Abstract
High levels of inflammatory cytokines in the genital tract suggest mucosal vulnerability and increased risk of HIV and sexually transmitted infection (STI) acquisition. Intravaginal douching is associated with bacterial vaginosis (BV) in women in the United States, and both douching and BV are linked to HIV and STI acquisition. This study evaluates inflammatory cytokines in the genital tract to increase understanding of the effects of both BV and intravaginal douching to the vaginal mucosa. A cross-sectional study of participants in the Miami WIHS investigated 72 reproductive age women (45 HIV+ and 27 high-risk HIV−) who completed intravaginal douching questionnaires and underwent collection of vaginal swabs and cervicovaginal lavages (CVLs). BV was assessed using the Nugent score. Inflammatory cytokines in the CVLs (interleukin [IL]-6, IL-8, IL-1α, IL-1β, soluble intracellular adhesion molecule-1 [sICAM-1], interferon [IFN]α2, chemokine C ligand 5 (CCL5), vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP1), tumor necrosis factor alpha (TNFα), and secretory leukocyte protease inhibitor [SLPI]) were measured. Fourteen (19%) women reported intravaginal douching; 24 (33%) had BV. BV, intravaginal douching, and HIV were associated with higher levels of inflammatory cytokines. After controlling for demographic and risk factors and HIV status, women who had BV and douched had higher levels of inflammatory cytokines than those without BV and who did not douche, or who only had BV or only douched. These findings suggest that BV and douching are associated with greater mucosal inflammation and may facilitate HIV acquisition and transmission. Although longitudinal studies are needed to determine temporal associations and causality, interventions to decrease rates of intravaginal douching and BV could significantly decrease women's risks of acquiring STIs and HIV and limit the spread of HIV.
Keywords: : vaginal douches, bacterial vaginosis, women and HIV, genital cytokines
Introduction
Cytokines and chemokines in the female reproductive tract (FRT) are released to induce an innate immune inflammatory response to sexually transmitted pathogens and resident members of the pathogenic vaginal microbiota.1 However, excess levels of inflammation lead to cellular damage and increased mucosal vulnerability to sexually transmitted infections (STI) and HIV.2–4 Interleukin (IL) IL-6, IL-8, IL-1α, IL-1β, soluble intracellular adhesion molecule-1 (sICAM-1), chemokine C ligand 5 (CCL5) (also known as Regulated on Activation Normal T cell Expressed and Secreted or RANTES), and vascular endothelial growth factor (VEGF) are considered prominent indicators of vaginal inflammation and, when expressed at high levels, are associated with FRT vulnerability to infection and HIV seroconversion.4,5 Secretory leukocyte protease inhibitor (SLPI) is an antimicrobial protein and decreased levels in the FRT are considered indicative of mucosal vulnerability.6–8 In women with HIV infection, high FRT inflammatory cytokines are associated with increased genital tract HIV RNA levels or HIV shedding and contribute to a greater risk of HIV transmission to sexual partners and newborns.3,9,10
Bacterial vaginosis (BV), the most common FRT infection, is characterized by a decrease in the number of vaginal lactobacilli and an increase in the number of anaerobic gram-negative bacilli.11,12 BV is associated with increased risk of obstetric and gynecological complications, STI and HIV acquisition, and HIV transmission to sexual partners and newborns.13,14 BV occurs in about 30% of reproductive age women, and up to 84% of BV is asymptomatic.15 Women with BV have higher levels of vaginal inflammatory cytokines and lower levels of SLPI than those without BV, and treatment for BV has been shown to decrease FRT inflammatory cytokines.6,16–20
Intravaginal cleansing is the practice of introducing substances inside the vagina for the purpose of hygiene, and the use of commercially available vaginal douches is common among women in the United States.21–23 Intravaginal cleansing is associated with a significant decrease in lactobacilli in the FRT, indicating the potential risk for the development of BV.24–27 In addition to promoting BV, intravaginal cleansing is associated with other obstetric and gynecological complications (pelvic inflammatory disease [PID] and ectopic pregnancy)21,28 and although definitive evidence is not conclusive, intravaginal cleansing may increase the risk of HIV acquisition.29–32
The mechanism by which BV and intravaginal cleansing facilitate HIV acquisition is not well understood and is likely mediated by changes in the inflammatory milieu of the FRT with recruitment of HIV target cells.33,34 The effect of introducing feminine hygiene products on the FRT inflammation has previously been evaluated in vitro, and it has been suggested that vaginal douches promote a proinflammatory environment in the presence of BV.35 The in vitro study showed a cause–effect relationship between commercially available vaginal douches and increased levels of inflammatory cytokines.35 A study determined that lime juice tampons but not douches caused a significant change in the levels of inflammatory cervicovaginal cytokines; however, this study was done in women at low risk of HIV and did not evaluate BV.34 No research has evaluated FRT inflammation in vivo in women who engage in vaginal cleansing using commercially available douches, nor if the combination of cleansing and BV increases FRT inflammatory markers and places women at a higher risk than vaginal cleansing or BV alone.
