Abstract
Tumor necrosis factor α (TNFα) is known to upregulate the expression of receptor activator of NF-κB ligand (RANKL). We investigated the role of the calcineurin/nuclear factor of activated T-cells (NFAT) signaling pathway in TNFα-induced RANKL expression in C2C12 and primary cultured mouse calvarial cells. TNFα-induced RANKL expression was blocked by the calcineurin/NFAT pathway inhibitors. TNFα increased NFAT transcriptional activity and subsequent RANKL promoter binding. Mutations in the NFAT-binding element (MT(N)) suppressed TNFα-induced RANKL promoter activity. TNFα increased prostaglandin E2 (PGE2) production, which in turn enhanced NFAT transcriptional activity and binding to the RANKL promoter. MT(N) suppressed PGE2-induced RANKL promoter activity. TNFα and PGE2 increased the expression of RANKL, NFAT cytoplasmic-1 (NFATc1), cAMP response element-binding protein (CREB), and cyclooxygenase 2 (COX2); which increment was suppressed by indomethacin, a COX inhibitor. Mutations in the CRE-like element blocked PGE2-induced RANKL promoter activity. PGE2 induced the binding of CREB to the RANKL promoter, whereas TNFα increased the binding of both CREB and NFATc1 to this promoter through a process blocked by indomethacin. The PGE2 receptor antagonists AH6809 and AH23848 blocked TNFα-induced expression of RANKL, NFATc1, and CREB; transcriptional activity of NFAT; and binding of NFATc1 or CREB to the RANKL promoter. These results suggest that TNFα-induced RANKL expression depends on PGE2 production and subsequent transcriptional activation/enhanced binding of NFATc1 and CREB to the RANKL promoter.
Keywords: TNFα, RANKL, PGE2, NFATc1, CREB
1. Introduction
Receptor activator of nuclear factor-κB ligand (RANKL), a critical regulator of osteoclastogenesis, is primarily produced by stromal cells or osteoblasts in bone tissue. RANKL, together with colony stimulating factor 1, induces the differentiation of osteoclasts from hematopoietic precursors and stimulates bone resorption of mature osteoclasts [1,2]. Various hormones and cytokines regulate RANKL expression in osteoblast or stromal cells through the activation of intracellular signaling pathways, including the cAMP/protein kinase A (PKA), calcineurin/nuclear factor of activated T-cells (NFAT), hedgehog, Wnt/β-catenin, and gp130/STAT pathways [3,4,5,6,7,8].
Tumor necrosis factor α (TNFα) is a multifunctional cytokine that regulates various cellular and biological processes such as cell proliferation, differentiation, apoptosis, immunity, and inflammation [9]. TNFα is known to directly induce bone resorption by activating mature osteoclasts and stimulating the proliferation and differentiation of osteoclast precursors or indirectly by inducing the expression of osteoclastogenic factors in stromal cells or osteoblasts [10,11,12,13].
Several mechanistic pathways have been proposed to determine how TNFα induces the expression of RANKL [14,15]. For example, p38 mitogen-activated protein kinase (MAPK) pathway activation mediates TNFα-induced RANKL expression and osteoclast differentiation in precursor bone marrow cells [16,17]. Cyclooxygenase (COX)/prostaglandin E (PGE) signaling is also considered a mechanistic pathway by which TNFα induces RANKL expression. Prostaglandin E2 (PGE2) belongs to the family of prostanoid, autocrine, and paracrine lipid mediators produced by cells following injury or cytokine or growth factor stimulation [18]. PGE2 has been described as a potent stimulator of osteoclastic bone resorption in the context of inflammatory diseases such as rheumatoid arthritis and osteomyelitis [19,20,21,22]. PGE2 elevates RANKL expression in cultured mouse primary osteoblasts [20,23] and human periodontal fibroblasts [24] and is known to bind to any of four G protein-coupled receptors (EP1, EP2, EP3, or EP4) in various target cells [24,25]. COX is a prostaglandin endoperoxide synthase that catalyzes prostaglandin synthesis. Expression of the COX isoform cyclooxygenase 2 (COX2), which is thought to mediate inflammatory events, is rapidly induced by proinflammatory mediators [26,27]. TNFα is known to induce COX2 expression and PGE2 production in human gingival fibroblasts via activation of the NFκB pathway [28]. Studies have also reported that TNFα increases RANKL expression through the COX2/PGE2/EP4/protein kinase A (PKA) signaling pathway [12,19,29].
We previously reported that the cAMP/PKA and calcineurin/NFAT signaling pathways must cooperate to induce parathyroid hormone-related protein (PTHrP)-induced RANKL expression in mouse osteoblastic cells [6]. The NFAT family comprises five members: NFAT cytoplasmic-1 (NFATc1) through NFAT5. Calcium signaling pathways dephosphorylate NFATc1 through NFATc4 via the activated calcineurin serine or threonine phosphatase. Dephosphorylated NFATs translocate to the nucleus and regulate the expression of target genes. The calcineurin/NFAT pathway plays an important role in bone resorption, and NFATc1 is a particularly critical transcription factor for osteoclast differentiation [30]. In mice, NFATc1 overexpression in osteoblasts led to increased osteoclast generation and bone resorption [31]. However, the role of the calcineurin/NFAT signaling pathway in TNFα-induced RANKL expression remains unexplored.
In the present study, we demonstrated that TNFα induces the transcriptional activation of NFAT via PGE2 production and that activation of the calcineurin/NFAT signaling pathway is involved in the TNFα/COX2/PGE2-mediated induction of RANKL expression.
2. Results
2.1. Calcineurin/Nuclear Factor of Activated T-Cells (NFAT) Signaling Is Involved in Tumor Necrosis Factor α (TNFα)-Induced Receptor Activator of Nuclear Factor-κB Ligand (RANKL) Expression
To confirm the effect of TNFα on RANKL expression in C2C12 cells, cells were incubated for 0, 1, 4, 6, 12, and 24 h in the presence of TNFα (10 ng/mL); subsequently, the RANKL expression patterns were examined. TNFα clearly upregulated the expression of both RANKL mRNA and protein, and TNFα-induced RANKL expression reached a peak at 24 h (Figure 1A). Therefore, we chose an incubation period of 24 h for the following experiments.
Figure 1.
