Abstract
Secondary abiotic mice generated by broad-spectrum antibiotic treatment provide a valuable tool for association studies with microbiota derived from different vertebrate hosts. We here generated human microbiota-associated (hma) mice by human fecal microbiota transplantation of secondary abiotic mice and performed a comprehensive survey of the intestinal microbiota dynamics in offspring of hma mice over 18 weeks following weaning as compared to their mothers applying both cultural and molecular methods. Mice were maintained under standard hygienic conditions with open cages, handled under aseptic conditions, and fed autoclaved chow and water. Within 1 week post weaning, fecal loads of commensal enterobacteria and enterococci had decreased, whereas obligate anaerobic bacteria such as Bacteroides/Prevotella species and clostridia were stably colonizing the intestines of hma offspring at high loads. Lactobacilli numbers were successively increasing until 18 weeks post weaning in both hma offspring and mothers, whereas by then, bifidobacteria were virtually undetectable in the former only. Interestingly, fecal lactobacilli and bifidobacteria were higher in mothers as compared to their offspring at 5 and 18 weeks post weaning. We conclude that the intestinal microbiota composition changes in offspring of hma mice, but also their mothers over time particularly affecting aerobic and microaerobic species.
Keywords: intestinal microbiota dynamics, secondary abiotic mice, gnotobiotic mice, fecal microbiota transplantation, human microbiota-associated mice, offspring of mice, intestinal ecology, culturomics
Introduction
The human intestinal microbiota is gaining increasing attention due to its association with a broad range of immunopathological conditions including metabolic diseases (e.g., obesity, metabolic syndrome, type 2 diabetes mellitus), autoimmune disorders (e.g., type 1 diabetes mellitus, rheumatoid arthritis, inflammatory bowel diseases), cancer, allergies, and neurodevelopmental and behavioral disorders (e.g., autism, depression, dementia) [1]. Thus, with respect to their microbiota, “humanized” mice provide rewarding in vivo models to unravel the molecular mechanisms underlying commensal bacteria–host interactions and its determinants in health and disease. In fact, human microbiota-associated (hma) formerly germfree mice that have been reared in isolators are widely applied nowadays to unravel the effects of (epi)genetic and environmental (including dietary) factors on intestinal microbiota and host (patho)physiology [2]. For instance, one study applying hma mice revealed that distinct microbial communities of the intestinal microbiota that had been derived from preterm infants differentially affected intestinal epithelial development with pivotal effects on developmental, defensive, and physiological processes within the intestinal epithelium [3]. In another report, germfree mice were reconstituted with microbiota of patients suffering from inflammatory bowel disease (IBD) such as Crohn’s disease and ulcerative colitis in order to investigate susceptibility to disease depending on abundances of distinct bacterial groups derived from diseased individuals [4].
Mice reassociated with human microbiota from different donors display an intestinal microbiome composition as well as metabolic profile that is similar to those observed in donors with preservation of distinct individual-specific features [5]. As an important prerequisite, the donor human microbiota could stably establish within the intestinal ecosystem in recipient mice with distinct similarities to the community composition in the respective donor suspensions [2, 6, 7], while some low-abundance Firmicutes, however, were shown to be absent in recipient mice resulting in an impaired response to infections [8]. Notably, commensal bacterial species appear to be critically adapted to specific hosts [8]. Hence, despite a highly specific coexistence and mutual interaction between microbiota species and host immunity, hma mice do not entirely mirror human microbiota–host interactions [9], given that a microbiota has been transplanted into a host with which it has not been co-evolved. Nevertheless, despite these limitations, the hma mouse model provides a unique tool to unravel microbiota–host interactions in health and disease.
One needs to take further into consideration, however, that studies with hma mice have been undertaken in isolator-raised germfree mice with a substantially underdeveloped immune system in most cases so far. Given that immunological differentiation and maturation are highly microbiota dependent, it becomes therefore obvious that comparisons of studies with germfree versus conventional mice are problematic per se [10]. This point of view is shared by recent studies reporting pivotal differences in phenotypes already under basal conditions and even more pronounced in various disease states when applying isolator-raised germfree and conventionally reared mice [11–14].
We therefore generated secondary abiotic (i.e., gnotobiotic) mice with a virtually depleted intestinal microbiota by broad-spectrum antibiotic treatment of conventionally reared mice exhibiting a fully developed immune system [10, 15, 16]. Following peroral fecal microbiota transplantations (FMTs), the complex human microbiota established within the gastrointestinal tract of these secondary abiotic mice [16, 17] and was stable for over 6 weeks [16]. By applying the hma mouse model, we could convincingly prove previously that the host-specific intestinal microbiota composition determines host susceptibily for and resistance against distinct enteropathogens including Campylobacter jejuni [16, 18–20]. Whereas conventionally colonized mice were protected from C. jejuni infection due to their physiological colonization resistance and expelled the pathogen within 48 h following high dose infection, C. jejuni could stably infect secondary abiotic mice even when reassociated with complex human intestinal microbiota [16]. Following reconstitution of secondary abiotic mice with murine microbiota, however, mice exhibited fully re-established colonization resistance against C. jejuni [16]. Our murine in vivo data are supported by recent human investigations, given that individuals exhibiting a reduced bacterial diversity in their intestines were susceptibile to C. jejuni infection [21]. Hence, our “humanized” murine in vivo model is well-suited to further dissect the molecular mechanisms underlying pathogen-commensal microbiota–host interactions.
