Abstract
Transfer RNAs (tRNAs) harbor a subset of post-transcriptional modifications required for structural stability or decoding function. N6-threonylcarbamoyladenosine (t6A) is a universally conserved modification found at position 37 in tRNA that pair A-starting codons (ANN) and is required for proper translation initiation and to prevent frame shift during elongation. In its absence, the synthesis of aberrant proteins is likely, evidenced by the formation of protein aggregates. In this work, our aim was to study the relationship between t6A-modified tRNAs and protein synthesis homeostasis machinery using Drosophila melanogaster. We used the Gal4/UAS system to knockdown genes required for t6A synthesis in a tissue and time specific manner and in vivo reporters of unfolded protein response (UPR) activation. Our results suggest that t6A-modified tRNAs, synthetized by the threonyl-carbamoyl transferase complex (TCTC), are required for organismal growth and imaginal cell survival, and is most likely to support proper protein synthesis.
Keywords: tRNA post-transcriptional modification, N6-threonylcarbamoyladenosine, unfolded protein response
1. Introduction
Transfer RNAs (tRNAs) are structured and stable nucleic acids, transcribed by RNA Polymerase III in eukaryotes. During protein synthesis, they act together with ribosomes as decoders of information contained in messenger RNAs (mRNAs), recognizing successive codons to add specific amino acids to nascent polypeptide chains [1]. After transcription, tRNAs are extensively processed and modified. A distinctive feature of tRNAs is the high level of post-transcriptional modification that is held, each one presents a subset of over 90 known modifications [2]. Depending on their position, they play structural roles [3] or are required for proper decoding activity stabilizing cognate base pairing or expediting wobble base pairing [4], thus increasing decoding capability [5]. Additionally, these kinds of modification prevent frame-shift [6], ensuring correct translation. Position 37 (adjacent to the anticodon) is frequently modified, even more so than the wobble position [7]. The most common modification present in purines at position 37 are N6-isopentenyladenosine (i6A), N6-isopenetyl-2-thiomethy-ladenosine (mS2i6A), 1-methylguanosine (m1G) and N6-threonylcarbamoyladenosine (t6A). The last is present in tRNAs that pair A-starting codons (ANN) (Figure 1) [2]. This modification is universally conserved and has a paramount role for tRNA decoding function, as has been shown in yeast [8,9], archea [10], bacteria [11], and, recently by our laboratory [12] and others [13], in Drosophila. This modification was identified over 40 years ago [14,15,16]; however, the enzymes that synthetize it were only recently identified [8,9]. Tcs1 (Yrdc) or Tcs2 (Sua5) catalyzes the formation of a l-threonyl-cabamoyl-AMP (TC-AMP) intermediate from bicarbonate, threonine and ATP. Next, the threonyl-carbamoyl transferase complex (TCTC, previously named KEOPS/EKC (kinase, endopeptidase and other proteins of small size/endopeptidase-like and kinase associated to transcribed chromatin)) is responsible for the last step of the reaction, transferring the TC-group to A37 in substrate tRNA [17]. This synthetic pathway is universally conserved [18]. In silico analyses have found Tcs2 (CG33786) and the TCTC subunit homologues in Drosophila; TCTC complex is composed of Tcs3 (Kae1), the catalytic subunit that physically interacts with Tcs5 (Prpk) and has regulatory functions over Tcs3; while Tsc6 (Pcc1) allows dimerization, two more subunits compose the yeast TCTC complex, Tcs4 (Qri7) and Tcs8 (Gon7), but there are no counterparts in Drosophila. Mutations in either the gene coding for TCTC subunits or Tcs2 eliminate t6A in tRNA and cause strong slow-growth phenotype in yeast [8], as well as cell and organismal size reductions in Drosophila [12,19]. At the molecular level, yeast lacking t6A present erroneous protein synthesis initiation at non-AUG codons and frameshifts during elongation [8], making the synthesis of misfolded and unfolded proteins very likely, as evidenced by the formation of protein aggregates [20]. Upon Tcs3 knockdown in Drosophila, a collection of signaling pathways, called the unfolded protein response (UPR), is activated in order to overcome stress caused by aberrant proteins in the lumen of the endoplasmic reticulum (ER), thus reestablishing protein homeostasis. Nonetheless, its chronic activation leads to apoptosis [21,22]. Chronic activation of the UPR is a likely explanation for the phenotypes observed upon loss of function of the TCTC subunits. In this work, our aim was to establish functional relationships between t6A-modified tRNAs and the protein synthesis homeostasis machinery in Drosophila. We show that a loss of function in the t6A synthetic machinery causes apoptosis and the UPR activation in imaginal cells, indicating that the role of t6A-modified tRNAs is to support correct protein synthesis.