Calcineurin/nuclear factor of activated T-cells (NFAT) activation is involved in tumor necrosis factor α (TNFα)-induced receptor activator of nuclear factor-κB ligand (RANKL) expression in C2C12 cells. (A) TNFα increased RANKL expression in a time-dependent manner. C2C12 cells were incubated in the presence of 10 ng/mL TNFα for the indicated time periods and subjected to quantitative reverse transcription-polymerase chain reaction (RT-PCR) and western blot analyses. Quantitative data are presented as means ± standard deviations (SD); (B) TNFα induces NFAT transcriptional activity. C2C12 cells were transfected with a reporter plasmid containing an NFAT response element, exposed to TNFα for 24 h, and subjected to a luciferase assay. Data are presented as firefly luciferase activity levels relative to Renilla activity; (C) Inhibition of the calcineurin/NFAT pathway blocked TNFα-mediated RANKL expression. C2C12 cells pretreated with FK506 (10 μg/mL) or cyclosporin A (10 μg/mL) were treated with TNFα for 24 h and subjected to RT-PCR and western blot analyses; (D) TNFα increases NFAT binding to the mouse RANKL promoter. C2C12 cells were incubated for 24 h with TNFα, after which a chromatin immunoprecipitation (ChIP) assay was performed using an antibody against NFATc1, with IgG serving as a negative control. The RANKL promoter region containing the NFAT binding element was amplified via PCR. Quantitative ChIP data were normalized to the input and are presented as values relative to vehicle-treated control samples (CON) (E) TNFα increased RANKL promoter–reporter activity in an NFAT binding element-dependent manner. C2C12 cells were transfected with a wild-type (RANKL-WT-luc) or NFAT-binding site mutant (RANKL-MT(N)-luc) RANKL promoter reporter, incubated for 24 h in the presence of TNFα or NFAT overexpression vector, and subjected to a luciferase assay (* p < 0.05, compared to control; # p < 0.05, compared to the indicated pair).
To determine whether the calcineurin/NFAT pathway is activated by TNFα, we used an NFAT reporter assay with reporter plasmid containing an NFAT response element [32]. TNFα induced an approximately 4.5-fold increase in NFAT reporter activity (Figure 1B). Next, we explored whether the calcineurin/NFAT pathway is involved in TNFα-induced RANKL expression. C2C12 cells were treated with TNFα for 24 h in the presence or absence of the calcineurin phosphatase inhibitors FK506 and cyclosporin A (CsA). Although FK506 did not affect the basal RANKL expression level, TNFα-induced expression of both RANKL mRNA and protein was blocked (Figure 1C). CsA also downregulated both the basal and TNFα-induced RANKL protein levels and significantly suppressed TNFα-induced RANKL mRNA expression despite increasing the basal RANKL mRNA expression (Figure 1C). These results indicate that calcineurin/NFAT pathway activation plays a role in TNFα-mediated RANKL induction in C2C12 cells.
Next, a chromatin immunoprecipitation (ChIP) assay was performed to investigate whether TNFα-activated NFAT could directly transactivate the RANKL gene by binding to its promoter. The PCR amplification result revealed that TNFα increased the NFATc1 binding to the RANKL promoter (Figure 1D). To further confirm that the binding of NFAT to the RANKL promoter was functionally important in TNFα-induced RANKL expression, luciferase (luc) reporter assays were conducted. RANKL promoter–reporter contains −2174 to +1 bp of the mouse RANKL gene [4].
When the RANKL-WT-luc reporter was used, TNFα induced an approximately three-fold increase in luciferase activity; however, the insertion of mutations into the NFAT binding element (−941 to −936 bp) partially inhibited the TNFα-mediated induction of RANKL promoter activity (Figure 1E). NFATc1 overexpression (positive control) resulted in an approximately two-fold induction that was completely blocked by mutations in the NFAT binding element (Figure 1E). These results indicate that TNFα-activated NFATc1 directly binds to and transactivates the RANKL promoter.
2.2. Inhibition of Calcineurin/NFAT Signaling Partially Reduces TNFα-Induced Prostaglandin E2 (PGE2) Production
Previous studies have demonstrated that TNFα increases RANKL expression through the COX2/PGE2/EP4/PKA signaling pathway [12,19,29] and that NFAT activation enhances both COX2 expression and PGE2 production [33,34]. Therefore, we examined whether calcineurin/NFAT signaling pathway activation would mediate TNFα-induced COX2 expression and PGE2 production.
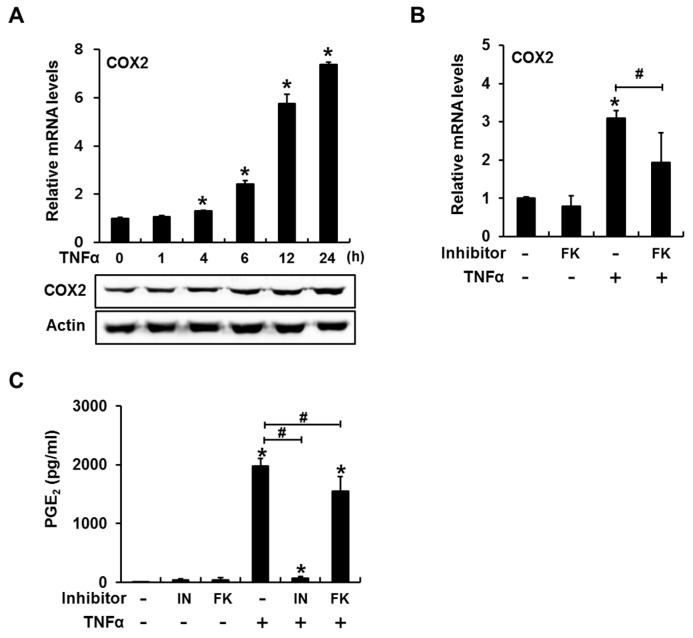
In C2C12 cells, TNFα treatment increased the expression levels of both COX2 mRNA and protein; these levels peaked at 24 h (Figure 2A). Consistent with this result, TNFα treatment for 24 h strongly induced PGE2 production, which was blocked by the COX inhibitor indomethacin (Figure 2C). FK506 did not significantly decrease basal COX2 mRNA expression or PGE2 production (Figure 2B,C). However, the addition of FK506 resulted in approximately 40% and 20% decreases in TNFα-induced COX2 mRNA expression and PGE2 production, respectively (Figure 2B,C). These results suggest that the calcineurin/NFAT signaling pathway plays a non-critical role in TNFα-induced COX2/PGE2 expression.
Figure 2.
The calcineurin/NFAT signaling pathway mediates TNFα-induced cyclooxygenase 2 (COX2) expression and prostaglandin E2 (PGE2) production in C2C12 cells. (A) TNFα stimulated COX2 mRNA and protein expression in a time-dependent manner; (B) TNFα-mediated COX2 expression decreased following inhibition of the calcineurin/NFAT signaling pathway. C2C12 cells pretreated with the calcineurin phosphatase inhibitor FK506 (10 μg/mL) were incubated in the presence or absence of TNFα for 24 h and subjected to RT-PCR analyses; (C) TNFα-induced PGE2 production was blocked by indomethacin, a COX inhibitor. Treatment with FK506 slightly suppressed TNFα-induced PGE2 production. C2C12 cells were incubated with FK506 or indomethacin (20 μM) in the presence of TNFα for 24 h and subjected to a PGE2 parameter assay (* p < 0.05, compared to control; # p < 0.05).