Generation of hma mice, however, is time-consuming, given that mice need to be at least 8 weeks of age prior quintuple antibiotic challenge for another 8 to 10 weeks. To assure stable bacterial colonization, mice should be further kept for at least 2 more weeks post FMT before inclusion into the respective experiment. Thus, breeding of hma mice would be a convenient measure, saving time and resources, but a stable human microbiota colonization of the murine gastrointestinal tract over time would be a pivotal prerequistite for experimental applications.
In the present study, we therefore investigated potential changes in intestinal microbiota composition in hma offspring mice and their corresponding mothers over time applying both comprehensive cultural (“culturomics”) and molecular methods.
Methods
Generation of secondary abiotic mice
C57BL/6j mice were reared and maintained under specific pathogen-free (SPF) conditions in the Forschungsinstitute für Experimentelle Medizin (Charité – University Medicine, Berlin, Germany). Secondary abiotic (i.e., gnotobiotic) mice with a virtually depleted microbiota were generated as described previously [15]. In brief, 8-week-old mice were transferred into sterile cages and subjected to a broad-spectrum antibiotic treatment for at least 8 weeks by adding ampicillin plus sulbactam (1 g/l; Ratiopharm, Germany), vancomycin (500 mg/l; Cell Pharm, Germany), ciprofloxacin (200 mg/l; Bayer Vital, Germany), imipenem (250 mg/l; MSD, Germany), and metronidazole (1 g/l; Fresenius, Germany) to the drinking water (ad libitum).
Generation of human microbiota-associated mice
Fresh fecal samples free of pathogenic bacteria, viruses, and parasites were collected from five individual healthy volunteers, dissolved in sterile phosphate buffered saline (PBS; Gibco, life technologies, UK), aliquoted, and stored at –80 °C as described previously [16]. Three days prior microbiota reassociation, the antibiotic cocktail was withdrawn and replaced by autoclaved drinking water. Immediately before FMT, individual fecal aliquots were thawed and pooled, and the main bacterial communities within the donor suspension were quantitatively assessed by cultural and molecular methods [16]. Then, secondary abiotic animals were subjected to peroral FMTs with 0.3 ml of the donor suspension by gavage on three consecutive days in order to generate hma mice. To assure proper establishment of the human microbiota in the murine host, mice were kept for at least 4 weeks before mating (two female mice and one age-matched male together in one cage). After successful fertilization, the murine father was separated from the pregnant mouse. Offspring were kept in the same cage with the mother until weaning by the age 3 weeks post partum. Mice were kept in open cages within an experimental semi-barrier (accessible only with lab coat, overshoes, caps, and sterile gloves) under standard conditions (22–24 °C room temperature, 55 ± 15% humidity, 12 h light/12 dark cycle). Mice were handled under aseptic conditions and had unlimited access to autoclaved tap water and chow (food pellets; Sniff, Soest, Germany). Altogether, 21 hma offspring (ten female and 11 male mice) from five litters and five hma mothers were included into the study.
Cultural survey of fecal microbiota
For comprehensive quantitative survey of intestinal microbiota composition applying “culturomics”, fecal samples were homogenized in sterile PBS and total bacterial loads, enterobacteria, enterococci, aerobic Gram-positive rods, Bacteroides/Prevotella spp., and Clostridium/Eubacterium spp. quantitatively assessed in serial dilutions on respective solid selective media as described earlier [15, 22, 23]. Bacteria were grown at 37 °C for at least 2 to 3 days under aerobic, microaerobic, and anaerobic conditions.
Molecular analysis of fecal microbiota
DNA was extracted from fecal samples as described previously [15, 24]. In brief, DNA was quantified by using Quant-iT PicoGreen reagent (Invitrogen, UK) and adjusted to 1 ng per microliter. Then, main bacterial groups abundant in the murine and human intestinal microbiota were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) with species-, genera-, or groupspecific 16S rRNA gene primers (Tib MolBiol, Germany) as described previously [16, 17, 25–27], and numbers of 16S rRNA gene copies per ng DNA of each sample were determined.
Statistical analysis
Medians and levels of significance were determined by Mann–Whitney U test (GraphPad Prism v5, La Jolla, CA, USA) as indicated. Two-sided probability (p) values ≤0.05 were considered significant.
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin). Animal welfare was monitored twice daily by assessment of clinical conditions.
