Abstract
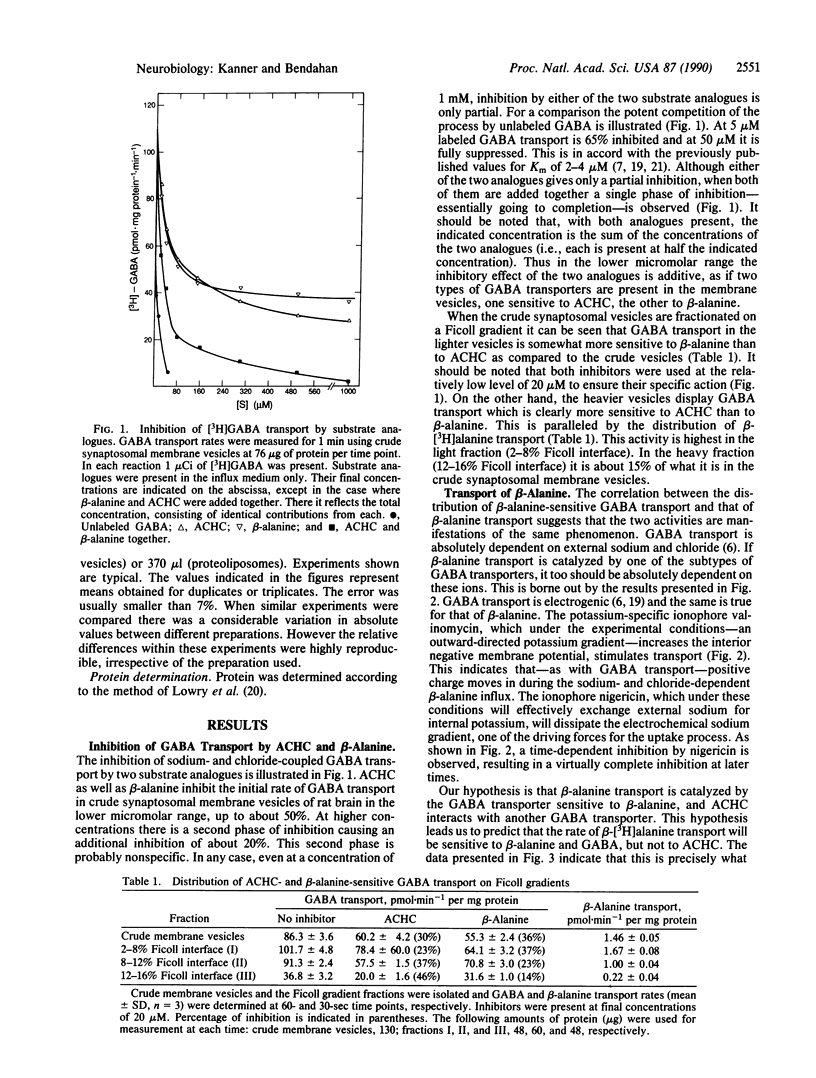
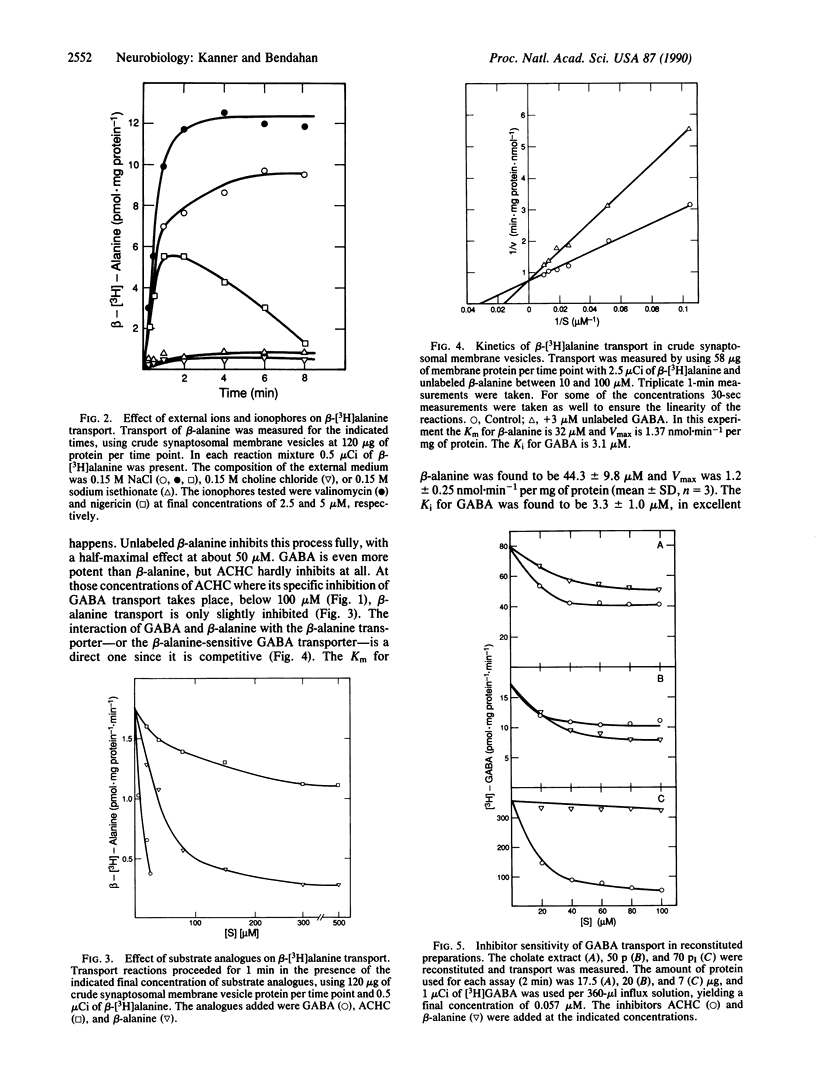
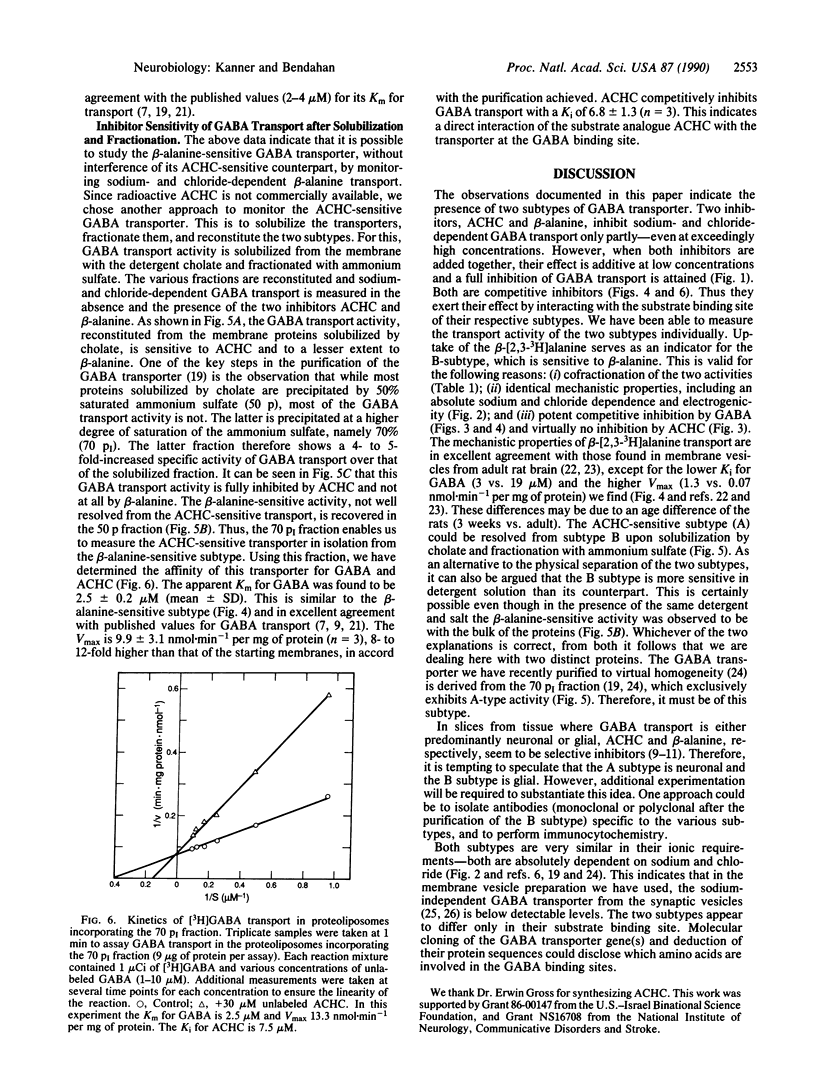
Electrogenic sodium- and chloride-dependent gamma-aminobutyric acid (GABA) transport in crude synaptosomal membrane vesicles is partly inhibited by saturating levels of either of the substrate analogues cis-3-aminocyclohexanecarboxylic acid (ACHC) or beta-alanine. However, both of them together potently and fully inhibit the process. Transport of beta-alanine, which exhibits an apparent Km of about 44 microM, is also electrogenic and sodium and chloride dependent and competitively inhibited by GABA with a Ki of about 3 microM. This value is very similar to the Km of 2-4 microM found for GABA transport. On the other hand, ACHC does not inhibit beta-alanine transport at all. Upon solubilization of the membrane proteins with cholate and fractionation with ammonium sulfate, a fraction is obtained which upon reconstitution into proteoliposomes exhibits 4- to 10-fold-increased GABA transport. This activity is fully inhibited by low concentrations of ACHC and is not sensitive at all to beta-alanine. GABA transport in this preparation exhibits an apparent Km of about 2.5 microM and it is competitively inhibited by ACHC (Ki approximately 7 microM). These data indicate the presence of two GABA transporter subtypes in the membrane vesicles: the A type, sensitive to ACHC, and the B type, sensitive to beta-alanine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agullo L., Jimenez B., Aragón C., Giménez C. Beta-alanine transport in synaptic plasma membrane vesicles from rat brain. Efflux, exchange and stoichiometry. Eur J Biochem. 1986 Sep 15;159(3):611–617. doi: 10.1111/j.1432-1033.1986.tb09929.x. [DOI] [PubMed] [Google Scholar]