2.3. PGE2 Enhances RANKL Promoter Activity in an NFAT Binding Element-Dependent Manner
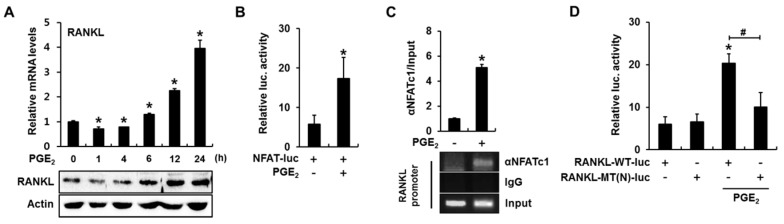
We next investigated whether calcineurin/NFAT signaling plays a role in PGE2-induced RANKL expression. When C2C12 cells were incubated in the presence of PGE2 (50 nM) for 1, 4, 6, 12, and 24 h, the levels of RANKL mRNA and protein were slightly decreased at an early time point, but remarkably increased in a time-dependent manner after 6 h (Figure 3A). PGE2 also induced an approximately three-fold increase in NFAT-luc reporter activity (Figure 3B). In addition, a ChIP assay with an NFATc1 antibody revealed that PGE2 enhanced NFATc1 binding to the RANKL promoter (Figure 3C). Furthermore, PGE2 significantly increased luciferase activity when the RANKL-WT-luc reporter, but not when the RANKL-MT(N)-luc, was used (Figure 3D). These results indicate that PGE2 activates the calcineurin/NFAT signaling pathway and that PGE2-induced NFAT directly activates RANKL transcription by binding to the RANKL promoter.
Figure 3.
PGE2 stimulates RANKL expression by activating the calcineurin/NFAT pathway in C2C12 cells. (A) PGE2 increased RANKL expression in a time-dependent manner. C2C12 cells were incubated in the presence of PGE2 (50 nM) for the indicated time periods, followed by quantitative RT-PCR and western blot analyses; (B) PGE2 increased NFAT transcriptional activity. C2C12 cells were transfected with a reporter plasmid containing an NFAT response element, treated with PGE2 for 24 h, and subjected to a luciferase assay; (C) PGE2 induced NFAT binding to the mouse RANKL promoter. C2C12 cells were treated with PGE2 for 24 h and a ChIP assay was performed; (D) PGE2 increased RANKL promoter-reporter activity in an NFAT binding element-dependent manner. C2C12 cells were transfected with RANKL-WT-luc or RANKL-MT(N)-luc, incubated for 24 h in the presence of PGE2, and subjected to a luciferase assay (* p < 0.05, compared to control; # p < 0.05).
2.4. Cyclooxygenase (COX) Inhibitor Blocks TNFα-Induced Binding of NFATc1 and cAMP Response Element-Binding Protein (CREB) to the RANKL Promoter
Significant increases in the mRNA and protein expression of RANKL, NFATc1, cAMP response element-binding protein (CREB), and COX2 were observed in C2C12 cells incubated with PGE2 for 24 h (Figure 4A). TNFα induced similar levels of gene expression, and this process was inhibited by indomethacin (Figure 4A). Further treatment with PGE2 in the presence of indomethacin and TNFα partially rescued the expression of RANKL, NFATc1, CREB, and COX2. These results suggest that COX/PGE2 mediates the TNFα-induced expression of RANKL, NFATc1, and CREB.
Figure 4.
The COX inhibitor indomethacin blocks TNFα-induced binding of NFATc1 and cAMP response element-binding protein (CREB) to the RANKL promoter in C2C12 cells. (A) PGE2 increased the expression of RANKL, NFATc1, CREB, and COX2, whereas indomethacin suppressed TNFα-mediated RANKL, NFATc1, CREB, and COX2 expression. Treatment with PGE2 in the presence of indomethacin and TNFα partially rescued the expression of RANKL, NFATc1, CREB, and COX2. C2C12 cells were incubated with the indicated reagents for 24 h and subjected to RT-PCR and western blot analyses; (B) PGE2 increased RANKL promoter–reporter activity in a CREB binding element-dependent manner. C2C12 cells were transfected with RANKL-WT-luc or a CREB-binding site mutant (RANKL-MT(C)-luc) RANKL promoter, incubated for 24 h in the presence of PGE2, and subjected to a luciferase assay; (C) PGE2 induced CREB binding to the mouse RANKL promoter. C2C12 cells were incubated for 24 h with PGE2 and subjected to a ChIP assay with CREB and control IgG antibodies. The RANKL promoter region containing the CREB-binding element was then amplified; (D) Indomethacin prevented TNFα-induced NFAT and CREB binding to the mouse RANKL promoter. C2C12 cells were incubated for 24 h with the indicated reagents and subjected to a ChIP assay (* p < 0.05, compared to control; # p < 0.05).
We previously demonstrated that PTHrP-induced cAMP/PKA signaling promoted NFAT transcriptional activity and that NFAT and CREB cooperate to transactivate the gene encoding RANKL in mice [6]. Because our above data demonstrate that PGE2 enhances the transcriptional activity of NFAT and its binding to the RANKL promoter, we next investigated whether PGE2, produced in response to TNFα, would enhance the binding of CREB to the RANKL promoter and subsequent transactivation. The RANKL promoter–reporter assay demonstrated that mutations inserted in the CRE-like element (−1093 to −1086 bp) prevented PGE2 from enhancing reporter activity (Figure 4B). In addition, a ChIP assay with a CREB antibody revealed that PGE2 enhanced the binding of CREB to the RANKL promoter (Figure 4C). Consistent with these results, TNFα significantly increased the binding of CREB and NFATc1 to the RANKL promoter in a process blocked by indomethacin (Figure 4D). These results suggest that TNFα-induced COX/PGE2 increases the binding of both CREB and NFAT to the RANKL promoter and transcriptional activation of the RANKL gene.
