Abstract
Oxidative stress occurs when cells are exposed to elevated levels of reactive oxygen species that can damage biological molecules. One bacterial response to oxidative stress involves disulfide bond formation either between protein thiols or between protein thiols and low-molecular-weight (LMW) thiols. Bacillithiol was recently identified as a major low-molecular-weight thiol in Bacillus subtilis and related Firmicutes. Four genes (bshA, bshB1, bshB2, and bshC) are involved in bacillithiol biosynthesis. The bshA and bshB1 genes are part of a seven-gene operon (ypjD), which includes the essential gene cca, encoding CCA-tRNA nucleotidyltransferase. The inclusion of cca in the operon containing bacillithiol biosynthetic genes suggests that the integrity of the 3′ terminus of tRNAs may also be important in oxidative stress. The addition of the 3′ terminal CCA sequence by CCA-tRNA nucleotidyltransferase to give rise to a mature tRNA and functional molecules ready for aminoacylation plays an essential role during translation and expression of the genetic code. Any defects in these processes, such as the accumulation of shorter and defective tRNAs under oxidative stress, might exert a deleterious effect on cells. This review summarizes the physiological link between tRNACys regulation and oxidative stress in Bacillus.
Keywords: bacillithiol, Bacillus, oxidative stress, tRNA
1. Introduction
Redox reactions are essential to the metabolic economy of living systems. However, when cells are exposed to high concentrations of reactive oxygen species, numerous biochemical and physiological pathways may be affected, thus disrupting cellular homeostasis. For these reasons, organisms have developed strategies to limit the effects of oxidative stress on biological components. One of such strategies involves protein protection through thiol regulation. Disulfide bonds are important in a number of metabolic pathways, one of which is the interaction between proteins and low-molecular-weight (LMW) thiols during oxidative stress. Diverse prokaryotic and eukaryotic organisms have the ability to synthesize glutathione, bacillithiol, or mycothiol as part of their stress responses. These LMW thiols play an essential role in the maintenance of a reducing environment in the cytosol.
Another important cellular strategy involved in stress responses occurs at the level of tRNA metabolism, where tRNAs charged with their specific amino acids are essential for the biosynthesis of stress-related proteins. In addition, there is evidence to suggest that, when cells do not have the capacity to effectively regulate the levels of reactive oxygen species, pathways are induced that lead to tRNA degradation as a mechanism for regulating the expression of particular genes. Although the role of tRNAs in cellular metabolism is extensive, in this minireview, we will focus on the physiological link between bacillithiol biosynthesis and tRNACys processing and regulation in Bacillus subtilis. Both molecules have molecular connections through the expression of genes from one operon (ypjD), which contains genes for both bacillithiol biosynthesis and tRNA maturation.
2. Bacillus under Oxidative Stress
Bacillus is a genus of Gram-positive bacteria, which are members of the family Bacillaceae. These bacteria are endospore formers and are obligate aerobes or facultative anaerobes [1,2]. Bacilli survive and disperse in the presence of other microorganisms in their natural environments. In the various environments in which they are found, Bacilli are subject to multiple sources of toxic oxidizing agents that induce internal production of reactive oxygen species [3]. Those conditions promote decisions between survival and cell death when damage to biomolecules is severe.
Under stress conditions, initiation signals result in the activation of the master transcription regulator, sigma factor σB. Activated σB regulates the transcription of approximately 200 genes in response to multiple physical, chemical, and other environmental stress stimuli [4]. Bacillus cells contain robust mechanisms to respond to and mitigate environmental stress. These responses include the neutralization of stress stimuli by disulfide bonds, which play a major role in stabilizing protein structures or are part of their catalytic site. In addition, many proteins possess cysteine residues which function as redox switches, e.g., ribonucleotide reductase, RNase A, RNase T, methionine sulfoxide reductase, alkylhydroperoxide reductase, arsenate reductase, and the global repressor of the peroxide regulon PerR [5,6,7,8].
