Abstract
The wobble uridine (U34) of transfer RNAs (tRNAs) for two-box codon recognition, i.e., tRNALysUUU, tRNAGluUUC, and tRNAGlnUUG, harbor a sulfur- (thio-) and a methyl-derivative structure at the second and fifth positions of U34, respectively. Both modifications are necessary to construct the proper anticodon loop structure and to enable them to exert their functions in translation. Thio-modification of U34 (s2U34) is found in both cytosolic tRNAs (cy-tRNAs) and mitochondrial tRNAs (mt-tRNAs). Although l-cysteine desulfurase is required in both cases, subsequent sulfur transfer pathways to cy-tRNAs and mt-tRNAs are different due to their distinct intracellular locations. The s2U34 formation in cy-tRNAs involves a sulfur delivery system required for the biosynthesis of iron-sulfur (Fe/S) clusters and certain resultant Fe/S proteins. This review addresses presumed sulfur delivery pathways for the s2U34 formation in distinct intracellular locations, especially that for cy-tRNAs in comparison with that for mt-tRNAs.
Keywords: the wobble uridine (U34), thio-modification of U34 (s2U34), iron-sulfur (Fe/S), cluster, cytosolic tRNA (cy-tRNA), mitochondrial tRNA (mt-tRNA)
1. Introduction
To date, over 100 different modifications have been found in RNA molecules from all domains of life [1] and, in particular, a variety of post-transcriptional modifications found in transfer RNAs (tRNAs) are known to have crucial roles for maintaining their structural stability and decoding accuracy, for effective codon recognition, and to completely contribute to protein translation [2,3,4].
Thio-modification in tRNAs involves the incorporation of sulfur into the carbon ring of purine or pyrimidine that occurs as a direct exchange of oxygen to sulfur [1]. Thio-modification of wobble uridine (U34) in tRNA is found at the second (C2) and/or fourth positions (C4) of the pyrimidine ring (termed s2U and s4U, respectively), and the s2U is often found in all domains of life, whereas the s4U is only found in eubacteria [5]. The mechanism behind the delivery of a sulfur atom to the U34 poses an intriguing problem because such intracellular sulfur delivery and transfer systems remain incompletely understood.
The s2 modification (thio-modification) is found at the U34 of the two-split box codon (lysine, glutamate, and glutamine) tRNAs. As mentioned above, thio-modification of U34 is found in all domains of life, and particularly in eukaryotes, this modification is found not only in cytosolic tRNAs (cy-tRNAs), but also in mitochondrial genome-encoded mitochondrial tRNAs (mt-tRNAs). The final products of the biosynthesized s2U34 have identical structures in tRNAs in both locations; however, the s2U34 formation pathways and participating components appear to be different. In particular, concerning the thio-modification of cy-tRNAs, to date, many proteins have been explored for their involvement in the sulfur delivery to the s2U34 of cy-tRNAs in yeast (Saccharomyces cerevisiae), nematode (Caenorhabditis elegans), and human cells [2]. Nevertheless, overall understanding of this process remains incomplete. Thus, in the present review, we point out remaining questions regarding thio-modification in different subcellular locations in eukaryotic cells, particularly emphasizing the intracellular sulfur transfer pathway to cy-tRNAs in eukaryotes that should be revealed in the future.
An additional topic in relation to the thio-modification of cy-tRNAs included in the present review is “hyper-modification” of U34. The U34 in both cy- and mt- tRNAs possess a methyl-derivative modification at C5 in addition to thio-modification at C2 of the same uridine [6]. This “hyper-modification” of U34 would be an additional important factor to maintain thio-modification of tRNAs. Thus, the relation between these modifications at U34 is also discussed.
2. Cysteine Desulfurase Nfs1 and Thio-Modification of tRNA
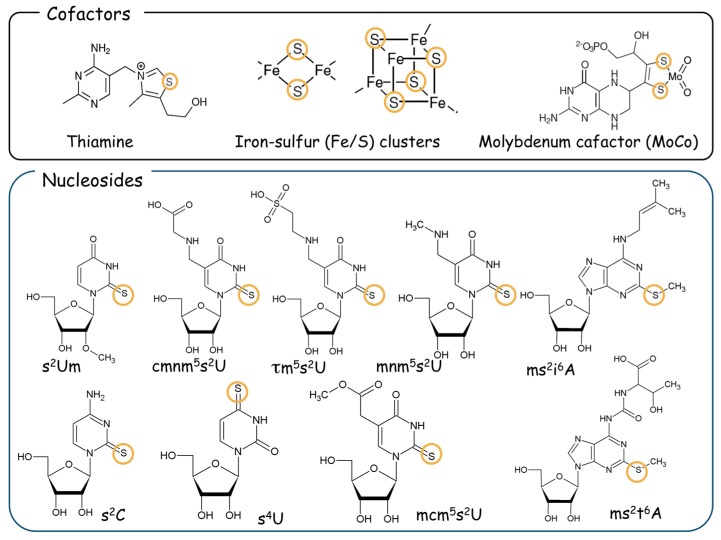
Bacterial l-cysteine desulfurase IscS is known to participate in the formation of s2U34 [7,8]. l-cysteine desulfurase (EC 2.8.1.7), is a pyridoxal-5′-phosphate (PLP)-containing protein. The substrate l-cysteine is initially bound to the PLP to form an intermediate with a Schiff base, and then a highly-conserved active site cysteine residue of this enzyme attacks the γ-sulfhydryl group of the PLP-cysteine intermediate, resulting in the abstraction of γ-SH from the intermediate to form a persulfide on the active-site cysteine residue of the enzyme. Such a persulfide formation in this enzyme is indispensable for biogenesis of the sulfur-bound small biomolecules (Figure 1).
Figure 1.
Sulfur-containing small molecules. Many biocofactors such as thiamine, iron-sulfur (Fe/S) clusters, and molybdenum cofactor (MoCo) contain sulfur atoms in their structure (upper column). Various sulfur-containing nucleosides are also identified. For example, the 2-thiocytidine derivatives are 2-thiocytidine (s2C) found in bacteria and 2-thio-2′-O-methyluridine (s2Um) found in humans. 4-thiouridine (s4U) is found in bacteria. Various types of the hyper-modified uridine, such as 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U), 5-methylaminomethyl-2-thiouridine (mnm5s2U), 5-taurinomethyl-2-thiouridine (τm5s2U), and 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) are found at U34. Various methylthio-adenosine derivatives, such as 2-methylthio- N6-isopentenyladenosine (ms2i6A) and 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A), are also found in both bacteria and eukaryotes. Sulfur atoms incorporated into the molecule in the biosynthetic pathway are shown with circles.
l-Alanine is formed from the substrate l-cysteine, and a sulfur atom removed from the substrate is attached to the active site of the enzyme [5,8]. The physiological functions of this enzyme are to form a protein-bound persulfide as an intermediate and to provide the persulfide-derived sulfur (sulfan sulfur) to form various intracellular small sulfur-containing molecules such as iron-sulfur (Fe/S) clusters, molybdopterin, thiamine thiazole, and nucleotides [9,10]. Likewise for bacteria, eukaryotic l-cysteine desulfurase Nfs1 is essential for the biogenesis of s2U34 in both cy-tRNAs and mt-tRNAs [11,12,13,14] (Figure 2). Eukaryotic Nfs1 is also identified as a member of the iron-sulfur cluster biogenesis (so-called “ISC”) proteins [15] and functions as a sulfur donor for the cluster scaffold in mitochondria [16].