This innovative study assesses the relative contribution of the use of vaginal douches and BV to changes in lower FRT mucosal inflammatory markers, to increase understanding of the damaging effects of vaginal cleansing on the vaginal mucosa. It was hypothesized that women engaging in vaginal douching who have BV will have higher levels of FRT inflammatory cytokines than those who do not engage in vaginal douching and do not have BV. Results have relevance to both women with HIV and those at risk of HIV infection.
Materials and Methods
Ethics statement
Before study-related procedures, recruitment, or data analysis, approval was obtained from the Institutional Review Board at the University of Miami Miller School of Medicine. Preceding enrollment and provision of informed consent, participants were provided detailed information on study aims and assured of the confidentiality of study records. Signed informed consent was obtained by the study coordinator from all participants before any study-related assessment.
Study procedures
The study utilized a cross-sectional design enrolling women with HIV or at risk for HIV infection, and it was conducted at the AIDS Clinical Research Unit (ACRU) and the Behavioral Research Unit at the University of Miami, FL. The study was undertaken in cooperation with the Miami Women and HIV Interagency Study (WIHS) and the Miami Center for AIDS Research (CFAR). As per WIHS design, HIV-infected and HIV-uninfected women were recruited on a 2:1 ratio, and within the WIHS, a subset of women were corecruited for the present study. HIV-uninfected women enrolled in the WIHS report at least one of the following risk factors in the prior year: injection drug use; diagnosis of an STI; unprotected intercourse with three or more men or protected with more than five men; or exchange of sex for drugs, money, or shelter. Eligibility criteria for participation were women 18 to 45 years of age and being sexually active in the 3 months previous to study enrollment. Pregnant women or women using contraceptive medications or intrauterine devices were excluded. The study coordinator conducted web-based assessment of demographic and sexual risk factors, medical history, and vaginal cleansing practices. On completion of the web-based assessment, genital samples were collected.
HIV testing
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test was used to determine HIV status for women without documentation of HIV status. Positive results were followed by a confirmatory HIV western blot. Participants known to be infected with HIV before the study presented documentation of positive HIV status, such as HIV western blot results, medical records, or two laboratory results with detectable HIV viral loads greater than 1000 copies/ml, and had a rapid test performed as confirmation.
Genital sample collection
Vaginal samples were collected by first inserting a vaginal speculum and then collecting with a vaginal cotton swab the secretions in the vaginal wall. The vaginal swab was placed on a glass slide and Gram stained for Nugent scoring. Following collection, the cervical os was examined and mucous was cleaned off. Cervicovaginal lavage (CVL) was then collected after installing 10 ml of sterile saline into the vaginal and cervical areas avoiding the os, and letting it rest for 60 s. CVL samples were transported on ice to the laboratory for analysis within 2 h of collection. CVLs were spun down for 10 min at 1000 g at 4°C and supernatants aliquoted and stored at −80°C for cytokine analysis.
Demographics and sexual risk factors
The demographic questionnaire assessed participant's age, race and ethnicity, marital status, educational level, employment, yearly income, alcohol use in the prior month, number of partners, history of exchanging sex for money, use of male condoms, and whether they had sexual activity the week before enrollment.
Medical history
The medical history questionnaires assessed whether a medical provider had previously diagnosed participants with STI (gonorrhea, syphilis, chlamydia, herpes, and trichomoniasis), PID, BV, and candida vaginitis, and self-reported date of their last menstrual period.
Vaginal cleansing and use of vaginal douches
The intravaginal cleansing questionnaire assessed whether participants had used a commercially available intravaginal douche in the past month using dichotomous response options (“yes,” “no”). Vaginal douching was limited to the use of commercial available douches and did not include the use of other products for vaginal cleansing. In addition to commercial douches, other products used for intravaginal cleansing (water alone, soap with water, vinegar, and homemade douches) were also assessed. Responses were not mutually exclusive.
Bacterial vaginosis
BV was diagnosed by Gram staining techniques of slides with vaginal secretions at the University of Miami microbiology laboratories within 24 h of collection. Staff were trained on proper procedures before analyzing the slides by the chief microbiologist and the same trained technicians were used to read the slides. Unclear cases were reviewed and confirmed by a second technician and the laboratory chief once a consensus was reached. The slides were judged based on Nugent criteria. A Nugent score of 4 or greater was diagnosed as abnormal vaginal flora, while a Nugent score of 7 or above was classified as BV.
Lower FRT inflammatory cytokines
Multiplex measurement in CVL samples for cytokines, sICAM-1, and CCL5
A panel containing 13 inflammatory (IL-6, IL-8, IL-1α, interleukin 1 receptor antagonist [IL-1Rα], IL-1β, interferon [IFN]α2, IL-10, IL-17α, VEGF, IL-4, interferon gamma-induced protein 10 [IP-10], monocyte chemoattractant protein-1 [MCP1], and tumor necrosis alpha [TNFα]) cytokines was measured in CVL samples using a customized MILLIPLEX™ Cytokine Human Ultrasensitive magnetic bead panel (EMD Millipore, Billerica, MA). A separate panel was used for both sICAM-1 and CCL5 measurement as suggested by the manufacturer. Undiluted samples were incubated overnight with cytokine-specific beads at 4°C with shaking. After washing, biotinylated detection antibodies and streptavidin-phycoerythrin were subsequently added. The beads were washed and acquired on a MAGPIX instrument (Luminex Corporation, Austin, TX). Median fluorescent intensity data were collected and analyzed with MILLIPLEX Analyst Software (EMD Millipore) and results were expressed as pg/ml. The lowest detection limit of standard curve for all analytes, except CCL5, was 3.2 pg/ml; for CCL5, the limit of standard curve was 2.44 pg/ml. Results for IL-10, IL-4, IL-17α, IP10, and IL-1Rα were not used for the analysis, given that most (∼80%) participants' values were below the detection cutoffs.