One important component of stress responses in Bacillus is the protection of exposed cysteine residues from fluctuations in the redox environment by bacillithiol (BSH), the α-anomeric glycoside of l-cysteinyl-d-glucosamine with l-malic acid. BSH is found in a wide range of bacteria including many Firmicutes [9].
3. Bacillithiol Biosynthesis and Function
Three loci are involved in bacillithiol biosynthesis (ypjD, yoyC, and ylbQ), all of which are expressed from canonical σA-dependent promoters. BSH biosynthesis (Figure 1) begins with the reaction of UDP-N-acetylglucosamine (UDP-GlcNAc) and l-malate to produce α-d-glucosaminyl l-malate (GlcNAc-Mal). This step is catalyzed by the enzyme BshA (N-acetyl-α-d-glucosaminyl l-malate synthase). The second step is deacetylation of GlcNAc-Mal to produce GlcN-Mal. This step is catalyzed by either of two redundant enzymes, BshB1 or BshB2 (N-acetyl-α-d-glucosaminyl l-malate deacetylase). The last step of BSH biosynthesis is achieved by coupling cysteine to GlcN-Mal, and is catalyzed by BshC (d-glucosaminyl l-malate cysteine ligase) [9,10]. BshA and BshC are essential in bacillithiol biosynthesis, while, in the absence of BshB1, bacillithiol production is reduced by up to 50%, indicative of the reduced activity of BshB2 [9,10].
Figure 1.
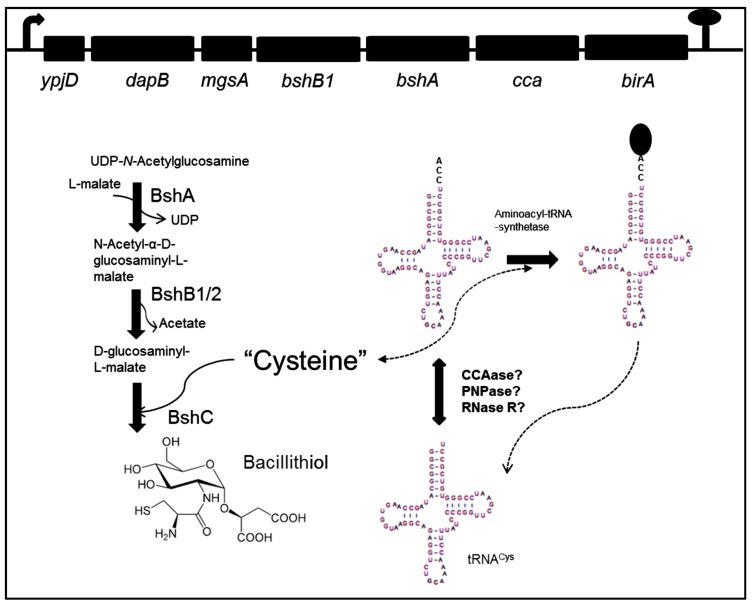
Possible relationship between tRNACys and bacillithiol biosynthesis. Genes involved in bacillithiol biosynthesis are indicated in the operon ypjD; bshB1 (N-acetyl-α-d-glucosaminyl l-malate deacetylase) and bshA (N-acetyl-α-d-glucosaminyl l-malate synthase). The bshB2 (N-acetyl-α-d-glucosaminyl l-malate deacetylase) gene is localized in the operon yoyC, while bshC (d-glucosaminyl l-malate cysteine ligase) is situated in the operon ylbQ. The cca (CCA, ATP (CTP): tRNA nucleotidyltransferase or CCAase) gene involved in tRNA maturation is indicated. A link between bacillithiol biosynthesis and tRNACys maturation or degradation could have physiological relevance for Bacillus subtilis under stress conditions. Thus, the free cysteine pool would be necessary both for the synthesis of bacillithiol and for the aminoacylation of the single tRNACys species in B. subtilis. The genes ypjD (pyrophosphohydrolase), dapB (dihydrodipicolinate reductase), mgsA (methylglyoxal synthase) and birA (biotin–protein ligase) are indicated in the operon. Polynucleotide phosphorylase (PNPase) and 3′-to-5′ processing exoribonuclease (RNase R) are indicated.
