Abstract
The progressive rise in multidrug-resistant (MDR) bacterial strains poses serious problems in the treatment of infectious diseases. While the number of newly developed antimicrobial compounds has greatly fallen, the resistance of pathogens against commonly prescribed drugs is further increasing. This rise in resistance illustrates the need for developing novel therapeutic and preventive antimicrobial options. The medicinal herb Nigella sativa and its derivatives constitute promising candidates. In a comprehensive literature survey (using the PubMed data base), we searched for publications on the antimicrobial effects of N. sativa particularly directed against MDR bacterial strains. In vitro studies published between 2000 and 2015 revealed that N. sativa exerted potent antibacterial effects against both Gram-positive and Gram-negative species including resistant strains. For instance, N. sativa inhibited the growth of bacteria causing significant gastrointestinal morbidity such as Salmonella, Helicobacter pylori, and Escherichia coli. However, Listeria monocytogenes and Pseudomonas aeruginosa displayed resistance against black cumin seed extracts. In conclusion, our literature survey revealed potent antimicrobial properties of N. sativa against MDR strains in vitro that should be further investigated in order to develop novel therapeutic perspectives for combating infectious diseases particularly caused by MDR strains.
Keywords: Nigella sativa, antimicrobial effects, multidrug-resistant (MDR) bacteria, medicinal plants, phytotherapy, herbal medicine
Introduction
Antimicrobial multidrug resistance
The rise in multidrug-resistant (MDR) bacteria has become a serious health problem and major challenge in developing treatment options of infectious diseases [1]. Understanding the molecular mechanisms underlying resistance development is fundamental for the attempts to overcome this issue. From a biomedical standpoint, resistance to treatment is a feature that is becoming increasingly common in bacteria, viruses, parasites, protozoa, and malignant tumor cells [2]. In the case of bacterial infections, common mechanisms leading to antibiotic resistance include the presence of drug-inactivating enzymes, modification of drug binding sites, changes to influx and efflux mechanisms, and alterations in enzyme pathways [3].
The β-lactams constitute a group of antibiotics including penicillins, cephalosporins, monobactams, and carbapenems which inactivate glycopeptide transpeptidases, thereby inhibiting bacterial cell wall synthesis. This leads to its main bactericidal properties via cell lysis [3, 4]. This interaction is particularly useful in treating bacteria with multiple layers of peptidoglycan such as Staphylococcus aureus and other Gram-positive bacteria [4]. However, in recent years, there has been an increase in resistance against staphylococci in hospital settings. For instance, nosocomial methicillin-resistant S. aureus (MRSA) has adapted through a DNA mutation leading to alteration of the drug target site. This results in ineffective drug binding and a decrease in its bactericidal activity [5].
Similar developments can be seen in enterococci commonly causing hospital acquired infections. The clinically relevant Enterococcus faecium and faecalis are part of the commensal microbiota lining the intestinal mucosa. Enterococci are able to spread antibiotic resistance properties through gene transfer to other susceptible bacteria. In consequence, vancomycin-resistant enterococci (VRE) have progressively become a serious problem in the clinical (particularly hospital) setting as this broad-spectrum antibiotic compound is commonly used as a reserve drug to treat intractable infections [6]. The decline in treatment options of bacterial infections has become critical in treating hospitalized patients and, therefore, calls for novel pharmacological therapeutical and preventive measures. One approach to tackle this issue is to explore the therapeutic properties of medicinal plants.
Nigella sativa – a medicinal plant
Plants have often laid the foundation of pharmaceutical drug development and allowed breakthroughs in treating diseases on a greater and more efficient scale. Notably, 60% of currently available antimicrobial and antitumoral drugs are derived from plants [7]. N. sativa is one of the oldest documented herbal medicinal plants and has been used for centuries in traditional Arabic medicine. Herbal treatment with this plant is already commonly applied and well known for its safety [8]. N. sativa seeds contain oil, protein, carbohydrate, fiber, and saponin. The fixed oil is composed of arachidonic acid, linoleic acid, oleic acid, almitoleic acid, palmitic acid, stearic acid, myristic acid, steroles, and eicosadenoic acid [9], whereas the essential oil of N. sativa entails nigellone, thymoquinone, thymohydroquinone, thymol, carvacrol, α- and β-pinene, d-limonene, d-citronellol, and p-cymeme [10, 11].