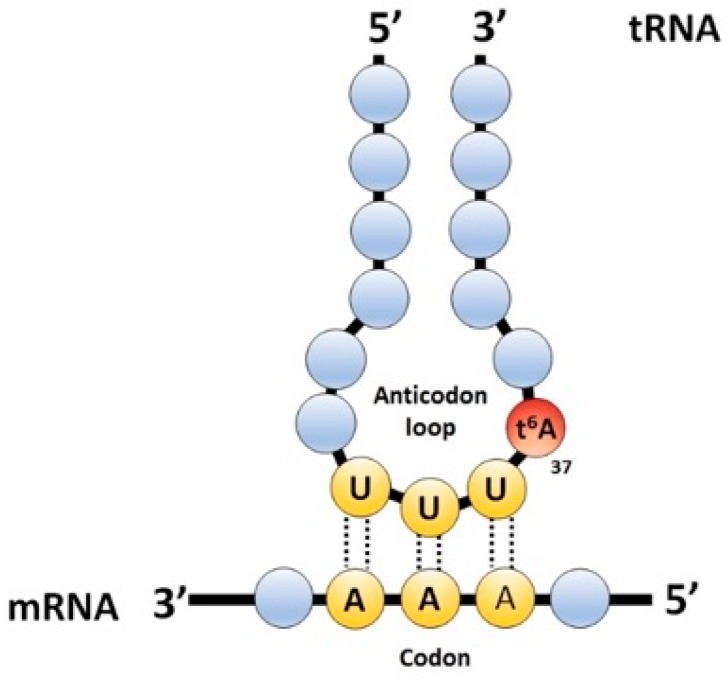
Figure 1.
N6-threonylcarbamoyladenosine (t6A) is harbored in position 37 of A-starting codons (ANN)-pairing transfer RNAs (tRNAs). The schematic representation of the anticodon loop of an ANN-pairing tRNA: t6A at position 37 is represented as a red full circle; the anticodon is represented in yellow; and the rest of the tRNA in light blue. Messenger RNA (mRNA) is represented at the bottom in light blue circles and the codon in yellow. Codon-anticodon interactions are depicted with dotted lines, and are stabilized by t6A, thus preventing intra-loop bonding between positions U33 and A37.
2. Results
We have previously shown that Drosophila mutants for tcs3 have extremely low levels of t6A-modified tRNAs. The phenotype of these mutants is too severe to establish functional relationships or underlying causes of phenotypes. In order to overcome this, we took a different approach using the binary Gal4/UAS system [23], which was originally created to study gene expression and was adapted to knockdown. Driver lines were used to express the yeast transcription factor Gal4 under a specific promoter. By itself, this does not present effects on cells, since it must bind to an upstream activating sequence (UAS) region to activate transcription. There is a plethora of flies with an UAS region upstream to a desired sequence to be expressed, either a coding sequence (i.e., green fluorescent protein (GFP)) or an inverted repeated (IR) to transcribe a RNA hairpin to activate specific RNA interference (RNAi) [24]. Gal4 and UAS lines were mated to knockdown Tcs2 and TCTC components either ubiquitously, or in a tissue-specific manner. A list of driver lines (Table 1) and UAS lines (Table 2) is provided.
Table 1.
List of Gal4 lines used.
| Name | Expression Pattern | Reference |
|---|---|---|
| tubulin (tub > Gal4) | Ubiquitous | BDSC (5138) |
| engrailed (en > Gal4) | Posterior compartment | BDSC (1973) |
| hedgehog (hh > Gal4) | Posterior compartment | Mullor et al. [55] |
| collagen type IV (cg > Gal) | Fat body (larval tissue) | BDSC (7011) |
| nubbin (nub > Gal4) | Wing pouch (imaginal disc) | BDSC (42699) |
BDSC: Bloomington Drosophila Stock Center.
Table 2.
List of upstream activating sequence (UAS) lines used.
| Name | Utility | Reference |
|---|---|---|
| UAS-Tcs2 IR | tcs2 knockdown | VDRC (dna13368) |
| UAS-Tcs3 IR | tcs3 knockdown | VDRC (106250) |
| UAS-Tcs5 IR | tcs5 knockdown | VDRC (dna7059) |
| UAS-Tcs6 IR | tcs6 knockdown | VDRC (4371) |
| UAS-GFP | GFP expression | BDSC (5137) |
| UAS-Tcs3 | Tcs3 expression | Rojas-Benitez et al. [12] |
| UAS-p35 | p35 expression | BDSC (5072) |
| UAS-BSKDN | Dominant negative BSK expression | BDSC (6409) |
| GFP-IR | GFP knockdown | BDSC (9331) |
| tub > Atf4 5’UTR::dsRed | UPR activation reporter | Kang et al. [36] |
| UAS-Xbp1::GFP | UPR activation reporter | Sone et al. [37] |
| UAS-Hsc70 | Hsc70 expression | BDSC (5843) |
VDRC: Vienna Drosophila Resource Center; BDSC: Bloomington Drosophila Stock Center.