The genes bshA and bshB1, whose products are involved in the first two steps of bacillithiol biosynthesis (Figure 1), are encoded in the same operon [9,10]. The operon begins with a putative pyrophosphohydrolase (ypjD), a dihydrodipicolinate reductase (dapB), and methylglyoxal synthase (mgsA). These are followed by the bshB1 and bshA genes. Downstream of bshA are two genes, cca and birA, encoding CCA-tRNA nucleotidyltransferase and biotin–protein ligase, respectively [11,12,13].
Once bacillithiol is synthetized during stress conditions, it is responsible for reducing intra- or intermolecular disulfide bonds in cytosolic proteins. It does this by attacking disulfide bonds and forming diverse BSH-disulfide products. This mechanism maintains a reducing cytoplasmic environment that protects exposed cysteine residues from oxidation and reduces accumulation of proteins with non-functional disulfide bonds [7,14].
Despite the established role of BSH in the maintenance of disulfide homeostasis, there is evidence to suggest that it is not essential for activating oxidative and disulfide stress responses for some stress-related genes in Bacillus subtilis. For instance, Nakano et al. [14] used a 2D gel fluorescence-based thiol-modification assay to identify reversibly oxidized proteins during the induction of disulfide stress in B. subtilis with diamide [diazenedicarboxylic acid bis(N,N-dimethylamide)], a specific oxidant for thiols. Their analysis demonstrated that the protein redox status, including that of the Spx protein, a master regulator of disulfide (thioldepletion) stress [14], remained unchanged in wild-type, bshA, bshB1, and bshB2 mutant strains [6,9]. Spx activity is controlled by a CXXC redox switch and, in its oxidized form, regulates transcription of target promoters, including those of the essential thioredoxin/thioredoxin reductase system encoded by trxA and trxB, and promoters for the operons ypjD, yoyC, and ylbQ encoding genes for bacillithiol synthesis [10]. Possible candidate molecules for the maintenance of protein redox status in the experiments just described are free cysteine and perhaps tRNACys (see below). With regard to a possible role for free cysteine, some redundancy in the function of LMW thiols in B. subtilis is expected [6]. In fact, both BSH and cysteine could regulate the activity of OhrR, a cysteine-based peroxide sensor [15].
Despite the apparent redundancy in disulfide stress protection mechanisms in B. subtilis, BSH null cells do show striking changes compared with the wild type, e.g., reduced efficiency of sporulation, increased sensitivity to high concentrations of NaCl and low pH, and dramatically increased sensitivity to the antibiotic fosfomycin [9]. The broad-spectrum activity of fosfomycin against bacteria is due to its function as a potent covalent inhibitor of MurA, a key enzyme involved in peptidoglycan biosynthesis. MurA catalyzes the transfer of enolpyruvate from phosphoenolpyruvate to uridine diphospho-N-acetylglucosamine. The covalent inhibition of MurA results from a nucleophilic attack by an active-site cysteine thiol at the C-2 position of fosfomycin [16]. One way to inactivate fosfomycin is through fosfomycin-resistance enzymes such as FosA, FosB, and FosX. FosA is a manganese-dependent glutathione transferase identified in various Gram-negative bacteria. FosA catalyzes the reaction between fosfomycin and glutathione (GSH), leading to GSH dependent ring opening at the sterically hindered C-1 carbon of fosfomycin to form an inactive GSH–fosfomycin conjugate. FosB is a divalent-metal-dependent thiol-S-transferase related to FosA that has been identified in many low-G+C Gram-positive bacteria. FosB catalyzes the formation of an inactive complex between BSH and fosfomycin by nucleophilic attack of BSH at the C-1 carbon of fosfomycin [17,18]. FosX is a metal-dependent hydrolase found in Mesorhizobium loti and Listeria monocytogenes, which catalyzes the hydrolysis of the antibiotic [16]. These activities are primarily responsible for conferring fosfomycin resistance on Bacillus subtilis and other Gram-positive bacteria.