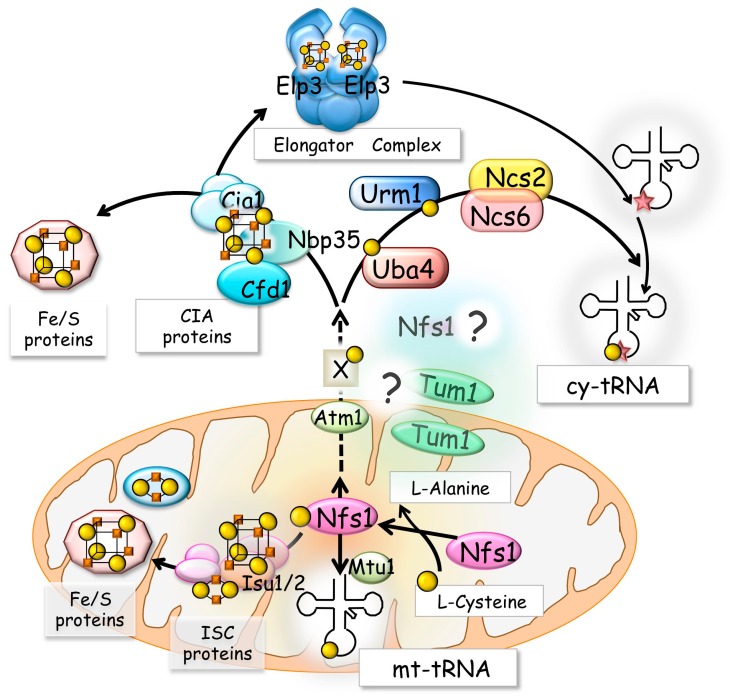
Figure 2.
Involvement of the l-cysteine desulfurase Nfs1 in transfer RNA (tRNA) modification and iron-sulfur (Fe/S) cluster biosynthesis in Saccharomyces cerevisiae. The l-cysteine desulfurase Nfs1, identified as a member of the iron-sulfur cluster biogenesis (so-called “ISC”) proteins, is essential for the biogenesis of s2U34 in both cytosolic transfer RNAs (cy-tRNAs) and mitochondrial transfer RNAs (mt-tRNAs). Mtu1 is a mitochondrial tRNA-specific 2-thiouridylase. Comparing to the case of humans, existence of the cytosolic Nfs1 and the dual location of Tum1 are unclear in yeast (see text in detail). Cytosolic Fe/S protein maturation requires mitochondrial Nfs1 and the cytosolic iron-sulfur cluster assembly (so-called “CIA”) proteins, Cfd1, Nbp35, and Cia1. A putative unknown sulfur carrier to be exported from mitochondria via a membrane-bound protein Atm1 is shown as X. Elongator complex function as a dimer of the complex of Elongator complex 1-6 (ELP1-6) proteins, and function in the C5 modification (marked with a star) of U34. Both a cytosolic ubiquitin-like protein (UBL), ubiquitin-related modifier 1 (Urm1), and its partner sulfurtransferase, ubiquitin-activating enzyme-like protein 4 (Uba4), are required for the formation of s2U34 in cy-tRNAs. Ncs6 and Ncs2 are also involved in the s2U34 formation in cy-tRNAs. The sulfur atoms delivered to the iron-sulfur (Fe/S) clusters and s2U34 in both mitochondrial (mt-) and cytosolic (cy-) tRNAs are shown with filled circles. Fe atoms of Fe/S clusters are shown with filled squares. See text in detail.
Yeast Nfs1 is essential for growth and is mainly located in the mitochondrial matrix [17,18]. When Nfs1 is depleted, thio-modifications of both mt-tRNAs and cy-tRNAs are strongly reduced, although thio-modified mt-tRNAs decrease rather faster than those of cy-tRNAs in yeast cells [11]. The fact that Nfs1 contributes to the formation of s2U34 in both intracellular locations appears consistent with the observation that yeast mitochondrial Nfs1 is also required for initial provision of sulfan sulfur in both mitochondrial and cytosolic Fe/S cluster biogenesis [19].
The mitochondrial Fe/S cluster formation system has been well studied and many proteins have been shown to be involved in this system [15] (Figure 2). Sulfur provided by the Nfs1 function is transferred to the scaffold IscU1/IscU2 hetero complex to form Fe/S clusters, following which it is delivered to various apo-Fe/S proteins after several steps of cluster transfer. On the other hand, a detailed molecular mechanism of the formation of s2U34 in mt-tRNAs remains to be elucidated. The s2U34 in mt-tRNAs is hyper-modified to cmnm5s2U (5-carboxymethylaminomethyl 2-thiouridine) and τm5s2U (5-taurinomethyl2-thiouridine) in yeast and humans, respectively [20,21,22]. Mtu1, a mitochondrial tRNA-specific 2-thiouridylase, is identified to be responsible for the generation of cmnm5s2U34 tRNA in yeast and τm5s2U34 in mammals [14] (Figure 2). Mtu1 is a eukaryotic orthologue of bacterial MnmA [14]. Escherichia coli MnmA is involved in the formation of s2U34 in the E. coli sulfur-relay system for tRNA thio-modification together with the TusA, TusBCD, and TusE proteins and MnmE [23,24]. However, in the yeast or human cases, no bacterial (Tus) orthologues have been identified to date, and mitochondrial ISC proteins except for Nfs1 appear not to be involved in the thio-modification of mt-tRNAs [11]. Therefore, an as-yet-unidentified sulfur transfer system that links mitochondrial Nfs1 and Mtu1 functions might exist in mitochondria.