Traditional medicine
In traditional Arabic herbal medicine, N. sativa has been used for a broad range of conditions including asthma, gastrointestinal dysfunctions, conjunctivitis, eczema, intrinsic hemorrhage, hypertension, and pyrexia [12]. In recent years, scientists have studied the therapeutic impact of N. sativa on cancer [13–15], hypertension [16], asthma [17], and gastrointestinal disease [18]. Some of these effects can be attributed to the extracts’ ability to increase activity of intrinsic enzymes with antioxidant function such as glutathione peroxidase and catalase. The oil itself acts as a free radical scavenger and impacts the activity of cytokines, chemokines, and prostaglandins and, furthermore, modulates B cell-dependent immune pathways [19].
Given its broad health beneficial effects, we here addressed the state of the art of antimicrobial effects of N. sativa and its derivates particularly against MDR bacterial strains applying a comprehensive literature survey.
Methods
Inclusion and exclusion criteria
Inclusion criteria were in vitro trials with bacterial strains and the effect of N. sativa on the inhibition of bacterial growth. In vivo studies and clinical trials were excluded given the small number of conducted studies so far. For the same reason, in vitro studies investigating the eradicative properties of N. sativa directed against viruses and fungi were also excluded.
Search strategy
An online literature search was systematically conducted from November 15 to December 23, 2016 on the MEDLINE database PubMed in order to find relevant publications discussing the link between N. sativa and its effects on resistant bacterial strains and considered all publications between 2000 and 2015. By utilizing Boolean logic through the advance search history option on the PubMed database, the following steps were conducted (as summarized in Fig. 1):
Fig. 1.
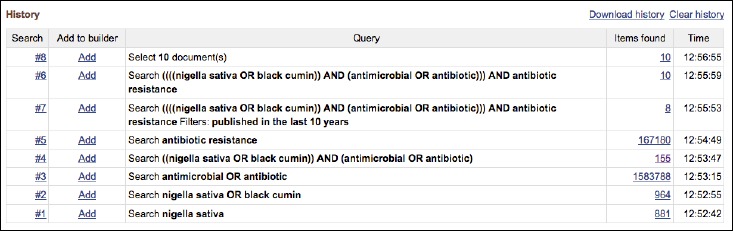
Boolean logic search results on PubMed
Firstly, “Nigella sativa OR black cumin” was searched, and the Boolean operator OR ensured that synonyms were included. This method was further enhanced by employing the MeSH (Medical Subject Headings) term, and therefore, other variations of the herbs’ name such as black caraway and kalonji could be found in the database. Secondly, the following search term “antimicrobial OR antibiotic” was included in order to find studies focussing on antibiotic properties. Thirdly, the term “antibiotic resistance” was used to ensure that investigations on resistant bacteria were included. Finally, to narrow down the search, all three search terms were united through the Boolean operator “AND” in following manner: “(Nigella sativa OR black cumin) AND (antimicrobial OR antibiotic) AND (antibiotic resistance)”.
In consequence, ten items were systematically found. By evaluating all ten articles according to the inclusion requirements, three were rejected since one article described a clinical trial, another study used Cuminum cyminum instead of the black seed herb, and the full article of an in vitro study was not available. Hence, a total of seven articles were included finally.
Data extraction
By using Microsoft Excel, information gained from respective articles was extracted according to the following criteria: type of extract used, scientific method, targeted bacteria (Gram-positive and Gram-negative), control, main findings, and references.
Results
Antibiotic properties of N. sativa
N. sativa oil and crude extract were analyzed for their effects on the growth of six Salmonella variants including S. paratyphi, S. enteritidis, S. typhimurium, S. heidelberg, and S. agona applying the well diffusion assay and broth dilution method [20]. By utilizing antibiotic susceptibility tests, all tested Salmonella strains were exposed to the synthetic antibiotic compounds ceftriaxone and ciprofloxacin and proven to be resistant. Remarkably, all resistant Salmonella strains were susceptible to N. sativa crude extract, while for the oil extract, this was the case in 90% of the strains. All of the against black seed oil tested Salmonella strains displayed distinct average inhibition zones, namely, 19 mm against S. paratyphi, 17 mm against S. enteritidis, 30 mm against S. typhimurium, 15 mm against S. heidelberg, and 23 mm againts S. agona [20].