4. Role of CCA-tRNA Nucleotidyltransferase
As is shown in Figure 1, cca, the gene encoding CCA, ATP (CTP): tRNA nucleotidyltransferase (CCAase), is a component of the operon that contains bshA and bshB1. The principal function of CCAase is the addition of CCA residues to the 3′ end of tRNAs and tRNA-like transcripts, during maturation or recycling of these molecules. tRNAs are synthesized as precursors that undergo a series of post-transcriptional modifications, such as 5′ and 3′ processing. All mature tRNA molecules possess a functional CCA sequence at the 3′ end, as this sequence is required for amino acid attachment. Thus, CCAase activity is necessary to maintain levels of mature tRNAs ready to be used in protein synthesis and other biological reactions [19,20,21].
Experimental evidence has confirmed that all three loci involved in BSH biosynthesis are induced approximately fivefold during oxidative stress in B. subtilis, under control of the master regulator of disulfide (thioldepletion) stress, Spx [10]. This observation suggests the possibility that CCAase is also over-expressed in similar conditions and its activity could be necessary to maintain mature tRNA levels during stress. However, expression of cca and regulation of CCA addition at the 3′ of tRNA during stress conditions has not received much attention, presumably because of the assumption that damaged tRNA molecules that might be produced under such conditions are degraded efficiently by RNases.
With regard to the latter possibility, there is evidence indicating that CCAase has the ability to distinguish between normal and damaged tRNAs, e.g., tRNAs with poly(A) tails or with CCACCA sequences, for efficient CCA addition at the 3′ end. Such aberrant tRNAs are generally marked for degradation by RNases, as an important mechanism in successful quality control [22,23]. Indeed, the cleavage of tRNAs by specific RNases in response to multiple physical, chemical, and other environmental stresses has been detected in prokaryotes and eukaryotes and raises the possibility that this cleavage of tRNA, principally in the anticodon loop, could be a potentially important causative factor in the protection of cells from these stresses [24]. For instance, cleavage of tRNALys in Escherichia coli by the PrrC nuclease, which is activated during bacteriophage T4 infection, has been reported [25]. Similarly, cleavage of tRNAs by RNase T2 and members of the RNase A family have been detected under stress conditions in yeast and mammalian cells [26,27]. tRNA cleavage during amino acid starvation in various biological systems has also been demonstrated [24]. All these reports indicate that tRNA cleavage is a common phenomenon in biological systems; its significance, regulation, and specific relationship to stress survival are unresolved questions.
5. A Link between Oxidative Stress, Bacillithiol, and tRNACys Maturation?
In Escherichia coli, all tRNA genes encode the 3′ CCA [28]. In B. subtilis, on the other hand, around 30% of tRNAs lack an encoded CCA at the 3′ end (Table 1). CCA must be added post-transcriptionally to those CCA-less tRNAs [29,30].
Table 1.
Distribution of tRNA isotypes with and without an encoded 3′ CCA in B. subtilis.
| Isotype | Ala | Gly | Pro | Thr | Val | Ser | Arg | Leu | Phe | Asn |
| With CCA | 4 | 7 | 3 | 2 | 4 | 5 | 4 | 2 | 2 | 3 |
| Without CCA | 2 | 0 | 0 | 3 | 1 | 0 | 3 | 6 | 1 | 1 |
| Isotype | Lys | Asp | Glu | His | Gln | Ile | Mel | Tyr | Cys | Trp |
| With CCA | 4 | 4 | 1 | 2 | 1 | 3 | 6 | 2 | 0 | 1 |
| Without CCA | 0 | 0 | 5 | 0 | 3 | 0 | 0 | 0 | 1 | 0 |
The presence of cca in the ypjD operon, along with bshA and bshB1 suggests the intriguing possibility that CCAase may be involved in the response of B. subtilis to oxidative stress. Evidence supporting this possibility was recently presented by Cruz Hernández et al. [29]. In that study, B. subtilis was subjected to oxidative stress generated by exposure to mercury, and the integrity of six tRNAs was then examined by electrophoretic mobility on Northern blots, finding that mercury exposure had no effect on the mobility of five of the six tRNAs, i.e., those for alanine, tryptophan, valine, leucine, and threonine. In contrast, northern blots revealed a shorter species of tRNACys that migrated faster than the mature tRNACys.