The formation of s2U34 in yeast cy-tRNAs is also strongly reduced when the expression of Nfs1 is completely repressed [11]. The explanation for the contribution of yeast Nfs1 to the formation of s2U34 in cytosol remains a focus of discussion, since, although Nfs1 is mainly localized in the mitochondrial matrix as mentioned above, the existence of extra-mitochondrial Nfs1 has been proposed [25,26]. In the case of the yeast S. cerevisiae, circumstantial evidence for possible dual localization of Nfs1 in the nuclear compartment in addition to the mitochondrial matrix was previously demonstrated by a genetic complement study [25] and by a mitochondrial peptidase Icp55-using experiment [26]. However, any role of yeast Nfs1 in the nucleus remains unclear. Yeast Nfs1 is a single copy nuclear gene product carrying a mitochondrial targeting signal that is cleaved off in the mitochondrial matrix. If any functional Nfs1 exists in cytosol, it might facilitate the thio-modification of cy-tRNAs, possibly for more direct initial provision of sulfan sulfur in cytosol. However, Nfs1 has not yet been biochemically identified in cytosol in yeast, nor has any sulfur transfer system using putative cytosolic Nfs1 been identified in vivo. Alternatively, if yeast Nfs1 is exclusively localized in mitochondria, but is required for the delivery of sulfur to cy-tRNAs, such hypothetical existence of functional Nfs1 in cytosol is not necessary; however, a certain sulfan sulfur delivery system from the mitochondrial matrix to cytosol must absolutely be required.
Differences might exist in human cases. Small amounts of human Nfs1 (NFS1) and other mitochondrial ISC proteins, ISCU (human orthologue of yeast IscU1/U2), NFU1, heat shock protein 20 (HSC20), and FXN (human orthologue of yeast frataxin), are detected in human cytosol [18,27,28,29,30,31,32,33]. When the plasmid-borne NFS1 gene was introduced and expressed in HeLa cells, NFS1 protein was detected in the cytosolic space by microscopic observation [34] and biochemical analyses [18]. However, it should be noted that cytosolically-expressed human NFS1 appears not to support the cytosolic Fe/S cluster assembly de novo without functional mitochondrially-localized NFS1 [16]. Therefore, it remains critically important to determine whether only cytoplasmic NFS1, rather than mitochondrial NFS1, is required for the formation of s2U34 in the human cytosol. Considering all of the above, Nfs1/NFS1 functions in both mitochondrial and cytosolic tRNAs thio-modification, most likely by providing the sulfur of the s2U34; however, the exact sulfur delivery pathway to tRNAs in distinct subcellular locations is complex and remains to be elucidated.
3. The Cytosolic UBL-UBA System and Thio-Modification of tRNA
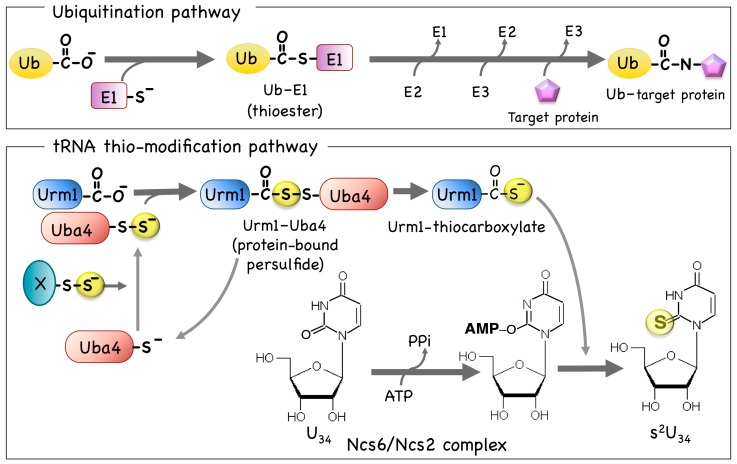
In the ubiquitin (Ub)-dependent protein degradation system, Ub is attached to its final target protein after several steps of the activated-Ub transfer between Ub and its partner UBA (ubiquitin activating enzyme-like proteins), that is, E1 (the ubiquitin activating enzyme), E2, and E3 enzymes. In Ub activation with its partner UBAs, the carboxyl terminal glycine of Ub binds to the active site cysteine residue of UBA by forming a thioester bond [35] (Figure 3). Many different ubiquitin-like protein (UBL)-UBA pairs are known to be involved in a variety of intracellular signal transduction systems [35] other than the protein degradation. Such UBLs show similar β-grasp fold structures and possess conserved glycine-glycine residues at the carboxyl terminal of the proteins [35]. UBA binds its partner UBL by a thioester bond, following which the UBL is transferred to another UBA protein of the same signal transduction stream, after which it is finally attached to the target protein [35]. The first reaction of the UBL-UBA system is thioester bond formation between the C-terminal glycine of UBL and the cysteine residue of the partner UBA [35]. Yeast ubiquitin related modifier 1 (Urm1) and its partner Uba4 form a unique UBL-UBA protein pair that was revealed to be required for the formation of s2U34 in cy-tRNAs [36,37,38,39,40]. A unique characteristic of Urm1 is that its final structure in sulfur transfer to the formation of s2U34 is not a protein-bound form, but rather its terminal thiocarboxylate formation, whereas the final form of other UBLs are protein-bound forms [35]. Interestingly, similar sets of the proteins are found in bacterial sulfur transfer systems such as thiazole formation in thiamine biosynthesis and molybdopterin (MPT) formation in molybdenum cofactor (MoCo) biosynthesis [36]. Thus, the Urm1-Uba4 system is thought to be an ancient sulfur transfer system that originally functioned in the bacterial sulfur transfer system found in the biogenesis of thiamine thiazole or MPT [36,39,41] (Figure 3).
Figure 3.
The UBL—ubiquitin-activating enzyme (E1)-like protein system related to the thio-modification of cytosolic transfer RNA (tRNA). The protein modifier system of ubiquitination found in eukaryotes (upper column) is compared with a plausible model for the sulfur transfer between Uba4 and Urm1, which is for the s2U34 formation in yeast cytosolic tRNA (lower column). In the ubiquitination pathway, ubiquitin (Ub) is first bound to the UBA protein E1 (a sulfhydryl of an active cysteine residue is shown in a yellow circle) and then, after several reactions, is finally bound to form a thioester bond. In the thio-modification pathway, a UBL protein Urm1 is first bound to the partner UBA protein, Uba4. A complex of Ncs6 and Ncs2 (the Ncs6/Ncs2 complex) is thought to be involved in the last step of the tRNA recognition and thio-modification of cy-tRNAs. Sulfur atoms transferred in this system are shown with filled circles.
