Abstract
While preexposure prophylaxis with oral tenofovir/emtricitabine reduces HIV acquisition rates, poor adherence to and acceptability of vaginal gels and the potential for evolving drug resistance have led to development of vaginal film formulations and other antiretroviral drugs, respectively, including the non-nucleoside reverse transcriptase inhibitor dapivirine. In this two-arm crossover study of a novel fast-dissolving dapivirine film and a previously studied semisolid dapivirine gel, 10 healthy women received a single 1.25 mg vaginal dose of each study product; one withdrew after the first dose. Clinical, pharmacokinetic, and antiviral pharmacodynamic assessments (ex vivo HIV-BaL challenge of tissue explants) were performed over 168 h postdose. Six of ten participants experienced mild to moderate adverse effects, similar between products, with no severe adverse events or adverse events attributed to study products. There were no statistically significant differences in plasma, cervicovaginal fluid (CVF), or cervical tissue dapivirine concentrations between the gel and film (all p > .05). CVF dapivirine concentrations were 1.5 and 6 log10 greater than tissue and plasma concentrations, respectively (p < .001). Both film and gel demonstrated reduced cervical tissue infectivity after ex vivo HIV challenge 5 h postdose, compared to baseline and 72-h postdose biopsies (p < .05 for gel, p = .06 for film). There was no difference in ex vivo explant HIV challenge between gel and film. The dapivirine film and gel performed similarly in terms of tolerability, pharmacokinetics, and antiviral effect. Dapivirine film may provide an alternative to pharmacokinetically comparable dapivirine gel formulations. Effectiveness remains to be tested.
Keywords: : HIV, preexposure prophylaxis, pharmacokinetics, pharmacodynamics, dapivirine, vaginal gel, vaginal film
Introduction
Infection with HIV remains a global health problem, with 2 million new HIV infections reported worldwide in 2014.1 Sexual transmission remains the most common mode of transmission, particularly for young women. A promising strategy to reduce sexual transmission of HIV is the use of preexposure prophylaxis (PrEP), during which a person takes or applies a drug or combination of drugs to reduce his or her risk of HIV acquisition. Randomized placebo controlled trials of PrEP using daily oral tenofovir/emtricitabine2 and pericoital 1% tenofovir vaginal gel3 demonstrated that high levels of product adherence result in reduced HIV acquisition.2–6 Poor adherence resulted in no protective effect of the same drug regimens.7–9
Alternative behavioral and biomedical strategies intended to improve adherence to PrEP regimens include the following: sustained release products that require infrequent dosing and provide long-term protection, or topical products that may be suitable for periodic dosing and provide alternatives to oral dosing desired by some.10–12 Sustained delivery product development includes the following: antiretroviral (ARV) vaginal rings replaced monthly; intramuscular injectable formulations of ARVs dosed every 2 months; and implantable ARV formulations with potential for yearly dosing as indicated by preclinical pharmacokinetic (PK) studies.13 Alternative topical approaches in development include the following: vaginal films and tablets, rectal gels, suppositories, and enemas; these efforts include tenofovir and several other candidate ARV compounds, including dapivirine.
Dapivirine (DPV) is a non-nucleoside reverse transcriptase inhibitor that has potent anti-HIV-1 activity, both in vitro and in vivo.14 While it has very poor oral absorption, making it impractical for oral dosing, DPV has shown promise as a topical microbicide. Studies investigating rings, films, and gels that deliver various doses of DPV have shown the products to be safe and well tolerated when dosed vaginally in women.15–22 A DPV gel developed for vaginal use proved safe and acceptable to men, when applied externally to the penis.23
Sustained delivery products are not necessary for persons with only periodic risk of HIV infection, and some individuals may choose to avoid the increased and sustained risk of systemic toxicity associated with injectable formulations. On-demand microbicide (topical formulation) products for use during periods of anticipated sexual exposure to HIV may be a suitable alternative for such persons. Most of the microbicide products evaluated for effectiveness have been aqueous-based gels applied pericoitally.3,8,9 While efficacy was low or absent in several randomized clinical trials of vaginal tenofovir gels with intent to treat analyses (CAPRISA 004, VOICE, FACTS 001), efficacy was demonstrated in the subset of individuals with high levels of adherence in all of these studies.24
