Abstract
Non-coding RNAs (ncRNAs) are a class of RNA molecules that do not encode proteins. Studies show that ncRNAs are not only involved in cell proliferation, apoptosis, differentiation, metabolism and other physiological processes, but also involved in the pathogenesis of diseases. Cardiac remodeling is the main pathological basis of a variety of cardiovascular diseases. Many studies have shown that the occurrence and development of cardiac remodeling are closely related with the regulation of ncRNAs. Recent research of ncRNAs in heart disease has achieved rapid development. Thus, we summarize here the latest research progress and mainly the molecular mechanism of ncRNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), in cardiac remodeling, aiming to look for new targets for heart disease treatment.
Keywords: non-coding RNA, microRNA, long non-coding RNA, circular RNA, cardiac remodeling
1. Introduction
Cardiovascular disease is the first leading cause of death which seriously threatens the health and quality of human life. Cardiac remodeling is the pathological process induced by the adaptive changes of cardiac insufficiency, and is closely related to the occurrence and development of many kinds of heart disease. Stress stimuli such as inflammation, pressure overload, oxidative injury and myocardial infarction (MI) can cause cardiac remodeling, the continued progress of which may ultimately develop to arrhythmia, heart failure, and even sudden death. Basically, the cardiac structure, function and phenotype have changed during cardiac remodeling, exhibiting as pathological cardiac hypertrophy with fetal gene re-expression, myocyte apoptosis, aging, necrosis, and extracellular matrix excessive fibrosis.
Cardiac remodeling involves complex molecular mechanisms. Thus, looking for key molecules that participate in the development process of cardiac remodeling is of great significance to elucidate the molecular mechanism, as well as to explore new ways of prevention and treatment of cardiovascular diseases. Non-coding RNAs (ncRNAs) are not only involved in cell proliferation, apoptosis, differentiation, metabolism and other important biological processes, but also closely related to the occurrence, development, treatment and diagnosis of diseases. In recent years, the research of ncRNAs in heart disease has been rapidly developed. More and more studies have revealed that ncRNAs play an important role in the development of cardiac remodeling. In this review, we focus on the latest research progress and mechanisms of ncRNAs, especially microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) in cardiac remodeling, aiming to find new therapeutic targets for heart disease treatment.
2. The Classification and Function of ncRNAs
ncRNAs are a class of RNA molecules that do not encode proteins and function directly at the RNA level. Based on the function, length and structure dissimilarity, ncRNAs can be divided into transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), guide RNAs (gRNAs), miRNAs, piwi-interacting RNAs (piRNAs), small interfering RNAs (siRNAs), lncRNAs, and circRNAs [1]. Table 1 shows the length range and main functions of each class of ncRNAs. At present, studies are mainly focused on the role of miRNAs, lncRNAs, piRNAs and circRNAs in the normal growth and development of body, physiological functions and pathological processes.
Table 1.
The classification and function of non-coding RNAs.
| ncRNAs | Length | Main Functions |
|---|---|---|
| tRNAs | 74~95 nt | Transfer amino acids to ribosomes during protein synthesis |
| rRNAs | 121~5000 nt | Ribosome components, directly involved in the synthesis of proteins in robosome |
| snRNAs | 100~300 nt | Process mRNA precursor (splicing and maturation) |
| snoRNAs | 100~300 nt | Guide chemical modifications of other RNAs, such as rRNAs, tRNAs and snRNAs |
| gRNAs | 55~70 nt | Participate in RNA editing |
| miRNAs | 19~23 nt | Negatively regulate gene expression by promote mRNA degradation or inhibit mRNA translation |
| piRNAs | 24~30 nt | Play roles in gametogenesis, maintain transposon silencing, translation suppression, epigenetic regulation |
| siRNAs | 21~25 nt | Silence complementary target mRNA |
| lncRNAs | >200 nt | Regulate gene expression in epigenetic, transcriptional, post-transcriptional levels, miRNA sponge |
| circRNAs | Circular | As competing endogenous RNA or miRNA sponge; regulate alternative splicing and parental gene expression |
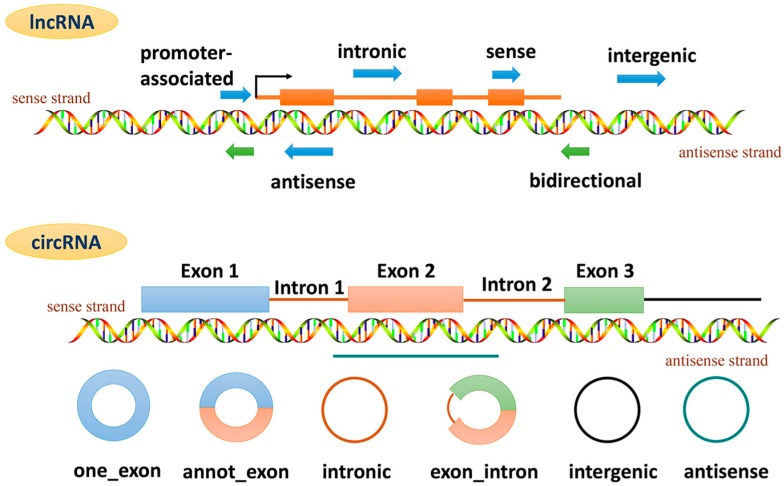
miRNAs are a class of endogenous single-strand ncRNA molecules with a length of 19–23 nucleotides, whose sequences are highly conserved among different species. Combined with the specific sites of the target mRNAs through base complementary, miRNAs can promote the degradation of mRNAs or inhibit the translation of mRNAs in the post transcriptional level, so as to exert its negative regulation on gene expression. Typically, an miRNA can regulate the expression of multiple genes, but a certain gene also can be precisely regulated by a plurality of miRNAs. lncRNAs, a class of RNA molecules more than 200 nucleotides long, generally do not encode proteins. According to their positions in the genome, lncRNAs can be classified into sense, antisense, intronic, intergenic, bidirectional and promoter-associated (Figure 1). lncRNAs have an mRNA-like structure, some with poly (A) tail, and show dynamic expression pattern and alternative splicing during differentiation. When compared with the coding gene, lncRNAs share less sequence conservation and show lower expression level [2]. lncRNAs can regulate gene expression in epigenetic, transcriptional and post-transcriptional levels, as well as directly modulate protein activity [3,4,5,6]. circRNAs are a class of RNA molecules derived from exon reverse splicing or intron lariat. Depending on their genomic structures, circRNAs can be divided into one_exon, annot_exon, intronic, exon_intron, intergenic and antisense (Figure 1). The expression of circRNAs is relatively stable because of their closed ring structure, which prevents them from being affected by RNA exonuclease, and is tissue- and developmental stage-specific [7]. circRNAs can play their regulatory function by competing endogenous RNA (ceRNA) mechanism, that is, acting as miRNA sponge in the cell [8]. In addition, circRNAs can regulate the linear splicing competition of pre-mRNAs and the transcription of parental genes [9,10]. piRNAs are small RNA molecules with a length of about 24–30 nucleotides. The investigation on piRNA is still at a preliminary stage, and current studies have found that piRNA binds to PIWI family proteins and forms a complex called piRNA-induced silencing complex (piRISC) which subsequently regulates the gene silencing pathway in germ cells [11].