Results
Generation of human microbiota-associated mice
Secondary abiotic mice with a virtually depleted intestinal murine microbiota following broad-spectrum antibiotic treatment were subjected to human fecal microbiota transplantations (FMTs) by gavage on three consecutive days. Both cultural and culture-independent (i.e., molecular) analyses revealed that the microbiota composition of the individual fecal suspensions used for FMT was comparable, given that bacterial loads of the main intestinal groups and species differed, if at all, by less than 0.5 and 1.0 order of magnitude (CFU/ml and gene numbers/ng DNA, respectively) (Fig. S1). Notably, bifidobacterial genes were abundant in relatively high number, whereas Mouse Intestinal Bacteroides were virtually undetectable in human fecal suspensions (Fig. S1B).
Fig. S1.
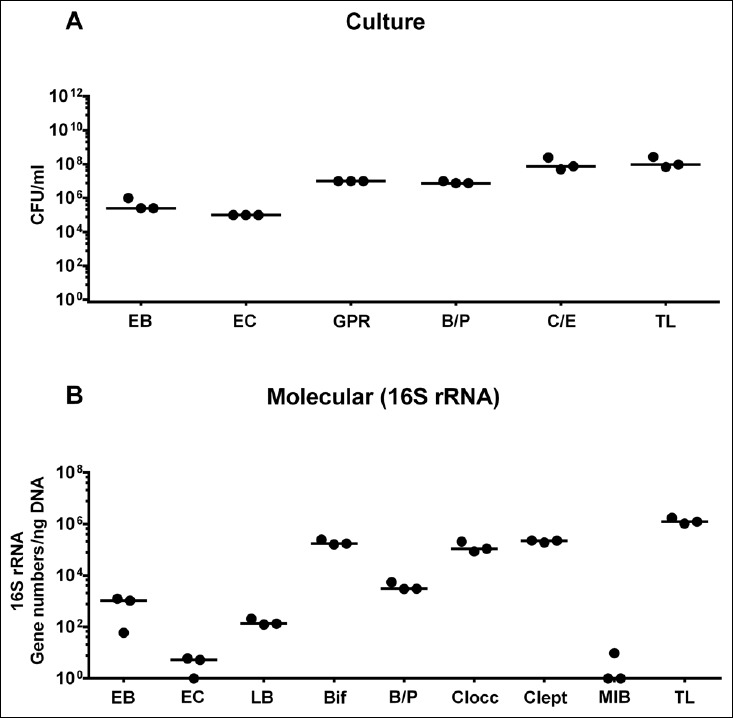
Microbiota composition of suspensions used for human fecal microbiota transplantation. Human microbiota-associated mice were generated by human fecal microbiota transplantation of secondary abiotic mice by gavage on three consecutive days (as described in methods). (A) Microbiota composition of suspensions used for gavage was quantitatively assessed by culture of enterobacteria (EB), enterococci (EC), Gram-positive rods (GPR), Bacteroides/Prevotella spp. (B/P), Clostridium/Eubacterium spp. (C/E), and total bacterial loads (TL) and expressed as colony forming units (CFU) per ml. (B) Microbiota survey was supplemented with quantitative molecular analysis of enterobacteria (EB), enterococci (EC), lactobacilli (LB), bifidobacteria (Bif), Bacteroides/Prevotella spp. (B/P), Clostridium coccoides group (Clocc), Clostridium leptum group (Clept), Mouse Intestinal Bacteroides (MIB), and total eubacterial loads (TL) by qRT-PCR (expressed as 16S rRNA gene numbers per ng DNA). Medians are shown
Intestinal microbiota dynamics in offspring of hma mice as assessed by culture
Following mating of female and male hma adult mice, we performed a comprehensive cultural survey of potential intestinal microbiota changes in the hma offspring on a weekly basis over 18 weeks starting by the age of 3 weeks at weaning. As controls, corresponding mothers were surveyed at defined time points (i.e., weaning, weeks 5, 14, and 18 post weaning). As expected, the fecal microbiota of (first generation) parental hma mice was substantially different from age- and sex-matched conventionally colonized counterparts (Fig. S2). Whereas total bacterial loads in fecal samples derived from both offspring and mothers remained constant over the entire observation period (Fig. 1), numbers of fecal enterobacteria (p < 0.001; Fig. 2A) and enterococci (p < 0.05–0.001; Fig. 3A) were lower in the offspring at all time points post weaning as compared to the initial time point. In the mothers, however, fecal counts of enterobacteria, but not enterococci, were lower 14 weeks post weaning versus weaning (p < 0.05; Fig. 2B), while enterococcal loads remained stable (n.s.; Fig. 3B). Interestingly, offspring of hma mice exhibited up to two order of magnitude lower fecal enterobacteria than their mothers at 5, 14, and 18 weeks post weaning (p < 0.01–0.001; Fig. 2), whereas intestinal enterococcal loads were lower in the offspring as compared to hma mothers as early as 5 weeks following weaning (p < 0.05; Fig. 3). Notably, hma offspring mice exhibited stable fecal loads of Gram-positive rods (approximately 108 CFU/g; Fig. 4A) that were higher as compared to those cultured from mothers at weaning and 18 weeks thereafter (p < 0.01 and p < 0.001, respectively; Fig. 4). Intestines of both hma offspring and mothers were stably colonized by high loads of obligate anaerobic species such as Bacteroides/Prevotella spp. and Clostridium/Eubacterium spp. (n.s.; Figs. 5 and 6). Hence, aerobic commensal bacterial species such as enterobacteria and enterococci were less abundant in the intestinal tract of hma offspring after weaning, whereas obligate anaerobic bacteria were rather stable intestinal colonizers.