2.5. The PGE2 Receptor Antagonists AH6809 and AH23848 Blocked TNFα-Induced Activation of NFAT and Binding of NFATc1 and CREB to the RANKL Promoter
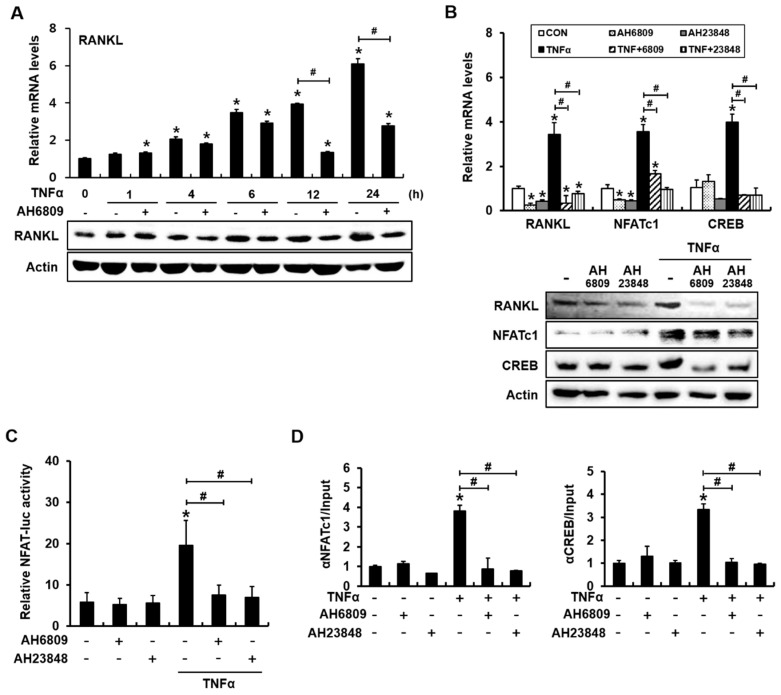
We next investigated whether PGE2 receptor antagonists would inhibit TNFα-induced RANKL expression and NFAT activation. AH6809 has the highest affinity for the EP2 receptor, but also acts as a weak antagonist against EP1 and prostaglandin D2 receptor 1 (DP1) receptors in the mouse [35]. AH23848 is a dual antagonist of the EP4 and thromboxane (TP1) receptors [36,37]. C2C12 cells were incubated with AH6809 (5 μM) in the presence of TNFα for 1, 4, 6, 12, and 24 h, and then the levels of RANKL mRNA and protein were examined. AH6809 did not significantly suppress TNFα-induced RANKL expression at an early time point (6 h or earlier), but inhibited the TNFα-mediated induction of RANKL thereafter (Figure 5A). Incubation for 24 h in the presence of AH6809 or AH23848 blocked the TNFα-mediated induction of RANKL, NFATc1, and CREB mRNA expression in C2C12 cells (Figure 5B). Compared with NFATc1 mRNA expression, TNFα-induced NFAT protein levels decreased moderately in the presence of AH6809 or AH23848 (Figure 5B). However, TNFα-induced NFAT transcriptional activity was abolished by the addition of AH6809 or AH23848 (Figure 5C). Furthermore, ChIP assays with antibodies to NFATc1 or CREB demonstrated that PGE2 receptor antagonists suppressed TNFα-induced binding of NFATc1 or CREB to the RANKL promoter (Figure 5D). These results indicate that TNFα-induced RANKL expression depends on the production of PGE2, which subsequently binds to EP2 or EP4 receptor and enhances the binding and transcriptional activity of NFATc1 and CREB in the mouse RANKL promoter.
Figure 5.
The PGE2 receptor antagonists AH6809 and AH23848 suppress the TNFα-induced activation of NFAT and binding of NFATc1 and CREB to the RANKL promoter in C2C12 cells. (A) PGE2 receptor antagonists significantly inhibited TNFα induction of RANKL only after incubation for 12 and 24 h; (B) AH6809 and AH23848 blocked TNFα-mediated RANKL, NFATc1, and CREB expression. AH6809 and AH23848 partially suppressed TNFα-induced NFATc1 protein expression. C2C12 cells were incubated with AH6809 (5 μM) or AH23848 (5 μM) in the presence or absence of TNFα for 24 h; (C) PGE2 receptor antagonists abolished TNFα-induced NFAT transcriptional activity; (D) PGE2 receptor antagonists prevented TNFα-induced NFAT and CREB binding to the mouse RANKL promoter (* p < 0.05, compared to control; # p < 0.05).
2.6. TNFα Enhances RANKL Expression in a PGE2 Production- and NFAT Activation-Dependent Manner in Primary Cultured Mouse Calvarial Cells
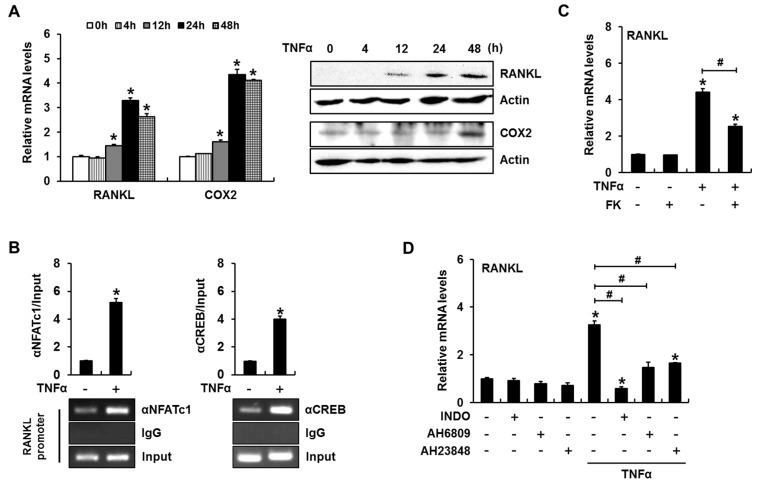
We next confirmed the roles of PGE2 and calcineurin/NFAT signaling with regard to TNFα-regulated RANKL expression in primary cultured mouse calvarial (MC) cells. Consistent with our observations in C2C12 cells, TNFα induced both RANKL and COX2 mRNA and protein expression (Figure 6A). PCR amplification of the DNA region containing the NFAT binding element or CRE-like region revealed that TNFα enhanced the binding of NFATc1 or CREB to the RANKL promoter (Figure 6B). FK506-mediated inhibition of NFAT activation significantly reduced TNFα-induced RANKL expression (Figure 6C). Indomethacin, AH6809, and AH23848 also inhibited TNFα-induced RANKL mRNA expression (Figure 6D). These results further suggest that PGE2 mediates TNFα-induced RANKL expression through a process that involves enhanced binding of NFAT and CREB to the RANKL promoter.
Figure 6.
PGE2 production and NFAT activation are necessary for TNFα-induced RANKL expression in primary cultured mouse calvarial cells. (A) TNFα enhanced the expression of RANKL and COX2. Calvarial cells were incubated in the presence of TNFα for the indicated time periods and subjected to RT-PCR and western blot analyses; (B) ChIP assays revealed that TNFα increased NFATc1 and CREB binding to their cognate binding sites in the mouse RANKL promoter; (C) A calcineurin/NFAT signaling inhibitor suppressed TNFα-induced RANKL expression. Calvarial cells were incubated for 24 h in the presence of FK506 and TNFα; (D) Treatment with a COX2 inhibitor or PGE2 receptor antagonists suppressed TNFα-induced RANKL expression. (* p < 0.05, compared to control; # p < 0.05).