Figure 1.
Long non-coding RNA (lncRNA) and circular RNA (circRNA) classification based on genomic location and context.
3. miRNAs and Cardiac Remodeling
In recent years, miRNAs have been the hotspot of life science research and the most widely studied ncRNAs. At present, more than 3000 miRNAs have been identified in human genome [12], which are involved in regulating the expression of more than 50% functional genes. miRNAs play an important role in maintaining cell homeostasis. Dysregulation of miRNAs can lead to a large number of diseases [13], including a variety of pathological processes of heart disease [14,15].
Thus far, many miRNA molecules involved in the regulation of cardiac hypertrophy and fibrosis have been identified. Olson’s group firstly find that the cardiac specific miR-208 encoded by the intron of α-MHC (myosin heavy chain 6, myh6) gene is abnormally expressed in pressure-induced cardiac hypertrophy and fibrosis, with the transformation of MHC gene from the adult type (α-MHC, myh6) to the fetal type (β-MHC, myh7) [16]. Knockout of miR-208 can inhibit cardiac hypertrophy induced by coarctation of the aorta. On the other hand, the first identified negative regulator of cardiac hypertrophy is miR-133 [17]. Overexpression of miR-133 can inhibit the hypertrophic response induced by pressure overload, and this anti-hypertrophic effect is achieved by inhibiting the expression of its target genes RhoA (Ras homolog gene family, member A), Cdc42 (cell division cycle 42) and Nelf-A/WHSC2 (Negative elongation factor complex member A/ Wolf-Hirschhorn Syndrome Candidate 2). miR-29 is one of the best studied anti myocardial fibrosis factors. Van Rooij et al. [18] have found that the level of miR-29 was significantly reduced under stress, making its targets such as collagen, elastin, fibrin and other extracellular matrix protein synthesis increased, thereby promoting the process of myocardial fibrosis. Over-expression of miR-29 can inhibit the synthesis of collagen and resist myocardial fibrosis. Clearly, the molecular mechanism of miRNA regulation is solely the inhibition of target mRNA expression, but the downstream targets are various and related to multiple signaling pathways in all aspects of life activities. Table 2 summaries the roles of recent identified miRNAs and their target genes in several aspects of pathological cardiac remodeling, such as hypertrophy, fibrosis and apoptosis. Moreover, the interaction between miRNAs and other ncRNAs makes it more interesting and worthy of research, which will be discussed in the following parts.
Table 2.
Effects of miRNAs in pathological cardiac remodeling.
| miRNAs | Effects | Target Genes/ Signaling Pathway | Pathological Conditions | Reference |
|---|---|---|---|---|
| Hypertrophy | ||||
| let-7a | Anti- | calmodulin | Ang II | [19] |
| miR-1 | Anti- | HADC4 | Thyroid hormone | [20] |
| miR-9 | Anti- | myocardin | Iso and Aldo | [21] |
| miR-10a | Anti- | Tbx5 | Ang II, TAC | [22] |
| miR-22 | Anti- | PURB, Sirt1, Hdac4 | Pressure overload | [23,24] |
| miR-23a | Pro- | MuRF1 | Iso and Aldo | [25] |
| miR-26a | Anti- | GATA4 | Ang II, TAC | [26] |
| miR-34 | Pro- | VEGF, vinculin, POFUT1, Notch1, SEMA4B | TAC | [27] |
| miR-101 | Anti- | Rab1a | Ang II, TAC | [28] |
| miR-155 | Pro- | Jarid2 | TAC, activated calcineurin Tg | [29] |
| miR-182 | Pro- | Bcat2, Foxo3, Adcy6/Akt | PIGF | [30] |
| miR-206 | Pro- | Foxp1 | YAP, pressure overload | [31] |
| miR-212/132 | Pro- | Foxo3 | Pressure overload | [32] |
| miR-223 | Pro- | ARC | Iso, TAC | [33] |
| miR-365 | Pro- | Skp2/ mTOR | Ang II | [34] |
| miR-378 | Anti- | MAPK1, IGFR1, GRB2, KSR1/MAPK | TAC | [35] |
| miR-489 | Anti- | MyD88 | Ang II, TAC | [36] |
| miR-497 | Anti- | Sirt4 | Ang II, TAC | [37] |
| miR-541 | Anti- | - | Ang II | [38] |
| Fibrosis | ||||
| miR-1 | Anti- | Fibullin-2/MAPK | Pressure overload | [39] |
| miR-21 | Pro- | Spry1/ERK-MAP kinase PTEN/SMAD7 | Pressure overload, Ang II | [40,41] |
| miR-22 | Anti- | TGFβRI | Ang II | [42] |
| miR-30 | Anti- | CTGF | Renin-2 Tg | [43] |
| miR-34a | Pro- | Smad4 | MI | [44] |
| miR-101 | Anti- | c-Fos/TGF-β1 | Ang II, CAL | [45] |
| miR-154 | Pro- | Atg7 | TAC | [46] |
| miR-200c | Pro- | DUSP-1/MAPK | High glucose | [47] |
| miR-433 | Pro- | AZIN1, JNK1/TGFβ/Smad3 | MI | [48] |
| Apoptosis/Necrosis | ||||
| miR-30 | Anti- | p53, Cyclophilin D | H2O2, I/R | [49,50] |
| miR-34a | Pro- | PPP1R10, ALDH2 | MI | [51,52] |
| miR-103/107 | Pro- | FADD | I/R, H2O2 | [53] |
| miR-188-3p | Anti- | Atg7 | I/R, A/R | [54] |
| miR-324-5p | Anti- | Mtfr1 | I/R, A/R | [55] |
| miR-361 | Pro- | PHB1 | M/I, H2O2 | [56] |
| miR-378 | Anti- | Caspase-3 | CAL/Hypoxia | [57] |
| miR-421 | Pro- | Pink1 | I/R, H2O2 | [58] |
| miR-484 | Anti- | Fission 1 | I/R, Anoxia, Dox | [59] |
| miR-499 | Anti- | CnAα, CnAβ | I/R, Anoxia | [60] |
| miR-532-3p | Pro- | ARC | Dox | [61] |
| miR-539 | Pro- | PHB2 | I/R, Anoxia | [62] |
| miR-761 | Anti- | MFF | I/R, H2O2 | [63] |
| miR-874 | Pro- | Caspase-8 | I/R, H2O2 | [64] |
| miR-2861 | Pro- | ANT1 | I/R, H2O2 | [65] |
4. lncRNAs and Cardiac Remodeling
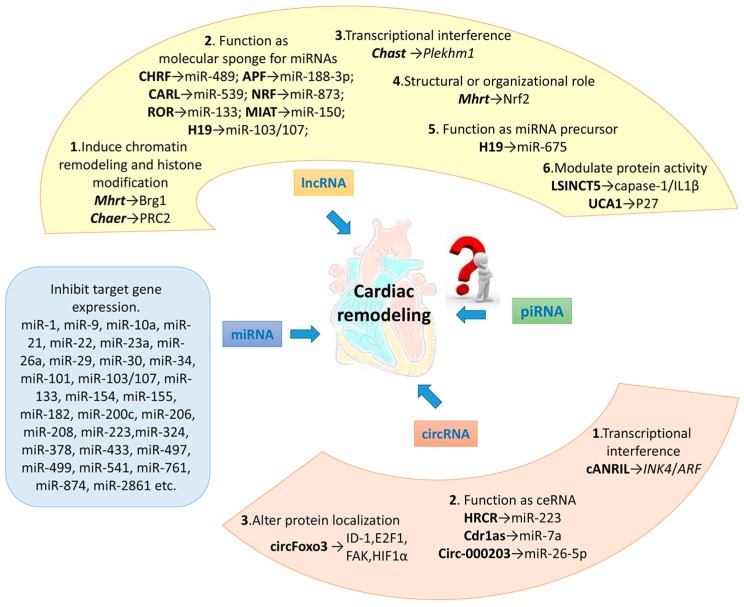
It is reported that only about 1.5% of the human genome can encode proteins, while the vast majority of the genome is in a state of non-transcribed or transcribes to ncRNAs [66]. As the next-generation high-throughput sequencing technology develops, a growing number of lncRNAs are identified. Thus far, a plurality of lncRNAs has been demonstrated to be involved in the regulation of cardiac remodeling via several different mechanisms (Figure 2). This makes lncRNAs function as potential therapeutic targets for the treatment of cardiac hypertrophy, heart failure and other cardiac disorders.