In another in vitro study, aqueous extracts of the black cumin seeds were used against seven strains of the Gram-negative pathogen Helicobacter pylori known to cause gastric and duodenal ulcers as well as gastroduodenal adenocarcinomas in 5–10% of infected individuals. Six of the strains had been isolated from hospitalized patients suffering from peptic ulcers [21]. The aqueous extract was produced by boiling N. sativa seeds in sterile distilled water (100 mg/ml) and filtered through sterile filter paper. Standard bacterial killing curves revealed that bacterial growth was completely inhibited in all H. pylori strains after 60 minutes incubation time [21].
In another set of experiments, the antibacterial activities of black cumin essential oil (EO) were tested against three Gram-positive and three Gram-negative bacterial strains, namely, S. aureus, Bacillus cereus, Listeria monocytogenes, and S. typhimurium, Escherichia coli, Pseudomonas aeruginosa, respectively [11]. The synthetic antibiotic ceftriaxone and a naturally derived antimicrobial named eugenol served as control compounds. For antibacterial survey, the agar disk diffusion method was applied with concentrations of 100, 200, 300, 400, and 500 μg compound per well. The EO microemulsion displayed its highest antibacterial activity in a minimum concentration of 100 μg EO per well against S. aureus and B. cereus with inhibition zones of 15.7 mm and 14.0 mm, respectively. As compared to eugenol microemulsion, black cumin microemulsion exerted higher antibiotic activity against S. aureus, B. cereus, and S. typhimurium in all concentrations [11].
No significant differences, however, could be observed in growth inhibitions against S. typhimurium at 300 μg/well exerted by N. sativa EO (18.0 mm inhibition zone) as compared to ceftriaxone (19.3 mm). Interestingly, at concentrations of 400 μg and 500 μg, the antibarterial efficacy of EO (26.5 mm) against respective bacterial strains was shown to be even more potent than ceftriaxone (23.7 mm). At 500 μg/well, N. sativa EO was found effective against E. coli as indicated by a mean inhibition zone of 30.3 mm, that was comparable to that exerted by ceftriaxone (31.3 mm) at the same concentration. L. monocytogenes and P. aeruginosa, however, were tested resistant to EO at all applied concentrations [11].
Another study using the agar diffusion method determined the growth of inhibition against S. aureus, two B. cereus strains, E. coli and P. aeruginosa by both N. sativa EO and crude oil extract applied in 500 mg/ml concentration [22]. The synthetic antibiotic gentamycin was used as negative control. The petroleum ether extract (500 mg/ml) exceeded the inhibition zone exerted by gentamycin in two tested B. cereus strains, namely, 40.0 ± 2.3 mm and 44.0 ± 0.3 mm against B. cereus strain ATCC 10987 and a B. cereus laboratory strain, respectively, following petroleum ether extract as compared to 30.0 ± 1.2 mm (against B. cereus ATCC 10987) and 25.0 ± 1.0 mm (against laboratory derived B. cereus strain) following gentamicin application. Both P. aeruginosa and S. aureus, however, were resistant to both plant extracts. Hence, the crude oil extract displayed higher antimicrobial activities as compared to the EO extract [22].
In a further study, the effect of black cumin seed oil was investigated in several concentrations on MDR S. aureus strains that had been isolated from diabetic wounds employing the well diffusion method [23]. Eight S. aureus isolates out of 19 strains tested were susceptible to N. sativa oil in its undiluted form, whereas another four isolates displayed susceptibility at concentrations ranging from 200 mg/ml to 800 mg/ml. The remaining isolates, that is, eleven in total, exhibited resistance to the oil in all dilutions. Thus, 62% of the tested isolates were sensitive to N. sativa oil [23].
Results from another in vitro experiment revealed a mean minimum bactericidal concentration of 819.2 μg/ml of N. sativa ethanolic extract against vancomycin-resistant S. aureus (VRSA). The five isolates were taken from clinical samples, and the agar well diffusion method was employed. At 20 mg/well of the ethanolic extract, the average inhibition zone for all five VRSA isolates was 22.2 mm [24].