3. Discussion
In the present study, we demonstrated that TNFα-induced RANKL expression involves both PGE2-induced activation of the calcineurin/NFAT pathway and subsequent binding of NFATc1 to the RANKL promoter. The following results of our study support the involvement of PGE2 and the calcineurin/NFAT signaling pathway in TNFα-induced RANKL expression: (i) TNFα stimulated COX2 expression and PGE2 production; (ii) a COX inhibitor or EP antagonists blocked TNFα-induced RANKL expression; (iii) TNFα-induced RANKL expression was significantly downregulated by inhibition of the calcineurin/NFAT pathway; (iv) TNFα enhanced the transcriptional activity of NFATc1 and binding of NFATc1 to the RANKL promoter; (v) TNFα-induced RANKL promoter–reporter activity was attenuated by mutations in the NFAT binding element; and (vi) a COX inhibitor or EP antagonists blocked TNFα-enhanced transcriptional activity and binding of NFAT to the RANKL promoter.
It has been well demonstrated that RANKL expression increases with TNFα treatment in a dose-dependent manner and 10 ng/mL of TNFα was used as a representative dose in several studies [38,39,40,41,42,43]. Therefore, we adopted a concentration of 10 ng/mL in the present study.
The ability of TNFα to stimulate PGE2 production via COX2 has been well demonstrated [28,44,45,46], and PGE2 is known to activate the cAMP/PKA signaling pathway in target cells [19]. Of the PGE2 receptor subtypes, EP4 has the highest expression in fibroblasts. Compared to control mice, EP4-deficient mice exhibited reduced inflammatory responses in a collagen-induced arthritis model, fewer osteoclasts, and reduced RANKL expression in osteoblast cells [20,47,48,49]. These reports suggest that EP4 is critical to the stimulation of bone resorption. Another report demonstrated that in MC osteoblastic cells, PGE2-induced cAMP production and RANKL expression are mainly mediated by EP4, although EP4 and EP2 must cooperate to ensure a full response to PGE2 [23]. In the present study, either an EP2 or EP4 antagonist completely blocked TNFα-induced RANKL expression. In addition, both antagonists abolished TNFα-induced NFAT reporter activity and the binding of NFATc1 and CREB to the RANKL promoter in C2C12 cells. In primary cultured MC cells, both antagonists had similar partial inhibitory effects on TNFα-induced RANKL expression. These results suggest that both EP2 and EP4 are involved in PGE2-induced RANKL expression. However, it is not clear why the responses of primary MC cells and C2C12 cells to EP antagonists differ.
In the present study, we did not examine the receptor subtypes involved in TNFα-induction of COX2 and PGE2 production. TNFα regulates cellular activities via the activation of either of two receptors: TNF receptor 1 (TNFR1) and TNFR2. TNFR1 is expressed ubiquitously, whereas TNFR2 expression is restricted to specific cell types, including immune cells and neurons [50]. Previous reports have demonstrated that TNFα induces COX2 expression and PGE2 production via TNFR1 in synovial fibroblasts [51,52]. Furthermore, induction of RANKL by TNFα was abolished in TNFR1-deficient mouse gingival epithelial cells [29]. These studies suggest that the activation of TNFR1 contributes to TNFα-induced activation of COX2 and production of PGE2 in C2C12 and mouse calvarial cells.
Although the PGE2-induced cAMP/PKA signaling is known to induce RANKL expression, the effector molecules downstream of PKA have not yet been clearly elucidated. Previously, PKA was shown to target CREB and thus increase the extent of CREB binding to distal enhancers of RANKL [53]. Assuming that PGE2/PKA signaling mediates TNFα-induced RANKL expression, we propose NFAT to be another transcription factor that acts downstream of PGE2 to induce RANKL transcription. Similar to a previous report in which CREB and NFATc1 cooperation was necessary to induce RANKL transcription via the PTHrP/cAMP/PKA pathway [6], TNFα-induced PGE2 enhanced the binding of CREB and NFATc1 to the mouse RANKL promoter, and mutations in either NFAT-binding element or the CRE-like element blocked PGE2-induced RANKL promoter activity. In addition, indomethacin and EP antagonists prevented TNFα-induced binding of NFATc1 and CREB to the RANKL promoter. These data support a role for NFATc1 as another mediator linking PGE2/PKA signaling to RANKL transcription.
A rich body of literature demonstrates that NFAT activation directly regulates COX expression and PGE2 production. Notably, NFAT activation regulates COX2-encoding genes in human colon carcinoma [33]. In this study, COX2 expression and PGE2 production were induced in response to NFAT stimuli and blocked when CsA or FK506 was used to inhibit calcineurin phosphatase activity. Lipopolysaccharide-induced NFAT activation was shown to regulate PGE2 synthesis in dendritic cells [34], and NFAT was found to induce COX2 transcription in human glioblastoma cells [54] and to regulate constitutive COX2 expression in the renal medulla [55]. Furthermore, PTH was shown to induce COX2 transcription through cross-talk between the cAMP/PKA and calcineurin/NFAT signaling pathways in murine osteoblastic cells [56]. Therefore, we determined whether inhibition of the calcineurin/NFAT pathway would suppress COX2-induced PGE2 production. FK506 partially suppressed TNFα-induced COX2 expression and PGE2 production. However, functional PGE2 inhibition by EP antagonists completely abolished the TNFα-induced transcriptional activity of NFAT, indicating that TNFα-induced NFAT activation is PGE2-dependent and subsequently contributes to the further induction of COX2 expression.
In the present study, TNFα-induced COX2 expression was obvious only after incubation for 4 h, although TNFα-induced RANKL expression was significant even after 1 h-incubation in C2C12 cells. In addition, PGE2 antagonist AH6809 did not significantly suppress TNFα-induced RANKL expression at an early time point (6 h or earlier). Given the data demonstrating the difference between the early vs. late response, it is suggested that there is a PGE2-independent pathway in RANKL induction of TNFα in the immediate response. Considering the continuing effect of TNFα and PGE2 on RANKL induction after 12 h, PGE2 seems to mediate the slow, delayed, long-term RANKL expression by TNFα, which is more likely to be associated with pathologic conditions such as inflammatory bone resorption.
In conclusion, the present study demonstrates that TNFα-induced RANKL expression depends on PGE2 production and subsequent transcriptional activation, as well as on the enhanced binding of NFATc1 and CREB to the RANKL promoter in mouse osteoblastic cells.