Measurement of SLPI levels in CVL
SLPI levels were measured in diluted samples by ELISA (Bio-Techne, Minneapolis, MN) with a sensitivity of detection of 25 pg/ml. Samples were tested using dilution ranges from 1:50, 1:100, to 1:300 to measure the actual SLPI levels in samples. Results were expressed as ng/ml.
Statistical analyses
Descriptive statistics were used to analyze demographic and medical history variables. Univariable Poisson models predicting cytokines were used to test for differences between BV, vaginal douching, HIV status, condom use, and race, and associations between cytokines and days since last menstruation, age, and frequency of sexual encounters. Vaginal douching was defined as having used commercial vaginal douches in the past month. Although other vaginal cleansing practices were assessed, due to collinearity, only vaginal douching with commercially available douches was included in the analysis as this practice is the most closely associated with the development of BV in the United States and products included in vaginal douches have previously shown a potential detrimental effect on the vaginal mucosa in the presence of BV.35 We chose not to combine different cleansing practices because all participants engaged in at least one type of cleansing practice, and thus would have resulted in zero-cells and hindered statistical analysis. For values below or above the detection limits, the natural log of the value calculated from the division of the cutoff value by the square root of 2 was used. All models were built using PROC GLIMMIX with a log link and back-transformed medians and standard errors. Then, using the same analytic procedure, multivariable Poisson models were built with BV, vaginal douching, HIV status, condom use, race, days since last menstruation, age, and frequency of sex in the past month as predictors. Last, an interaction of BV and vaginal douching was tested for all cytokines, if both predictors were significant at p < .10 in multivariable models, also using a Poisson regression model with interaction. To follow-up significant interaction effects, medians were plotted and all possible six pairwise comparisons were tested for significance. Statistical Analysis System (SAS) v9.4 for Windows was used for all analyses.
Results
Demographics, medical history, douching history, and Nugent score (Table 1)
Table 1.
Sociodemographic Characteristics, Medical History, Use of Vaginal Douches, and Bacterial Vaginosis (N = 72)
| Characteristic | All | HIV− (n = 27) | HIV+ (n = 45) | X2/t/Z, p |
|---|---|---|---|---|
| Mean age (SD) | 34.17 (7.30) | 34.88 (6.32) | 34.80 (7.87) | 0.22, .829 |
| Race, n (%) | ||||
| Black | 55 (76.4) | 19 (70.4) | 36 (80.0) | |
| White | 12 (16.7) | 5 (18.5) | 7 (15.6) | |
| Mixed or other | 5 (6.9) | 3 (11.1) | 2 (4.4) | 1.51, .468 |
| Ethnicity, n (%) | ||||
| Hispanic | 13 (18.1) | 8 (29.6) | 5 (11.1) | |
| Non-Hispanic | 46 (63.9) | 16 (59.3) | 30 (66.7) | |
| Haitian | 7 (9.7) | 0 (0.0) | 7 (15.6) | |
| Other | 6 (8.3) | 3 (11.1) | 3 (6.7) | 8.02, .035 |
| Marital status, n (%) | ||||
| Never married | 28 (40.6) | 12 (46.2) | 16 (37.2) | |
| Stable partnership | 8 (11.6) | 2 (7.7) | 6 (14.0) | |
| Unstable partnership | 33 (47.8) | 12 (46.2) | 21 (48.8) | 0.84, .692 |
| Educational level, n (%) | ||||
| High school or less | 51 (71.8) | 15 (55.6) | 36 (81.8) | |
| More than high school | 20 (28.2) | 12 (44.4) | 8 (18.2) | 5.70, .017 |
| Employed, n (%) | ||||
| Yes | 16 (23.2) | 8 (30.8) | 8 (18.6) | |
| No | 53 (76.8) | 18 (69.2) | 35 (81.4) | 1.35, .246 |
| Yearly household income (USD), n (%) | ||||
| ≤1200 | 50 (72.5) | 14 (53.8) | 36 (83.7) | |
| >1200 | 19 (27.5) | 12 (46.2) | 7 (16.3) | 7.25, .007 |
| Alcohol use last month, n (%) | ||||
| Yes | 36 (52.2) | 17 (65.4) | 19 (44.2) | |
| No | 33 (47.8) | 9 (34.6) | 24 (55.8) | 2.92, .088 |
| Partners past 5 years, mean (SD) | 10.67 (21.29) | 16.93 (28.50) | 6.91 (14.59) | 1.12, .262 |
| Condom use past month, n (%) | ||||
| Always | 35 (50.0) | 9 (33.3) | 26 (60.5) | |
| Sometimes | 13 (18.6) | 4 (14.8) | 9 (20.9) | |
| Never | 22 (31.4) | 14 (51.9) | 8 (18.6) | 8.61, .014 |
| Lifetime exchange of sex for compensation, n (%) | ||||