The smaller tRNACys species observed in conditions of oxidative stress had an electrophoretic mobility identical to that previously reported for tRNACys lacking the CCA end, as assessed using synthetic CCA-less tRNACys as a molecular marker [30]. It must be noted that the purification and sequencing of the smaller tRNACys species were not performed in this study. However, the analysis of some fragments of tRNACys obtained by reverse transcription polymerase chain reaction (RT-PCR) did demonstrate the presence of tRNACys species that had been modified by the addition of poly(A) or heteropolymeric 3′ tails, suggesting that these were immature or damaged species, targeted for degradation [30]. Thus, it seems likely that the smaller tRNA produced by oxidative stress induced by mercury exposure is CCA-less tRNACys. After 4 h of exposure to mercury, the shorter tRNACys species accounted for approximately 10% of total tRNACys (mature tRNACys plus the shorter species).
Cruz Hernández et al. [29] also examined the effects of oxidative stress on the accumulation of the shorter tRNACys species in B. subtilis mutants lacking the ribonucleases polynucleotide phosphorylase (PNPase) and RNase R. They observed a significant increase in the relative amount of the shorter tRNACys species in the pnp mutant and in the pnp rnr double mutant such that, by 6 h following exposure to mercury, the shorter tRNACys species accounted for 70% of the total tRNACys observed. The shorter tRNACys species was not observed in the rnr single mutant, suggesting that PNPase may be involved in either the generation of mature tRNACys via its polymerization activity or in the degradation of the CCA-less tRNACys via phosphorolysis. This suggestion is supported by results obtained from studies in E. coli. Reuven et al. demonstrated the accumulation of shorter species of tRNACys, and other tRNA species, in mutants lacking CCAase, poly(A) polymerase, and PNPase [31].
Of additional interest was the observation by Cruz Hernandez et al. that the relative amount of the shorter tRNACys species decreased after 6 h of oxidative stress [29]. By 10 h post-exposure to mercury, the relative level of the shorter species had decreased to less than 1% of the total in the wild-type strain and to ca. 10% of the total in the pnp mutant strain. The decrease in the relative amount of the putative CCA-less species with time of exposure to mercury can be rationalized via the operation of heavy metal detoxification mechanisms that are activated under conditions of heavy metal-induced oxidative stress. The failure to observe the shorter tRNACys species in the rnr mutant remains unexplained.
How might tRNA maturation or degradation be affected by oxidative stress? The results obtained by Cruz Hernández et al. [29] demonstrate a clear role for PNPase in the maturation, repair, or degradation of tRNACys during stress conditions, a role which has been verified in various species, from bacteria to humans [32,33,34,35]. However, the observation of the putative CCA-less tRNACys species also suggests an important role for CCAase itself in the production of mature tRNACys during oxidative stress. CCAase activity is presumably necessary to produce mature tRNAs that are competent to participate in protein synthesis, but the results of Cruz Hernández et al. also raise the interesting question of whether there is a relationship between cca expression and the expression of the genes involved in BSH synthesis, as proposed in Figure 1. It is also possible that expression of the genes involved in BSH biosynthesis affects the production of cysteine (Figure 1), increasing the activity of cysteine biosynthetic genes, which are controlled by different regulators, including CymR, CysL, and SpX [6,7]. This increase would make cysteine available for BSH synthesis and for the function of the free amino acid as an LMW thiol.
