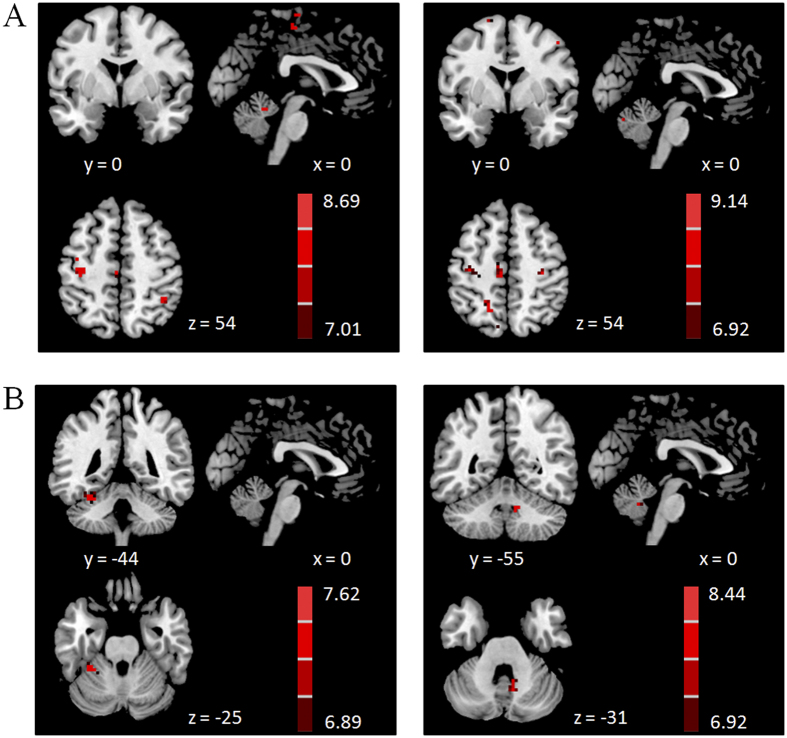
Between-group comparisons showed that in performance of the tapping task, the RVM had enhanced connectivity with the left M1, bilateral cerebellum, right inferior parietal lobule and left paracentral lobule in PD patients. During performing the dual-task, the RVM had increased connectivity with the bilateral M1, right cerebellum, left superior parietal lobule, and left paracentral lobule in PD patients (Fig. 5A). There was no between-group difference while performing the counting task.
Figure 5. Brain regions more connected with the ROIs in PD patients.
Brain areas more connected with the RVM (A) or LCV (B) in PD patients than in controls during performing tapping task (left column), and dual-task (right column). Two-sample t-test, P < 0.05, FWE corrected. T-value bars are shown on the right.
Connectivity in the LCV
In both groups, the LCV had functional connectivity with the posterior lobe of the bilateral cerebellum, SMA-proper, and PMC during execution of the single tapping task. In performing the single counting task, the LCV had connectivity with the bilateral cerebellum, pre-SMA, and PMC. During performance of the dual-task, the LCV showed connectivity with the bilateral cerebellum, pre-SMA, SMA-proper, PMC, and parietal cortex (Supplementary Fig. 2, Supplementary Tables 3 and 4).
The between-group comparison showed that the LCV had enhanced connectivity with the left anterior lobe of cerebellum during performing the tapping task, and had increased connectivity with the right anterior lobe of cerebellum in performance of the dual-task in PD patients compared to controls (Fig. 5B). There was no between-group difference while performing the counting task.
We did not find any brain regions additionally connected with the RVM or LCV in dual-task compared with the single component tasks in both groups.
Connectivity in the precuneus
The results of connectivity in the precuneus during performance of dual-task were showed in Supplementary Fig. 3. The between-group comparison showed that the precuneus had enhanced connectivity with the right anterior lobe of cerebellum, right anterior cingulate gyrus, and left superior parietal lobule in PD patients compared to controls (Supplementary Fig. 3C; two sample t-test, P < 0.05, FWE corrected). We did not find increased connectivity in controls than in patients.
Discussion
In the current study, we investigated the role of the cerebellum in dual-tasking in PD patients. We found that the right cerebellar vermis and left lobule V of cerebellar anterior lobe were additionally activated for execution of a dual motor and cognitive task in healthy controls. In contrast, there were not any cerebellar regions additionally activated while performing this dual-task in PD patients. In addition, the dual-tasking related neural connectivity pattern was different between PD patients and controls. Our findings suggest that the cerebellum might play a role in dual-tasking deficiency in PD.