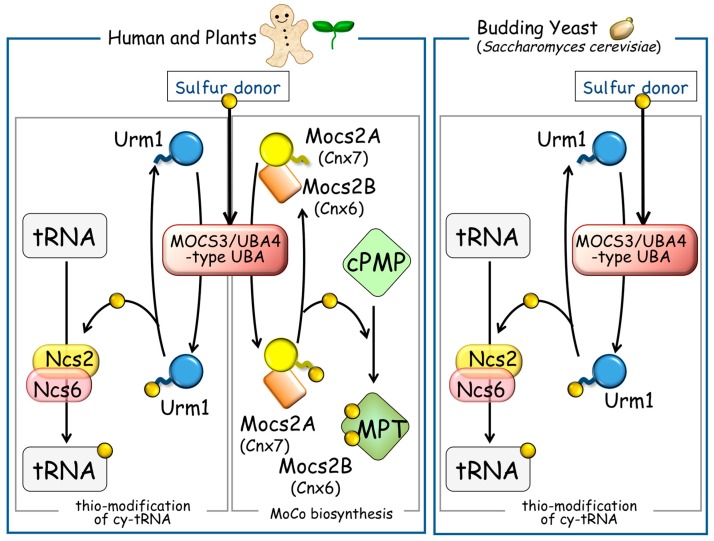
The Uba4/MOCS3-type UBA proteins including yeast Uba4 are found in all domains of life [42,43]. Human and plant Uba4/MOCS3-type UBA proteins, namely molybdenum cofactor synthesis protein 3 (MOCS3) and Cnx5, respectively, were firstly identified as sulfurtransferases required to construct an MPT in MoCo biosynthesis [44]. MoCo biosynthesis is also found in various organisms, but not in some fungi including S. cerevisiae and many parasitic protozoa [44,45]. Therefore, Uba4 appears to be a sulfurtransferase specific to the thio-modification of cy-tRNAs in the yeast S. cerevisiae. On the other hand, the Urm1-type UBL proteins are also ubiquitously found in eukaryotes and archaea [42,43]; however, an additional UBL protein (molybdenum cofactor synthesis protein 2A (MOCS2A)-type UBL proteins) exists that is only found in MoCo-producing organisms. Plant Cnx7 belongs to the UBL of this type and is required for MoCo synthesis, but not for thio-modification of cy-tRNAs. Thio-modification in yeast ∆URM1 cells is restored only by the Urm1-type UBL protein Urm1 (Arabidopsis thaliana Urm11 and Urm12), but not by MOCS2A-type UBL protein Cnx7 [42]. In addition, human MOCS3 has the ability to transfer sulfur that is attached to its C-terminal rhodanese-like domain to two distinct acceptor proteins, MOCS2A and URM1 in human cells [13,37,38]. These results indicate the distinctive role of the Urm1-type and the MOCS-type UBL proteins [42] (Figure 4).
Figure 4.
Sulfur delivery to the U34 in cy-tRNAs and for molybdopterin (MPT) biosynthesis in eukaryotes. Multicellular eukaryotes (human and plant) possess two types of ubiquitin-like (UBL proteins, for MoCo biosynthesis, and for the thio-modification of tRNA, both of which associate with the common Uba4/MOCS3-type UBA proteins (left panel). On the other hand, the yeast Saccharomyces cerevisiae contains no MoCo biosynthesis-related proteins and only Urm1 for thio-modification of tRNAs associates to Uba4 (right panel). Sulfur atoms to be delivered are shown with filled circles.
Yeast Uba4 and human MOCS3 contain the N-terminal MoeB/E1-like domain (MoeB is an E. coli orthologue of the human MOCS3) and the C-terminal rhodanese-like domain [36,46]. The N-terminal MoeB/E1-like domain of the Uba4/MOCS3-type UBA proteins can bind the C-terminally adenylated Urm1 via a thioester bond that is commonly found in the activation mechanism of various UBL proteins [35]. The C-terminal rhodanese-like domain of these Uba4/MOCS3-type UBA proteins can provide sulfur to Urm1 to ultimately form a thiocarboxylate at the conserved C-terminal glycine of Urm1 [46,47]. Although the sulfur transfer mechanism from Uba4 to Urm1 remains incompletely understood, by analogy, similar to the bacterial acyldisulfide linkage formation between ThiS and ThiF (tRNA sulfurtransferase F) [48,49,50] found in the biosynthesis of thiamine thiazole [51,52], a protein-bound persulfide might be formed as an intermediate structure in the sulfur transfer between Uba4 and Urm1.
A combination study of the split-enhanced green fluorescent protein system and fluorescence resonance energy transfer analysis showed that NFS1 without a mitochondrial localization signal can interact with MOCS3 in the cytosol of HeLa cells when the N-terminal signal-truncated NFS1 is ectopically expressed [34]. In addition, an in vitro biochemical assay showed that human NFS1 could transfer sulfur to the C-terminal domain of MOCS3 [34]. On the other hand, the Uba4 without its N-terminal MoeB/E1 domain cannot complement thio-modification of cy-tRNA, thereby indicating that the whole region of Uba4 is indispensable for the s2U34 formation in cy-tRNA in yeast [36]. Thus, the direct interaction between NFS1 with MOCS3 in the sulfur transfer in human cytosol in vivo remains to be investigated.
Yeast Tum1 (Yor251c) is a double rhodanese domain-containing sulfurtransferase. The rhodanese superfamily is a versatile sulfur carrier protein catalyzing sulfur transfer reactions in various metabolic and regulatory pathways [53,54]. The s2U34 formation in cy-tRNAs is partially reduced when the Tum1 gene is disrupted, indicating that a Tum1-mediated sulfur transfer to the s2U34 in cy-tRNAs is an alternative or redundant pathway besides another sulfur transfer pathway of thio-modification in cy-tRNAs [38,40,55]. Results of an in vitro sulfur transfer assay between Tum1 and Nfs1 [40] imply a possibility that extra-mitochondrial Nfs1 might exist and function in the sulfur transfer system of the cy-tRNA thio-modification pathway. However, the exact subcellular localization of yeast Tum1 remains unclear, and as described above, conclusive evidence for the existence of cytosolic Nfs1 and its direct involvement in cytosolic sulfur transfer reactions remains lacking in yeast cells [56,57]. Therefore, the Tum1-mediated sulfur transfer pathway in cytosol remains unclear. On the other hand, two forms of human Tum1 (TUM1) are found in both cytosol and mitochondria in HeLa cells [58], indicating that they may correspond to the observed dual localization of human Nfs1 (NFS1) [34]. Moreover, the isolated NFS1 and the rhodanese domain of Uba4/MOCS3 and of TUM1 can be interactive in vitro [59]. However, no direct evidence of mitochondrial-cytosolic sulfur transfer mediated by Tum1, nor the interaction between NFS1 and TUM1 in mitochondria, has yet been demonstrated. Thus, the direct involvement of TUM1 in s2U34 formation in human cy-tRNAs remains to be clarified.
4. Fe/S Proteins and the Formation of s2U34 in Cytosol
Cytosolic Fe/S protein maturation requires mitochondrial Nfs1 and the cytosolic iron-sulfur cluster assembly (so-called “CIA”) proteins [56,57,60]. Interestingly, thio-modification of cy-tRNAs in yeast requires the CIA proteins Cfd1, Nbp35, and Cia1 in addition to Nfs1 [12]. Considering that mitochondrial Nfs1 and CIA proteins are required for both the cytosolic Fe/S protein maturation and the cy-tRNA thio-modification, certain cytosolic Fe/S protein(s) might be directly involved in the formation of s2U34 in cy-tRNAs (Figure 2).