2.1. t6A Synthetic Machinery is Required for Larval Growth
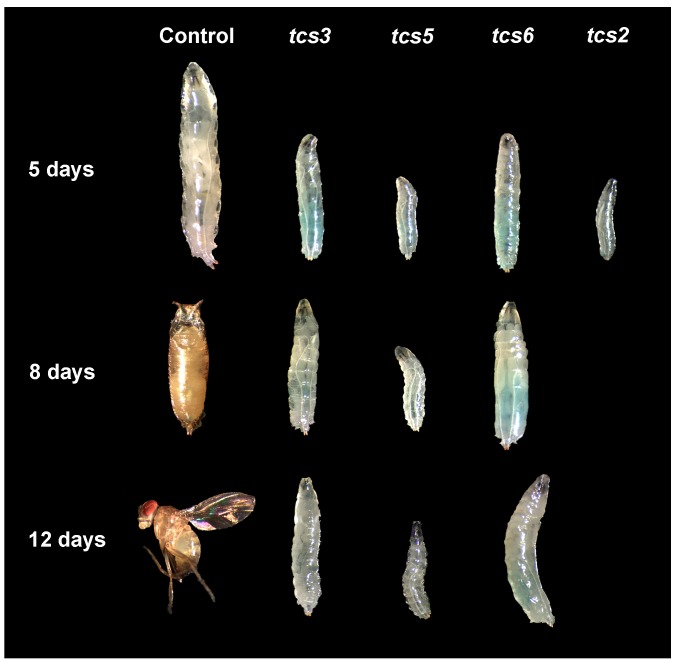
To shed light on t6A-modified tRNA function in Drosophila using the tubulin driver (tub > Gal4), we knocked-down genes required for t6A synthesis using different validated UAS-IR constructs [25,26,27]. Reduced larval size was observed by Day 5 after egg laying (AEL) (Figure 2). By Day 8, the AEL control individuals had pupariated, but when TCTC components or tcs2 were silenced, smaller larvae were observed with no pupa features. In the case of the tcs2 knockdown, larvae were small by Day 5 and died by Day 8 AEL. In the controls, by Day 12 adults are observed, while in the loss of function only larvae were present. These results, which are consistent with previous data, suggest that all silenced genes, namely t6A-modified tRNAs, are required for animal growth.
Figure 2.
Ubiquitous knockdown of t6A modification system results in reduced larval size. Ubiquitous knock down of components of the threonyl-carbamoyl transferase complex (TCTC) and tcs2 was achieved using the Gal4/UAS system. For this, the tub > Gal4 driver was used and animal size was evaluated at three different time points. The figure shows the representative images of the individuals depicted at different times after egg laying (AEL).
2.2. Tcs3 is Required for Imaginal Cell Survival
Ubiquitous knockdown of tcs2 and the TCTC subunits caused a severe phenotype, a condition for which underlying causes are difficult to establish. To overcome this, we made a tissue-specific knockdown. The Drosophila adult structures arise from groups of highly proliferative cells called imaginal discs where wings are formed from wing imaginal discs [28]. We silenced tcs3, the catalytic subunit of the TCTC, in the posterior compartment of the developing wing imaginal discs using the engrailed driver (en > Gal4) [29], so that any effect on wing development could be later analyzed in the adult structures. This allowed us to evaluate functional relationships between genes using a morphological trait in wings (area of sector D) (Figure 3A, green-colored area). A collapse between veins IV and V (Figure 3B,H) was regularly observed when tcs3 was silenced. In order to test knockdown specificity, we crossed flies in which tcs3 was knocked-down with null mutants for tcs3, generating a heterozygous mutant background in which the sector D area was further reduced (data not shown). In addition, we simultaneously silenced tcs3 and expressed the Tcs3 coding sequence, a complete reversion of the phenotype was observed (Figure 3C,H). These results showed that the observed phenotype was specific to the tcs3 knockdown.
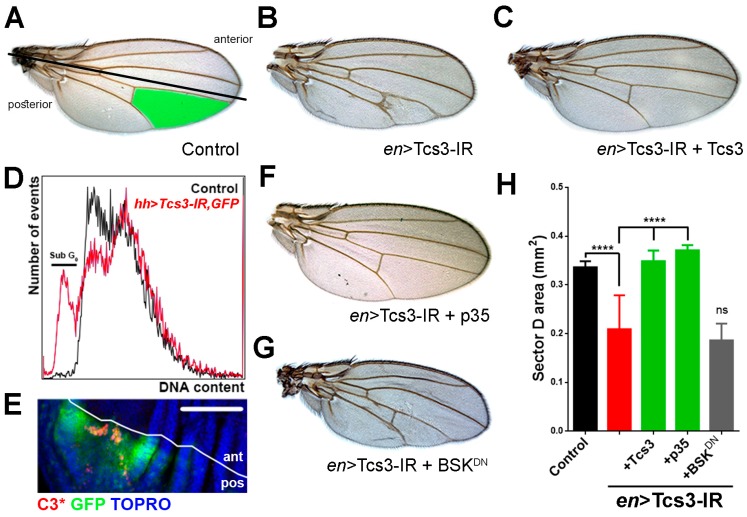
Figure 3.
tcs3 knockdown causes apoptosis in imaginal cells. (A) Using the Gal4/UAS system tcs3 was knocked-down in the wing disc posterior compartment (engrailed driver, en > Gal4; bottom part of the wing where the boundary between the anterior and posterior compartments is indicated with a black line). Representative pictures of the observed phenotypes are shown. Control (en > Gal4/+); (B) tcs3 knock down (en > Tcs3-IR); (C) co-expression of Tcs3 and Tsc3-IR; (D) hedgehog driver (hh > Gal4) was used to knock down tcs3 and express GFP in the wing posterior compartment. Wing discs were disaggregated and cells analyzed by flow cytometry. Control cells (GFP-, anterior compartment) and GFP+ (hh > Tcs3-IR, GFP) were analyzed for DNA content (DRAQ5). The sub G1 population is indicated by a black line over the curve; (E) cleaved Caspase-3 (C3*) was detected by immunofluorescence in wing discs in which tcs3 was silenced in the GFP expressing domain using the hedgehog driver. A white line indicates the boundary of the anterior (ant) and posterior (pos) compartments; bar 100 µm; (F) Simultaneous tcs3 knockdown and p35 expression or (G) BSK dominant negative (BSKDN) expression. (H) Quantification of the area of sector D in the wings of these animals (green section in control) (n = 15, ANOVA, p < 0.005).