4. Materials and Methods
4.1. Reagents and Antibodies
Recombinant human TNFα was purchased from R&D Systems (Minneapolis, MN, USA). FK506, cyclosporin A, SB203580, H89, PGE2, and indomethacin were purchased from Sigma (St. Louis, MO, USA). AH6809 and AH23848 were purchased from Cayman Chemical (Ann Arbor, MI, USA). RANKL antibody was purchased from R&D Systems. Antibodies to NFATc1, COX2, and β-actin and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). CREB antibody was purchased from Cell Signaling Technology (Danvers, MA, USA).
4.2. Cell Culture
C2C12 cells, a murine mesenchymal cell line that can be differentiated into osteoblasts [57], were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
MC cells were isolated from the frontal and parietal bones of neonatal ICR mice, as previously described [58]. Before preparation of MC cells, animals were weaned in mouse gang cages following Institutional Animal Care and Use Committee policies. The animal study was reviewed and approved by the Special Committee on Animal Welfare, Seoul National University, Seoul, Republic of Korea (approval no. SNU-20140228-1-5). MC cells were cultured in α-minimum essential medium (α-MEM) supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. DMEM, α-MEM, and FBS were obtained from Hyclone (Walkersville, MD, USA).
4.3. Plasmid Construction
Construction of the NFATc1 expression vector and a NFAT reporter plasmid has been described in previous publications [32,59]. Construction of a RANKL promoter-luciferase reporter plasmid (RANKL-WT: −2174 to +1 bp of the mouse RANKL promoter) and a function-defective mutant reporter containing mutations in the NFAT-binding site (RANKL-MT-N; GGAAAA⟶GCttAA) [4] or in the CRE-like element (RANKL-MT-C; TGAGGTCA⟶TGAGGagg) have been described in previous publications [6,60,61].
4.4. Reverse Transcription-Polymerase Chain Reaction
Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed as described previously [6]. PCR primers with the following sequences were synthesized by Cosmogenetech (Seoul, Korea): RANKL-forward (F) 5′-CAG AAG ATG GCA CTC ACT GCA-3′, RANKL-reverse (R) 5′-CAC CAT CGC TTT CTC TGC TCT-3′; NFATc1-F 5′-AAT AAC ATG CGA GCC ATC ATC-3′, NFATc1-R 5′-TCA CCC TGG TGT TCT TCC TC-3′; CREB-F 5′-AGC TGC CAC TCA GCC GGG TA-3′, CREB-R 5′-TGG TGC TCG TGG GTG CTG TG-3′; COX2-F 5′-CCA GCA CTT CAC CCA TCA GTT-3′; COX2-R 5′-ACC CAG GTC CTC GCT TAT GA-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-F 5′-TCA ATG ACA ACT TTG TCA AGC-3′; and GAPDH-R 5′-CCA GGG TTT CTT ACT CCT TGG-3′. GAPDH was used as a reference to normalize each sample for quantification.
4.5. Western Blot Analysis
The western blot analysis was performed as described previously [6]. Immune complexes were visualized using EZ-Western Lumi Pico (Daeillab Service Co., Seoul, Korea), and chemiluminescence was detected using a MicroChemi device (DNR, Jerusalem, Israel).
4.6. Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation (ChIP) assay was performed as described previously [4]. Cross-linked DNA fragments were subjected to pre-clearing with blocked protein G agarose, and immunoprecipitation was performed with an NFATc1 or CREB antibody or species-matched control IgG. After purification, DNA sequences were analyzed by PCR amplification of the mouse RANKL promoter region encompassing either the NFAT-binding element (amplified region: −1070 to −858 bp) or CRE-like element (amplified region: −1147 to −967 bp). PCR primers with the following sequences were used: NFAT-binding element forward, 5′-GCA AGC TCC AGG CCA GCC TAG-3′ and reverse, 5′-CCA ATA AGA CGG CTC AGC TG-3′; CRE-like element forward, 5′-AGG AGG CAG AGA TGG CAG AG-3′ and reverse, 5′-ACA CGC GCG CGC GCA AAT A-3′.
4.7. Luciferase Reporter Assay
The luciferase reporter assay was performed as described previously [6]. In order to normalize transfection efficiency, Renilla luciferase plasmid was co-transfected with RANKL promoter reporters.
4.8. PGE2 Assay
Cells were incubated for 24 h in medium supplemented with the indicated reagents, after which supernatants were collected. The concentration of PGE2 in the supernatant was determined using Parameter PGE2 (R&D Systems) according to the manufacturer’s instructions.
4.9. Statistical Analysis
Statistical significance was determined using Student’s t-test. For the multiple comparisons, one way ANOVA was performed. When significant main effects were detected, post hoc analyses were conducted with the least squares means error test. Differences were considered significant at p < 0.05. Data were analyzed by using the SAS program (version 9.1; SAS Institute, Cary, NC, USA).
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2012R1A1A2002638).