tRNAs are generally considered to be relatively stable molecules in cells because they have a complex secondary structure, contain protective nucleotide sequences at their 3′ ends and because they are associated with components of the translational machinery that reduce the action of RNases [36,37]. Nevertheless, there is evidence that supports global changes in the tRNA pool in different cellular states or under various growth conditions [38,39]. For instance, it has been shown in E. coli that starvation for a single amino acid results in reduced levels of the corresponding aminoacylated tRNA and degradation of this tRNA is accelerated during the first 20 minutes following the initiation of starvation [38,39]. Given these observations, we speculate that, during stress conditions in B. subtilis, cysteine depletion or other effects on components of the translation machinery, such as aminoacyl-tRNA synthetases, may increase uncharged tRNACys accumulation. This situation could lead to an increased rate of degradation of the uncharged tRNACys by various RNases, resulting in the buildup of CCA-less tRNACys species. Thus, under these conditions, increased levels of CCAase may be necessary for CCA repair.
If such mechanisms are operative in B. subtilis, there must be some feature of them that limits their effects to tRNACys, at least among the tRNAs examined by Cruz Hernández et al. [29]. One possible reason for this specificity might involve the requirement for cysteine in the formation of bacillithiol and the possible link between tRNACys and cysteine levels (S-thiolation). Further work will be necessary to explore these hypotheses.
6. Concluding Remarks
Available evidence suggests the interesting possibility that there may be a biologically significant relationship between the maturation or decay of tRNACys and the response of B. subtilis to oxidative stress. This evidence suggests the further possibility that tRNACys may play a role in the organism’s metabolic processes other than its role in translation. For example, tRNACys might function as a store of cysteine for bacillithiol biosynthesis or might otherwise play a role in the protection of cysteine residues from oxidation in various stress-related proteins. The combination of these effects would lead to the rapid readjustment of aminoacylated tRNACys levels, ensuring tRNA quality control to maintain cell integrity. These possibilities await experimental verification.
Acknowledgments
This work was partially financed by the Universidad Autónoma de Querétaro, México (project Fovin-UAQ, 2013, Clave 1299-Nue 3408 to J.C.G.).
Author Contributions
All authors have contributed to the experimental design, and the drafting and revision of the manuscript. Julio Alfonso Cruz Medina, José Humberto Valenzuela Soto, Sergio Pacheco Hernández, and Sergio Romero Gómez were responsible for figure and table design, editing, and preparation. All authors approved the reviewed final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Slepecky R.A., Hemphill H.E. The Prokaryotes. Springer; Berlin/Heidelberg, Germany: 2006. The genus Bacillus—nonmedical; pp. 530–562. [Google Scholar]
- 2.Mols M., Abee T. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 2011;13:1387–1394. doi: 10.1111/j.1462-2920.2011.02433.x. [DOI] [PubMed] [Google Scholar]
- 3.Zuber P. Management of oxidative stress in Bacillus. Annu. Rev. Microbiol. 2009;63:575–597. doi: 10.1146/annurev.micro.091208.073241. [DOI] [PubMed] [Google Scholar]
- 4.Reder A., Höper D., Gerth U., Hecker M. Contributions of Individual σB-Dependent General Stress Genes to Oxidative Stress Resistance of Bacillus subtilis. J. Bacteriol. 2012;194:3601–3610. doi: 10.1128/JB.00528-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbig A.F., Helmann J.D. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 2001;41:849–859. doi: 10.1046/j.1365-2958.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 6.Hochgräfe F., Mostertz J., Albrecht D., Hecker M. Fluorescence thiol modification assay: Oxidatively modified proteins in Bacillus subtilis. Mol. Microbiol. 2005;58:409–425. doi: 10.1111/j.1365-2958.2005.04845.x. [DOI] [PubMed] [Google Scholar]