| Yes | 27 (38.0) | 12 (44.4) | 15 (34.1) | |
| No | 44 (62.0) | 15 (55.6) | 29 (65.9) | 0.76, .363 |
| History of gonorrhea, n (%) | ||||
| No | 58 (80.6) | 25 (92.6) | 33 (73.3) | |
| Yes | 14 (19.4) | 2 (7.4) | 12 (26.7) | 4.00, .046 |
| History of syphilis, n (%) | ||||
| No | 59 (81.9) | 26 (96.3) | 33 (73.3) | |
| Yes | 13 (18.1) | 1 (3.7) | 12 (26.7) | 6.01, .014 |
| History of chlamydia, n (%) | ||||
| Yes | 50 (69.4) | 20 (74.1) | 30 (66.7) | |
| No | 22 (30.6) | 7 (25.9) | 15 (33.3) | 0.44, .602 |
| History of PID, n (%) | ||||
| No | 66 (91.7) | 26 (96.3) | 40 (88.9) | |
| Yes | 6 (8.3) | 1 (3.7) | 5 (11.1) | 1.09, .400 |
| History of herpes, n (%) | ||||
| No | 65 (90.3) | 25 (92.6) | 40 (88.9) | |
| Yes | 7 (9.7) | 2 (7.4) | 5 (11.1) | 0.510, .704 |
| History of trichomoniasis, n (%) | ||||
| No | 53 (73.6) | 20 (74.1) | 33 (73.3) | 0.005, 1.000 |
| Yes | 19 (26.4) | 7 (25.9) | 12 (26.7) | |
| History of BV, n (%) | ||||
| No | 58 (80.6) | 21 (77.8) | 37 (82.2) | |
| Yes | 14 (19.4) | 6 (22.2) | 8 (17.8) | 0.213, .761 |
| History of candida vaginitis, n (%) | ||||
| No | 38 (52.8) | 9 (33.3) | 29 (64.4) | |
| Yes | 34 (47.2) | 18 (66.7) | 16 (35.6) | 6.554, .015 |
| Use of commercial douches in past month, n (%) | ||||
| No | 58 (80.6) | 21 (77.8) | 37 (82.2) | |
| Yes | 14 (19.4) | 6 (22.2) | 8 (17.8) | 0.21, .645 |
| Bacterial vaginosis, n (%) | ||||
| No | 48 (66.7) | 18 (66.7) | 30 (66.7) | |
| Yes | 24 (33.3) | 9 (33.3) | 15 (33.3) | 0.00, 1.000 |
Bold numbers indicate significant p value <0.05.
BV, bacterial vaginosis; PID, pelvic inflammatory disease.
Participants were 72 women with a mean age of 34.17 (SD = 7.30). Three-fourths (76%) of the women were black and participants were primarily (64%) non-Hispanic. Nearly half (48%) of the women were in an unstable partnership. Most women had completed high school or less (72%), were unemployed (77%), and had a yearly household income of less than 1,200 (73%). On average, participants had 11 partners in the past 5 years (SD = 21.29), and almost two-fifths (38.0%) of the women reported having exchanged sex for compensation in the past. Only half (50.0%) reported consistent condom use in the past month, and an average of 5 (SD = 7.17) sexual encounters were reported in the past month. The median number of days from menstruation was 14 days (SD = 6.9).
Many of the participants reported a prior STI. Nearly one-fifth (19.4%) of participants reported having a history of gonorrhea, and 18% of syphilis, 31% of chlamydia, 8% of PID, 10% of herpes, 26% of trichomoniasis, 19% of BV, and 47% of candida vaginitis. Further details of demographic characteristics and medical history are reported in Table 1.
About two-thirds of women were infected with HIV (45% or 63%). All women reported at least one type of intravaginal cleansing practice. Twenty-seven (37%) women reported the use of water, 22 (31%) used soap with water, 10 (14%) used vinegar, and 8 (11%) used homemade douches made with soap or vinegar. Douching with a commercially available douche in the prior month was reported by 14 women (19%).
More than two-thirds of the sample (48 women) had Nugent scores consistent with abnormal vaginal flora (Nugent score = 4–6), and one-third (24) had a BV Nugent score (Nugent score ≥7). None had a normal Nugent score of 0–3, which is not unexpected in a population at high risk for HIV.
The demographic and risk factors, medical history, douching history, and BV are shown in Table 1.
Associations of lower FRT soluble inflammatory markers (IL-6, IL-8, IL1α, IL1β, SLPI, sICAM-1, IFNα2, CCL5, VEGF, MCP1, and TNFα) by demographic and risk factors, date from menstrual period, use of vaginal douches, BV, and HIV status
In bivariate analyses and multivariable analysis (using age, race, condom use, frequency of sexual encounters, days since last menstruation, vaginal douching, HIV status, and BV as predictors), most inflammatory cytokines were higher in younger women, women of white race, those who reported consistent condom use, had higher number of sexual encounters, shorter time from menstruation, used vaginal douches, had HIV infection, or had BV. Table 2 presents the unadjusted and adjusted models.