- Bevan S., Chiu S. Y., Gray P. T., Ritchie J. M. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., King A. C. Influence of membrane potential on the sodium-dependent uptake of gamma-aminobutyric acid by presynaptic nerve terminals: experimental observations and theoretical considerations. J Membr Biol. 1976 Dec 28;30(2):153–173. doi: 10.1007/BF01869665. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fykse E. M., Fonnum F. Uptake of gamma-aminobutyric acid by a synaptic vesicle fraction isolated from rat brain. J Neurochem. 1988 Apr;50(4):1237–1242. doi: 10.1111/j.1471-4159.1988.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Hell J. W., Maycox P. R., Stadler H., Jahn R. Uptake of GABA by rat brain synaptic vesicles isolated by a new procedure. EMBO J. 1988 Oct;7(10):3023–3029. doi: 10.1002/j.1460-2075.1988.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Bloom F. E. Studies of the uptake of 3 H-gaba and ( 3 H)glycine in slices and homogenates of rat brain and spinal cord by electron microscopic autoradiography. Brain Res. 1972 Jun 8;41(1):131–143. doi: 10.1016/0006-8993(72)90621-x. [DOI] [PubMed] [Google Scholar]
- Johnston T. P., McCaleb G. S., Clayton S. D., Frye J. L., Krauth C. A., Montgomery J. A. Synthesis of analogues of N-(2-chloroethyl)-N'-(trans-4-methylcyclohexyl)-N-nitrosourea for evaluation as anticancer agents. J Med Chem. 1977 Feb;20(2):279–290. doi: 10.1021/jm00212a019. [DOI] [PubMed] [Google Scholar]