Fig. S2.
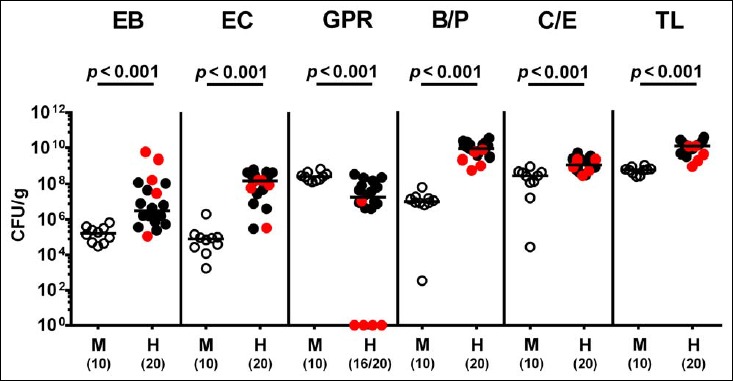
Microbiota composition in first generation parental human microbiota-associated and conventionally colonized mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Microbiota composition was assessed in fecal samples derived from first generation (parental) hma mice (H, filled circles) as compared to conventionally colonized counterparts (M, open circles) applying culturomics. Absolute numbers of enterobacteria (EB), enterococci (EC), Gram-positive rods (GPR), Bacteroides/Prevotella spp. (B/P), Clostridium/Eubacterium spp. (CE), and total bacterial loads (TL) are expressed as colony forming units per gram (CFU/g) feces. Medians, significance levels (p values) determined by Mann–Whitney U test, and numbers of culture-positive samples out of the total number of analyzed animals (in parentheses) are indicated. Mothers of hma offspring mice are depicted in red
Fig. 1.
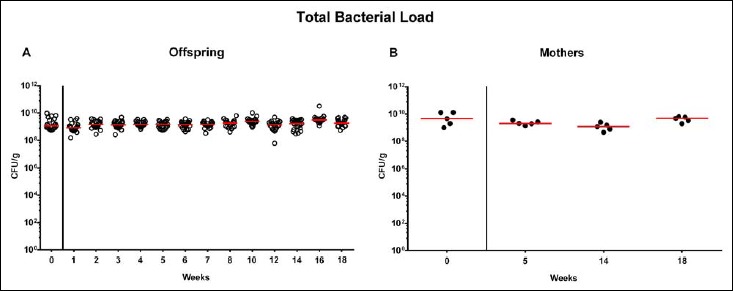
Total bacterial fecal loads in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Individual total bacterial loads were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians are shown. Data were pooled from five litters
Fig. 2.
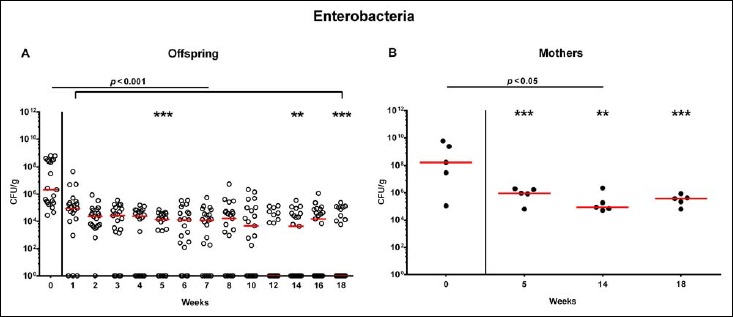
Fecal loads of enterobacteria in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Enterobacteria were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (**p < 0.01; ***p < 0.001). Data were pooled from five litters
Fig. 3.
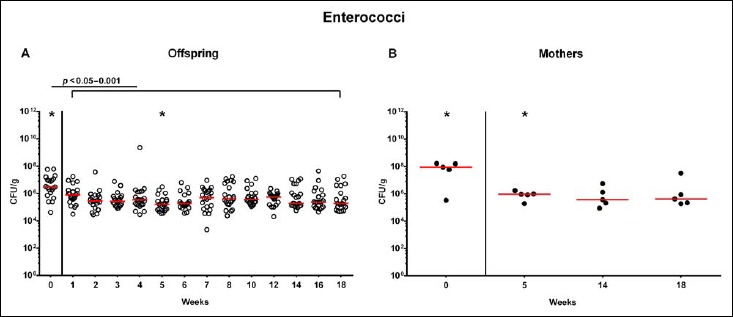
Fecal loads of enterococci in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Enterococci were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (*p < 0.05). Data were pooled from five litters
Fig. 4.