When people attempt to perform two tasks at the same time, impaired performance tends to occur, which is defined as dual-task interference. In the current study, most subjects did not show significant dual-task interference after practice, which supports previous findings that practice can diminish dual-task interference8,12,15,16,17,18,19,20. While five patients could not perform the dual-task properly even after training, most patients performed the dual-task at the same level as that in controls. This finding is consistent with previous observations that PD patients have more difficulty in performing dual-task; however, they can perform some relatively simple dual-tasks correctly after training8,20.
In agreement with our previous study12, we found that in healthy subjects, the RVM and LCV were additionally activated in performing a dual-task (combining a motor task and a cognitive counting task) compared with single tasks (Fig. 2). Whether the cerebellum is also involved in other types of dual-tasks would need further investigation. In contrast, no regions in the cerebellum were additionally activated in the dual-task in PD patients. We found that during performance of the single tapping task, the RVM and LCV were already activated in PD patients (Fig. 1A), and were more activated in PD patients than in controls (Fig. 3A). Hyperactivation in the cerebellum in PD patients has been extensively reported20,21,22,23,24. However, the nature of this phenomenon remains unclear. A common explanation is that it is a reflection of the compensatory effect14,20,24. Performance of a dual-task with high accuracy indicates that the component tasks are performed automatically25,26. It is known that PD patients have great difficulty in performing movements automatically, and require more brain activity, which might be to compensate for basal ganglia dysfunction in order to perform automatic movements20. However, more activation in the cerebellum might also represent a primary pathophysiological change of PD14, as a consequence of the inability to inhibit contextually inappropriate circuits secondary to abnormal basal ganglia outflow27. Our findings indicate that due to either compensatory or pathological effects, the RVM and LCV were already activated in the single tapping task. Presumably, the limitation of neural resources in these cerebellar regions has been reached even with single tasks in PD patients, and these regions could not be further recruited in dual tasks.
Functional connectivity reflects the integration within functionally specialized networks for a given task28. In healthy controls, the RVM and LCV mainly connected with motor-related regions, e.g. the M1 and SMA-proper, during performance of the tapping task. In contrast, these regions had more connections with cognitive-related areas, e.g. the pre-SMA, while performing the counting-task. The SMA-proper is primarily involved in movement behaviors, while the pre-SMA is associated with cognitive behaviors29,30,31,32,33. The pre-SMA is certainly also involved in motor behavior. However, anatomical and physiological evidence suggests that the pre-SMA is more like a prefrontal area than a motor area, and provides cognitive, sensory or motivational inputs for motor behavior34. In performing the dual-task, the RVM and LCV had connectivity with both motor and cognitive-related areas (e.g. SMA-proper and pre-SMA), but did not connect to any regions that were not connected in the single component tasks. Our previous study found that the RVM and LCV had strengthened connectivity with motor and cognitive networks during the practice of dual-task12. These findings suggest that the role of the RVM and LCV is likely to integrate the motor and cognitive networks, and may also adjust these networks to be more efficient in order to perform the dual motor and cognitive task properly12.
In PD patients, the pattern of network connectivity in the RVM and LCV was similar with that in controls while performing the counting task. In contrast, the pattern of connectivity while performance of the single tapping task was different between the two groups, especially in the RVM. The RVM had connections not only with the motor-related regions, but also with the pre-SMA and prefrontal cortex. The prefrontal cortex is known to be involved in generating new movements35,36, task rehearsal37, attention to action9,38, and in monitoring of motor performance39,40. In addition, the connectivity between the RVM and motor-related areas, e.g. the M1, was increased in PD patients. As a consequence, in PD patients, it might be difficult for the RVM to recruit more motor, cognitive and attentional associated networks for the dual-task.
These cerebellar regions also showed enhanced connectivity during performance of the dual-task, which is possibly a compensatory effort41,42,43. However, the modulation of network connectivity might be a reflection of pathological effects. In healthy subjects, these cerebellar regions have distinct connectivity profiles while performing different tasks. The re-organization of networks may reduce the segregation of neural circuits, and may disrupt the balance between the networks. A recent study found that in PD patients with freezing of gait, the worse dual-task performance was correlated with the altered balance between cognitive and motor networks11. Presumably, the modulated connectivity profile and disrupted balance between the motor and cognitive networks might result in the difficulty of these cerebellar regions in integrating these networks while performing dual-tasks.