As the observed veins were malformed, we wondered if this phenotype was produced by apoptosis occurring in the wing imaginal cells. To investigate this, we simultaneously knocked-down tcs3 and expressed GFP in the posterior compartment of the wing imaginal discs using the hedgehog driver (hh > Gal4), and analyzed the DNA content in the control (GFP-) and GFP-expressing/tsc3 knockdown cells by flow cytometry (Figure 3D). In cells where tcs3 was knocked-down, we detected a reduction in the G0/G1 cell population and a new population of cells in the sub-G0/G1 region, which exhibited a deficit in DNA content, a feature consistent with apoptotic cells [30]. In order to confirm this, we detected cleaved Caspase-3 in the wing imaginal discs by immunohistochemistry. Positive patches for cleaved Caspase-3 were present only in the posterior compartment, indicating that apoptosis was induced only in the region where tcs3 was silenced (Figure 3E). In order to prove whether apoptosis was the underlying cause of the phenotype upon tcs3 knockdown, we simultaneously overexpressed the anti-apoptotic protein p35 [31,32] where a reversion of the phenotype similar to the expressed Tcs3 was observed (Figure 3F,H). In contrast, the overexpression of a dominant negative form of Basket (BSKDN), the Drosophila orthologue of c-Jun N-terminal kinase (JNK) required apoptosis activation in several stressful conditions [33], did not rescue the phenotype (Figure 3E,H). Thus, our results showed that Tcs3 is required for the survival of imaginal cells and suggest that apoptosis of imaginal cells, independent of BSK, is the underlying cause of the phenotype.
2.3. Activation of UPR upon Silencing of t6A Synthetic Machinery
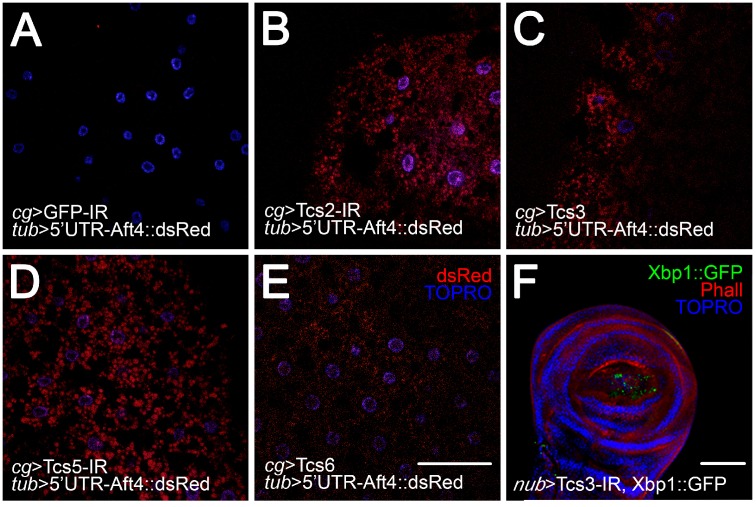
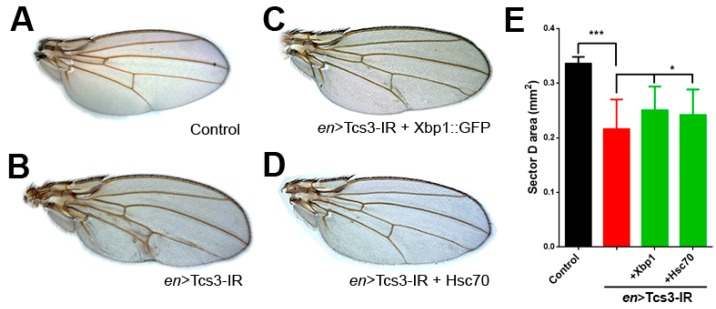
t6A is a structural feature that allows the correct recognition of ANN codons by its cognate tRNA. It has been suggested that its absence causes incorrect protein synthesis initiation and enhances the chance of frameshifting during translation in yeast [8]. Under these conditions, the synthesis of misfolded and unfolded proteins is very likely, as evidenced by the formation of protein aggregates [20]. When unfolded proteins populate the lumen of the ER it leads to ER stress, which activates the UPR, a collection of signaling pathways aiming to reestablish protein synthesis homeostasis [34] if this is not accomplished, apoptosis is induced [35]. In previous studies, we showed that tcs3 or tcs5 knockdown activated the UPR [21]. This analysis was extended to the complete set of t6A synthetic machinery genes in non-proliferative cells, the fat body (Figure 4A–E) and evaluated the UPR induction using an in vivo reporter of PERK/Atf4 activation. Atf4 mRNA translation control occurs due to several small upstream open reading frames (uORFs) in the 5’ UTR. The last one overlaps Atf4 coding sequence in a different reading frame, therefore inhibiting its translation in the unstressed cells where upon ER stress, the main reading frame is translated [22]. In the reporter, Atf4 5’UTR is fused to dsRed ORF, then in stressed cells dsRed will be translated [36]. In control animals, no dsRed signal was detected (Figure 4A); however, when tcs2 (Figure 4B) or TCTC subunits were silenced (Figure 4C–E), a signal was detected, indicating that the UPR is active via the PERK/ATF4 pathway, confirming and extending our previous results for tcs3 and tcs5 [21]. In addition, we wondered whether the UPR activation upon tcs3 silencing also took place in the imaginal cells (proliferative cells). In order to prove this, we used the nubbin driver (nub > Gal4) to knockdown tcs3 and simultaneously express a different in vivo UPR activation reporter, in which a Xbp-1::GFP fusion protein was translated only after non-canonical splicing of Xbp-1::GFP mRNA is induced upon Ire1 activation by ER stress [37]. Upon tcs3 knockdown, we detected GFP in the nub > Gal4 territory (Figure 4F), indicating the UPR was active in these cells. We wondered if chronic ER stress and the UPR activation were the underlying causes of the observed phenotype in wings. To address this, we simultaneously silenced tcs3 and overexpressed Xbp-1::GFP (Figure 5C) or Hsc70 (Figure 5D), the Drosophila orthologue of BIP/GRP78 (the main ER chaperone) [38], where both partially reverted the phenotype (Figure 5E). These results show that Tcs3 is required for organismal growth and the survival of imaginal cells in Drosophila, and most likely supports proper protein synthesis.