Figure 2.
Summary of the ncRNAs and their molecular mechanisms in cardiac remodeling.
4.1. lncRNA and Epigenetic Regulation
4.1.1. lncRNA and Chromatin Remodeling
In transverse aortic constriction (TAC)-induced pressure overload mice model, Han et al. [67] have indicated that cardiac-specific lncRNA Mhrt, which is the antisense transcript of myosin heavy chain 7 (Myh7), can exert its cardioprotective function through lncRNA-chromatin interaction mechanism. Pathological stress activates the chromatin remodeling factor Brg1 to form a complex composed of Brg1-HDAC-Parp, which directly binds to the promoter region of Mhrt and inhibits the transcription and expression of Mhrt. The helicase domain of Brg1 is capable of binding with both lncRNA Mhrt and chromatinized DNA targets, but not naked DNA. Mhrt inhibits the binding of Brg1 to its genomic DNA targets by competitively binding with helicase to prevent the occurrence of chromatin remodeling. Mhrt and Brg1 form a complete feedback circuit that plays a crucial role in the protection of the heart. Restored expression of Mhrt under pathological conditions can protect the heart from excessive hypertrophy and heart failure [67].
4.1.2. lncRNA and Histone Methylation
In a recent study, a cardiac-enriched lncRNA named Chaer (cardiac-hypertrophy-associated epigenetic regulator) has been demonstrated to be both necessary and sufficient for the development of cardiac hypertrophy [68]. It was identified from the dysregulated lncRNAs in heart failure mice induced by pressure overload. The transcript of Chaer was found to be predominantly located in the nucleus and highly conserved among mice, rats and humans. In Chaer-KO mice, hypertrophy of the heart was significantly attenuated after TAC operation. Moreover, the pathological fibrosis was weakened and the heart function was protected. Mechanistically, a 66-mer motif in Chaer can directly interact with the enhancer of zeste homolog 2 (EZH2) subunit of polycomb repressor complex 2 (PRC2), and interfere with PRC2 targeting to genomic loci, thereby inhibiting histone H3 lysine 27 methylation in the promoter regions of cardiac hypertrophy-associated genes. The interaction between Chaer and PRC2 is transiently induced after hormonal or stress stimulation, and is a prerequisite for epigenetic reprogramming and induction of cardiac hypertrophy-associated genes. It is essential that the inhibition of Chaer expression in heart prior to, but not after, the onset of stress overload can significantly reduce cardiac hypertrophy and heart dysfunction. In addition, the level of the human transcript CHAER was significantly increased in dilated cardiomyopathy compared with that in normal hearts. In human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), CHAER specially interacts with EZH2 and has rapamycin sensitivity. Overexpression of CHAER exhibits a similar hypertrophy phenotype with mouse homolog, indicating a cross-species regulatory function of Chaer.
4.2. lncRNA as Molecular Sponge for miRNA
The molecular mechanism of lncRNAs as ceRNAs in regulating cardiac remodeling has been extensively studied. In Ang II-induced hypertensive mice, cardiac hypertrophy related lncRNA named CHRF can inhibit the expression and activity of miR-489 as an endogenous molecular sponge, and subsequently up-regulate the level of myeloid differentiation factor88 (MyD88), which is a direct target of miR-489, to promote cardiac hypertrophy [36]. In cardiomyocytes treated with phenylephrine (PE), the expression level of lncRNA-ROR (or lincRNA-ST8SIA3) is significantly increased, knock-down of which reduces the hypertrophic response [69]. It is revealed that lncRNA-ROR functions as miR-133 sponge, and overexpression of miR-133 can reverse the pro-hypertrophic effect of lncRNA-ROR. In addition, lncRNA myocardial infarction associated transcript (MIAT) has been demonstrated to be a pro-hypertrophic regulator in cardiac hypertrophy induced by Ang II via acting as a molecular sponge for miR-150 [70].
Cardiomyocytes autophagy plays an important role in maintaining homeostasis, myocardial cell size, as well as cardiac structure and function. Wang et al. [54] have identified a lncRNA that can regulate autophagy in cardiac myocytes and named it autophagy promoting factor (APF). The results show that miR-188-3p can suppress autophagy-induced cardiomyocyte death or MI by targeting autophagy-related protein 7 (ATG7). APF acts as miR-188-3p sponge to regulate the level of ATG7 and thus plays a role in regulating autophagy and MI [54].
Myocardial cell death is the cytological basis of many cardiovascular diseases, inhibition of which can improve cardiac function. lncRNA plays an important role in ischemia-reperfusion (I/R) injury induced cardiac remodeling by participating in the regulation of myocardial apoptosis. A cardiac apoptosis-related lncRNA called CARL is able to upregulate the level of prohibitin 2 (PHB2) by competitively binding with PHB2 upstream negative regulator miR-539, thereby inhibiting cardiomyocyte apoptosis and MI injury-induced cardiac remodeling [62].
In the past, cell necrosis was thought to be a passive form of cell death. However, recent studies have demonstrated that certain types of necrosis are regulated by signaling pathways such as receptor-interacting serine/threonine-protein kinase (RIPK) 1 and 3. In the process of MI and hydrogen peroxide (H2O2)-induced myocardial necrosis, the levels of miR-103/107 are upregulated, which subsequently suppress the expression of Fas-Associated protein with Death Domain (FADD). FADD can interact with receptor-interacting protein 1 (RIP1) to inhibit the formation of RIP1/RIP3 complex, thereby promoting the development of myocardial necrosis. lncRNA H19 serving as the miR-103/107 sponge can reduce the endogenous miR-103/107 levels, inhibit the cell necrosis and MI size induced by ischemic injury, as well as the myocardial fibrosis and cardiac remodeling, thus improving cardiac function [53]. Additionally, a lncRNA named necrosis-related factor (NRF) has been revealed to participate in the regulation of cardiomyocytes programmed necrosis by directly targeting miR-873 and consequently suppressing the expression of RIPK1/RIPK3, which are miR-873 downstream targets [71].