The enhanced activity and connectivity also indicate that the efficiency of neural systems is reduced in PD patients. The recruitment of more brain resources can help maintain the performance of some simple dual-tasks. However, when performing more complex dual-task, the limitation of brain capacity might be exceeded. This might be a reason contributing to the difficulty in performing the more complex dual-tasks in PD patients.
Besides the cerebellar regions, the precuneus was additionally activated in the dual-task compared to the single component tasks in both groups. This finding is consistent with previous reports on healthy controls12,44,45 and PD patients8. The precuneus has been associated with attention46, orientation, monitoring47, working memory48 and preparation of movements49. It has been suggested that the additionally activated precuneus in performing dual-tasks is likely due to increased demands of attention than in performing single tasks44. A recent study showed that in PD patients with freezing of gait, worse dual-task performance was correlated with increased connectivity between the precuneus and dorsal putamen11. We found that the precuneus had enhanced connectivity with some areas in PD patients compared to controls. Whether the precuneus plays a substantial role in dual-task deficiency in PD needs to be clarified in future.
There were some limitations in the current study. First, we used a relatively low-resolution fMRI scanning protocol. In recent years, some high-resolution fMRI (e.g. 1.5 mm3 in plane resolution) scanning protocols have been developed. Future studies with high-resolution fMRI scanning will be helpful to investigation on cerebellum. Second, we applied a relatively easy paradigm to investigate dual-task related neural correlates. This paradigm is working well in distinguishing dual-task related activity from single task activations12. However, as PD patients also can perform this dual-task correctly after training, this paradigm may not sensitive to detect dual-task deficiency in PD. An improved paradigm might be developed in future. Third, as head motion has significant impact on connectivity results50, we include head motion parameters as regressors to minimize the influence of head motion on our results. However, as head motion may be task-correlated, whether head motion correlated with the task behaviors should be examined. As we only measured the correction of finger tapping and counting, this correlation analysis cannot be performed in the current study, which needs to be examined in future studies. Forth, we chose patients at a relatively mild stage to ensure that most patients could perform the dual-task properly after training. It is possible that PD patients at more advanced stage have more difficulty in performing dual-task, and neural changes in more advanced patients may be different from that in patients at mild stage. Moreover, we only focused on the cerebellum in the current study. It has been shown that some cortical regions (e.g. the SMA and PMC) are also involved in dual-task deficiency in PD8,10,11,51. In addition, several studies have shown that the dorsolateral prefrontal cortex (DLPFC) is involved in dual-tasking52,53. Therefore, the cerebellum is likely one of the reasons contributing to dual-task deficiency in PD. More studies are warranted to fully understand this problem.
In conclusion, the present study found that the right cerebellar vermis and left lobule V of cerebellar anterior lobe were additionally activated for dual-task performance in healthy controls. In contrast, there was no cerebellar region additionally activated while performing dual-task in PD patients. In addition, these cerebellar regions had enhanced connectivity with motor and cognitive associated networks in PD patients. Our findings suggest that the limited cerebellar resources and the difficulty of these cerebellar regions in integrating motor and cognitive networks might contribute to dual-tasking deficiency in PD patients.
Methods
Subjects
Twenty-five PD patients were involved in this study. The diagnosis of PD was based on the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria54. Patients were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS)55, the Hoehn and Yahr disability scale56, and Mini-Mental State Examination while off their medications. Bradykinesia/rigidity was the predominant symptom and was more severe on the right side in every patient. To avoid disturbance of the fMRI signal, all patients were chosen to have at most a mild tremor (no more than mild degree in the items 3.15 to 3.18 of the UPDRS examination). Twenty age and gender matched healthy subjects were recruited as the control group. All patients and control subjects were right-handed according to the Edinburgh Inventory57. Five patients were excluded because they could not perform the dual-task properly. In addition, two patients and two controls had excessive head motion during the fMRI acquisition, and their datasets were discarded. The demographics and clinical details from the remaining controls and patients are shown in Table 3. The experiments were performed according to the Declaration of Helsinki and were approved by the Institutional Review Board of Xuanwu Hospital. All subjects gave their written informed consent for the study.