In vitro reconstitution of the mcm5s2U34 in tRNA shows that mcm5 formation at C5 is required for s2 formation [40]. Elongator complex (ELP), which is composed of six subunits (Elp1, Elp2, Elp3, Elp4, Elp5, and Elp6) is required for the formation of 5-methoxycarbonylmethyl (mcm5) or 5-carbamoylmethyl (ncm5) at C5 of U34 [61], and, intriguingly, the active subunit Elp3 contains a [4Fe-4S] cluster in addition to S-adenosylmethyonine [62]. Recently resolved structures of Elp3 and Elp4, Elp5, and Elp6 multi-subunit complex (Elp456) indicate the structural importance of Elp3 in tRNA binding ability and interaction with the (Elp456) [63,64]. Yeast ∆ELP3 cells exhibit a reduced thio-modification level of cytosolic tRNAs and lack the entire C5 modification of these tRNAs [36,37,40], resulting in reduced decoding efficiency in protein translation [61]. Therefore, Elp3 containing its Fe/S cluster might be a key enzyme to construct the entire “hyper-modified” U34 structure, including s2U34 formation of cytosolic tRNAs to fulfill their activity. Further studies to clarify the direct mechanistic relation of the Fe/S protein involving C5 modification and thio-modification of cytosolic tRNAs should be performed.
The recent finding from bacterial homologue of the yeast cytosolic 2-thiouridine synthetase (Ncs6) might show the implication of the requirement of another Fe/S protein in the s2U34 formation in cy-tRNAs. ThiI (bacterial s4U synthetase)-like protein recently found in Methanococcus maripaludis was designated as the putative bacterial Ncs6 by sequence similarity to eukaryotic Ncs6, and was revealed to contain a [3Fe-4S] cluster essential for the tRNA thio-modification of M. maripaludis [65,66]. Other yeast cytosolic 2-thiouridine synthetases Ncs2 and Ncs6, in addition to Uba4, Urm1, and Tum1, are necessary to construct the s2U34 in vitro [40]. Yeast Ncs6-lacking cells show depletion of thio-modification in cy-tRNAs [36,38,39,40]. Ncs6 contains a nucleotide binding motif (PP-loop motif) (SGGxDS) [55], resembling a catalytic motif of N-type ATP pyrophosphatases also found in bacterial MnmA (required for bacterial s2U34 formation [8,23]) and in bacterial ThiI (required for bacterial s4U8 formation [5,67]). In general, ATP pyrophosphatases are thought to be important to activate the target positions of pyrimidine bases by forming acyl-adenylate intermediates. In Schizosaccharomyces pombe and C. elegans, C. elegans Ncs6 orthologue (Ctu1) and C. elegans Ncs2 orthologue (Ctu2) are physically associated with the s2U34 formation of tRNAs [68]. S. cerevisiae Ncs6 and human orthologue ATP binding protein 3 (ATPBD3) bind Urm1 and URM1, respectively [69]. From these observations, a complex of Ncs6 and Ncs2 (the Ncs6/Ncs2 complex) is thought to be involved in the last step of the tRNA recognition and thio-modification of cy-tRNAs. Although which protein is an actual final sulfur donor to the U34 of the tRNA molecule and how the Ncs6/Ncs2 complex recognizes both Urm1 and the target tRNA and catalyzes the thio-modification reaction remain to be elucidated, investigations of the putative Fe/S-containing Ncs6 might reveal important implications of understanding the molecular mechanisms of thio-modification of eukaryotic cy-tRNAs [69].
5. Perspectives
The entire modification of U34, including thio-modification, is essential for the accuracy of codon-anticodon pairing and for effective decoding in protein translation, thereby contributing to a fine-tuning in protein productivity in cells [70]. Deficiency of the wobble U34 modifications in cy-tRNAs causes ribosome stalling at the A-site during translation [71], indicating that the wobble U34 modification including s2U34 formation is responsible for maintaining the efficiency of protein translation in diverse cellular functions. Structural stability in and around the anticodon loop would be important to guarantee such translation efficiency [72]. For example, when s2 and/or mcm5 modification at U34 together with A37 or ψ (pseudouridine)38/39 modifications are lacking, the morphologies of yeast cells are altered and growth defects are exhibited [73]. This indicates that the cooperative interaction of these tRNA modifications is essential for cell homeostasis. Deficiency of the wobble U34 modification might also affect degradation/decay of the tRNA, as has been proposed for other types of tRNA modifications [74].
Translation quality control in regard to the U34 modification of tRNA appears to influence diverse physiological effects in eukaryotic cells. When either of Uba4, Urm1, or Ncs6 is lacking, yeast cells cannot exhibit invasive growth as a stress response, which normally occurs under glucose starvation conditions [75]. Elevated growth temperature of yeast cells was shown to affect the tRNA modification status and cause changes to their morphology [74]. Yeast NCS2, UBA4 (=NCS3), NCS6, and ELP2 (=NCS10) were originally designated as genes whose mutations exhibit the synthetic lethality of ∆CLA4 cells [76]. Cla4 functions as a Cdc42-activated signal-transducing kinase and is involved in various physiological functions, including yeast morphology formation, budding, and metabolic control [77,78,79,80]. From this evidence, U34 modification in cytosolic tRNAs appears to be related to such variations in the morphological and physiological status of the cells.
In humans, pathogenic defects related to U34 modification in both mt-tRNAs and cy-tRNAs are reported. In regard to mitochondrial diseases that are known to involve mitochondrial tRNA modification [81], for example, patients with myoclonus epilepsy associated with ragged-red fibers (MERFF) syndrome bear a mutation (A8344G) in the T-arm of the mt-RNA gene, and the tRNA modification in the patients is impaired [82,83]. Another case is a reduction of respiration enzyme activity in the defect of a mitochondrial tRNA-modifying enzyme GTP-binding protein 3 (GTPBP3) that is involved in the C5 of U34 (τm5U) in mt-tRNAs [84]. In regard to pathogenic defects concerning the U34 modification of cy-tRNAs, in familial dysautonomia, for example, patients exhibit reduced levels of mcm5s2U in cy-tRNAs [85].