4.3. lncRNA Regulates the Expression of Adjacent Protein Coding Gene
Viereck et al. [72] have identified a cardiac-specific, lncRNA molecule capable of promoting cardiac hypertrophy from differential lncRNA expression profiles in sham and TAC-operated mouse hearts, and named it Chast (cardiac hypertrophy-associated Transcript). Mechanistically, the hypertrophy promoting factor NFAT can bind to Chast promoter and activate the transcription of Chast. Chast plays an important role in regulating the level of its adjacent gene Plekhm1, which is an autophagy regulatory factor, thus blocking the autophagy of cardiomyocytes and promoting cardiac hypertrophy. Although lncRNAs are less conserved and have tissue-specificity compared to miRNAs, the researchers have observed high expression levels of the Chast human homolog CHAST in hypertrophic heart tissue of patients with aortic stenosis, suggesting that CHAST can be used as a drug target for the treatment of cardiac hypertrophy. It has also been demonstrated that silencing of Chast with antisense oligonucleotide inhibitors can alleviate cardiac hypertrophy induced by coarctation of the aorta and improve cardiac function.
4.4. lncRNA and Protein Complexes Bind to the Gene Promoter Region, Regulating Gene Expression
Doxorubicin (Dox) is one of the most effective broad-spectrum anticancer drugs. However, it can induce cardiotoxicity that promotes cardiomyocytes apoptosis and cardiomyopathy. As described above, lncRNA Mhrt is a heart protective regulator against pathological cardiac remodeling [67]. Recently, researchers have reported that obestatin can attenuate Dox-induced cardiac dysfunction via upregulating Mhrt expression [73]. Mechanistically, Mhrt can positively regulate the expression of Nrf2 (Nuclear factor erythroid 2-related factor 2), a signaling pathway which is involved in preventing cardiac remodeling and heart failure [73,74]. Further, they prove that Mhrt prompts combination of H3 histone and Nrf2 promoter, thereby enhancing the transcription of Nrf2 and increasing Nrf2 protein level.
4.5. lncRNA as miRNA Precursor
As one of the earliest discovered imprinted gene, lncRNA H19 has been shown to play an important role in mammalian embryogenesis and tumorigenesis. The first exon of H19 is able to encode miR-675. A novel study reveals that H19 functions as a negative regulator in pathological cardiac hypertrophy mediated by miR-675 [75]. Overexpression of H19 can reduce the increase of cell size and hypertrophy-relate gene levels induced by PE, whereas knock-down of H19 promotes cardiomyocyte hypertrophy. Moreover, miR-675 overexpression or knock-down reveals its inhibitive effects on myocardial hypertrophy. Then rescue and mutation experiments were performed to demonstrate that miR-675 can mediate the function of H19 to inhibit cardiomyocyte hypertrophy. Finally, Ca2+/calmodulin-dependent protein kinase II δ-isoform (CaMKIIδ) has been identified to be a direct target of H19/miR-675 axis, which is involved in the regulation of cardiac hypertrophy. These findings reveal a new pathway: H19 exerts its cardiomyocyte hypertrophy negative regulatory role as miR-675 precursor, the mature form of which directly inhibits the expression of CaMKIIδ.
4.6. lncRNA Modulates Protein Activity
A recently identified lncRNA, Urothelial carcinoma-associated 1 (UCA1), is reported to be an anti-apoptosis regulator, the levels of which are markedly decreased in cardiac I/R injury rat models and cardiomyocytes treated with H2O2 [76]. Knock-down of UCA1 reduces the viability of cardiomycytes and promotes cell apoptosis. Mechanistically, UCA1 can directly suppress p27 at the protein level, while overexpression of p27 activates the caspase-3 apoptotic pathway. Taken together, the precise molecular mechanism is that downregulation of UCA1 in primary cardiomyocytes exhibits pro-apoptotic function partially through stimulating p27 protein expression, revealing the important role of UCA1 in I/R injury induced cardiac remodeling. Similarly, lncRNA LSINCT5 is involved in the regulation of myocardial apoptosis induced by B-type-natriuretic peptide (BNP) via activating the capase-1/interleukin (IL)-1β signaling pathway [77].
4.7. Others
lncRNA BC088254 was identified from differentially expressed lncRNA microarray data in cardiac hypertrophic rats and was upregulated after TAC. Bioinformatics and coexpression network analyses suggest that lncRNA BC088254 has a relationship with phb2 and can weakly affect the expression of phb2, but the result still needs to be verified [78]. Two lncRNAs named MI-associated transcript 1 (MIRT1) and 2 (MIRT2) are revealed to be significantly increased in MI mice and relevant to multiple genes known to be involved in cardiac remodeling, such as Icam1, Tgfb1, etc. [79]. Furthermore, through gain and loss of function investigations, cardiac fibroblast-enriched lncRNAs n379599, n379519, n384648, n380433 and n410105 are indicated to participate in the TGF-β signaling pathway to regulate the fibrosis related genes expression in ischemic cardiomyopathy [80]. At the late stage of diabetes, hearts undergo hypertrophy and other remodeling events, which lead to heart dysfunction and failure eventually. Zhang et al. [81] report that downregulation of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) can inhibit the apoptosis of cardiomyocytes and improve heart function in diabetic rats. On the contrary, another team proves that MALAT1 is not necessary during pressure overload-induced cardiac remodeling and failure in mice [82]. These findings suggest the specific functions of lncRNAs and the importance of validating the proposed lncRNAs in relevant disease models.
5. circRNAs and Cardiac Remodeling
As early as the 1990s, scientists had discovered the existence of circRNAs [83]. However, circRNAs were once considered to be produced by RNA splicing errors or splicing process by-products due to technique and approach limitations at that time. In 2013, it was revealed that circRNAs function as molecular sponge for miRNAs [8], making circRNAs become a new hotspot in the field of ncRNAs after miRNAs and lncRNAs.
Due to the advanced sequencing technology and data analysis methods, a growing number of circRNAs have been uncovered from human and murine hearts [84,85,86]. The first circRNA profiling study has identified 575 circRNA species from adult murine hearts and showed that several circRNAs are relevant for the cardiovascular disease pathogenesis [85]. The comparative analyses of the circRNA expression from rats (neonatal and adult), mice (sham or after TAC) and humans (failing, non-failing) have detected more than 9000 candidate circRNAs from each species [86]. Recently, a detailed annotation and analysis of genome-wide circRNA expression uncovered 15,318 cardiac circRNAs in the human heart [84]. All of these provide a good basis for studying the role of circRNAs in heart disease.
5.1. circRNA Regulates the Adjacent Gene Expression
Although the functional research on circRNA is still in its infancy, emerging evidence suggests that circRNAs possess important biological functions and have a close relationship with cardiovascular diseases. Genome wide association studies (GWAS) have shown that the single nucleotide polymorphisms (SNPs) in 9p21.3, the neighboring chromosome sequence of INK4/ARF gene, are associated with susceptibility to atherosclerotic vascular disease (ASVD). CircRNA cANRIL is the antisense transcript of INK4/ARF gene. It can regulate the expression of INK4/ARF and increase the risk of ASVD [87].