Table 2.
Unadjusted and Adjusted Models Predicting IL-6, IL-8, IL-1α, IL-1β, SLPI, sICAM-1, IFNα2, CCL5, VEGF, MCP1, and TNFα (n = 72)
|
IL-6 Mean |
IL-8 Mean |
IL-1α Mean |
IL-1β Mean |
SLPI Mean |
sICAM-1 Mean |
IFNα2 Mean |
CCL5 Mean |
VEGF Mean |
MCP1 Mean |
TNFα Mean |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||
| Estimate | −0.049 | −0.018 | −0.017 | 0.017 | −0.022 | −0.034 | 0.002 | −0.027 | −0.004 | −0.007 | −0.003 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .813 | <.001 | <.001 | <.001 | .152 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .336 | .002 | <.001 | <.001 | .681 |
| Race | |||||||||||
| Black/African American (BA) | 4.62 | 822.46 | 3572.02 | 76.57 | 139984 | 155.30 | 91.43 | 0.92 | 225.73 | 105.71 | 5.45 |
| White (WH) | 37.38 | 1601.14 | 2928.20 | 124.56 | 313357 | 305.98 | 91.36 | 8.64 | 237.95 | 220.07 | 5.71 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .429 | <.001 | .007 | <.001 | .132 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.002 | <.001 | .981 | <.001 | .017 | <.001 | .769 |
| Condom use in the past month | |||||||||||
| Always | 18.97 | 1169.11 | 2707.34 | 106.36 | 264025 | 269.31 | 92.63 | 6.44 | 249.84 | 172.57 | 6.03 |
| Sometimes/never | 9.10 | 1126.39 | 3863.42 | 89.67 | 166140 | 176.44 | 90.17 | 1.24 | 214.99 | 134.81 | 5.17 |
| Unadjusted p | .021 | <.001 | <.001 | <.001 | <.001 | <.001 | .645 | <.001 | <.001 | <.001 | .116 |
| Adjusted pa | <.001 | <.001 | <0.001 | <.001 | <.001 | <.001 | .324 | <.001 | <.001 | <.001 | .179 |
| Frequency of sexual encounters | |||||||||||
| Estimate | 0.023 | −0.042 | 0.004 | 0.042 | −0.006 | 0.019 | 0.009 | −0.060 | 0.009 | −0.029 | 0.022 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .047 | <.001 | <.001 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .004 |
| Days since last menstruation | |||||||||||
| Estimate | −0.0057 | −0.0010 | 0.0004 | −0.0006 | −0.0005 | −0.0013 | −0.0006 | −0.0012 | −0.0003 | −0.0004 | 0.0001 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .350 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .066 | <.001 | <.001 | .456 |
| User of vaginal douches | |||||||||||
| No | 23.62 | 1335.42 | 2326.49 | 60.86 | 241849 | 205.30 | 82.46 | 6.60 | 215.24 | 199.90 | 5.18 |
| Yes | 7.31 | 986.11 | 4495.87 | 156.72 | 181374 | 231.46 | 101.30 | 1.21 | 249.56 | 116.38 | 6.01 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .327 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | .044 | <.001 | <.001 | <.001 | <.001 | .335 |
| HIV status | |||||||||||
| Uninfected | 18.80 | 1085.25 | 3378.31 | 104.54 | 205496 | 188.37 | 84.72 | 5.89 | 229.46 | 133.22 | 5.76 |
| Infected | 9.18 | 1213.43 | 3096.10 | 91.23 | 213459 | 252.26 | 98.59 | 1.35 | 234.09 | 174.63 | 5.41 |
| Unadjusted p | <.001 | .345 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .059 | <.001 | .878 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .249 | <.001 | .589 |
| BV | |||||||||||
| No | 11.26 | 1016.03 | 2380.28 | 64.01 | 205496 | 159.79 | 87.76 | 2.54 | 229.82 | 215.00 | 4.01 |
| Yes | 15.33 | 1296.10 | 4394.28 | 149.00 | 213459 | 297.37 | 95.18 | 3.14 | 233.72 | 108.21 | 7.76 |
| Unadjusted p | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .001 | <.001 | <.001 |
| Adjusted pa | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .007 | .168 | .364 | <.001 | <.001 |
Adjusted means (for categorical variables) or exponentiated coefficients/estimates (for continuous variables) are presented.
Bold numbers indicate significant p value <0.05.
Multivariable Poisson regression models included age (continuous), race, condom use, frequency of sexual encounters (continuous), days since last menstruation (continuous), vaginal douching, HIV status, and BV as predictors.
Unit: picograms per milliliter (pg/ml).