One limitation of the vaginal gel identified in acceptability studies included the participant experience with the product, as the gel often leaked from the vagina after application, as well as the bulkiness of the applicator that complicated product storage and transport.25 A quick dissolving vaginal film dosage form—far smaller in size, less packaging to dispose of postapplication, less volume to leak from the vagina, and less volume to dilute innate endogenous antibacterial and antiviral properties of vaginal fluid—may overcome some limitations of gel products which impact adherence. The Listerine® breath mint strips are a familiar and acceptable fast-dissolving film formulation for oral dosing. Vaginal films, like the nonoxynol-9 contraceptive film, have proven more acceptable than gel formulations.26,27
For this study (FAME 02B), we used a soft, flexible, translucent DPV vaginal film composed of a polyvinyl alcohol base, with an individual unit size of 1″ × 2″ and 70 μm thick. In vitro studies show the films dissolve rapidly upon exposure to an aqueous environment, releasing more than half of the DPV within 10 min.28 Each individual film contains 1.25 mg of DPV and is comparable to phase 1 studies of DPV gels (0.05% with administration of 2.5 g/2.8 mL). Studies of DPV vaginal gel (Gel 4759, also studied in companion study, FAME 02, and first reported in Nel et al.19) have shown that these products are safe, generally acceptable to women (apart from leakiness noted above), and lead to low systemic DPV concentrations.17–19,22
The current study describes the multicompartment pharmacokinetics (blood, cervical tissue, and cervicovaginal fluid [CVF]) and ex vivo pharmacodynamics (HIV tissue explant challenge) over 1 week following a single dose of DPV film compared to a DPV gel formulation. The companion study, FAME 02, involved 1 week of daily dosing of the same DPV film and gel products, but with additional safety assessments.22
Materials and Methods
Study design and participants
This was a two-arm, single site randomized crossover study of two DPV formulations, conducted at the Drug Development Unit (DDU) of the Johns Hopkins Hospital in Baltimore, MD. The protocol was approved by the Johns Hopkins Medicine Institutional Review Board. Ten healthy HIV-uninfected women between the ages of 18 and 45 years were recruited to participate. After ensuring eligibility, participants were randomized to receive either DPV vaginal gel (0.05%, 2.5 g/2.5 mL volume, total 1.25 mg applicator dose) followed by DPV vaginal film (1.25 mg/film) or film followed by gel.
At the first study visit, the DPV gel product was applied by an investigator using a polyethylene vaginal applicator (HTI Plastics, Lincoln, NE). The DPV film product was applied in the mid-vaginal region by a gynecologist during a speculum examination. The participant remained recumbent for ∼30 min after each dose. Over the next 12 h, serial blood samples for DPV concentration were collected, and samples of CVF and cervical biopsies were collected 5 h after dosing. CVF samples were taken from three locations within the lower female genital tract—mid vagina, posterior vaginal fornix, and external cervical os using a Dacron swab (Cardinal Health, McGraw Park, IL). Cervical biopsies were selected to align with companion FAME 02 study; they are easier to collect and are associated with the highest concentrations of HIV surrogates in our prior studies.29
Participants returned to the DDU to provide blood samples at 24, 48, 72, and 168 h after dosing. Cervical biopsies were repeated at the 72-h visit, and CVF samples were collected at 72 and 168 h. A final safety assessment for the first study product was conducted 14 days after dosing, after which the second formulation was dosed at a similar time during the participant's next menstrual cycle. The second study product was followed by the same sampling schedule. A final set of cervical biopsies was collected from each participant several weeks after completing the second product dosing, to serve as a negative control for ex vivo explant assessments.
Clinical assessment
Participants were assessed for adverse events (AEs) at each study visit, and if detected, each AE was assigned a grade based on the Division of AIDS (DAIDS) Table for Grading Adult and Pediatric Adverse Events, Version 1.0 and the Female Genital Grading Table for Use in Microbicide Studies (Appendix 1 to DAIDS Table for Grading Adult and Pediatric Adverse Events, Version 1.0).30,31
Pharmacokinetic sample analysis
Plasma DPV concentrations were measured using a validated ultra-performance liquid chromatographic–tandem mass spectrometric (LC-MS/MS) method that has been previously described.32 The lower limit of quantification (LLOQ) for this assay is 20 pg/mL, with coefficient of variation (%CV) for intra- and interassay precision and accuracy ranging from 5.23 to 13.89.
CVF samples were analyzed for DPV concentration using validated LC-MS/MS analysis, with LLOQ of 0.250 ng/swab, corresponding to median 0.01 ng/mg based on CVF sample volumes. Cervical tissue DPV concentration was assessed, following homogenization and protein precipitation, using a validated LC-MS/MS method with a LLOQ of 0.05 ng/sample, corresponding to median 0.07 ng/mg based on biopsy weights.21 Tissue DPV quantification was performed using calibrators prepared in human plasma and matrix-specific tissue quality control samples.