Table 3. Demographics and clinical details of the subjects (mean ± SD).
| PD patients | Controls | |
|---|---|---|
| Age (years) | 62.50 ± 6.95 (50–75) | 62.28 ± 6.76 (50–74) |
| Sex | 7 female, 11 male | 7 female, 11 male |
| Disease Duration (years) | 4.17 ± 1.86 | |
| UPDRS motor score | 18.94 ± 5.84 | |
| Hoehn and Yahr staging | 1.64 ± 0.59 | |
| Mini-Mental State Examination | 29.06 ± 1.11 | 29.28 ± 0.96 |
| L-dopa dose (mg/day) | 347.22 ± 101.06 |
The comparisons between the PD patients and controls were analyzed with two-sample t-tests (p > 0.05).
Task
All subjects were asked to perform two single tasks and one dual-task. The paradigm was similar to that in a recent study12. Single tasks contained a motor task and a number counting (cognitive) task. The motor task was a self-paced tapping task in which subjects briskly tapped their right index and middle fingers alternatively at 1 Hz frequency, with amplitude of about 2.5 cm. For the counting task, a random series of the numbers 2, 3, 5, and 8 were presented on a screen and subjects were asked to identify the number of times they saw a specified target number. The numbers were presented at an irregular interval (average interval 1.5 s). In the dual-task, subjects performed the tapping and counting tasks simultaneously. As a first priority, we asked the subjects to perform the motor task correctly, and then a second priority, to count the target numbers correctly.
Before the fMRI scanning, subjects were given one hour practice in order to perform the single and dual-tasks correctly. The successful performance of the dual-task was determined by the execution of three dual-task trials in a row without errors (each trial lasted 3 min), and without behavioral difference from the single tasks. As mentioned above, five patients were excluded because they could not perform the dual-task properly after one hour’s practice.
fMRI Procedure
fMRIs were performed on a 3 T MR scanner (Trio system; Siemens Magnetom scanner, Erlangen, Germany). A standard head coil was used with foam padding to restrict head motion. All subjects underwent high- resolution MRI scanning, which included a 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) scan (TR = 1600 ms, TE = 2.13 ms, 176 sagittal slices, slice thickness = 1.0 mm, field of view (FOV) = 224 mm × 256 mm, voxel size of 1 mm3 isotropic), and a T2-weighted MRI scan (TR = 5000 ms, TE = 87 ms, 35 axial slices, slice thickness = 4 mm, FOV = 256 mm × 256 mm, voxel size = 1 × 1 × 4 mm3). Blood-oxygen-level dependent data were acquired with gradient-echo echo-planar sequences (TR = 2000 ms, TE = 40 ms, 33 axial slices, slice thickness = 3.5 mm, no gap, Flip angle = 90°, FOV = 256 mm × 256 mm, matrix size = 64 × 64). As it has been shown that heart rate has influence on fMRI signal58, we monitored heart rate in each subject during fMRI scanning.
An electrical response device that could work inside the MRI scanner was fixed to each subject’s right hand. The response device had 2 buttons, corresponding to the index and middle fingers of the right hand and was used to record finger movements during fMRI scanning. For each subject, there were three runs in fMRI scanning session. Each run was 8 min. All runs were block-designed and contained two conditions, which were defined as the “rest” and “task” condition, respectively. Each condition lasted 30 s and was repeated eight times. In the rest condition, subjects were asked to relax and focus on the screen in front of them without moving or thinking while inside the scanner. The task condition in each run contained one of the right hand tapping, number counting, or dual-tasks. Visual signals were presented on the screen to inform the subjects to switch from rest to task condition and vice versa. The subjects viewed the visual signals through a mirror built into the head coil. Task order of these three runs was randomized across subjects in each session. After scanning each run, subjects were asked to report the whole number of the target number in that run.
Behavioral data analysis
The behavioral measurements included the accuracy and frequency for finger-tapping, and number of target numbers in each run. Wrong button presses for motor task or incorrect numbers being reported for the counting task were considered errors. The accuracy in each single and dual-task were entered into a repeated-measures ANOVA to calculate the within- and between-group differences in performance between single and dual-tasks.