5.2. circRNA Functions as ceRNA
One important functionary mechanism of circRNAs is as molecular sponges for miRNAs. Wang et al. have identified a heart-related circRNA, HRCR, which functions as molecular sponge of miR-223 and protects the heart from pathological hypertrophy [33]. This is a new signal pathway, which is composed of HRCR/miR-223/ARC, participating in the regulation of cardiac hypertrophy and heart failure. Results show that miR-223 transgenic mice can spontaneously undergo cardiac hypertrophy and heart failure, while miR-223 knockout mice can resist the pathological cardiac hypertrophy induced by isoprenaline (Iso). miR-223 can suppress the expression of the anti-apoptotic protein ARC through binding to its gene 3′ untranslated region. In general, HRCR can adsorb the endogenous miR-223, then inhibit its function and up-regulate the expression of its downstream target ARC, thus playing a role in inhibiting pathological cardiac hypertrophy [33]. In a similar study, circRNA Cdr1as, which is the antisense of cerebellar degeneration-related protein 1 transcript, functions as miR-7a sponge and is proved to be able to promote apoptosis and MI development in heart [88]. Overexpression of miR-7a can protect cardiomyocytes from hypoxia-induced apoptosis by directly inhibiting the apoptotic process related target genes PARP and SP1. Both in vitro and in vivo experiments illustrate that overexpression of Cdr1as aggravates MI injuries by sponging miR-7a and thus upregulating the levels of PARP and SP1, while miR-7a co-overexpression significantly attenuates the changes Cdr1as-induced [88]. Recently, circRNA_000203 has been identified from cardiac fibroblasts to have the pro-fibrosis effect as miR-26-5p sponge, thus blocking the interactions between miR-26-5p and its target fibrosis-associated genes Col1a2 and CTGF [89]. These proved pathways consisting of HRCR/miR-223/ARC, Cdr1as/miR-7a/ SP1 and circRNA_000203/miR-26-5p/ Col1a2, CTGF in cardiac myocytes and fibroblasts provide new therapeutic targets for the treatment of cardiac remodeling related diseases.
5.3. circRNA Binds to Specific Proteins, Altering Their Cellular Localization
Yang et al. [90] have reported that circRNA circ-Foxo3, encoded by the forkhead box transcription factor 3 (Foxo3) and mainly localized in the cytoplasm, is able to interact with and suppress the aging-associated proteins, resulting in increased severity of cardiomyopathy. Circ-Foxo3 is. In the model of cardiomyopathy induced by Dox, overexpressed circ-Foxo3 can interact with the anti-senescent protein ID-1, transcription factor E2F1 and the anti-stress proteins FAK, HIF1α, which are in turn restricted to be translocated to the nucleus to exert their functions, leading to cardiac senescence and apoptosis; whereas silencing endogenous circ-Foxo3 inhibits senescence and relieves cardiomyopathy [90]. Later, they demonstrate that the oral Ganoderma spore oil possesses cardiovascular protective effect through regulating circ-Foxo3 expression [91]. Evidence reveals that Ganoderma treatment can suppress the expression level of circ-Foxo3, reduce left ventricular hypertrophy and improve cardiac function generated by TAC. Despite all of this, the regulatory mechanism of circRNAs and their role in cardiac remodeling remain to be fully discovered.
6. Conclusions and Prospective
Evidence for miRNAs as important disease regulating factors has been well developed. It has given great expectation to using miRNAs as therapeutic drug targets. Thus far, clinical trials have been carried out on several miRNA candidates to treat tumor disease. For instance, inhibitor of miR-122 (Miravirsen) for the treatment of hepatitis C has entered the second phase of clinical trial, and mimics of miR-34a (MRX34) are being tested in a phase I clinical trial in patients with hepatocellular carcinoma or hepatic metastases [92,93]. More and more miRNAs are demonstrated to regulate cardiac remodeling, but there is still no reports regarding miRNAs as drug targets used in the treatment of cardiovascular diseases. Thus, it is undoubtedly very promising for the application of miRNAs in cardiovascular disease diagnosis, treatment and prognosis. Nevertheless, in-depth understanding of the mechanism of miRNAs in the development of cardiovascular diseases will still be the focus of future research. Recently, Wang et al. [94] have revealed that the excessive reactive oxygen species (ROS) in cardiac myocytes can modified miR-184 to oxidized miR-184, which promotes apoptosis by suppressing the expression of its non-native targets Bcl-xL and Bcl-w and increases the susceptibility of the heart to I/R injury. Indeed, this novel mechanism of ROS in regulating heart disease offers a new research angle for miRNAs.
As new regulatory molecules, the mechanisms of lncRNAs and circRNAs are more complex than that of miRNAs. For instance, they function as molecular scaffolds to regulate gene expression by binding with transcription factors or chromatin remodeling complex proteins, act as mRNA and miRNA endogenous sponges, participate in epigenetic regulation by directly binding to gene promoter region, or regulate mRNA stability of the neighboring genes and so on. Similarly, further in-depth research of lncRNAs and circRNAs in heart diseases, especially the mechanism investigation, is urgently requested. Remarkably, as an important class of small RNA molecules, the function of piRNA is gradually being recognized. Current studies have revealed that piRNA mainly plays a role in germ cells. However, few studies have reported on somatic cells, even the relationship between piRNA and heart disease, which is a new field well worth exploring.
Acknowledgments
This work was supported by the Shandong Provincial Natural Science Foundation of China (ZR2016HB30), Qingdao Postdoctoral Application Research Funded Project (2015143) and China Postdoctoral Science Foundation Funded Project (2016M592132).