Author Contributions
Jeong-Hwa Baek and Hyung-Ryong Kim conceived and designed the experiments; Hyun-Jung Park performed the experiments; Hyun-Jung Park, Kyunghwa Baek, and Jeong-Hwa Baek analyzed the data; Hyun-Jung Park contributed reagents, materials, and analysis tools; Hyun-Jung Park and Kyunghwa Baek wrote the paper; Kyunghwa Baek and Jeong-Hwa Baek revised the manuscript contents; and Jeong-Hwa Baek and Hyung-Ryong Kim approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Udagawa N., Takahashi N., Jimi E., Matsuzaki K., Tsurukai T., Itoh K., Nakagawa N., Yasuda H., Goto M., Tsuda E., et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: Receptor activator of NF-κB ligand. Bone. 1999;25:517–523. doi: 10.1016/S8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 2.Wada T., Nakashima T., Hiroshi N., Penninger J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Fu Q., Manolagas S.C., O’Brien C.A. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol. Cell. Biol. 2006;26:6453–6468. doi: 10.1128/MCB.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H.L., Bae O.Y., Baek K.H., Kwon A., Hwang H.R., Qadir A.S., Park H.J., Woo K.M., Ryoo H.M., Baek J.H. High extracellular calcium-induced NFATc3 regulates the expression of receptor activator of NF-κB ligand in osteoblasts. Bone. 2011;49:242–249. doi: 10.1016/j.bone.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Mak K.K., Bi Y.M., Wan C., Chuang P.T., Clemens T., Young M., Yang Y.Z. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev. Cell. 2008;14:674–688. doi: 10.1016/j.devcel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Park H.J., Baek K., Baek J.H., Kim H.R. The cooperation of CREB and NFAT is required for PTHrP-induced RANKL expression in mouse osteoblastic cells. J. Cell. Physiol. 2015;230:667–679. doi: 10.1002/jcp.24790. [DOI] [PubMed] [Google Scholar]
- 7.Spencer G.J., Utting J.C., Etheridge S.L., Arnett T.R., Genever P.G. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J. Cell. Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y.H., Heulsmann A., Tondravi M.M., Mukherjee A., Abu-Amer Y. Tumor necrosis factor-α (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 9.Cope A.P., Liblau R.S., Yang X.D., Congia M., Laudanna C., Schreiber R.D., Probert L., Kollias G., McDevitt H.O. Chronic tumor necrosis factor alters T-cell responses by attenuating T-cell receptor signaling. J. Exp. Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma Y., Kaji K., Katogi R., Takeshita S., Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 11.Kitazawa R., Kimble R.B., Vannice J.L., Kung V.T., Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994;94:2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner U.H., Ohlin A. Tumor necrosis factors α and β can stimulate bone resorption in cultured mouse calvariae by a prostaglandin-independent mechanism. J. Bone Miner. Res. 1993;8:147–155. doi: 10.1002/jbmr.5650080205. [DOI] [PubMed] [Google Scholar]
- 13.Thomson B.M., Mundy G.R., Chambers T.J. Tumor necrosis factors α and β induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 14.Kitaura H., Kimura K., Ishida M., Kohara H., Yoshimatsu M., Takano-Yamamoto T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013;2013:181849. doi: 10.1155/2013/181849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W., Amcheslavsky A., Takeshita S., Drissi H., Bar-Shavit Z. TNF-α expression is transcriptionally regulated by RANK ligand. J. Cell. Physiol. 2005;202:371–378. doi: 10.1002/jcp.20127. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M., Sudo T., Saito T., Osada H., Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL) J. Biol. Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 17.Rossa C., Ehmann K., Liu M., Patil C., Kirkwood K.L. MKK3/6-p38 MAPK signaling is required for IL-1β and TNF-α-induced RANKL expression in bone marrow stromal cells. J. Interferon Cytokine Res. 2006;26:719–729. doi: 10.1089/jir.2006.26.719. [DOI] [PubMed] [Google Scholar]
- 18.Narumiya S., Sugimoto Y., Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1111/j.1749-6632.1994.tb52729.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y., Mizoguchi T., Take I., Kurihara S., Udagawa N., Takahashi N. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J. Biol. Chem. 2005;280:11395–11403. doi: 10.1074/jbc.M411189200. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Okada Y., Pilbeam C.C., Lorenzo J.A., Kennedy C.R., Breyer R.M., Raisz L.G. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–2061. doi: 10.1210/en.141.6.2054. [DOI] [PubMed] [Google Scholar]
- 21.Miyaura C., Inada M., Matsumoto C., Ohshiba T., Uozumi N., Shimizu T., Ito A. An essential role of cytosolic phospholipase a2α in prostaglandin E2-mediated bone resorption associated with inflammation. J. Exp. Med. 2003;197:1303–1310. doi: 10.1084/jem.20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trebino C.E., Stock J.L., Gibbons C.P., Naiman B.M., Wachtmann T.S., Umland J.P., Pandher K., Lapointe J.M., Saha S., Roach M.L., et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. USA. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzawa T., Miyaura C., Inada M., Maruyama T., Sugimoto Y., Ushikubi F., Ichikawa A., Narumiya S., Suda T. The role of prostaglandin e receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 24.Fujino H., Xu W., Regan J.W. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J. Biol. Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen M., Solle M., Audoly L.P., Tilley S.L., Stock J.L., McNeish J.D., Coffman T.M., Dombrowicz D., Koller B.H. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J. Immunol. 2002;169:4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 26.Hempel S.L., Monick M.M., Hunninghake G.W. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J. Clin. Investig. 1994;93:391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell J.A., Belvisi M.G., Akarasereenont P., Robbins R.A., Kwon O.J., Croxtall J., Barnes P.J., Vane J.R. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: Regulation by dexamethasone. Br. J. Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakao S., Ogtata Y., Shimizu E., Yamazaki M., Furuyama S., Sugiya H. Tumor necrosis factor α (TNF-α)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFκB in human gingival fibroblasts. Mol. Cell. Biochem. 2002;238:11–18. doi: 10.1023/A:1019927616000. [DOI] [PubMed] [Google Scholar]
- 29.Fujihara R., Usui M., Yamamoto G., Nishii K., Tsukamoto Y., Okamatsu Y., Sato T., Asou Y., Nakashima K., Yamamoto M. Tumor necrosis factor-α enhances RANKL expression in gingival epithelial cells via protein kinase a signaling. J. Periodontal Res. 2014;49:508–517. doi: 10.1111/jre.12131. [DOI] [PubMed] [Google Scholar]
- 30.Sitara D., Aliprantis A.O. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol. Rev. 2010;233:286–300. doi: 10.1111/j.0105-2896.2009.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winslow M.M., Pan M., Starbuck M., Gallo E.M., Deng L., Karsenty G., Crabtree G.R. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev. Cell. 2006;10:771–782. doi: 10.1016/j.devcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Chuvpilo S., Jankevics E., Tyrsin D., Akimzhanov A., Moroz D., Jha M.K., Schulze-Luehrmann J., Santner-Nanan B., Feoktistova E., Konig T., et al. Autoregulation of NFATc1/a expression facilitates effector t cells to escape from rapid apoptosis. Immunity. 2002;16:881–895. doi: 10.1016/S1074-7613(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 33.Duque J., Fresno M., Iniguez M.A. Expression and function of the nuclear factor of activated T-cells in colon carcinoma cells: Involvement in the regulation of cyclooxygenase-2. J. Biol. Chem. 2005;280:8686–8693. doi: 10.1074/jbc.M413076200. [DOI] [PubMed] [Google Scholar]