Imaging data analysis
Data Preprocessing
Image analysis was performed with SPM8 software (Wellcome Institute of Cognitive Neurology, London, UK). fMRI data were slice-time corrected and aligned to the first image of each run for motion correction. After spatial normalization, images were resampled into voxels that were 3 × 3 × 3 mm in size, and smoothed with a 6 mm Gaussian smoothing kernel. Each participant’s movement parameters were examined. As described previously, two patients and two controls had excessive head motion (more than 1 mm maximum translation in x, y or z, or 1° of maximum angular rotation about each axis), and their datasets were discarded.
Brain Activity Analysis
Data were first analyzed for each single participant separately on a voxel-by-voxel basis using the general linear model approach for the time series. We defined a model using a fixed effect boxcar design convolved with a hemodynamic response function for analysis of task-dependent activation. We added the head motion parameters as regressors to optimally control for the motion effects. In addition, heart rate was used as a regressor to reduce its influence on activation in the cerebellum58. At the first-level, a contrast representing the effect of the “task” condition compared with the “rest” condition was calculated in each participant. These contrast images were used in the second level for random-effects analyses.
At the second level, first, a one-sample t-test model was used to identify the brain activity in each condition in each group. Then, we used one-way ANOVA to explore the brain regions that were additionally activated in the dual-task compared to single tasks in each group (dual-task–tapping–counting)8,12,59. Additionally, a two-sample t-test model was used to explore the difference between patients and normal subjects in each condition.
Brain Activity in the Cerebellum
The activations specific to the cerebellum were further analyzed with the Spatially Unbiased Infra-tentorial Template toolbox (SUIT Version 3.2)60,61. We used the high resolution T1- images to isolate the cerebellum from the rest of the brain, and normalized to the SUIT template using a nonlinear deformation. Then, we used unsmoothed functional data to calculate the contrasts in each subject and in each condition with the same procedures for the whole brain activity analysis. These contrast images were resliced using the deformation found in the last step and smoothed. At the second level, the activations in the cerebellum in each condition were analyzed. The results were overlaid on the high-resolution SUIT atlas of the human cerebellum60,61.
Brain Deactivation Analysis
In addition, we analyzed deactivation during performance of the dual-task. The deactivation means more voxel intensity in the “rest” condition than that in the “task” condition. The deactivation in each group was measured in each group by a one-sample t-test. Then, a two-sample t-test model was used to explore the difference of deactivation between the groups.
Network Connectivity Analysis
According to the results from brain activity analysis in this study, the right cerebellar vermis (RVM), left lobule V of the cerebellar anterior lobe (LCV) and precuneus were additionally activated in the dual-task rather than in the single component tasks in healthy controls. These three areas were chosen as the regions of interest (ROIs) for functional connectivity analysis. The ROIs were centered at the voxels showing the maximum magnitude of activation within these regions, with a radius of 5 mm. We only chose the smoothed data at task conditions for connectivity analysis. The images were filtered with a high-pass filter (128 seconds). We measured functional connectivity with partial correlation analysis by a toolkit REST (www.restfmri.net). The white matter and cerebrospinal fluid signals were regressed out. A seed reference time course was obtained within each ROI. Correlation analysis was carried out between the seed reference and the whole brain in a voxel-wise manner in each ROI. The individual results were entered into a random effect one-sample t-test to determine brain regions showing significant connectivity with each ROI within each group in each condition. Then, a two-sample t-test model was used to explore the difference between patients and normal subjects in each condition. Finally, we used one-way ANOVA to examine if there were any brain regions that were additionally connected with the ROIs in the dual-task compared to single tasks in each group. A family-wise error (FWE) corrected threshold of P < 0.05 was used for all brain activity and connectivity analysis. Extent threshold was 10 voxels.
Additional Information
How to cite this article: Gao, L. et al. The cerebellum in dual-task performance in Parkinson’s disease. Sci. Rep. 7, 45662; doi: 10.1038/srep45662 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by grant from the National Science Foundation of China (81571228).
Footnotes
The authors declare no competing financial interests.
Author Contributions Linlin Gao: execution of the research, analysis of the data, writing of the manuscript; Jiarong Zhang, Yanan Hou: execution of the research, analysis of the data; Mark Hallett, Piu Chan: conception of the research, advice on data analysis; Tao Wu: conception, organization of the research, and critique of the manuscript.