Abbreviations
| Adcy6 | Adenylate cyclase 6 |
| ALDH2 | Aldehyde dehydrogenase 2 |
| Aldo | Aldosterone |
| AMPK | AMP-activated protein kinase |
| Ang II | Angiotensin II |
| ANT1 | Adenine nucleotide translocase 1 |
| APF | Autophagy promoting factor |
| A/R | Anoxia/reoxygenation |
| ASVD | Atherosclerotic vascular disease |
| Bcat2 | Branched chain amino acid transaminase 2 |
| BNP | B-type-natriuretic peptide |
| CAL | Coronary artery ligation |
| calcineurin-NFAT | Calcineurin-nuclear factor of activated T cells |
| CaMKIIδ | Ca2+/calmodulin-dependent protein kinase II δ-isoform |
| CARL | Cardiac apoptosis related lncRNA |
| Cdc42 | Cell division cycle 42 |
| Cdr1as | Antisense of cerebellar degeneration-related protein 1 |
| ceRNA | Competing endogenous RNA |
| Chaer | Cardiac-hypertrophy-associated epigenetic regulator |
| Chast | Cardiac hypertrophy-associated Transcript |
| CHRF | Cardiac hypertrophy related lncRNA |
| circRNAs | Circular RNAs |
| CnA | Calcineurin catalytic subunit |
| CTGF | Connective tissue growth factor |
| Dox | Doxorubicin |
| EZH2 | Zeste homolog 2 |
| FADD | Fas-Associated protein with Death Domain |
| Foxo3 | Forkhead box transcription factor 3 |
| Foxp1 | Forkhead box protein P1 |
| GATA4 | GATA Binding Protein 4 |
| GRB2 | Growth factor receptor-bound protein 2 |
| GWAS | Genome wide association studies |
| HADC4 | Histone Deacetylase 4 |
| hiPSC-CMs | Human induced pluripotent stem cell–derived cardiomyocytes |
| H2O2 | Hydrogen peroxide |
| HRCR | Heart-related circRNA |
| IGFR-1 | Insulin-like growth factor receptor 1 |
| IL | Interleukin |
| I/R | Ischemia-reperfusion |
| Iso | Isoprenaline |
| JAK-STAT | Janus Kinase- Signal transducers and activators of transcription |
| Jarid2 | Jumonji, AT rich interactive domain 2 |
| KSR1 | Kinase suppressor of ras 1 |
| lncRNAs | Long non-coding RNAs |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| MAPKs | Mitogen-activated protein kinases |
| MFF | Mitochondrial fission factor |
| MI | Myocardial infarction |
| MIAT | Myocardial infarction associated transcript |
| miRNAs | MicroRNAs |
| MuRF1 | Muscle specific ring finger protein 1 |
| MyD88 | Myeloid differentiation factor88 |
| Myh7 | Myosin heavy chain 7 |
| ncRNAs | Non-coding RNAs |
| Nelf-A | Negative elongation factor complex member A |
| NF-κB | Nuclear factor-κB |
| NRF | Necrosis-related factor |
| PE | Phenylephrine |
| PHB2 | Prohibitin 2 |
| PI3K/Akt | Phosphatidylinositol 3-kinase/Akt |
| PIGF | Placental growth factor |
| Pink1 | PTEN Induced Putative Kinase 1 |
| piRISC | piRNA-induced silencing complex |
| piRNAs | Piwi-interacting RNAs |
| PKC | Protein kinase C |
| POFUT1 | Protein O-fucosyltranferase 1 |
| PPP1R10 | Protein Phosphatase 1 Regulatory Subunit 10 |
| PRC2 | Polycomb repressor complex 2 |
| PTEN | Phosphatase And Tensin Homolog |
| PURB | Purine-rich element binding protein B |
| Rab1a | Ras-related protein Rab-1A |
| RhoA | Ras homolog gene family, member A |
| RIPK | Receptor-interacting serine/threonine-protein kinase |
| ROS | Reactive oxygen species |
| rRNAs | Ribosomal RNAs |
| SEMA4B | Semaphorin 4B |
| siRNAs | Small interfering RNAs |
| Sirt4 | Sirtuin 4 |
| Skp2 | S-Phase Kinase-Associated Protein 2 |
| SMAD7 | SMAD Family Member 7 |
| snoRNAs | Small nucleolar RNA |
| SNPs | Single nucleotide polymorphisms |
| snRNAs | Small nuclear RNAs |
| Spry1 | Sprouty homolog 1 |
| TAC | Transverse aortic constriction |
| Tbx 5 | T-box 5 |
| Tg | Transgenic |
| tRNAs | Transfer RNAs |
| TNF-α | Tumor necrosis factor-α |
| UCA1 | Urothelial carcinoma-associated 1 |
| VEGF | Vascular endothelial growth factors |
| WHSC2 | Wolf-Hirschhorn Syndrome Candidate 2 |
| YAP | Yes-associated protein |
Author Contributions
Jinning Gao wrote the manuscript and designed the figures; Wenhua Xu contributed in modifying the manuscript; Jianxun Wang, Kun Wang and Peifeng Li conceived the idea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 2.Johnsson P., Lipovich L., Grander D., Morris K.V. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim. Biophys. Acta. 2014;1840:1063–1071. doi: 10.1016/j.bbagen.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J., Bi C., Clark B.S., Mady R., Shah P., Kohtz J.D. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes. Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltran M., Puig I., Pena C., Garcia J.M., Alvarez A.B., Pena R., Bonilla F., de Herreros A.G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes. Dev. 2008;22:756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willingham A.T., Orth A.P., Batalov S., Peters E.C., Wen B.G., Aza-Blanc P., Hogenesch J.B., Schultz P.G. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 7.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 8.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microrna sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 9.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 10.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. CircRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P., Kang J.Y., Gou L.T., Wang J., Xue Y., Skogerboe G., Dai P., Huang D.W., Chen R., Fu X.D., et al. MIWI and piRNA-mediated cleavage of messenger RNAs in mouse testes. Cell Res. 2015;25:193–207. doi: 10.1038/cr.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Londin E., Loher P., Telonis A.G., Quann K., Clark P., Jing Y., Hatzimichael E., Kirino Y., Honda S., Lally M. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc. Natl. Acad. Sci. USA. 2015;112:E1106–E1115. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Q., Wang Y., Hao Y., Juan L., Teng M., Zhang X., Li M., Wang G., Liu Y. MiR2disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–D104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small E.M., Olson E.N. Pervasive roles of micrornas in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thum T., Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ. Res. 2015;116:751–762. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 16.Van Rooij E., Sutherland L.B., Qi X., Richardson J.A., Hill J., Olson E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 17.Care A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., Bang M.L., Segnalini P., Gu Y., Dalton N.D., et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 18.Van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X., Sun F., Luo S., Zhao W., Yang T., Zhang G., Gao M., Lu R., Shu Y., Mu W., et al. Let-7a is an antihypertrophic regulator in the heart via targeting calmodulin. Int. J. Biol. Sci. 2017;13:22–31. doi: 10.7150/ijbs.16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diniz G.P., Lino C.A., Moreno C.R., Senger N., Barreto-Chaves M.L. MicroRNA-1 overexpression blunts cardiomyocyte hypertrophy elicited by thyroid hormone. J. Cell. Physiol. 2017 doi: 10.1002/jcp.25781. [DOI] [PubMed] [Google Scholar]