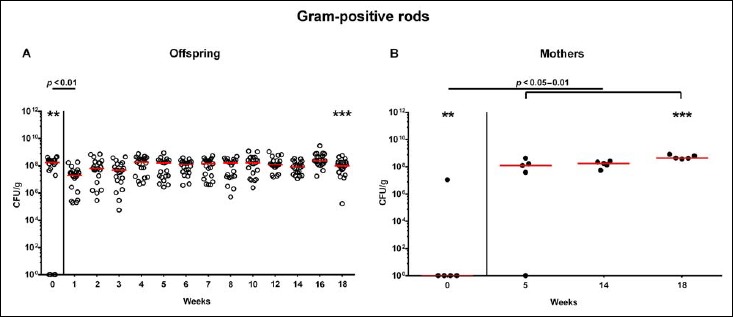
Fecal loads of aerobic Gram-positive rods in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Aerobic Gram-positive rods were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (**p < 0.01; ***p < 0.001). Data were pooled from five litters
Fig. 5.
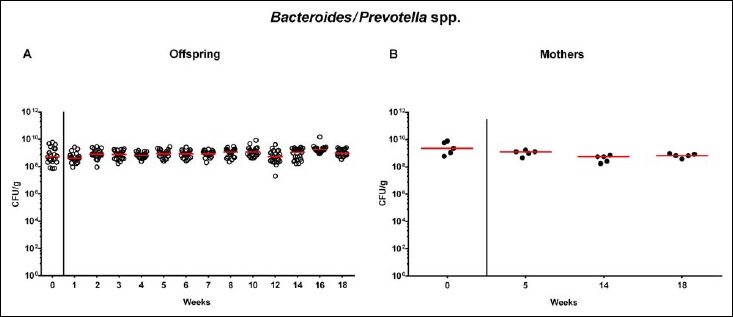
Fecal loads of Bacteroides/Prevotella species in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Bacteroides/Prevotella spp. were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians are shown. Data were pooled from five litters
Fig. 6.
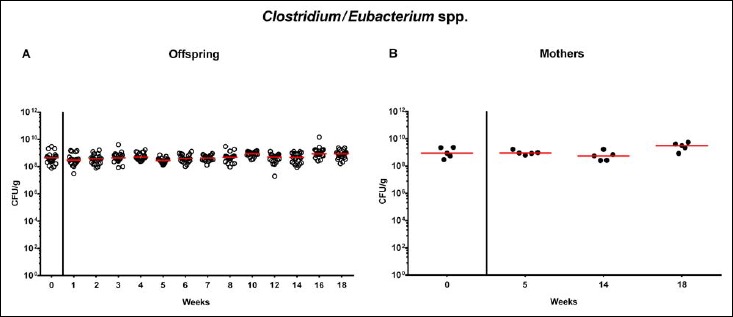
Fecal loads of Clostridium/Eubacterium species in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Clostridium/Eubacterium spp. were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points as indicated by culture and expressed as colony forming units per gram feces (CFU/g). Medians are shown. Data were pooled from five litters
Molecular survey of intestinal microbiota dynamics in hma offspring
In order to also assess fastidiuos and uncultivable intestinal bacteria, we additionally applied quantitative molecular (i.e., 16S rRNA based) methods. In line with results obtained from culture, total eubacterial loads were stable over time in feces of both hma offspring and mothers (n.s.; Fig. 7), whereas enterobacterial and enterococcal gene numbers had decreased in hma offspring mice as early as 1 week post weaning (p < 0.001; Fig. 8A and Fig. 9A). In mothers, less enterobacterial 16S rRNA could be detected at 18 weeks post weaning as compared to the starting point (p < 0.01; Fig. 8B), while enterococci were virtually undetectable (Fig. 9B). Furthermore, enterobacterial gene numbers were lower in feces derived from offspring as compared to mothers at week 5 post weaning (p < 0.05; Fig. 8). In hma offspring and mothers, fecal gene numbers of lactobacilli had increased until 18 weeks following weaning (p < 0.001 and p < 0.01, respectively; Fig. 10) with up to 4 orders of magnitude higher numbers in mothers than offspring at weaning as well as weeks 5 and 18 post weaning (p < 0.05–0.01; Fig. 10). Interestingly, in hma offspring mice, fecal bifidobacteria could be detected at very low levels only (i.e., close to the detecting limit) (Fig. 11A), whereas in mothers, bifidobacterial gene numbers were vitually undetectable at the time point of weaning, but up to 4 log orders of magnitude higher as compared to hma offspring 5 and 18 weeks post weaning (p < 0.001; Fig. 11B). Fecal gene numbers of Bacteroides/Prevotella spp. (Fig. 12), of Mouse Intestinal Bacteroides (Fig. 13), and of the Clostridium leptum group (Fig. 14) were stable over time and did not differ between offspring and mothers. At weaning, hma offspring harbored approximately one order of magnitude lower fecal Clostridium coccoides group gene numbers as compared to their mothers (p < 0.005; Fig. 15). As early as 1 week after weaning, gene numbers of the C. coccoides group were slightly lower in feces taken from hma offspring, but higher by 5 weeks post weaning as compared to weaning (p < 0.001; Fig. 15A). Hence, molecular analyses confirmed cultural results and further revealed increasing lactobacilli loads until 18 weeks post weaning, whereas by then, bifidobacteria were virtually undetectable in hma offspring, but not mothers.