CCL5, chemokine C ligand 5; IL, interleukin; IFN, interferon; MCP1, monocyte chemotactic protein-1; sICAM-1, soluble intracellular adhesion molecule-1; SLPI, secretory leukocyte protease inhibitor; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Inflammatory cytokines in women with BV and engaging in vaginal douches
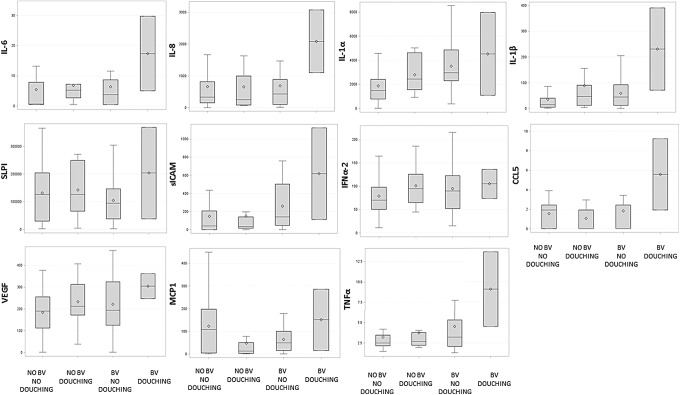
Following multivariable models, interactions of BV and vaginal douching were tested for all cytokines (IL-6, IL-8, IL-1α, IL-1β, SLPI, sICAM, IFNα2, CCL5, VEGF, MCP1, and TNFα), controlling for HIV status. Significant interaction effects between commercial douching and BV were found for IL-8, IL1α, IL1β, SLPI, IFNα2, sICAM, CCL5, VEGF, MCP1, and TNFα. Interactions were plotted and are presented in Figure 1, along with their respective follow-up pairwise comparisons. That is, although levels of inflammatory cytokines were different depending on whether the woman had BV, this varied as a function of whether the woman used vaginal douches, such that levels of mediators of inflammation were highest in women who had BV and douched when compared to women who did not have BV and did not douche, douched and did not have BV, and had BV and did not douche. All interactions were tested, including HIV status, condom use, race, days since last menstruation, age, and frequency of sexual encounters as controls. Analysis to assess the relationship of inflammatory cytokines in women with BV engaging in intravaginal cleansing other than commercial douches was conducted and no significant associations were found.
FIG. 1.
Inflammatory cytokines (pg/ml) in women engaging in vaginal douching with and without BV. Box plots report minimum, first interquartile range, median, third interquartile range, and maximum values; the mean is identified by open squares. IL-6: Levels of IL-6 were not significantly different between groups. IL-8: No BV and no douching was significantly different from no BV and douching and BV and douching; no BV and douching was significantly different from BV and no douching and BV and douching; BV and no douching was significantly different than BV and douching (all p < .01). IL-1α, IL-1β, SLPI, MCP1: All groups were significantly different from each other (all p < .05). sICAM-1: No BV and no douching was significantly different from no BV and douching, BV and no douching, and BV and douching; no BV and douching was significantly different from BV and no douching, and BV and douching; BV and no douching was significantly different than BV and douching (all p < .01). IFNα2: No BV and no douching was significantly different from no BV and douching, and BV and no douching (p < .05); no BV and douching was significantly different than BV and no douching. CCL5: No BV and douching was significantly different than BV and no douching and BV and douching (p < .05). VEGF: No BV and no douching was significantly different from no BV and douching, BV and no douching, and BV and douching; no BV and douching was significantly different from BV and douching; BV and no douching was significantly different than BV and douching, and BV and douching (all p < .01). TNFα: No BV and no douching was significantly different than BV and no douching, and BV and douching (p < .01); no BV and douching was significantly different than BV and douching (p < .05). All models controlled for age (continuous), race, condom use, frequency of sexual encounters (continuous), days since last menstruation (continuous), vaginal douching, HIV status, and BV. BV, bacterial vaginosis; CCL5, chemokine C ligand 5; IFN, interferon; MCP1, monocyte chemotactic protein-1; sICAM-1, soluble intracellular adhesion molecule-1; SLPI, secretory leukocyte protease inhibitor; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Discussion
This study examined the levels of inflammatory cytokines in the FRT of women with HIV or at risk for HIV infection, and the relative contribution of the use of commercial vaginal douches and BV to distal FRT inflammation. Results indicated that most genital tract inflammatory cytokines were higher in younger women, women of white race, those who reported consistent condom use, had higher number of sexual encounters, shorter time from menstruation, used vaginal douches, had HIV infection, or had BV. In addition, distal FRT inflammation was increased in women who used vaginal douches and had BV when compared to those who did not use vaginal douches and/or did not have BV, regardless of demographic and risk factors and HIV status.
In the United States, the use of commercially available vaginal douches for vaginal cleansing has previously been identified as one of the main risk factors for developing BV, and BV has been associated with HIV acquisition.14,36,37 In addition to HIV, BV has also been associated with STI acquisition, HIV transmission to sexual partners and newborns, preterm delivery, and spontaneous abortions.38 Therefore, both vaginal cleansing and BV are important contributors to poor women's health outcomes and risk of STI and HIV acquisition.21,28,39
The mechanism by which vaginal cleansing and BV increase the risk of HIV acquisition is not well understood, as it is not clear if the risk of using vaginal douches is independent or mediated by BV. It has been postulated that vaginal douches promote a change in the vaginal flora facilitating BV and an increase in the FRT inflammatory milieu with the associated release of inflammatory cytokines. Such an inflammatory milieu favors recruitment and activation of HIV host cells, the expression of HIV receptors, and tissue damage, all increasing mucosal vulnerability to HIV during vaginal intercourse.35
The increase of inflammatory cytokines in the FRT in women with BV and in HIV-infected women has previously been described.6,9,19 However, this is the first study to include the effect of vaginal cleansing with commercial douches as a potential contributor to FRT inflammation. Higher levels of cytokines identified in women who both engage in vaginal douching and have BV suggest that the combination of both factors impacts markers of inflammation in a greater magnitude than BV alone, causing the highest damage to the FRT mucosa and the highest potential for HIV acquisition.