Figure 4.
Unfolded protein response (UPR) is activated in larval and imaginal cells deficient of t6A modified tRNAs. In vivo UPR activation reporters were used. (A–E) Construct in which the 5’UTR of Atf4 mRNA was fused with dsRed and expressed ubiquitously; when the PERK/Atf4 branch of the UPR is activated, dsRed is translated. In this reporter background, t6A synthetic machinery was knocked-down exclusively in the fat body using collagen type IV driver (cg > Gal4). Nuclei were stained with TO-PRO3. Bar 100 µm; (F) tcs3 was knocked-down using the nubbin > Gal4 driver, simultaneously an Ire1 branch UPR reporter was expressed. Upon UPR activation, the Xbp-1::GFP message was spliced and the fusion protein synthetized. F-actin was stained with phalloidin (Phall) and nuclei with TO-PRO3 (TOPRO). Bar 100 µm.
Figure 5.
Enhancement of protein folding capacity of cells partially reverted wing phenotype. (A) Control wings (en > Gal4/+), using the en > Gal4 driver we simultaneously knocked-down tcs3 (B) and overexpressed (C) Xbp1::GFP or (D) Hsc70. (E) Quantification of the area of sector D (n = 15, ANOVA, p < 0.005).
3. Discussion
Nucleic acids in cells are post-transcriptionally modified, a phenomenon known for several decades. In RNA, over 90 modifications have been identified, most of them found in tRNAs [39]. Modifications present in the body of tRNA are usually relevant for folding and stability [40,41], while the ones present in the anticodon wobble position 34 and base 37, adjacent to the anticodon are required for proper decoding capabilities. Some of these modifications such as N6-threonylcarbamoyladenosine (t6A) are universally conserved and its synthesis requires a multi-enzymatic pathway, which is present in all domains of life, suggesting that it is part of the minimal translation machinery [42] and could be considered as a primordial modification [43]. The study of this modification would allow us to understand not only how the translation machinery works, but also how it has evolved as this modification would have eased the evolution of highly-accurate translation systems [44]. Intensive study of tRNA modification using metazoan models is imperative as tRNA modification have been linked to numerous human pathologies (reviewed in references [45,46]).
Our group [12] and others [13] have observed that tcs3 mutants lacked imaginal discs and larval tissues were smaller, indicating that different cell types could have particular requirements for t6A-modified tRNAs; consistently Drosophila tcs3 [47] and human TCS3/OSGEP [48] are differentially expressed in different tissues, supporting a tissue-specific demand. Accordingly, silencing the TCTC components in differentiated Drosophila photoreceptors did not cause any phenotype [13], suggesting that differentiated cells have a low demand for t6A-modified tRNAs; in contrast, highly proliferative cells such as the ones that the wing imaginal discs in Drosophila are composed, seem to have a high demand for these tRNAs. There could be a cell cycle-dependent requirement of t6A-modified tRNAs even in the same cell type, as we observed only G0/G1 cells die by apoptosis, which is consistent with the severe target of rapamycin (TOR) inhibition in tcs3 mutants [12] as its activity promotes G1/S progression [49].
One could propose that the absence of t6A has a global and disastrous effect on translation; however, this appears not to be the case. Even with twice the erroneous translation start in non-AUG codons and doubling the translational ambiguities, only a subset of genes was affected, suggesting that the pleiotropic phenotype observed was caused not by a global translational problem, but depended on codon-specific defects [20]. Modified bases in tRNAs modulate protein synthesis [50]. Codon bias contributes to establish protein level in order to adjust production rate of a particular proteins to changing requirements (e.g., stressful conditions, cell cycle progression, differentiation, etc.), and tRNA modifications are regulated, resulting in preferential translation of mRNAs coding the required proteins [51,52]. Different cell types, each one having particular translational requirements, would be differentially affected, as each will have particular codon biases and requirements for modified tRNAs. For instance, imaginal cells die when t6A-modified tRNAs levels are diminished; on the contrary, larval tissue deals with this by lowering their anabolic rate and survive. Ribosome profiling combined with proteomics from different tissues would help to elucidate this in Drosophila.