- 34.Zanoni I., Ostuni R., Barresi S., di Gioia M., Broggi A., Costa B., Marzi R., Granucci F. CD14 and NFAT mediate lipopolysaccharide-induced skin edema formation in mice. J. Clin. Investig. 2012;122:1747–1757. doi: 10.1172/JCI60688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X., Kundu N., Rifat S., Walser T., Fulton A.M. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez T., Moreno J.J. Role of EP(1) and EP(4) PGE(2) subtype receptors in serum-induced 3T6 fibroblast cycle progression and proliferation. Am. J. Physiol. Cell Physiol. 2002;282:C280–C288. doi: 10.1152/ajpcell.00128.2001. [DOI] [PubMed] [Google Scholar]
- 38.Collin-Osdoby P., Rothe L., Anderson F., Nelson M., Maloney W., Osdoby P. Receptor activator of NF-κB and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J. Biol. Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 39.Wada N., Maeda H., Yoshimine Y., Akamine A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-κB ligand in periodontal ligament fibroblasts through the induction of interleukin-1β and tumor necrosis factor-α. Bone. 2004;35:629–635. doi: 10.1016/j.bone.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y.S., Min K.S., Lee H.D., Oh H.W., Kim E.C. Effect of cytosolic phospholipase A2 on proinflammatory cytokine-induced bone resorptive genes including receptor activator of nuclear factor kappa B ligand in human dental pulp cells. J. Endod. 2010;36:636–641. doi: 10.1016/j.joen.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Goto H., Hozumi A., Osaki M., Fukushima T., Sakamoto K., Yonekura A., Tomita M., Furukawa K., Shindo H., Baba H. Primary human bone marrow adipocytes support TNF-α-induced osteoclast differentiation and function through RANKL expression. Cytokine. 2011;56:662–668. doi: 10.1016/j.cyto.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Wei S., Kitaura H., Zhou P., Ross F.P., Teitelbaum S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005;115:282–290. doi: 10.1172/JCI200523394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ainola M., Mandelin J., Liljestrom M., Konttinen Y.T., Salo J. Imbalanced expression of RANKL and osteoprotegerin mRNA in pannus tissue of rheumatoid arthritis. Clin. Exp. Rheumatol. 2008;26:240–246. [PubMed] [Google Scholar]
- 44.Chen C.C., Sun Y.T., Chen J.J., Chiu K.T. TNF-α-induced cyclooxygenase-2 expression in human lung epithelial cells: Involvement of the phospholipase c-γ2, protein kinase c-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J. Immunol. 2000;165:2719–2728. doi: 10.4049/jimmunol.165.5.2719. [DOI] [PubMed] [Google Scholar]
- 45.Ke J., Long X., Liu Y., Zhang Y.F., Li J., Fang W., Meng Q.G. Role of NF-κB in TNF-α-induced COX-2 expression in synovial fibroblasts from human tmj. J. Dent. Res. 2007;86:363–367. doi: 10.1177/154405910708600412. [DOI] [PubMed] [Google Scholar]
- 46.Medeiros L.R., Freitas L.B., Rosa D.D., Silva F.R., Silva L.S., Birtencourt L.T., Edelweiss M.I., Rosa M.I. Accuracy of magnetic resonance imaging in ovarian tumor: A systematic quantitative review. Am. J. Obstet. Gynecol. 2011;204:67.e1–67.e10. doi: 10.1016/j.ajog.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 47.McCoy J.M., Wicks J.R., Audoly L.P. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Investig. 2002;110:651–658. doi: 10.1172/JCI0215528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyaura C., Inada M., Suzawa T., Sugimoto Y., Ushikubi F., Ichikawa A., Narumiya S., Suda T. Impaired bone resorption to prostaglandin E2 in prostaglandin e receptor EP4-knockout mice. J. Biol. Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 49.Wei X., Zhang X., Zuscik M.J., Drissi M.H., Schwarz E.M., O’Keefe R.J. Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J. Bone Miner. Res. 2005;20:1136–1148. doi: 10.1359/JBMR.050206. [DOI] [PubMed] [Google Scholar]
- 50.Kalliolias G.D., Ivashkiv L.B. Tnf biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alaaeddine N., DiBattista J.A., Pelletier J.P., Cloutier J.M., Kiansa K., Dupuis M., Martel-Pelletier J. Osteoarthritic synovial fibroblasts possess an increased level of tumor necrosis factor-receptor 55 (TNF-r55) that mediates biological activation by TNF-α. J. Rheumatol. 1997;24:1985–1994. [PubMed] [Google Scholar]
- 52.Alsalameh S., Amin R.J., Kunisch E., Jasin H.E., Kinne R.W. Preferential induction of prodestructive matrix metalloproteinase-1 and proinflammatory interleukin 6 and prostaglandin E2 in rheumatoid arthritis synovial fibroblasts via tumor necrosis factor receptor-55. J. Rheumatol. 2003;30:1680–1690. [PubMed] [Google Scholar]
- 53.Kim S., Yamazaki M., Zella L.A., Meyer M.B., Fretz J.A., Shevde N.K., Pike J.W. Multiple enhancer regions located at significant distances upstream of the transcriptional start site mediate RANKL gene expression in response to 1,25-dihydroxyvitamin D3. J. Steroid Biochem. 2007;103:430–434. doi: 10.1016/j.jsbmb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Wang Z., Li J., Zhang W., Ren F., Yue W. Nfatc1 activation promotes the invasion of u251 human glioblastoma multiforme cells through COX-2. Int. J. Mol. Med. 2015;35:1333–1340. doi: 10.3892/ijmm.2015.2124. [DOI] [PubMed] [Google Scholar]
- 55.Kirkby N.S., Chan M.V., Zaiss A.K., Garcia-Vaz E., Jiao J., Berglund L.M., Verdu E.F., Ahmetaj-Shala B., Wallace J.L., Herschman H.R., et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. P. Natl. Acad. Sci. USA. 2016;113:434–439. doi: 10.1073/pnas.1517642113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H., Chikazu D., Voznesensky O.S., Herschman H.R., Kream B.E., Drissi H., Pilbeam C.C. Parathyroid hormone induction of cyclooxygenase-2 in murine osteoblasts: Role of the calcium-calcineurin-NFAT pathway. J. Bone Miner. Res. 2010;25:819–829. doi: 10.1359/jbmr.091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K.S., Kim H.J., Li Q.L., Chi X.Z., Ueta C., Komori T., Wozney J.M., Kim E.G., Choi J.Y., Ryoo H.M., et al. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000;20:8783–8792. doi: 10.1128/MCB.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y.H., Kim J.M., Kim S.N., Kim G.S., Baek J.H. P44/42 MAPK activation is necessary for receptor activator of nuclear factor-κB ligand induction by high extracellular calcium. Biochem. Biophys. Res. Commun. 2003;304:729–735. doi: 10.1016/S0006-291X(03)00661-2. [DOI] [PubMed] [Google Scholar]
- 59.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 60.Elefteriou F., Ahn J.D., Takeda S., Starbuck M., Yang X., Liu X., Kondo H., Richards W.G., Bannon T.W., Noda M., et al. Leptin regulation of bone resorption by the sympathetic nervous system and cart. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 61.Mori K., Kitazawa R., Kondo T., Maeda S., Yamaguchi A., Kitazawa S. Modulation of mouse RANKL gene expression by Runx2 and PKA pathway. J. Cell. Biochem. 2006;98:1629–1644. doi: 10.1002/jcb.20891. [DOI] [PubMed] [Google Scholar]