Furthermore, antibacterial activities of black caraway ethanol extract against MRSA strains were surveyed. Following exposure to a concentration of 4 mg in the agar diffusion assay, all MRSA strains were shown to be susceptible to the extract with a minimum inhibitory concentration ranging from 0.2 to 0.5 mg/ml [25]. The above-mentioned findings are summarized in Table 1.
Table 1.
Antibacterial effects of Nigella sativa
| Extract | Method | Gram-positive bacteria | Gram-negative bacteria | Control | Main findings | References |
|---|---|---|---|---|---|---|
| Aqueous extract of seeds | Standard killing curve | Not available | H. pylori | H. pylori with sterile distilled water | 100% of inhibition of growth after 60-min incubation against all seven strains. | [21] |
| Oil and crude extract | Well diffusion assay and broth dilution method | Not available | Six strains of Salmonella | Antibiotic susceptibility test against 50 and 100 μg/ml ciprofloxacin and ceftriaxone | All Salmonella strains were susceptible to the crude extract. 90% of resistant Salmonella strains were sensitive to the oil extract. |
[20] |
| Essential oil extract in microdilution | Agar well diffusion | S. aureus, B. cereus, L. monocytogenes | S. typhimurium, E. coli, P. aeruginosa | Eugenol microemulsion and ceftriaxone | EO showed its high antibacterial activity in the concentration of 100 μg EO/well against S. aureus and B. cereus. At 500 μg EO/well effective against E. coli (20.2 mm) and S. typhimurium (26.5 mm). L. monocytogenes and P. aeruginosa were resistant to EO at all concentrations. |
[11] |
| Essential oil and petroleum ether extract | Agar well diffusion | S. aureus, two strains of B. cereus | E. coli and P. aeruginosa | Gentamycin sulfate | The petroleum ether extract (500 mg/ml) exceeded the zone of inhibition of gentamycin in both tested strains of B. cereus. P. aeruginosa was resistant to both plant extracts. |
[22] |
| Oil extract | Disc diffusion and well diffusion method | MRSA | Not available | Antibiotic susceptibility test | 62% of tested MRSA strains were susceptible to N. sativa oil. | [23] |
| Ethanolic extract | Broth microdilution test and agar well diffusion test | VRSA | Not available | Linezolid, moxifloxacin, clindamycin | Highest inhibition of growth (26 mm) was achieved at 20 mg/well (ethanolic extract) for five VRSA isolates. | [24] |
| Ethanolic extract | Disc and agar dilution method | MRSA | Not available | Unknown | All MRSA strains were susceptible to the ethanolic extract at 4 mg/disc. | [25] |
Antibacterial constituents of N. sativa
The chemical constituents of N. sativa essential oil gained by hydrodistillation were analyzed by gas chromatography [11]. The antimicrobial properties of black cumin seed oil were shown to depend on the concentration of the active compounds thymoquinone and thymohydroquinone. Thymoquinone made up 52.6% of the EO extract, thus, constituting the main active component, whereas p-cymene, the second dominating compound, was found to make up to 25% of the EO [11].
Notably, thymoquinone displays a critical antibiotic role as it is able to decrease bacterial resistance to distinct antimicrobial compounds. One common mechanism of resistance is an increased antibiotic clearance from the cell via efflux pumps, thereby decreasing the intracellular concentration of the respective antimicrobial compound. Thymoquinone was described to inhibit the activity of bacterial efflux pumps and consequently to interrupt an important process leading to antibiotic resistance [11]. Besides, thymoquinone exerted potent antibiofilm directed properties against both Gram-positive and Gram-negative pathogens [11].
Discussion
Main findings of the literature survey
Our comprehensive literature survey revealed that the medicinal herb N. sativa exhibited antimicrobial properties against bacterial strains that were shown to be resistant against commonly used synthetic antibiotic compounds. Both Gram-positive and Gram-negative bacteria were susceptible to N. sativa at different concentrations. Enteropathogens such as Salmonella, H. pylori, and E. coli strains were inhibited in their growth. The same applies to B. cereus, S. aureus, and, strikingly, to MDR S. aureus strains including MRSA and even VRSA. However, L. monocytogenes and P. aeruginosa were shown to exert resistances directed against black cumin seed extracts. Such resistances against N. sativa may arise given the distinct adaptability and metabolic versatility inherent in certain bacterial strains [11]. The positive effects in the conducted in vitro studies, however, strongly support the application of this medicinal plant as a potential drug for combating bacterial infectious diseases.