Fig. 7.
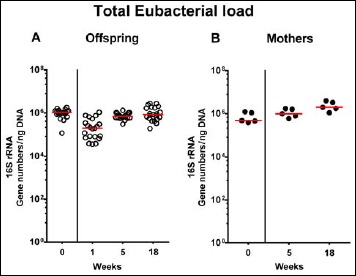
Fecal total eubacterial gene numbers in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Individual total eubacterial loads were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians are shown. Data were pooled from five litters
Fig. 8.
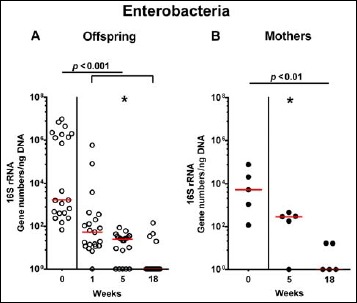
Fecal gene numbers of enterobacteria in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Enterobacteria were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (*p < 0.05). Data were pooled from five litters
Fig. 9.
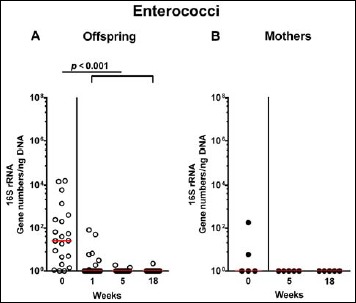
Fecal gene numbers of enterococci in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Enterococci were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Data were pooled from five litters
Fig. 10.
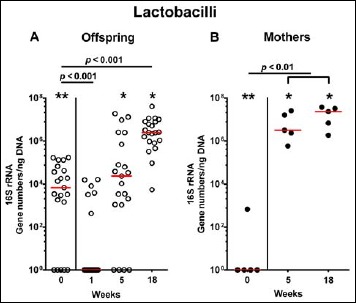
Fecal gene numbers of lactobacilli in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Lactobacilli were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (*p < 0.05; **p < 0.01). Data were pooled from five litters
Fig. 11.
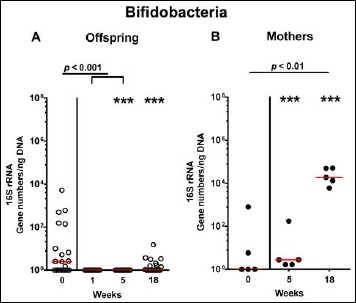
Fecal gene numbers of bifidobacteria in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Bifidobacteria were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (***p < 0.001). Data were pooled from five litters
Fig. 12.
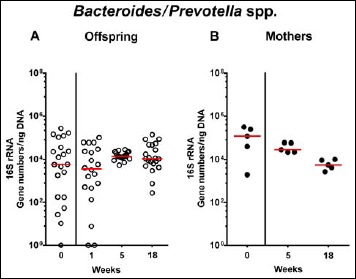
Fecal gene numbers of Bacteroides/Prevotella species in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Bacteroides/Prevotella spp. were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians are shown. Data were pooled from five litters
Fig. 13.
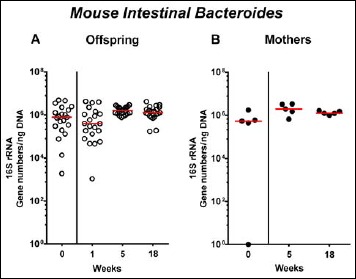
Fecal gene numbers of Mouse Intestinal Bacteroides in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Mouse Intestinal Bacteroides were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians are shown. Data were pooled from five litters
Fig. 14.
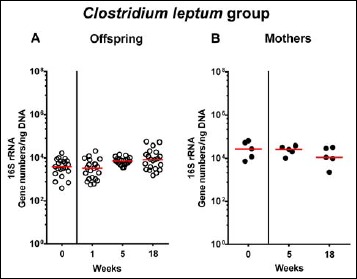
Fecal gene numbers of Clostridium leptum group in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Clostridium leptum were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians are shown. Data were pooled from five litters
Fig. 15.
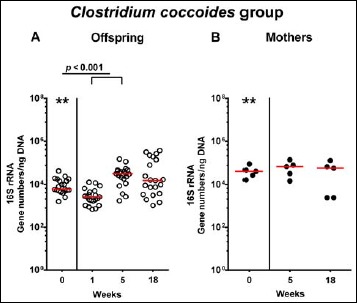
Fecal gene numbers of Clostridium coccoides group in offspring of human microbiota-associated mice. Human microbiota-associated (hma) mice were generated by human fecal microbiota transplantation of secondary abiotic mice as described in methods. Clostridium coccoides were quantitatively assessed in fecal samples derived from (A) offspring (open circles; n = 21) of hma mice and (B) their mothers (closed circles; n = 5) at defined time points by qRT-PCR and expressed as 16S rRNA gene numbers per ng DNA. Medians and significance levels (p values) determined by Mann–Whitney U test are shown. Significant differences between offspring and mothers at a defined time point (as determined by Mann–Whitney U test) are indicated by asterisks (**p < 0.01). Data were pooled from five litters
Discussion
During the past years, the endogenous intestinal microbiota has gained increasing attention regarding their impact in health and disease. Microbial colonization and host immune development and maturation run in parallel and are essentially involved in regulation of intestinal (patho) physiology [2, 28]. Thus, with respect to their microbiota, “humanized” mice comprise an elegant tool to perform in vivo proof-of-principle experiments (quasi “clinical trials in mice”) that unravel the effects of environmental and genetic factors on intestinal microbiota and host (patho)physiology in health and disease [2].