References
- Benecke R., Rothwell J. C., Dick J. P., Day B. L. & Marsden C. D. Performance of simultaneous movements in patients with Parkinson’s disease. Brain 109 (Pt 4), 739–757 (1986). [DOI] [PubMed] [Google Scholar]
- Castiello U. & Bennett K. M. The bilateral reach-to-grasp movement of Parkinson’s disease subjects. Brain 120 (Pt 4), 593–604 (1997). [DOI] [PubMed] [Google Scholar]
- Brown R. G. & Marsden C. D. Dual task performance and processing resources in normal subjects and patients with Parkinson’s disease. Brain 114 (Pt 1A), 215–231 (1991). [PubMed] [Google Scholar]
- Woodward T. S., Bub D. N. & Hunter M. A., Task switching deficits associated with Parkinson’s disease reflect depleted attentional resources. Neuropsychologia 40, 1948–1955 (2002). [DOI] [PubMed] [Google Scholar]
- Benecke R., Rothwell J. C., Dick J. P., Day B. L. & Marsden C. D. Simple and complex movements off and on treatment in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 50, 296–303 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhaidat J., Kerr A., Evans J. J., Pilling M. & Skelton D. A. Validity of simple gait-related dual-task tests in predicting falls in community-dwelling older adults. Arch Phys Med Rehabil 95, 58–64 (2014). [DOI] [PubMed] [Google Scholar]
- Spildooren J. et al. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord 25, 2563–2570 (2010). [DOI] [PubMed] [Google Scholar]
- Wu T. & Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 79, 760–766 (2008). [DOI] [PubMed] [Google Scholar]
- Wu T. et al. Attention to Automatic Movements in Parkinson’s Disease: Modified Automatic Mode in the Striatum. Cereb Cortex 25, 3330–3342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S. et al. Dual-task interference and brain structural connectivity in people with Parkinson’s disease who freeze. J Neurol Neurosurg Psychiatry 86, 786–792 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort G. et al. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience 317, 36–46 (2016). [DOI] [PubMed] [Google Scholar]
- Wu T., Liu J., Hallett M., Zheng Z. & Chan P. Cerebellum and integration of neural networks in dual-task processing. Neuroimage 65, 466–475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A. C., Dum R. P. & Strick P. L. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107, 8452–8456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. & Hallett M. The cerebellum in Parkinson’s disease. BRAIN 136, 696–709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. & Pashler H. Is dual-task slowing instruction dependent? J Exp Psychol Hum Percept Perform 27, 862–869 (2001). [PubMed] [Google Scholar]
- Ruthruff E., Johnston J. C. & Van Selst M. Why practice reduces dual-task interference. J Exp Psychol Hum Percept Perform 27, 3–21 (2001). [PubMed] [Google Scholar]
- Ruthruff E., Johnston J. C., Van Selst M., Whitsell S. & Remington R. Vanishing dual-task interference after practice: has the bottleneck been eliminated or is it merely latent? J Exp Psychol Hum Percept Perform 29, 280–289 (2003). [DOI] [PubMed] [Google Scholar]
- Schumacher E. H. et al. Virtually perfect time sharing in dual-task performance: uncorking the central cognitive bottleneck. Psychol Sci 12, 101–108 (2001). [DOI] [PubMed] [Google Scholar]
- Van Selst M., Ruthruff E. & Johnston J. C. Can practice eliminate the psychological refractory period effect? J Exp Psychol Hum Percept Perform 25, 1268–1283 (1999). [DOI] [PubMed] [Google Scholar]
- Wu T. & Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. BRAIN 128, 2250–2259 (2005). [DOI] [PubMed] [Google Scholar]
- Jahanshahi M. et al. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118 (Pt 4), 913–933 (1995). [DOI] [PubMed] [Google Scholar]
- Rascol O. et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 120 (Pt 1), 103–110 (1997). [DOI] [PubMed] [Google Scholar]
- Catalan M. J., Ishii K., Honda M., Samii A. & Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain 122 (Pt 3), 483–495 (1999). [DOI] [PubMed] [Google Scholar]
- Yu H., Sternad D., Corcos D. M. & Vaillancourt D. E. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35, 222–233 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. E. Attention to action. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 351, 1473–1479 (1996). [DOI] [PubMed] [Google Scholar]
- Wu T., Kansaku K. & Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91, 1690–1698 (2004). [DOI] [PubMed] [Google Scholar]
- Mink J. W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50, 381–425 (1996). [DOI] [PubMed] [Google Scholar]
- Friston K. J. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2, 56–78 (1994). [Google Scholar]