Limitations of the literature survey
Conclusions should be rather carefully drawn from the afore-discussed papers given the lack of consistencies in variables such as drug concentrations and other confounding constituents. This limitation is due to the natural variation in the concentrations of compounds found in plant-derived extracts. Furthermore, the relatively small number of papers exhibit another limitation.
Future perspectives and in vivo applications
The antimicrobial activities exerted by thymoquinone may be potentiated by synergistically acting agents present in the oil such as p-cymene, which needs to be further explored. Fully understanding the mechanisms underlying the antimicrobial effects of thymoquinone and other components that might be relevant to the distinct antimicrobial impact will help to formulate effective synthetic antibiotic compounds, which will provide novel therapeutic options of combating bacterial infections caused even by MDR strains.
In order to achieve this, further in vitro studies applying comparable extracts under standardized methods are required for allowing more conclusive statements regarding the potent antimicrobial effects of N. sativa and its derivatives in pharmacological formulations.
Important steps towards potential pharmacological application were elucidating antimicrobial efficacies of N. sativa in in vivo studies. In this context, one previous study addressed the impact of N. sativa seed on intestinal E. coli colonization and jejunal morphology in laying hens [26]. The in vivo data revealed that best results for intestinal health were achieved following application of 2% N. sativa, resulting in a significant reduction in ileal E. coli loads [26]. Moreover, thymoquinone sufficiently preserved intestinal epithelial barrier function and prevented from bacterial translocation. These effects were supported by another in vivo study including rats with intestinal obstruction, a condition associated with motility dysfunction, intestinal bacterial overgrowth, and mucosal disruption [27]. Upon intraperitoneal treatment with 10 mg thymoquinone per kg body weight, rats displayed a decrease in oxidative stress and inflammatory cytokines and were prevented from inflammatory damage to both liver and intestine [27].
Furthermore, in a clinical trial, patients suffering from non-ulcer dyspepsia were subjected to N. sativa in order to treat underlying H. pylori infection and compared to a cohort receiving a well-established triple therapy with clarithromycin, amoxicillin, and omeprazole. The patients were randomly included into the following groups: 1) triple therapy; 2) 1 g N. sativa plus 40 mg omeprazole; 3) 2 g N. sativa plus 40 mg omeprazole; 4) 3 g N. sativa plus 40 mg omeprazole, for 4 weeks, respectively. Remarkably, the combination of 2 g N. sativa plus 40 mg omeprazole was shown to be most effective in eradicating H. pylori [28]. Future clinical research should further expand these promising findings to diseases caused by other microbial pathogens.
In conclusion, our literature survey revealed potent antimicrobial properties of N. sativa against MDR bacteria in vitro providing promising perspectives for the development of novel therapeutic options combating infections that are particularly caused by MDR bacterial strains.
Funding Statement
Funding sources: There has been no source of funding.