Since generation of hma mice is very challenging and time-consuming, we addressed in the present study how the intestinal microbiota were changing in offspring of hma mice and their mothers over time. To the best of our knowledge, this is the first comprehensive kinetic survey of intestinal microbiota composition in offspring of hma mice that had been generated by FMT of seconday abiotic mice with a fully developed immune system applying both “culturomics” and molecular methods. We were able to show that within 1 week post weaning (i.e., by the age of 4 weeks), fecal loads of commensal enterobacteria and enterococci had decreased, whereas obligate anaerobic bacteria such as Bacteroides/Prevotella spp. and clostridia were stably colonizing the intestines of hma offspring at high loads. This is well in line with an earlier study in offspring of hma isolator-raised Balb/c mice [29]. The authors demonstrated that facultative anaerobes such as enterobacteria and streptococci could be cultured from the intestinal tract as early as the first day post partum, whereas anaerobes were undetectable in the first week of life. Furthermore, most of the parental intestinal bacteria colonized the intestines of the offspring of hma mice until weaning (i.e., 3 weeks post partum) [29]. As in our survey, numbers in intestinal enterobacteria and enterococci were declining after weaning, but were still comparable to maternal loads [29, 30]. In our study, lactobacilli numbers were successively increasing until 18 weeks post weaning in both hma offspring and mothers, whereas by then, bifidobacteria were detectable in the latter only. Interestingly, fecal lactobacilli and bifidobacteria were higher in mothers as compared to their offspring at 5 and 18 weeks post weaning. Hirayama and colleagues showed that bifidobacteria were eliminated from the intestinal tract of some hma mice following FMT which was depending on the respective human fecal donor suspension [31]. If established in the intestines, however, bifidobacteria virtually never became predominant in hma offspring despite high intestinal loads in their mothers [29]. Applying culture-independent molecular methods, Collins and coworkers were able to show that isolator-raised C57BL/6 mice that had been subjected to peroral human FMT maintained a stable human microbiota in their intestines over several generations upon housing under conventional SPF conditions [32].
One needs to take into consideration, however, that the murine intestinal microbiota is highly depending on housing conditions [27]. In our study, “humanized” mice were maintained under standard hygienic conditions with open cages, handled under aseptic conditions and fed autoclaved chow and water, thus, not mimicking human conditions at all. Particularly, the diet is a pivotal determinant of intestinal microbiota composition. In an elegant earlier study, Hirayama et al. could show that intestinal enterobacterial loads increased upon feeding hma mice a diet with high meat contents [29]. Change to a standard chow high in fibre and gran, however, could reverse intestinal enterobacterial numbers back to basal levels. This was further supported by our own study, where secondary abiotic mice had been fed a Western (“Cafeteria”) diet for 10 weeks. Upon Western diet, mice developed an intestinal microbiota that was comparable to that observed in hma mice as indicated by higher total bacterial loads in the colonic lumen as well as higher counts of distinct bacterial commensals including enterobacteria (such as E. coli) and Clostridium/Eubacterium spp. as compared to mice on conventional chow, whereas lactobacilli were lower in the large intestines of the former [33]. In addition, feeding a Western diet to secondary abiotic for 10 weeks resulted in a weight gain of more than 40%. Interestingly, like in overweight mice on Western diet, distinct features of intestinal microbiota composition such as increased intestinal enterobacterial loads could be determined in obese (ob/ob) mice if compared to lean wildtype controls, both on conventional chow [33]. Hence, among a plethora of factors including (patho)physiological conditions, nutrition is one important (but not exclusive) determinant of intestinal microbiota composition. When studying human microbiota–host interactions in mice, not only the intestinal microbiota composition itself but also the respective dietary regimen as its pivotal determinant should resemble human conditions.
We conclude that, when taking both the strengths and limitations of the applied model into consideration, hma secondary abiotic mice including their offspring provide valuable tools in dissecting the molecular mechanisms underlying host commensal–immune interactions in health and disease.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Alexandra Bittroff-Leben, Ines Puschendorf, Gernot Reifenberger, and the staff of the animal research facility at Charité – University Medicine Berlin for excellent technical assistance.