Many of the enzymes involved in tRNA thio-modification are also associated with additional physiological functions other than tRNA modification. Urm1 also functions as the “Urm1-related protein conjugation system” (so-called “urmylation”) besides thio-modification of tRNAs. urmylation is related to target of rapamycin (TOR) pathway-related signal transduction and cellular morphological changes under various nutritional stress conditions [74,86,87]. In addition, intracellular levels of sulfur-containing amino acids assimilated as nutrients can regulate the translation ability by changing the status of the thio-modification of tRNAs, thereby balancing the metabolic homeostasis in the cell [88]. Human URM1-decifient mutants result in the G1 arrest in cell cycle and exhibit multinuclear morphology [38]. The Uba4/MOCS3-type UBA proteins in multicellular eukaryotes have dual functions in sulfur transfer to tRNAs, as well as to MPT. Elongator complex, including Elp3, is also a multifunctional enzyme complex. Biosynthetic pathways of tRNA thio-modification and Fe/S protein biosynthesis in distinct intracellular locations appear to be deeply related and are indispensable for cell homeostasis.
Acknowledgments
We would like to thank the editor Valérie de Crécy-Lagard for inviting and giving us the chance to write in this special issue on tRNA modification.
Author Contributions
Y.N., M.N., and T.Y. all contributed to the writing of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. MODOMICS: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Yacoubi B., Bailly M., de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modification of transfer RNAs. Annu. Rev. Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 3.Jackman J.E., Alfonzo J.D. Transfer RNA modifications: Nature’s combinatorial chemistry playground. Wiley Interdiscip. Rev RNA. 2013;4:35–48. doi: 10.1002/wrna.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauhon C.T., Kambampati R. The iscS gene in Escherichia coli is required for the biosynthesis of 4-thiouridine, thiamin, and NAD. J. Biol. Chem. 2000;275:20096–20103. doi: 10.1074/jbc.M002680200. [DOI] [PubMed] [Google Scholar]
- 6.Grosjean H., de Crécy-Lagard V., Marck C. Deciphering synonymous codons in the three domains of life: Co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010;584:252–264. doi: 10.1016/j.febslet.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson K., Lundgren H.K., Hagervall T.G., Björk G.R. The Cysteine Desulfurase IscS Is Required for Synthesis of All Five Thiolated Nucleosides Present in tRNA from Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2002;184:6830–6835. doi: 10.1128/JB.184.24.6830-6835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambampati R., Lauhon C.T. MnmA and IscS Are Required for in Vitro 2-Thiouridine Biosynthesis in Escherichia coli. Biochemistry. 2003;42:1109–1117. doi: 10.1021/bi026536+. [DOI] [PubMed] [Google Scholar]
- 9.Lauhon C.T. Requirement for IscS in biosynthesis of all thionucleosides in Escherichia coli. J. Bacteriol. 2002;184:6820–6829. doi: 10.1128/JB.184.24.6820-6829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler D. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 2006;6:825–840. doi: 10.1111/j.1574-6976.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakai Y., Umeda N., Suzuki T., Nakai M., Hayashi H., Watanabe K., Kagamiyama H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 2004;279:12363–12368. doi: 10.1074/jbc.M312448200. [DOI] [PubMed] [Google Scholar]
- 12.Nakai Y., Nakai M., Lill R., Suzuki T., Hayashi H. Thio Modification of Yeast Cytosolic tRNA Is an Iron-Sulfur Protein-Dependent Pathway. Mol. Cell Biol. 2007;27:2841–2847. doi: 10.1128/MCB.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury M.M., Dosche C., Löhmannsröben H.G., Leimkühler S. Dual Role of the Molybdenum Cofactor Biosynthesis Protein MOCS3 in tRNA Thiolation and Molybdenum Cofactor Biosynthesis in Humans. J. Biol. Chem. 2012;287:17297–17307. doi: 10.1074/jbc.M112.351429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeda N., Suzuki T., Yukawa M., Ohya Y., Shindo H., Watanabe K., Suzuki T. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J. Biol. Chem. 2005;280:1613–1624. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- 15.Lill R., Srinivasan V., Mühlenhoff U. The role of mitochondria in cytosolic-nuclear iron–sulfur protein biogenesis and in cellular iron regulation. Curr. Opin. Microbiol. 2014;22:111–119. doi: 10.1016/j.mib.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Biederbick A., Stehling O., Rösser R., Niggemeyer B., Nakai Y., Elsässer H.P., Lill R. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol. Cell. Biol. 2006;26:5675–5687. doi: 10.1128/MCB.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakai Y., Yoshihara Y., Hayashi H., Kagamiyama H. cDNA cloning and characterization of mouse nifS-like protein, m-Nfs1: Mitochondrial localization of eukaryotic NifS-like proteins. FEBS Lett. 1998;433:143–148. doi: 10.1016/S0014-5793(98)00897-7. [DOI] [PubMed] [Google Scholar]
- 18.Land T., Rouault T.A. Targeting of a Human Iron-Sulfur Cluster Assembly Enzyme, nifs, to Different Subcellular Compartments Is Regulated through Alternative AUG Utilization. Mol. Cell. 1998;2:807–815. doi: 10.1016/S1097-2765(00)80295-6. [DOI] [PubMed] [Google Scholar]
- 19.Kispal G., Csere P., Prohl C., Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18:3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin R.P., Sibler A.P., Gehrke C.W., Kuo K., Edmonds C.G., McCloskey J.A., Dirheimer G. 5-[[(Carboxymethyl)amino]methyl]uridine is found in the anticodon of yeast mitochondrial tRNAs recognizing two-codon families ending in a purine. Biochemistry. 1990;29:956–959. doi: 10.1021/bi00456a016. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002;21:6581–6589. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T., Suzuki T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2014;42:7346–7357. doi: 10.1093/nar/gku390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Numata T., Fukai S., Ikeuchi Y., Suzuki T., Nureki O. Structural basis for sulfur relay to RNA mediated by heterohexameric TusBCD complex. Structure. 2006;2:357–366. doi: 10.1016/j.str.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Nakai Y., Nakai M., Hayashi H., Kagamiyama H. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 2001;276:8314–8320. doi: 10.1074/jbc.M007878200. [DOI] [PubMed] [Google Scholar]