- 21.Wang K., Long B., Zhou J., Li P.F. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J. Biol. Chem. 2010;285:11903–11912. doi: 10.1074/jbc.M109.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Zhai G., Ji Y., Jing H. MicroRNA-10a targets T-box 5 to inhibit the development of cardiac hypertrophy. Int. Heart J. 2017;58:100–106. doi: 10.1536/ihj.16-020. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z.P., Chen J., Seok H.Y., Zhang Z., Kataoka M., Hu X., Wang D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013;112:1234–1243. doi: 10.1161/CIRCRESAHA.112.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurha P., Abreu-Goodger C., Wang T., Ramirez M.O., Drumond A.L., van Dongen S., Chen Y., Bartonicek N., Enright A.J., Lee B., et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation. 2012;125:2751–2761. doi: 10.1161/CIRCULATIONAHA.111.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Z., Murtaza I., Wang K., Jiao J., Gao J., Li P.F. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Wang Z., Xiao W. microRNA-26a protects against cardiac hypertrophy via inhibiting GATA4 in rat model and cultured cardiomyocytes. Mol. Med. Rep. 2016;14:2860–2866. doi: 10.3892/mmr.2016.5574. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo B.C., Gao X.M., Winbanks C.E., Boey E.J., Tham Y.K., Kiriazis H., Gregorevic P., Obad S., Kauppinen S., Du X.J., et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. USA. 2012;109:17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L., Yuan M., Zhou R., Bai Q., Zhang W., Zhang M., Huang Y., Shi L. MicroRNA-101 inhibits rat cardiac hypertrophy by targeting Rab1a. J. Cardiovasc. Pharmacol. 2015;65:357–363. doi: 10.1097/FJC.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 29.Seok H.Y., Chen J., Kataoka M., Huang Z.P., Ding J., Yan J., Hu X., Wang D.Z. Loss of microRNA-155 protects the heart from pathological cardiac hypertrophy. Circ. Res. 2014;114:1585–1595. doi: 10.1161/CIRCRESAHA.114.303784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N., Hwangbo C., Jaba I.M., Zhang J., Papangeli I., Han J., Mikush N., Larrivee B., Eichmann A., Chun H.J., et al. miR-182 modulates myocardial hypertrophic response induced by angiogenesis in heart. Sci. Rep. 2016;6 doi: 10.1038/srep21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Del Re D.P., Nakano N., Sciarretta S., Zhai P., Park J., Sayed D., Shirakabe A., Matsushima S., Park Y., et al. miR-206 mediates YAP-induced cardiac hypertrophy and survival. Circ. Res. 2015;117:891–904. doi: 10.1161/CIRCRESAHA.115.306624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ucar A., Gupta S.K., Fiedler J., Erikci E., Kardasinski M., Batkai S., Dangwal S., Kumarswamy R., Bang C., Holzmann A., et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012;3 doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K., Long B., Liu F., Wang J.X., Liu C.Y., Zhao B., Zhou L.Y., Sun T., Wang M., Yu T., et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016 doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 34.Wu H., Wang Y., Wang X., Li R., Yin D. MicroRNA-365 accelerates cardiac hypertrophy by inhibiting autophagy via the modulation of Skp2 expression. Biochem. Biophys. Res. Commun. 2017;484:304–310. doi: 10.1016/j.bbrc.2017.01.108. [DOI] [PubMed] [Google Scholar]
- 35.Ganesan J., Ramanujam D., Sassi Y., Ahles A., Jentzsch C., Werfel S., Leierseder S., Loyer X., Giacca M., Zentilin L., et al. miR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127:2097–2106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 36.Wang K., Liu F., Zhou L.Y., Long B., Yuan S.M., Wang Y., Liu C.Y., Sun T., Zhang X.J., Li P.F. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 2014;114:1377–1388. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y., Zhang X., Fan S., Cui G., Shen Z. MicroRNA-497 inhibits cardiac hypertrophy by targeting Sirt4. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0168078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Li N., Long B., Fan Y.Y., Liu C.Y., Zhou Q.Y., Murtaza I., Wang K., Li P.F. Cardiac hypertrophy is negatively regulated by miR-541. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakikes I., Chaanine A.H., Kang S., Mukete B.N., Jeong D., Zhang S., Hajjar R.J., Lebeche D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenzen J.M., Schauerte C., Hubner A., Kolling M., Martino F., Scherf K., Batkai S., Zimmer K., Foinquinos A., Kaucsar T., et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 2015;36:2184–2196. doi: 10.1093/eurheartj/ehv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., et al. MicroRNA-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 42.Hong Y., Cao H., Wang Q., Ye J., Sui L., Feng J., Cai X., Song H., Zhang X., Chen X. miR-22 may suppress fibrogenesis by targeting TGFβR I in cardiac fibroblasts. Cell. Physiol. Biochem. 2016;40:1345–1353. doi: 10.1159/000453187. [DOI] [PubMed] [Google Scholar]
- 43.Duisters R.F., Tijsen A.J., Schroen B., Leenders J.J., Lentink V., van der Made I., Herias V., van Leeuwen R.E., Schellings M.W., Barenbrug P., et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Qi Y., Du J.Q., Zhang D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets. 2014;18:1355–1365. doi: 10.1517/14728222.2014.961424. [DOI] [PubMed] [Google Scholar]
- 45.Pan Z., Sun X., Shan H., Wang N., Wang J., Ren J., Feng S., Xie L., Lu C., Yuan Y., et al. miR-101 Inhibited Post-Infarct Cardiac Fibrosis and Improved Left Ventricular Compliance via FOS/TGFβ1 Pathway. Circulation. 2012;126:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 46.Bernardo B.C., Nguyen S.S., Gao X.M., Tham Y.K., Ooi J.Y., Patterson N.L., Kiriazis H., Su Y., Thomas C.J., Lin R.C., et al. Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci. Rep. 2016;6 doi: 10.1038/srep22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh G.B., Raut S.K., Khanna S., Kumar A., Sharma S., Prasad R., Khullar M. MicroRNA-200c modulates DUSP-1 expression in diabetes-induced cardiac hypertrophy. Mol. Cell. Biochem. 2017;424:1–11. doi: 10.1007/s11010-016-2838-3. [DOI] [PubMed] [Google Scholar]
- 48.Tao L., Bei Y., Chen P., Lei Z., Fu S., Zhang H., Xu J., Che L., Chen X., Sluijter J.P., et al. Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics. 2016;6:2068–2083. doi: 10.7150/thno.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., Donath S., Li Y., Qin D., Prabhakar B.S., Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6 doi: 10.1371/annotation/4050116d-8daa-4b5a-99e9-34cdd13f6a26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K., An T., Zhou L.Y., Liu C.Y., Zhang X.J., Feng C., Li P.F. E2F1-regulated miR-30b suppresses Cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ. 2015;22:743–754. doi: 10.1038/cdd.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan F., Sun A., Zhao H., Liu X., Zhang W., Jin X., Wang C., Ma X., Shen C., Zou Y., et al. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr. Pharm. Des. 2013;19:4865–4873. doi: 10.2174/13816128113199990325. [DOI] [PubMed] [Google Scholar]
- 52.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Treguer K., Carmona G., Bonauer A., et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 53.Wang J.X., Zhang X.J., Li Q., Wang K., Wang Y., Jiao J.Q., Feng C., Teng S., Zhou L.Y., Gong Y., et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting fadd. Circ. Res. 2015;117:352–363. doi: 10.1161/CIRCRESAHA.117.305781. [DOI] [PubMed] [Google Scholar]
- 54.Wang K., Liu C.Y., Zhou L.Y., Wang J.X., Wang M., Zhao B., Zhao W.K., Xu S.J., Fan L.H., Zhang X.J., et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat. Commun. 2015;6 doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 55.Wang K., Zhang D.L., Long B., An T., Zhang J., Zhou L.Y., Liu C.Y., Li P.F. NfAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K., Liu C.Y., Zhang X.J., Feng C., Zhou L.Y., Zhao Y., Li P.F. miR-361-regulated prohibitin inhibits mitochondrial fission and apoptosis and protects heart from ischemia injury. Cell Death Differ. 2015;22:1058–1068. doi: 10.1038/cdd.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang J., Song X.W., Tian J., Chen H.Y., Li D.F., Wang J.F., Ren A.J., Yuan W.J., Lin L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17:410–423. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang K., Zhou L.Y., Wang J.X., Wang Y., Sun T., Zhao B., Yang Y.J., An T., Long B., Li N., et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat. Commun. 2015;6 doi: 10.1038/ncomms8619. [DOI] [PubMed] [Google Scholar]