References
- 1.Fernández J, Bert F, Nicolas-Chanoine MH: The challenges of multi-drug-resistance in hepatology. J Hepatol 65, 1043–1054 (2016) [DOI] [PubMed] [Google Scholar]
- 2.Avner BS, Fialho AM, Chakrabarty AM: Overcoming drug resistance in multi-drug resistant cancers and microorganisms: a conceptual framework. Bioengineered 3, 262–270 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Gao J, Tang W: Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci Trends 10, 14 –21 (2016) [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Bradford PA: β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6(8), pii: a025247 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joo HS, Otto M: Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim Biophys Acta 1848, 3055–3061 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes K, Bardossy AC, Zervos M: Vancomycin-resistant enterococci: epidemiology, infection prevention, and control. Infect Dis Clin North Am 30, 953–965 (2016) [DOI] [PubMed] [Google Scholar]
- 7.Calixto JB: Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 33, 179–189 (2000) [DOI] [PubMed] [Google Scholar]
- 8.Burits M, Bucar F: Antioxidant activity of Nigella sativa essential oil. Phytother Res 14, 323–328 (2000) [DOI] [PubMed] [Google Scholar]
- 9.El-Tahir KE-DH, Bakeet DM: The black seed Nigella sativa Linnae21us-A mine for multi cures: a plea for urgent clinical evaluation of its volatile oil. J Taibah Univ Med Sci 1, 1–19 (2006) [Google Scholar]
- 10.Nickavar B, Mojab F, Javidnia K, Amoli MA: Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z Naturforsch C 58(9–10), 629–631 (2003) [DOI] [PubMed] [Google Scholar]
- 11.Shaaban HA, Sadek Z, Edris AE, Saad-Hussein A: Analysis and antibacterial activity of Nigella sativa essential oil formulated in microemulsion system. J Oleo Sci 64, 223–232 (2015) [DOI] [PubMed] [Google Scholar]
- 12.Ali BH, Blunden G: Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17, 299–305 (2003) [DOI] [PubMed] [Google Scholar]
- 13.Salim EI, Fukushima S: Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer 45,195–202 (2003) [DOI] [PubMed] [Google Scholar]
- 14.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK: Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res 64, 622–630 (2004) [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S, Kaseb AO, Wang Z, Kong DM, Mussop P, Subhash S, Fazlul HM, Ramzi M: Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res 69, 5575–5583 (2009) [DOI] [PubMed] [Google Scholar]
- 16.Dehkordi FR, Kamkhah AF: Antihypertensiveeffect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol 22, 447–452 (2008) [DOI] [PubMed] [Google Scholar]
- 17.Boskabady M, Mohsenpoor N, Takaloo L: Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine 17, 707–713 (2010) [DOI] [PubMed] [Google Scholar]
- 18.El-Abhar H, Abdallah D, Saleh S: Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J Ethnopharmacol 84, 251–258 (2003) [DOI] [PubMed] [Google Scholar]
- 19.Gholamnezhad Z, Keyhanmanesh R, Boskabady MH: Anti-inflammatory, antioxidant, and immunomodulatory aspects of Nigella sativa for its preventive and bronchodilatatory effects on obstructive respiratory diseases: a review of basic and clinical evidence. J Funct Food 17, 910–927 (2015) [Google Scholar]
- 20.Sarwar A, Latif Z: GC-MS characterisation and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Nat Prod Res 29, 447–451 (2015) [DOI] [PubMed] [Google Scholar]
- 21.O’Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, Bassett C: Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol 11, 7499–7507 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacha K, Tariku Y, Gebreyesus F, Zerihun S, Mohammed A, Weiland-Bräuer N, Schmitz RA, Mulat M: Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: implication for development of potent antimicrobial agents. BMC Microbiol 16, 139 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emeka LB, Emeka PM, Khan TM: Antimicrobial activity of Nigella sativa L. seed oil against multi-drug resistant Staphylococcus aureus isolated from diabetic wounds. Pak J Pharm Sci 28, 1985–1990 (2015) [PubMed] [Google Scholar]
- 24.Liaqat F, Sheikh AA, Nazir J, Hussain T, Rabbani M, Shaheen AY, Muhammed J: Report-isolation identification and control of vancomycin resistant Staphylococcus aureus. Pak J Pharm Sci 28, 997–1004 (2015) [PubMed] [Google Scholar]
- 25.Hannan A, Saleem S, Chaudhary S, Barkaat M, Arshad MU: Anti-bacterial activity of Nigella sativa against clinical isolates of methicillin resistant Staphylococcus aureus. J Ayub Med Coll Abbottabad 20, 72–74 (2008) [PubMed] [Google Scholar]
- 26.Boka J, Mahdavi A, Samie A, Jahanian R: Effect of different levels of black cumin (Nigella sativa L.) on performance, intestinal Escherichia coli colonization and jejunal morphology in laying hens. J Anim Physiol Anim Nutr 98, 373–383 (2014) [DOI] [PubMed] [Google Scholar]
- 27.Kapan M, Tekin R, Onder A, Firat U, Evliyaoglu O, Taskesen F, Arikanoglu Z: Thymoquinone ameliorates bacterial translocation and inflammatory response in rats with intestinal obstruction. Int J Surg 10, 484–488 (2012) [DOI] [PubMed] [Google Scholar]
- 28.Salem EM, Yar T, Bamosa A, Al-Quorain A, Yasawy MI, Alsulaiman RM, Randhawa MA: Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol 16, 207–214 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]