As imaginal cells were more affected than other cell types, we silenced tcs3 in the posterior wing compartment using the Gal4 driver engrailed and observed a reduction in the sector D area, a phenotype we demonstrated was caused specifically by tcs3 knockdown. This change in morphological trait allowed us to establish functional relationships. The reduction in sector D was rescued by blocking apoptosis with p35, an anti-apoptotic protein [31]. We also asked what activated apoptosis when t6A-modified tRNAs levels were diminished. One possible explanation for the observed phenotype is the chronic activation of the UPR. Silencing either component of the TCTC activated the UPR in larval and imaginal tissue. We noticed that when cellular folding capacity was enhanced—either by overexpression of Xbp1 or Hsc70—a partial rescue of the phenotype was observed. This indicates that at least in part, the phenotype was caused by aberrant protein overload at the ER. Another component of the phenotype that was not explored further, was the observation of differential ORF selection by the levels of t6A-modified tRNAs. A fact that, independently from the UPR activation, could explain the Atf4-5’UTR::dsRed reporter activation. Nonetheless, our previous results, using a different reporter, showed that the UPR was active when tcs3 or tcs5 was silenced [21]. Thus, diminished levels of t6A-modified tRNAs would have at least two different impacts on protein synthesis: making it error-prone and to favor or inhibit specific ORF translation, creating a disturbance in protein synthesis homeostasis.
A series of unanswered questions remain about t6A-modified tRNAs and its relationship with protein synthesis and other cellular processes, for instance, in previous studies we showed that t6A-modified tRNAs were related, by an unknown mechanism, to TOR activity [12] and endocytosis [19]. Other modifications present in the anticodon loop, such as methoxycarbonylmethyl-2-thiouridine (mcm5s2U) also affect translation efficiency and compromise TOR activity [53] and activate GCN4-dependent transcription [54], common features that have been observed in mutant yeast for TCTC subunits [8,12,19]. Therefore, these observations suggest that there are unknown molecular mechanisms that link tRNA modification to stress-response mechanisms and other central components of cellular physiology. How tcs3 expression and more importantly, how tRNA modifications are regulated in different cell types or through cell cycle are questions that require further research.
4. Materials and Methods
4.1. Fly Husbandry and Fly Stocks
Animals were raised at low density at 25 °C on standard meal containing wheat flour (50 g/L); fresh yeast (100 g/L); agar-agar (11 g/L); dextrose monohydrate (80 g/L); propionic acid (6 mL/L); and Nipagin (1.56 g/L). Stocks were obtained from the Bloomington Drosophila Stock Center (BDSC) and the Vienna Drosophila Resource Center (VDRC) [25]. The Atf4 5’UTR::dsRed reporter is described in reference [36] and Xpb1::GFP is described in reference [37].
4.2. Morphological, Morphometric and Statistical Analysis
Adults of the corresponding genotypes were crossed for 24 h in vials with standard meal, then withdrawn and incubated for the indicated periods of time. Pictures of the larvae were taken with a Nikon SMZ800 stereoscope (Tokyo, Japan) using a Micrometrics 519CU OM camera and Micrometrics SE Premium 4 software (Unitron, New York, NY, USA). Drosophila wings were mounted in a 1:1 mixture of lactic acid/ethanol as described in reference [19] and photographed under an Olympus BX51 microscope (Tokyo, Japan) using a Moticam 2500 digital camera (Motic. XiangAn, Xiamen, China). The wing area was measured using Adobe Photoshop CS5 Extended (Adobe, San José, CA, USA). All data presented are mean ± standard deviation (s.d.) and were subjected to a one-way ANOVA test using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). p values lower than 0.05 were considered to be significant, unless otherwise indicated.
4.3. Immunofluorescence
Larvae were dissected and fixed as described in reference [56]. Confocal images were captured using a Zeiss LSM510 Meta confocal microscope (Oberkochen, Germany). Nuclei were stained with TO-PRO-3 (1:200, phalloidin (1:200, Invitrogen, Carlsbad, CA, USA) and cleaved Caspase-3 (1:200 Cell Signaling, Danvers, MA, USA).
4.4. Flow Cytometry
Larvae were dissected in the early third stage and wing imaginal discs were transferred to microtubes with 500 µL of Trypsin/EDTA (Sigma, St. Louis, MO, USA) and 10 µM DRAQ5 (Biostatus, Loughborough, UK). For disaggregation, imaginal discs were incubated a 25 °C with constant shaking for 2 h, and, to stop digestion, fetal bovine serum was added to a final concentration of 2%. Cells were filtered through a 0.35 µm mesh (Nythal, Heiden, Switzerland) and immediately analyzed in a FACSCanto flow cytometer (BD, Franklin Lakes, NJ, USA). Data were analyzed using FlowJo (TreeStar, Ashland, OR, USA).
5. Conclusions
t6A-modified tRNAs are required in Drosophila melanogaster for correct protein synthesis in order to support organismal growth and cell survival of imaginal cells.
Acknowledgments
This work was funded by FONDECYT 1140522 and FONDAP 15090007 grants to Alvaro Glavic and FONDECYT POSTDOC 3160326 to Diego Rojas-Benítez. We would like to thank The Company of Biologists and International Union of Biochemistry and Molecular Biology for travel funding to Diego Rojas-Benítez. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Vienna Drosophila Resource Center were used in this study.