- 59.Wang K., Long B., Jiao J.Q., Wang J.X., Liu J.P., Li Q., Li P.F. miR-484 regulates mitochondrial network through targeting Fis1. Nat. Commun. 2012;3 doi: 10.1038/ncomms1770. [DOI] [PubMed] [Google Scholar]
- 60.Wang J.X., Jiao J.Q., Li Q., Long B., Wang K., Liu J.P., Li Y.R., Li P.F. mR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 61.Wang J.X., Zhang X.J., Feng C., Sun T., Wang K., Wang Y., Zhou L.Y., Li P.F. MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang K., Long B., Zhou L.Y., Liu F., Zhou Q.Y., Liu C.Y., Fan Y.Y., Li P.F. Carl lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat. Commun. 2014;5 doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 63.Long B., Wang K., Li N., Murtaza I., Xiao J.Y., Fan Y.Y., Liu C.Y., Li W.H., Cheng Z., Li P. miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic. Biol. Med. 2013;65:371–379. doi: 10.1016/j.freeradbiomed.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang K., Liu F., Zhou L.Y., Ding S.L., Long B., Liu C.Y., Sun T., Fan Y.Y., Sun L., Li P.F. miR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang K., Long B., Li N., Li L., Liu C.Y., Dong Y.H., Gao J.N., Zhou L.Y., Wang C.Q., Li P.F. MicroRNA-2861 regulates programmed necrosis in cardiomyocyte by impairing adenine nucleotide translocase 1 expression. Free Radic. Biol. Med. 2016;91:58–67. doi: 10.1016/j.freeradbiomed.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 66.Encode Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han P., Li W., Lin C.H., Yang J., Shang C., Nurnberg S.T., Jin K.K., Xu W., Lin C.Y., Lin C.J., et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z., Zhang X.J., Ji Y.X., Zhang P., Deng K.Q., Gong J., Ren S., Wang X., Chen I., Wang H., et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 2016;22:1131–1139. doi: 10.1038/nm.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang F., Zhou X., Huang J. Long non-coding RNA-ROR mediates the reprogramming in cardiac hypertrophy. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0152767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X.H., Yuan Y.X., Rao S.L., Wang P. LncRNA miat enhances cardiac hypertrophy partly through sponging miR-150. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3653–3660. [PubMed] [Google Scholar]
- 71.Wang K., Liu F., Liu C.Y., An T., Zhang J., Zhou L.Y., Wang M., Dong Y.H., Li N., Gao J.N., et al. The long noncoding RNA NFR regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23:1394–1405. doi: 10.1038/cdd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viereck J., Kumarswamy R., Foinquinos A., Xiao K., Avramopoulos P., Kunz M., Dittrich M., Maetzig T., Zimmer K., Remke J., et al. Long noncoding RNA chast promotes cardiac remodeling. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf1475. [DOI] [PubMed] [Google Scholar]
- 73.Li H.Q., Wu Y.B., Yin C.S., Chen L., Zhang Q., Hu L.Q. Obestatin attenuated doxorubicin-induced cardiomyopathy via enhancing long noncoding Mhrt RNA expression. Biomed. Pharmacother. 2016;81:474–481. doi: 10.1016/j.biopha.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Zhou S., Sun W., Zhang Z., Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu L., An X., Li Z., Song Y., Li L., Zuo S., Liu N., Yang G., Wang H., Cheng X., et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc. Res. 2016;111:56–65. doi: 10.1093/cvr/cvw078. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Zhou D., Li G., Ming X., Tu Y., Tian J., Lu H., Yu B. Long non coding RNA-UCA1 contributes to cardiomyocyte apoptosis by suppression of p27 expression. Cell. Physiol. Biochem. 2015;35:1986–1998. doi: 10.1159/000374006. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X., Sha M., Yao Y., Da J., Jing D. Increased B-type-natriuretic peptide promotes myocardial cell apoptosis via the B-type-natriuretic peptide/long non-coding RNA LSINCT5/caspase-1/interleukin 1β signaling pathway. Mol. Med. Report. 2015;12:6761–6767. doi: 10.3892/mmr.2015.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X., Zhang L., Liang J. Unraveling the expression profiles of long noncoding RNAs in rat cardiac hypertrophy and functions of lncRNA BC088254 in cardiac hypertrophy induced by transverse aortic constriction. Cardiology. 2016;134:84–98. doi: 10.1159/000443370. [DOI] [PubMed] [Google Scholar]
- 79.Zangrando J., Zhang L., Vausort M., Maskali F., Marie P.Y., Wagner D.R., Devaux Y. Identification of candidate long non-coding RNAs in response to myocardial infarction. BMC Genom. 2014;15:460. doi: 10.1186/1471-2164-15-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang Z.P., Ding Y., Chen J., Wu G., Kataoka M., Hu Y., Yang J.H., Liu J., Drakos S.G., Selzman C.H., et al. Long non-coding RNAs link extracellular matrix gene expression to ischemic cardiomyopathy. Cardiovasc. Res. 2016;112:543–554. doi: 10.1093/cvr/cvw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang M., Gu H., Xu W., Zhou X. Down-regulation of lncRNA mMALAT1 reduces cardiomyocyte apoptosis and improves left ventricular function in diabetic rats. Int. J. Cardiol. 2016;203:214–216. doi: 10.1016/j.ijcard.2015.10.136. [DOI] [PubMed] [Google Scholar]
- 82.Peters T., Hermans-Beijnsberger S., Beqqali A., Bitsch N., Nakagawa S., Prasanth K.V., de Windt L.J., van Oort R.J., Heymans S., Schroen B. Long non-coding RNA Malat-1 is dispensable during pressure overload-induced cardiac remodeling and failure in mice. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0150236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Capel B., Swain A., Nicolis S., Hacker A., Walter M., Koopman P., Goodfellow P., Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 84.Tan W.L., Lim B.T., Anene-Nzelu C.G., Ackers-Johnson M., Dashi A., See K., Tiang Z., Lee D.P., Chua W.W., Luu T.D., et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 2017;113:298–309. doi: 10.1093/cvr/cvw250. [DOI] [PubMed] [Google Scholar]
- 85.Jakobi T., Czaja-Hasse L.F., Reinhardt R., Dieterich C. Profiling and validation of the circular RNA repertoire in adult murine hearts. Genom. Proteom. Bioinform. 2016;14:216–223. doi: 10.1016/j.gpb.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werfel S., Nothjunge S., Schwarzmayr T., Strom T.M., Meitinger T., Engelhardt S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016;98:103–107. doi: 10.1016/j.yjmcc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Burd C.E., Jeck W.R., Liu Y., Sanoff H.K., Wang Z., Sharpless N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geng H.H., Li R., Su Y.M., Xiao J., Pan M., Cai X.X., Ji X.P. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang C.M., Zhang M., Huang L., Hu Z.Q., Zhu J.N., Xiao Z., Zhang Z., Lin Q.X., Zheng X.L., Yang M., et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 2017;7 doi: 10.1038/srep40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du W.W., Yang W., Chen Y., Wu Z.K., Foster F.S., Yang Z., Li X., Yang B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2016 doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 91.Xie Y.Z., Yang F., Tan W., Li X., Jiao C., Huang R., Yang B.B. The anti-cancer components of ganoderma lucidum possesses cardiovascular protective effect by regulating circular RNA expression. Oncoscience. 2016;3:203–207. doi: 10.18632/oncoscience.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs. 2016:1–9. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y., et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 94.Wang J.X., Gao J., Ding S.L., Wang K., Jiao J.Q., Wang Y., Sun T., Zhou L.Y., Long B., Zhang X.J., et al. Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol. Cell. 2015;59:50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]