Funding Statement
Funding sources: This work was supported by grants from the German Research Foundation (DFG) to S.B. (SFB633, TP A7), M.M.H. (SFB633, TP B6), E.V.K. and I.E. (SFB633, Immuco).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1.Nguyen TL, Vieira-Silva S, Liston A, Raes J: How informative is the mouse for human gut microbiota research? Dis Model Mech 8, 1–16 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI: The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1, 6ra14 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Lu L, Sun J, Petrof EO, Claud EC: Preterm infant gut microbiota affects intestinal epithelial development in a humanized microbiome gnotobiotic mouse model. Am J Physiol Gastrointest Liver Physiol 311, G521–32 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natividad JM Pinto-Sanchez MI Galipeau HJ Jury J Jordana M Reinisch W et al.: Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm Bowel Dis 21, 1883–1893 (2015) [DOI] [PubMed] [Google Scholar]
- 5.Marcobal A Kashyap PC Nelson TA Aronov PA Donia MS Spormann A et al.: A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J 7, 1933–1943 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006) [DOI] [PubMed] [Google Scholar]
- 7.Seedorf H Griffin NW Ridaura VK Reyes A Cheng J Rey FE et al.: Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159, 253–266 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H Pamp SJ Hill JA Surana NK Edelman SM Troy EB et al.: Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149, 1578–1593 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P: Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev 40, 117–132 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiebiger U, Bereswill S, Heimesaat MM: Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (Bp) 6, 253–271 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazebrouck S Przybylski-Nicaise L Ah-Leung S Adel-Patient K Corthier G Wal JM et al.: Allergic sensitization to bovine beta-lactoglobulin: comparison between germ-free and conventional BALB/c mice. Int Arch Allergy Immunol 148, 65–72 (2009) [DOI] [PubMed] [Google Scholar]
- 12.Taurog JD Richardson JA Croft JT Simmons WA Zhou M Fernandez-Sueiro JL et al.: The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 180, 2359–2364 (1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ: T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol 150, 91–97 (1997) [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen AK, Hansen CH, Krych L, Nielsen DS: Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol 20, 17727–17736 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimesaat MM Bereswill S Fischer A Fuchs D Struck D Niebergall J et al.: Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177, 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 16.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, et al. : Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6, e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimesaat MM, Boelke S, Fischer A, Haag LM, Loddenkemper C, Kuhl AA, et al. : Comprehensive postmortem analyses of intestinal microbiota changes and bacterial translocation in human flora associated mice. PLoS One 7, e40758 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masanta WO Heimesaat MM Bereswill S Tareen AM Lugert R Gross U et al.: Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013, 526860 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter T Bereswill S Glunder G Haag LM Hanel I Heimesaat MM et al.: [Campylobacteriosis of man: livestock as reservoir for Campylobacter species]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 54, 728–734 (2011) [DOI] [PubMed] [Google Scholar]
- 20.Heimesaat MM, Bereswill S: Murine infection models for the investigation of Campylobacter jejuni–host interactions and pathogenicity. Berl Munch Tierarztl Wochenschr 128, 98–103 (2015) [PubMed] [Google Scholar]
- 21.Kampmann C, Dicksved J, Engstrand L, Rautelin H: Composition of human faecal microbiota in resistance to Campylobacter infection. Clin Microbiol Infect 22, 61 e1–8 (2016) [DOI] [PubMed] [Google Scholar]
- 22.Heimesaat MM Fischer A Jahn HK Niebergall J Freudenberg M Blaut M et al.: Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 56, 941–948 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, et al. : Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Tolllike receptors 2 and 4. PLoS One 2, e662 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bereswill S Kuhl AA Alutis M Fischer A Mohle L Struck D et al.: The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog 6, 19 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimesaat MM Nogai A Bereswill S Plickert R Fischer A Loddenkemper C et al.: MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010) [DOI] [PubMed] [Google Scholar]
- 26.Rausch S, Held J, Fischer A, Heimesaat MM, Kuhl AA, Bereswill S, et al. : Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One 8, e74026 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoene-Reineke C, Fischer A, Friese C, Briesemeister D, Gobel UB, Kammertoens T, et al. : Composition of intestinal microbiota in immune-deficient mice kept in three different housing conditions. PLoS One 9, e113406 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez JM Murphy K Stanton C Ross RP Kober OI Juge N et al.: The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 26, 26050 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirayama K, Miyaji K, Kawamura S, Itoh K, Takahashi E, Mitsuoka T: Development of intestinal flora of humanflora-associated (HFA) mice in the intestine of their offspring. Exp Anim 44, 219–222 (1995) [DOI] [PubMed] [Google Scholar]
- 30.Hirayama K: Ex-germfree mice harboring intestinal microbiota derived from other animal species as an experimental model for ecology and metabolism of intestinal bacteria. Exp Anim 48, 219–227 (1999) [DOI] [PubMed] [Google Scholar]
- 31.Hirayama K, Kawamura S, Mitsuoka T: Development and stability of human faecal flora in the intestine of ex-germfree mice. Microb Ecol Health Dis 4, 95–99 (1991) [Google Scholar]
- 32.Collins J, Auchtung JM, Schaefer L, Eaton KA, Britton RA: Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome 3, 35 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bereswill S, Plickert R, Fischer A, Kuhl AA, Loddenkemper C, Batra A, et al. What you eat is what you get: Novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur J Microbiol Immunol (Bp) 1, 237–248 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]