- 7.Leichert L.I., Scharf C., Hecker M. Global characterization of disulfide stress in Bacillus subtilis. J. Bacteriol. 2003;185:1967–1975. doi: 10.1128/JB.185.6.1967-1975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan M., Welker E., Wedemeyer W.J., Scheraga H.A. Oxidative folding of proteins. Acc. Chem. Res. 2000;33:805–812. doi: 10.1021/ar000063m. [DOI] [PubMed] [Google Scholar]
- 9.Gaballa A., Newton G.L., Antelmann H., Parsonage D., Upton H., Rawat M., Claiborne A., Fahey R.C., Helmann J.D. Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli. Proc. Natl. Acad. Sci. USA. 2010;107:6482–6486. doi: 10.1073/pnas.1000928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaballa A., Antelmann H., Hamilton C.J., Helmann J.D. Regulation of Bacillus subtilis bacillithiol biosynthesis operons by Spx. Microbiology. 2013;159(Pt 10):2025–2035. doi: 10.1099/mic.0.070482-0. [DOI] [PubMed] [Google Scholar]
- 11.Bower S., Perkins J., Yocum R.R., Serror P., Sorokin A., Rahaim P., Howitt C.L., Prasad N., Ehrlich S.D., Pero J. Cloning and characterization of the Bacillus subtilis birA gene encoding a repressor of the biotin operon. J. Bacteriol. 1995;177:2572–2575. doi: 10.1128/jb.177.9.2572-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K., Ehrlich S.D., Albertini A., Amati G., Andersen K.K., Arnaud M., Asai K., Ashikaga S., Aymerich S., Bessieres P., et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raynal L.C., Krisch H.M., Carpousis A.J. The Bacillus subtilis nucleotidyltransferase is a tRNA CCA-Adding enzyme. J. Bacteriol. 1998;180:6276–6282. doi: 10.1128/jb.180.23.6276-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano S., Küster-Schöck E., Grossman A.D., Zuber P. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2003;100:13603–13608. doi: 10.1073/pnas.2235180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.W., Soonsanga S., Helmann J.D. A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. USA. 2007;104:8743–8748. doi: 10.1073/pnas.0702081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts A.A., Sharma S.V., Strankman A.W., Duran S.R., Rawat M., Hamilton C.J. Mechanistic studies of FosB: A divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus. Biochem. J. 2013;451:69–79. doi: 10.1042/BJ20121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao M., Bernat B.A., Wang Z., Armstrong R.N., Helmann J.D. FosB a Cysteine-Dependent Fosfomycin Resistance Protein under the Control of ςW, an Extracytoplasmic-Function ςfactor in Bacillus subtilis. J. Bacteriol. 2001;183:2380–2383. doi: 10.1128/JB.183.7.2380-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigsby R.E., Fillgrove K.L., Beihoffer L.A., Armstrong R.N. Fosfomycin resistance proteins: A nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily. Methods Enzymol. 2005;401:367–379. doi: 10.1016/S0076-6879(05)01023-2. [DOI] [PubMed] [Google Scholar]
- 19.Schürer H., Schiffer S., Marchfelder A., Mörl M. This Is the End: Processing, Editing and Repair at the tRNA 3-Terminus. Biol. Chem. 2001;382:1147. doi: 10.1515/BC.2001.144. [DOI] [PubMed] [Google Scholar]