In interpreting study findings, some limitations must be discussed: (1) the study's small sample size limits the generalizability of the results; (2) BV was assessed by using Nugent criteria and more sophisticated culture or microbiome analysis could have differentiated more microbial factors associated with inflammation in women who douche; (3) the results only apply to vaginal douching using commercial products and cannot be generalized to other vaginal cleansing practices or combination of similar practices; (4) our study did not have the power to detect differences related to levels of endogenous or exogenous sex steroid hormones and we did not determine the phase of the menstrual cycle. We, however, included the number of days from the last menstruation as a control variable; and (5) because of the cross-sectional and noninterventional nature of our study, we are limited on inferring causality based on associations. Longitudinal studies with a larger sample size and frequent assessments are needed to account for hormonal variations associated with menstrual cycles, and to establish a baseline level of cytokines and changes in cytokines over time. In addition, women who are not at risk for HIV should be included in future studies as controls.
Public health efforts should be undertaken to inform women of the potential damaging effects of douching on the vaginal mucosal surface and the risks associated with vaginal douches as well as other intravaginal cleansing practices, including the risk of HIV acquisition. Both BV and vaginal douching are difficult to influence; BV is often asymptomatic and screening for asymptomatic BV has not shown to be beneficial in the general population, and douching is a common behavior among women.15,36 This study provides further evidence to support continued research evaluating interventions to decrease the use of vaginal douches and BV in both HIV-infected and HIV-uninfected women, as such interventions could be of relevance in women's health programs, especially in those that focus on STI and HIV prevention.
Acknowledgments
This study was funded by the National Institutes of Health, the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine [P30AI073961], Miami Women's Interagency HIV Infection Study (WIHS) [U01AI103397], and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [K23HD074489]. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Disclaimer
Data in this article were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I–WIHS IV).
Authors' Contributions
All authors have seen and approved the content of this article, have contributed significantly to the work, and declare no conflict of interest. No writing assistance was sought in preparing this manuscript. M.L.A., S.P., S.P., D.L.J., and M.A.F. contributed to the conception and design of the work. M.L.A. and V.J.R. contributed to the acquisition of the data. S.P., M.R., and O.M. contributed performing laboratory analysis. M.L.A., V.J.R., and K.A. contributed to the analysis and interpretation of the data. R.N.F. contributed with advice on interpretation of the cytokine analyses. M.L.A., R.N.F., S.P., D.L.J., S.P., and M.A.F. drafted the manuscript and revised it critically for important intellectual content. All authors have contributed to the final version of this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fahey JV, Schaefer TM, Channon JY, Wira CR: Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod 2005;20:1439–1446 [DOI] [PubMed] [Google Scholar]
- 2.Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ: High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis 2000;182:467–473 [DOI] [PubMed] [Google Scholar]
- 3.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A: Markers of local immunity in cervico-vaginal secretions of HIV infected women: Implications for HIV shedding. Sex Transm Infect 2004;80:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison C, Fichorova RN, Mauck C, et al. : Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014;66:109–117 [DOI] [PubMed] [Google Scholar]
- 5.Masson L, Mlisana K, Little F, et al. : Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: A cross-sectional study. Sex Transm Infect 2014;90:580–587 [DOI] [PubMed] [Google Scholar]
- 6.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP: Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol 2000;183:1243–1248 [DOI] [PubMed] [Google Scholar]
- 7.Novak RM, Donoval BA, Graham PJ, et al. : Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol 2007;14:1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkus J, Agnew K, Lawler R, Mitchell C, Hitti J: Effects of pregnancy and bacterial vaginosis on proinflammatory cytokine and secretory leukocyte protease inhibitor concentrations in vaginal secretions. J Pregnancy 2010;2010:385981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear GT, Zariffard MR, Chen HY, et al. : Positive association between HIV RNA and IL-6 in the genital tract of Rwandan women. AIDS Res Hum Retroviruses 2008;24:973–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauck C, Chen PL, Morrison CS, et al. : Biomarkers of cervical inflammation and immunity associated with cervical shedding of HIV-1. AIDS Res Hum Retroviruses 2016;32:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin HL, Richardson BA, Nyange PM, et al. : Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863–1868 [DOI] [PubMed] [Google Scholar]