- Deiber M. P., Honda M., Ibanez V., Sadato N. & Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81, 3065–3077 (1999). [DOI] [PubMed] [Google Scholar]
- Jenkins I. H., Jahanshahi M., Jueptner M., Passingham R. E. & Brooks D. J. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123 (Pt 6), 1216–1228 (2000). [DOI] [PubMed] [Google Scholar]
- Kurata K., Tsuji T., Naraki S., Seino M. & Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J Neurophysiol 84, 1667–1672 (2000). [DOI] [PubMed] [Google Scholar]
- Sakai K. et al. What and when: parallel and convergent processing in motor control. J Neurosci 20, 2691–2700 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Mushiake H., Saito N. & Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 93, 8694–8698 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N. & Strick P. L. Imaging the premotor areas. Curr Opin Neurobiol 11, 663–672 (2001). [DOI] [PubMed] [Google Scholar]
- Deiber M. P. et al. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84, 393–402 (1991). [DOI] [PubMed] [Google Scholar]
- Jueptner M. et al. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77, 1313–1324 (1997). [DOI] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Evans A. C. & Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA 90, 873–877 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik E. C. et al. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex 23, 2677–2689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J. B. et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex 11, 260–266 (2001). [DOI] [PubMed] [Google Scholar]
- Hoshi E. & Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol 17, 234–242 (2007). [DOI] [PubMed] [Google Scholar]
- Palmer S. J., Li J., Wang Z. J. & McKeown M. J. Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 166, 1110–1118 (2010). [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S., de la Fuente-Fernandez R., Galley S. & McKeown M. J. Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol 23, 407–412 (2010). [DOI] [PubMed] [Google Scholar]
- Wu T. et al. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 55, 204–215 (2011). [DOI] [PubMed] [Google Scholar]
- Wenderoth N., Debaere F., Sunaert S. & Swinnen S. P. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. EUR J Neurosci 22, 235–246 (2005). [DOI] [PubMed] [Google Scholar]
- Mochizuki H. et al. Brain activity associated with dual-task management differs depending on the combinations of response modalities. Brain Res 1172, 82–92 (2007). [DOI] [PubMed] [Google Scholar]
- Culham J. C., Cavanagh P. & Kanwisher N. G. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron 32, 737–745 (2001). [DOI] [PubMed] [Google Scholar]
- Gusnard D. A., Raichle M. E. & Raichle M. E. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2, 685–694 (2001). [DOI] [PubMed] [Google Scholar]
- Callicott J. H. et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 9, 20–26 (1999). [DOI] [PubMed] [Google Scholar]
- Astafiev S. V. et al. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23, 4689–4699 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. G. et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S. J., Lee P. W., Wang Z. J., Au W. L. & McKeown M. J. Theta, beta But not alpha-band EEG connectivity has implications for dual task performance in Parkinson’s disease. Parkinsonism Relat Disord 16, 393–397 (2010). [DOI] [PubMed] [Google Scholar]
- D’Esposito M. et al. The neural basis of the central executive system of working memory. Nature 378, 279–281 (1995). [DOI] [PubMed] [Google Scholar]
- Herath P., Klingberg T., Young J., Amunts K. & Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex 11, 796–805 (2001). [DOI] [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Kilford L. & Lees A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55, 181–184 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A. E. F. S. Assessment of Parkinson’s disease. In: Quantification of Neurological Deficit, TL M. (ed.), Boston: Butterworths, pp. 285–309 (1989). [Google Scholar]
- Hoehn M. M. & Yahr M. D. Parkinsonism: onset, progression, and mortality. 1967. Neurology 57, S11–S26 (2001). [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]
- Schlerf J., Ivry R. B. & Diedrichsen J. Encoding of sensory prediction errors in the human cerebellum. J Neurosci 32, 4913–4922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szameitat A. J., Schubert T. & Muller H. J. How to test for dual-task-specific effects in brain imaging studies—an evaluation of potential analysis methods. Neuroimage 54, 1765–1773 (2011). [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138 (2006). [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J. H., Flavell J., Cussans E. & Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.