Author Contributions
Diego Rojas-Benítez and Cristián Eggers conceived, performed the experiments, and wrote the paper. Alvaro Glavic conceived experiments and helped draft and revise the article for critically important intellectual content.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Phizicky E.M., Hopper A.K. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Yacoubi B., Bailly M., de Crecy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer rnas. Ann. Rev. Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 3.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 4.Novoa E.M., Pavon-Eternod M., Pan T., de Pouplana L.R. A role for tRNA modifications in genome structure and codon usage. Cell. 2012;149:202–213. doi: 10.1016/j.cell.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M., Guo J., Cha J., Chae M., Chen S., Barral J.M., Sachs M.S., Liu Y. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature. 2013;495:111–115. doi: 10.1038/nature11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front. Genet. 2014;5:144. doi: 10.3389/fgene.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machnicka M.A., Olchowik A., Grosjean H., Bujnicki J.M. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 2014;11:1619–1629. doi: 10.4161/15476286.2014.992273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugeron M.C., Lenstra T.L., Frizzarin M., El Yacoubi B., Liu X., Baudin-Baillieu A., Lijnzaad P., Decourty L., Saveanu C., Jacquier A., et al. Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 2011;39:6148–6160. doi: 10.1093/nar/gkr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J.P., Iwata-Reuyl D., Murzin A.G., de Crecy-Lagard V. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011;30:882–893. doi: 10.1038/emboj.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrochia L., Crozat E., Hecker A., Zhang W., Bareille J., Collinet B., van Tilbeurgh H., Forterre P., Basta T. In vitro biosynthesis of a universal t6A tRNA modification in archaea and eukarya. Nucleic Acids Res. 2013;41:1953–1964. doi: 10.1093/nar/gks1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutsch C., El Yacoubi B., de Crecy-Lagard V., Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J. Biol. Chem. 2012;287:13666–13673. doi: 10.1074/jbc.M112.344028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas-Benitez D., Thiaville P.C., de Crecy-Lagard V., Glavic A. The levels of a universally conserved trna modification regulate cell growth. J. Biol. Chem. 2015;290:18699–18707. doi: 10.1074/jbc.M115.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C.J., Smibert P., Zhao X., Hu J.F., Ramroop J., Kellner S.M., Benton M.A., Govind S., Dedon P.C., Sternglanz R., et al. An extensive allelic series of drosophila kae1 mutants reveals diverse and tissue-specific requirements for t6A biogenesis. RNA. 2015;21:2103–2118. doi: 10.1261/rna.053934.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins B.N., Keller E.B. The enzymatic synthesis of N-(purin-6-ylcarbamoyl)threonine, an anticodon-adjacent base in transfer ribonucleic acid. Biochemistry. 1974;13:4622–4628. doi: 10.1021/bi00719a024. [DOI] [PubMed] [Google Scholar]
- 15.Korner A., Soll D. N-(purin-6-ylcarbamoyl)threonine: Biosynthesis in vitro in transfer RNA by an enzyme purified from Escherichia coli. FEBS Lett. 1974;39:301–306. doi: 10.1016/0014-5793(74)80135-3. [DOI] [PubMed] [Google Scholar]
- 16.Chheda G.B., Hong C.I., Piskorz C.F., Harmon G.A. Biosynthesis of N-(purin-6-ylcarbamoyl)-l-threonine riboside. Incorporation of l-threonine in vivo into modified nucleoside of transfer ribonucleic acid. Biochem. J. 1972;127:515–519. doi: 10.1042/bj1270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrochia L., Guetta D., Hecker A., Forterre P., Basta T. Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A trna modification. Nucleic Acids Res. 2013;41:9484–9499. doi: 10.1093/nar/gkt720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiaville P.C., Iwata-Reuyl D., de Crecy-Lagard V. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t(6)A), a universal modification of tRNA. RNA Biol. 2014;11:1529–1539. doi: 10.4161/15476286.2014.992277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibar C., Cataldo V.F., Vasquez-Doorman C., Olguin P., Glavic A. Drosophila p53-related protein kinase is required for PI3K/TOR pathway-dependent growth. Development. 2013;140:1282–1291. doi: 10.1242/dev.086918. [DOI] [PubMed] [Google Scholar]
- 20.Thiaville P.C., Legendre R., Rojas-Benitez D., Baudin-Baillieu A., Hatin I., Chalancon G., Glavic A., Namy O., de Crecy-Lagard V. Global translational impacts of the loss of the trna modification t6A in yeast. Microb. Cell. 2016;3:29–45. doi: 10.15698/mic2016.01.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas-Benitez D., Ibar C., Glavic A. The drosophila EKC/KEOPS complex: Roles in protein synthesis homeostasis and animal growth. Fly. 2013;7:168–172. doi: 10.4161/fly.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetz C., Chevet E., Oakes S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 24.Kim K., Lee Y.S., Harris D., Nakahara K., Carthew R.W. The RNAi pathway initiated by Dicer-2 in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 2006;71:39–44. doi: 10.1101/sqb.2006.71.008. [DOI] [PubMed] [Google Scholar]
- 25.Dietzl G., Chen D., Schnorrer F., Su K.C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 26.Perkins L.A., Holderbaum L., Tao R., Hu Y., Sopko R., McCall K., Yang-Zhou D., Flockhart I., Binari R., Shim H.S., et al. The transgenic RNAi project at harvard medical school: Resources and validation. Genetics. 2015;201:843–852. doi: 10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y., Comjean A., Roesel C., Vinayagam A., Flockhart I., Zirin J., Perkins L., Perrimon N., Mohr S.E. FlyRNAi.Org—The database of the drosophila RNAi screening center and transgenic RNAi project: 2017 update. Nucleic Acids Res. 2017;45:D672–D678. doi: 10.1093/nar/gkw977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morata G. How drosophila appendages develop. Nat. Rev. Mol. Cell Biol. 2001;2:89–97. doi: 10.1038/35052047. [DOI] [PubMed] [Google Scholar]