- 26.Naamati A., Regev-Rudzki N., Galperin S., Lill R., Pines O. Dual Targeting of Nfs1 and Discovery of Its Novel Processing Enzyme, Icp55. J. Biol. Chem. 2009;284:30200–30208. doi: 10.1074/jbc.M109.034694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y., Ghosh M.C., Tong W.H., Rouault T.A. Human ISD11 is essential for both iron-sulfur cluster assembly and maintenance of normal cellular iron homeostasis. Hum. Mol. Genet. 2009;18:3014–3025. doi: 10.1093/hmg/ddp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong W.H., Rouault T.A. Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab. 2006;3:199–210. doi: 10.1016/j.cmet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Rouault T.A., Tong W.H. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- 30.Rouault T.A., Tong W.H. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;8:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong W.H., Jameson G.N., Huynh B.H., Rouault T.A. Subcellular compartmentalization of human Nfu, an iron-sulfur cluster scaffold protein, and its ability to assemble a [4Fe-4S] cluster. Proc. Natl. Acad. Sci. USA. 2003;100:9762–9767. doi: 10.1073/pnas.1732541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhrigshardt H., Singh A., Kovtunovych G., Ghosh M., Rouault T.A. Characterization of the human HSC20, an unusual DnaJ type III protein, involved in iron-sulfur cluster biogenesis. Hum. Mol. Genet. 2010;19:3816–3834. doi: 10.1093/hmg/ddq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia H., Cao Y., Dai X., Marelja Z., Zhou D., Mo R., Al-Mahdawi S., Pook M.A., Leimkühler S., Rouault T.A., et al. Novel Frataxin Isoforms May Contribute to the Pathological Mechanism of Friedreich Ataxia. PLoS ONE. 2012;7:e47847. doi: 10.1371/journal.pone.0047847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marelja Z., Mullick Chowdhury M., Dosche C., Hille C., Baumann O., Löhmannsröben H.G., Leimkühler S. The L-Cysteine Desulfurase NFS1 Is Localized in the Cytosol where it Provides the Sulfur for Molybdenum Cofactor Biosynthesis in Humans. PLoS ONE. 2013;8:e60869. doi: 10.1371/journal.pone.0060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulman B.A., Harper J.W. Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009;10:319–331. doi: 10.1038/nrm2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakai Y., Nakai M., Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J. Biol. Chem. 2008;283:27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 37.Huang B., Lu J., Byström A.S. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14:2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlieker C.D., Van der Veen A.G., Damon J.R., Spooner E., Ploegh H.L. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc. Natl. Acad. Sci. USA. 2008;105:18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leidel S., Pedrioli P.G., Bucher T., Brost R., Costanzo M., Schmidt A., Aebersold R., Boone C., Hofmann K., Peter M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 40.Noma A., Sakaguchi Y., Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Veen A.G., Ploegh H.L. Ubiquitin-like proteins. Annu. Rev. Biochem. 2012;81:323–357. doi: 10.1146/annurev-biochem-093010-153308. [DOI] [PubMed] [Google Scholar]
- 42.Nakai Y., Harada A., Hashiguchi Y., Nakai M., Hayashi H. Arabidopsis Molybdopterin Biosynthesis Protein Cnx5 Collaborates with the Ubiquitin-like Protein Urm11 in the Thio-modification of tRNA. J. Biol. Chem. 2012;287:30874–30884. doi: 10.1074/jbc.M112.350090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranda H.V., Nembhard N., Su D., Hepowit N., Krause D.J., Pritz J.R., Phillips C., Söll D., Maupin-Furlow J.A. E1- and ubiquitin-like proteins provide a direct link between protein conjugation and sulfur transfer in archaea. Proc. Natl. Acad. Sci. USA. 2011;108:4417–4422. doi: 10.1073/pnas.1018151108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendel R.R. The molybdenum cofactor. J. Biol. Chem. 2013;288:13165–13172. doi: 10.1074/jbc.R113.455311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Gladyshev V.N. Molybdoproteomes and evolution of molybdenum utilization. J. Mol. Biol. 2008;379:881–899. doi: 10.1016/j.jmb.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krepinsky K., Leimkühler S. Site-directed mutagenesis of the active site loop of the rhodanese-like domain of the human molybdopterin synthase sulfurase MOCS3. Major differences in substrate specificity between eukaryotic and bacterial homologs. FEBS J. 2007;274:2778–2787. doi: 10.1111/j.1742-4658.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz J., Chowdhury M.M., Hänzelmann P., Nimtz M., Lee E.Y., Schindelin H., Leimkühler S. The sulfurtransferase activity of Uba4 presents a link between ubiquitin-like protein conjugation and activation of sulfur carrier proteins. Biochemistry. 2008;47:6479–6489. doi: 10.1021/bi800477u. [DOI] [PubMed] [Google Scholar]
- 48.Dorrestein P.C., Zhai H., McLafferty F.W., Begley T.P. The biosynthesis of the thiazole phosphate moiety of thiamin: The sulfur transfer mediated by the sulfur carrier protein ThiS. Chem. Biol. 2004;11:1373–1381. doi: 10.1016/j.chembiol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Xi J., Ge Y., Kinsland C., McLafferty F.W., Begley T.P. Biosynthesis of the thiazole moiety of thiamin in Escherichia coli: Identification of an acyldisulfide-linked protein—Protein conjugate that is functionally analogous to the ubiquitin/E1 complex. Proc. Natl. Acad. Sci. USA. 2001;98:8513–8518. doi: 10.1073/pnas.141226698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehmann C., Begley T.P., Ealick S.E. Structure of the Escherichia coli ThiS-ThiF complex, a key component of the sulfur transfer system in thiamin biosynthesis. Biochemistry. 2006;45:11–19. doi: 10.1021/bi051502y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J., Zhang J., Wang L., Zhou J., Huang H., Wu J., Zhong Y., Shi Y. Solution structure of Urm1 and its implications for the origin of protein modifiers. Proc. Natl. Acad. Sci. USA. 2006;103:11625–11630. doi: 10.1073/pnas.0604876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerscher O., Felberbaum R., Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 53.Bordo D., Bork P. The rhodanese/Cdc25 phosphatase superfamily. Sequence–structure–function relations. EMBO Rep. 2002;3:741–746. doi: 10.1093/embo-reports/kvf150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cipollone R., Ascenzi P., Visca P. Common themes and variations in the rhodanese superfamily. IUBMB Life. 2007;59:51–59. doi: 10.1080/15216540701206859. [DOI] [PubMed] [Google Scholar]
- 55.Björk G.R., Huang B., Persson O.P., Byström A.S. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13:1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul V.D., Lill R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim. Biophys. Acta. 2015;1853:1528–1539. doi: 10.1016/j.bbamcr.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 57.Lill R. Function and Biogenesis of Iron-Sulphur Proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 58.Wiedemann N., Urzica E., Guiard B., Müller H., Lohaus C., Meyer H.E., Ryan M.T., Meisinger C., Mühlenhoff U., Lill R., et al. Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 2006;25:184–195. doi: 10.1038/sj.emboj.7600906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marelja Z., Stöcklein W., Nimtz M., Leimkühler S. A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J. Biol. Chem. 2008;283:25178–25185. doi: 10.1074/jbc.M804064200. [DOI] [PubMed] [Google Scholar]
- 60.Netz D.J., Pierik A.J., Stümpfig M., Mühlenhoff U., Lill R. The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 2007;3:278–286. doi: 10.1038/nchembio872. [DOI] [PubMed] [Google Scholar]