- Jones G. P., Neal M. J. Selective inhibition of neuronal GABA uptake by cis-1,3-aminocyclohexane carboxylic acid. Nature. 1976 Nov 18;264(5583):281–284. doi: 10.1038/264281a0. [DOI] [PubMed] [Google Scholar]
- Kanner B. I. Active transport of gamma-aminobutyric acid by membrane vesicles isolated from rat brain. Biochemistry. 1978 Apr 4;17(7):1207–1211. doi: 10.1021/bi00600a011. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Bendahan A., Radian R. Efflux and exchange of gamma-aminobutyric acid and nipecotic acid catalysed by synaptic plasma membrane vesicles isolated from immature rat brain. Biochim Biophys Acta. 1983 May 26;731(1):54–62. doi: 10.1016/0005-2736(83)90397-8. [DOI] [PubMed] [Google Scholar]
- Kanner B. I. Bioenergetics of neurotransmitter transport. Biochim Biophys Acta. 1983 Dec 30;726(4):293–316. doi: 10.1016/0304-4173(83)90013-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I. Ion-coupled neurotransmitter transport. Curr Opin Cell Biol. 1989 Aug;1(4):735–738. doi: 10.1016/0955-0674(89)90042-2. [DOI] [PubMed] [Google Scholar]
- Kanner B. I. Modulation of neurotransmitter transport by the activity of the action potential sodium ion channel in membrane vesicles from rat brain. Biochemistry. 1980 Feb 19;19(4):692–697. doi: 10.1021/bi00545a013. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Keynan S., Kanner B. I. gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry. 1988 Jan 12;27(1):12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J. Neurotransmitter uptake: a tool in identifying neurotransmitter-specific pathways. Life Sci. 1973 Dec 16;13(12):1623–1634. doi: 10.1016/0024-3205(73)90110-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neal M. J., Bowery N. G. Cis-3-aminocyclohexanecarboxylic acid: a substrate for the neuronal GABA transport system. Brain Res. 1977 Dec 9;138(1):169–174. doi: 10.1016/0006-8993(77)90793-4. [DOI] [PubMed] [Google Scholar]
- Radian R., Bendahan A., Kanner B. I. Purification and identification of the functional sodium- and chloride-coupled gamma-aminobutyric acid transport glycoprotein from rat brain. J Biol Chem. 1986 Nov 25;261(33):15437–15441. [PubMed] [Google Scholar]
- Radian R., Kanner B. I. Reconstitution and purification of the sodium- and chloride-coupled gamma-aminobutyric acid transporter from rat brain. J Biol Chem. 1985 Sep 25;260(21):11859–11865. [PubMed] [Google Scholar]
- Radian R., Kanner B. I. Stoichiometry of sodium- and chloride-coupled gamma-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1983 Mar 1;22(5):1236–1241. doi: 10.1021/bi00274a038. [DOI] [PubMed] [Google Scholar]
- Schon F., Kelly J. S. Selective uptake of (3H)beta-alanine by glia: association with glial uptake system for GABA. Brain Res. 1975 Mar 21;86(2):243–257. doi: 10.1016/0006-8993(75)90700-3. [DOI] [PubMed] [Google Scholar]
- Zafra F., Aragon M. C., Valdivieso F., Gimenez C. beta-Alanine transport into plasma membrane vesicles derived from rat brain synaptosomes. Neurochem Res. 1984 May;9(5):695–707. doi: 10.1007/BF00964516. [DOI] [PubMed] [Google Scholar]