- 29.Brower D.L. Engrailed gene expression in Drosophila imaginal discs. EMBO J. 1986;5:2649–2656. doi: 10.1002/j.1460-2075.1986.tb04547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermes I., Haanen C., Reutelingsperger C. Flow cytometry of apoptotic cell death. J. Immunol. Methods. 2000;243:167–190. doi: 10.1016/S0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsubo T., Kamada S., Tsujimoto Y. Inhibition of apoptosis by a baculovirus p35 gene. Nihon Rinsho. J. J. Clin. Med. 1996;54:1907–1911. [PubMed] [Google Scholar]
- 32.Hay B.A., Wolff T., Rubin G.M. Expression of baculovirus p35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 33.Igaki T., Kanda H., Yamamoto-Goto Y., Kanuka H., Kuranaga E., Aigaki T., Miura M. Eiger, a TNF superfamily ligand that triggers the drosophila JNK pathway. EMBO J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hetz C. The biological meaning of the UPR. Nat. Rev. Mol. Cell Biol. 2013;14:404. doi: 10.1038/nrm3606. [DOI] [PubMed] [Google Scholar]
- 35.Demay Y., Perochon J., Szuplewski S., Mignotte B., Gaumer S. The PERK pathway independently triggers apoptosis and a Rac1/Slpr/JNK/Dilp8 signaling favoring tissue homeostasis in a chronic ER stress drosophila model. Cell Death Dis. 2014;5:e1452. doi: 10.1038/cddis.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang K., Ryoo H.D., Park J.E., Yoon J.H., Kang M.J. A Drosophila reporter for the translational activation of ATF4 marks stressed cells during development. PLoS ONE. 2015;10:e0126795. doi: 10.1371/journal.pone.0126795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sone M., Zeng X., Larese J., Ryoo H.D. A modified upr stress sensing system reveals a novel tissue distribution of IRE1/XBP1 activity during normal Drosophila development. Cell Stress Chaperones. 2013;18:307–319. doi: 10.1007/s12192-012-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer M.P., Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. CMLS. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. Modomics: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helm M. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006;34:721–733. doi: 10.1093/nar/gkj471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandrov A., Chernyakov I., Gu W., Hiley S.L., Hughes T.R., Grayhack E.J., Phizicky E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 42.Grosjean H., Breton M., Sirand-Pugnet P., Tardy F., Thiaucourt F., Citti C., Barre A., Yoshizawa S., Fourmy D., de Crecy-Lagard V., et al. Predicting the minimal translation apparatus: Lessons from the reductive evolution of mollicutes. PLoS Genet. 2014;10:e1004363. doi: 10.1371/journal.pgen.1004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarian C., Townsend H., Czestkowski W., Sochacka E., Malkiewicz A.J., Guenther R., Miskiewicz A., Agris P.F. Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem. 2002;277:16391–16395. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- 44.McKenney K.M., Alfonzo J.D. From prebiotics to probiotics: The evolution and functions of tRNA modifications. Life. 2016;6:13. doi: 10.3390/life6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres A.G., Batlle E., de Pouplana L.R. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014;20:306–314. doi: 10.1016/j.molmed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Kirchner S., Ignatova Z. Emerging roles of trna in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98–112. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 47.St Pierre S.E., Ponting L., Stefancsik R., McQuilton P., FlyBase C. Flybase 102—Advanced approaches to interrogating FlyBase. Nucleic Acids Res. 2014;42:D780–D788. doi: 10.1093/nar/gkt1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 49.Wu M.Y., Cully M., Andersen D., Leevers S.J. Insulin delays the progression of Drosophila cells through G2/M by activating the dTOR/dRaptor complex. EMBO J. 2007;26:371–379. doi: 10.1038/sj.emboj.7601487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duechler M., Leszczynska G., Sochacka E., Nawrot B. Nucleoside modifications in the regulation of gene expression: Focus on trna. Cell. Mol. Life Sci. CMLS. 2016;73:3075–3095. doi: 10.1007/s00018-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endres L., Dedon P.C., Begley T.J. Codon-biased translation can be regulated by wobble-base tRNA modification systems during cellular stress responses. RNA Biol. 2015;12:603–614. doi: 10.1080/15476286.2015.1031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klassen R., Grunewald P., Thuring K.L., Eichler C., Helm M., Schaffrath R. Loss of anticodon wobble uridine modifications affects tRNAlys function and protein levels in Saccharomyces cerevisiae. PLoS ONE. 2015;10:e0119261. doi: 10.1371/journal.pone.0119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zinshteyn B., Gilbert W.V. Loss of a conserved trna anticodon modification perturbs cellular signaling. PLoS Genet. 2013;9:e1003675. doi: 10.1371/journal.pgen.1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullor J.L., Guerrero I. A gain-of-function mutant of patched dissects different responses to the hedgehog gradient. Dev. Biol. 2000;228:211–224. doi: 10.1006/dbio.2000.9862. [DOI] [PubMed] [Google Scholar]
- 56.Cruz C., Glavic A., Casado M., de Celis J.F. A gain-of-function screen identifying genes required for growth and pattern formation of the Drosophila melanogaster wing. Genetics. 2009;183:1005–1026. doi: 10.1534/genetics.109.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]