- 61.Karlsborn T., Mahmud A.K., Tükenmez H., Byström A.S. Loss of ncm5 and mcm5 wobble uridine side chains results in an altered metabolic profile. Metabolomics. 2016;12:177. doi: 10.1007/s11306-016-1120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paraskevopoulou C., Fairhurst S.A., Lowe D.J., Brick P., Onesti S. The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 2006;3:795–806. doi: 10.1111/j.1365-2958.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 63.Glatt S., Létoquart J., Faux C., Taylor N.M., Séraphin B., Müller C.W. The Elongator subcomplex Elp456 is a hexameric RecA-like ATPase. Nat. Struct. Mol. Biol. 2012;19:314–320. doi: 10.1038/nsmb.2234. [DOI] [PubMed] [Google Scholar]
- 64.Glatt S., Zabel R., Kolaj-Robin O., Onuma O.F., Baudin F., Graziadei A., Taverniti V., Lin T.Y., Baymann F., Séraphin B., et al. Structural basis for tRNA modification by Elp3 from Dehalococcoides mccartyi. Nat. Struct. Mol. Biol. 2016;9:794–802. doi: 10.1038/nsmb.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Long F., Wang L., Söll D., Whitman W.B. The putative tRNA 2-thiouridine synthetase Ncs6 is an essential sulfur carrier in Methanococcus maripaludis. FEBS Lett. 2014;588:873–877. doi: 10.1016/j.febslet.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Vinyard D.J., Reesbeck M.E., Suzuki T., Manakongtreecheep K., Holland P.L., Brudvig G.W., Söll D. A [3Fe-4S] cluster is required for tRNA thiolation in archaea and eukaryotes. Proc. Natl. Acad. Sci. USA. 2016;113:12703–12708. doi: 10.1073/pnas.1615732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmer L.D., Leung M.H., Downs D.M. The cysteine desulfhydrase CdsH is conditionally required for sulfur mobilization to the thiamine thiazole in Salmonella enterica. J. Bacteriol. 2014;22:3964–3970. doi: 10.1128/JB.02159-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dewez M., Bauer F., Dieu M., Raes M., Vandenhaute J., Hermand D. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc. Natl. Acad. Sci. USA. 2008;105:5459–5464. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van der Veen A.G., Schorpp K., Schlieker C., Buti L., Damon J.R., Spooner E., Ploegh H.L., Jentsch S. Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc. Natl. Acad. Sci. USA. 2011;108:1763–1770. doi: 10.1073/pnas.1014402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinshteyn B., Gilbert W.V. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 2013;9:e1003675. doi: 10.1371/journal.pgen.1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nedialkova D.D., Leidel S.A. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell. 2015;161:1606–1618. doi: 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durant P.C., Bajji A.C., Sundaram M., Kumar R.K., Davis D.R. Structural Effects of Hypermodified Nucleosides in the Escherichia coli and Human tRNALys Anticodon Loop: The Effect of Nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry. 2005;44:8078–8089. doi: 10.1021/bi050343f. [DOI] [PubMed] [Google Scholar]
- 73.Klassen R., Ciftci A., Funk J., Bruch A., Butter F., Schaffrath R. tRNA anticodon loop modifications ensure protein homeostasis and cell morphogenesis in yeast. Nucleic Acids Res. 2016;44:10946–10959. doi: 10.1093/nar/gkw705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damon J.R., Pincus D., Ploegh H.L. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol. Biol. Cell. 2015;26:270–282. doi: 10.1091/mbc.E14-06-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goehring A.S., Rivers D.M., Sprague G.F., Jr. Urmylation: A ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol. Biol. Cell. 2003;11:4329–4341. doi: 10.1091/mbc.E03-02-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goehring A.S., Mitchell D.A., Tong A.H., Keniry M.E., Boone C., Sprague G.F., Jr. Synthetic Lethal Analysis Implicates Ste20p, a p21-activated Protein Kinase, in Polarisome Activation. Mol. Biol. Cell. 2003;4:1501–1516. doi: 10.1091/mbc.E02-06-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cvrcková F., De Virgilio C., Manser E., Pringle J.R., Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 78.Benton B.K., Tinkelenberg A., Gonzalez I., Cross F.R. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol. Cell. Biol. 1997;17:5067–5076. doi: 10.1128/MCB.17.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartholomew C.R., Hardy C.F. p21-activated kinases Cla4 and Ste20 regulate vacuole inheritance in Saccharomyces cerevisiae. Eukaryot. Cell. 2009;8:560–572. doi: 10.1128/EC.00111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin M., Unden H., Jacquier N., Schneiter R., Just U., Höfken T. The Cdc42 effectors Ste20, Cla4, and Skm1 down-regulate the expression of genes involved in sterol uptake by a mitogen-activated protein kinase-independent pathway. Mol. Biol. Cell. 2009;20:4826–4837. doi: 10.1091/mbc.E09-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirchner S., Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98–112. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 82.Florentz C., Sohm B., Tryoen-Tóth P., Pütz J., Sissler M. Human mitochondrial tRNAs in health and disease. Cell. Mol. Life Sci. 2003;60:1356–1375. doi: 10.1007/s00018-003-2343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yasukawa T., Kirino Y., Ishii N., Holt I.J., Jacobs H.T., Makifuchi T., Fukuhara N., Ohta S., Suzuki T., Watanabe K. Wobble modification deficiency in mutant tRNAs in patients with mitochondrial diseases. FEBS Lett. 2005;579:2948–2952. doi: 10.1016/j.febslet.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 84.Martínez-Zamora A., Meseguer S., Esteve J.M., Villarroya M., Aguado C., Enríquez J.A., Knecht E., Armengod M.E. Defective Expression of the Mitochondrial-tRNA Modifying Enzyme GTPBP3 Triggers AMPK-Mediated Adaptive Responses Involving Complex I Assembly Factors, Uncoupling Protein 2, and the Mitochondrial Pyruvate Carrier. PLoS ONE. 2015;10:e0144273. doi: 10.1371/journal.pone.0144273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karlsborn T., Tükenmez H., Chen C., Byström A.S. Familial dysautonomia (FD) patients have reduced levels of the modified wobble nucleoside mcm5s2U in tRNA. Biochem. Biophys. Res. Commun. 2014;454:441–445. doi: 10.1016/j.bbrc.2014.10.116. [DOI] [PubMed] [Google Scholar]
- 86.Rubio-Texeira M. Urmylation controls Nil1p and Gln3p-dependent expression of nitrogen-catabolite repressed genes in Saccharomyces cerevisiae. FEBS Lett. 2007;581:541–550. doi: 10.1016/j.febslet.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 87.Goehring A.S., Rivers D.M., Sprague G.F., Jr. Attachment of the ubiquitin-related protein Urm1p to the antioxidant protein Ahp1p. Eukaryot. Cell. 2003;2:930–936. doi: 10.1128/EC.2.5.930-936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laxman S., Sutter B.M., Wu X., Kumar S., Guo X., Trudgian D.C., Mirzaei H., Tu B.P. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154:416–429. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]