- 20.Neuenfeldt A., Just A., Betat H., Mörl M. Evolution of tRNA nucleotidyltransferases: A small deletion generated CC-adding enzymes. Proc. Natl. Acad. Sci. USA. 2008;105:7953–7958. doi: 10.1073/pnas.0801971105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mörl M., Marchfelder A. The final cut: The importance of tRNA 3′-processing. EMBO Rep. 2001;2:17–20. doi: 10.1093/embo-reports/kve006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Reimers S., Pandit S., Deutscher M.P. RNA quality control: Degradation of defective transfer RNA. EMBO J. 2002;21:1132–1138. doi: 10.1093/emboj/21.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilusz J.E., Whipple J.M., Phizicky E.M., Sharp P.A. tRNAs marked with CCACCA are targeted for degradation. Science. 2011;334:817–821. doi: 10.1126/science.1213671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson D.M., Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–219. doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Levitz R., Chapman D., Amitsur M., Green R., Snyder L., Kaufmann G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 1990;9:1383–1389. doi: 10.1002/j.1460-2075.1990.tb08253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu H., Feng J., Liu Q., Sun F., Tie Y., Zhu J., Xing R., Sun Z., Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 27.Thompson D.M., Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell Biol. 2009;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Y.M. CCA addition to tRNA: Implications for tRNA quality control. IUBMB Life. 2010;62:251–260. doi: 10.1002/iub.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz Hernandez A., Millan E.S., de Jesus Romero Gomez S., Antonio Cervantes Chavez J., Garcia Martinez R., Pastrana Martinez X., Gomez J.L., Jones G.H., Guillen J.C. Exposure of Bacillus subtilis to mercury induces accumulation of shorter tRNA Cys species. Metallomics. 2013;5:398–403. doi: 10.1039/c3mt20203h. [DOI] [PubMed] [Google Scholar]
- 30.Campos-Guillen J., Arvizu-Gomez J.L., Jones G.H., Olmedo-Alvarez G. Characterization of tRNACys processing in a conditional Bacillus subtilis CCase mutant reveals the participation of RNase R in its quality control. Microbiology. 2010;156(Pt 7):2102–2111. doi: 10.1099/mic.0.034652-0. [DOI] [PubMed] [Google Scholar]
- 31.Reuven N.B., Zhou Z., Deutscher M.P. Functional overlap of tRNA nucleotidyltransferase, poly(A) polymerase I, and polynucleotide phosphorylase. J. Biol. Chem. 1997;272:33255–33259. doi: 10.1074/jbc.272.52.33255. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa H., Kuwano M., Sekiguchi M. Specific Binding of 8-Oxoguanine-Containing RNA to Polynucleotide Phosphorylase Protein. Biochemistry. 2001;40:9977–9982. doi: 10.1021/bi010595q. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa H., Sekiguchi M. Human polynucleotide phosphorylase protein in response to oxidative stress. Biochemistry. 2006;45:6749–6755. doi: 10.1021/bi052585l. [DOI] [PubMed] [Google Scholar]
- 34.Wu J., Li Z. Human polynucleotide phosphorylase reduces oxidative RNA damage and protects HeLa cell against oxidative stress. Biochem. Biophys. Res. Commun. 2008;372:288–292. doi: 10.1016/j.bbrc.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J., Jiang Z., Liu M., Gong X., Wu S., Burns C.M., Li Z. Polynucleotide phosphorylase protects Escherichia coli against oxidative stress. Biochemistry. 2009;48:2012–2020. doi: 10.1021/bi801752p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo Y., Deutscher M.P. The physiological role of RNaseT can be explained by its unusual substrate specificity. J. Biol. Chem. 2002;277:29654–29661. doi: 10.1074/jbc.M204252200. [DOI] [PubMed] [Google Scholar]
- 37.Dutta T., Malhotra A., Deutscher M.P. How a CCA Sequence Protects Mature tRNAs and tRNA Precursors from Action of the Processing Enzyme RNase BN/RNase Z. J. Biol. Chem. 2013;288:30636–30644. doi: 10.1074/jbc.M113.514570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong J., Xiao C., Gu W., Du G., Sun X., He Q., Zhang G. Transfer RNAs Mediate the Rapid Adaptation of Escherichia coli to Oxidative Stress. PLoS Genet. 2015;11:e1005302. doi: 10.1371/journal.pgen.1005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svenningsen S.L., Kongstad M., Stenum T.S., Muñoz-Gómez A.J., Sørensen M.A. Transfer RNA is highly unstable during early aminoacid starvation in Escherichia coli. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw1169. [DOI] [PMC free article] [PubMed] [Google Scholar]