- 12.Witkin SS, Linhares IM, Giraldo P: Bacterial flora of the female genital tract: Function and immune regulation. Best Pract Res Clin Obstet Gynaecol 2007;21:347–354 [DOI] [PubMed] [Google Scholar]
- 13.Sweet RL: Gynecologic conditions and bacterial vaginosis: Implications for the non-pregnant patient. Infect Dis Obstet Gynecol 2000;8:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS: Bacterial vaginosis and HIV acquisition: A meta-analysis of published studies. AIDS (London, England) 2008;22:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koumans EH, Sternberg M, Bruce C, et al. : The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007;34:864–869 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell C, Balkus J, Agnew K, Lawler R, Hitti J: Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. J Womens Health (Larchmt) 2009;18:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudin MH, Landers DV, Meyn L, Hillier SL: Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: A randomized trial. Obstet Gynecol 2003;102:527–534 [DOI] [PubMed] [Google Scholar]
- 18.Wasiela M, Krzeminski Z, Kalinka J, Brzezinska-Blaszczyk E: [Correlation between levels of selected cytokines in cervico-vaginal fluid of women with abnormal vaginal bacterial flora]. Med Dosw Mikrobiol 2005;57:327–333 [PubMed] [Google Scholar]
- 19.Beigi RH, Yudin MH, Cosentino L, Meyn LA, Hillier SL: Cytokines, pregnancy, and bacterial vaginosis: Comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. J Infect Dis 2007;196:1355–1360 [DOI] [PubMed] [Google Scholar]
- 20.Donders GG, Bosmans E, Dekeersmaecker A, Vereecken A, Van Bulck B, Spitz B: Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol 2000;182:872–878 [DOI] [PubMed] [Google Scholar]
- 21.Martino JL, Vermund SH: Vaginal douching: Evidence for risks or benefits to women's health. Epidemiol Rev 2002;24:109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aral SO, Mosher WD, Cates W, Jr: Vaginal douching among women of reproductive age in the United States: 1988. Am J Public Health 1992;82:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson T, Merchant J, Grimley D, Oh MK: Vaginal douching among adolescent and young women: More challenges than progress. J Pediatr Adolesc Gynecol 2004;17:249–255 [DOI] [PubMed] [Google Scholar]
- 24.Juliano C, Piu L, Gavini E, Zanetti S, Fadda G: In vitro antibacterial activity of antiseptics against vaginal lactobacilli. Eur J Clin Microbiol Infect Dis 1992;11:1166–1169 [DOI] [PubMed] [Google Scholar]
- 25.Pavlova S, Tao L: In vitro inhibition of commercial douche products against vaginal microflora. Infect Dis Obstet Gynecol 2000;8:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brotman RM, Klebanoff MA, Nansel TR, et al. : A longitudinal study of vaginal douching and bacterial vaginosis—A marginal structural modeling analysis. Am J Epidemiol 2008;168:188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonck K, Kaul R, Keli F, et al. : Sexually transmitted infections and vaginal douching in a population of female sex workers in Nairobi, Kenya. Sex Transm Infect 2001;77:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholes D, Daling JR, Stergachis A, Weiss NS, Wang S, Grayston JT: Vaginal douching as a risk factor for acute pelvic inflammatory disease. Obstet Gynecol 1993;81:601–606 [PubMed] [Google Scholar]
- 29.McClelland RS, Lavreys L, Hassan WM, Mandaliya K, Ndinya-Achola JO, Baeten JM: Vaginal washing and increased risk of HIV-1 acquisition among African women: A 10-year prospective study. AIDS 2006;20:269–273 [DOI] [PubMed] [Google Scholar]
- 30.Myer L, Kuhn L, Stein ZA, Wright TC, Jr., Denny L: Intravaginal practices, bacterial vaginosis, and women's susceptibility to HIV infection: Epidemiological evidence and biological mechanisms. Lancet Infect Dis 2005;5:786–794 [DOI] [PubMed] [Google Scholar]
- 31.van de Wijgert JH, Morrison CS, Cornelisse PG, et al. : Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2008;48:203–210 [DOI] [PubMed] [Google Scholar]
- 32.Low N, Chersich MF, Schmidlin K, et al. : Intravaginal practices, bacterial vaginosis, and HIV infection in women: Individual participant data meta-analysis. PLoS Med 2011;8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcaide ML, Strbo N, Romero L, et al. : Bacterial vaginosis is associated with loss of gamma delta T cells in the female reproductive tract in women in the Miami Women Interagency HIV Study (WIHS): A cross sectional study. PLoS One 2016;11:e0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauck CK, Lai JJ, Weiner DH, et al. : Toward early safety alert endpoints: Exploring biomarkers suggestive of microbicide failure. AIDS Res Hum Retroviruses 2013;29:1475–1486 [DOI] [PubMed] [Google Scholar]
- 35.Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN: Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis 2013;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M: Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol 2013;121:773–780 [DOI] [PubMed] [Google Scholar]
- 37.Taha TE, Hoover DR, Dallabetta GA, et al. : Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of HIV. AIDS 1998;12:1699–1706 [DOI] [PubMed] [Google Scholar]
- 38.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P: Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol 2003;189:139–147 [DOI] [PubMed] [Google Scholar]
- 39.Rajamanoharan S, Low N, Jones SB, Pozniak AL: Bacterial vaginosis, ethnicity, and the use of genital cleaning agents: A case control study. Sex Transm Dis 1999;26:404–409 [DOI] [PubMed] [Google Scholar]