HIV exposure and HIV-1 p24 measurement
Two cervical biopsies from each study product and time point (5 h, 72 h) and the no drug control were collected in L15 media (Mediatech, Manassas, VA), 1 × Pen/Strep/Glutamine, 1 × Amphotericin B, and 10% FBS (Gemini BioProducts, Woodland, CA). Biopsies were individually transferred to single wells of a 48-well plate containing 1 mL culture medium—DMEM (Mediatech) with 10% human AB serum (Gemini BioProducts), 1 × Pen/Strep/Glutamine (Gemini BioProducts), 1 × Nonessential amino acids (Mediatech), and 100 units IL-2 (Roche). Biopsies were exposed to HIV-1 BaL at final concentration of 5 × 104 TCID50/mL and incubated for 2 h at 37°C and 5% CO2 in 100% humidity.
After infection, biopsies were washed four times with 1 mL HBSS, weighed, and transferred to separate wells of a 48-well plate (1 biopsy/well) containing 1 mL fresh culture medium (described above). The day of infection was denoted as day 0. Plates were incubated for 14 days at 37°C and 5% CO2 in 100% humidity. 0.7 mL culture medium was harvested on days 4, 7, 10, and 14 and replaced with fresh medium. Harvested medium was stored frozen at −20°C until analysis. HIV-1 p24 concentration in the harvested medium was measured using the PerkinElmer Alliance HIV-1 p24 ELISA Kit according to manufacturer's instructions. Cumulative p24 produced by biopsies over 14 days was used for analysis.
Statistical analysis
Plasma values were analyzed calculating area under the concentration-time curve from zero to last sample (AUClast), peak concentration (Cmax), and time to peak concentration (Tmax) with noncompartmental methods using Phoenix WinNonlin® software, version 6.4 (Pharsight/Certara, Princeton, NJ). Crude maximum half-life estimates for CVF and cervical tissue DPV were also calculated using imputation of LLOQ/2 where concentration fell below the LLOQ. [Note: these are poor estimates due to very sparse sampling (two tissue and three CVF samples) with highly uncertain time to both Cmax and LLOQ. Guided somewhat by plasma Tmax data, the calculated estimates are highly conservative and are very likely longer than the true elimination half-life.] Concentration-time data are presented using SigmaPlot®, version 13 (Systat Software, Inc., San Jose, CA).
Descriptive statistics of data and comparisons between the two formulations and between different matrices were performed using the Wilcoxon rank sum test using Stata®, version 12 (StataCorp LP, College Station, TX). Differences in CVF among the three lower female genital tract sites were tested first with the Friedman test, followed by post hoc paired comparisons (Wilcoxon test). A p-value of less than .05 was considered statistically significant in comparison testing and correlation.
The DPV concentration–response (cumulative p24) relationship was explored through (1) testing correlation of DPV tissue concentration and cumulative p24 (Spearman correlation coefficient), (2) linear least-squares regression modeling (using raw and log-log transform of p24 and DPV concentration) with participant and formulation (film vs. gel) as covariates, and (3) a series of pharmacodynamic models using the Hill equation to estimate concentration at which antiviral effect is 50% of maximum (IC50), maximum inhibitory effect (Imax), baseline effect (no drug), and sigmoidicity (Hill coefficient) using Phoenix WinNonlin, version 6.4 (Pharsight/Certara). Correlation and pharmacodynamic modeling were performed by both excluding all Below Limit of Quantitation (BLQ) DPV concentrations and imputing those concentrations as LLOQ.
Results
Subjects
Participants were mean [standard deviation (SD)] 28.7 (±8.3) years of age. Half of participants (n = 5) were African American, with non-Hispanic white, Hispanic, Asian, and mixed race among the other 5. One participant withdrew from the study after her first product dosing (film) and did not have any samples collected after the first day. Pharmacokinetic and pharmacodynamic data are presented from the remaining 9 participants who have complete data, while AE data are presented from all 10 enrolled participants.
Adverse events
A total of 24 AEs were recorded throughout the study, all of which occurred in 6 of 10 participants (i.e., 4 participants experienced no AEs). The AEs were reported within the week following dosing, except for one AE at baseline (known history of iron-deficiency anemia) and two identified at the follow-up visit that occurred between the first and second dosing visits. Reported AEs included: headache, diarrhea, periorbital edema, upper respiratory infection, phlebotomy site bruising, urinary tract infection, mononucleosis, and dehydration; laboratory abnormalities included hypoglycemia, hyperglycemia, anemia, hypokalemia, and increased neutrophil count.
No serious AEs (SAEs) were recorded for any participant, and the majority of AEs were Grade 1 (only 6 of 24 were Grade 2, which occurred in two participants). Of the AEs that occurred during dosing intervals (n = 21), they were evenly distributed between the two products (11 occurred with gel, 10 occurred with film). All AEs were determined to be “not related” to study product exposure.
Pharmacokinetics
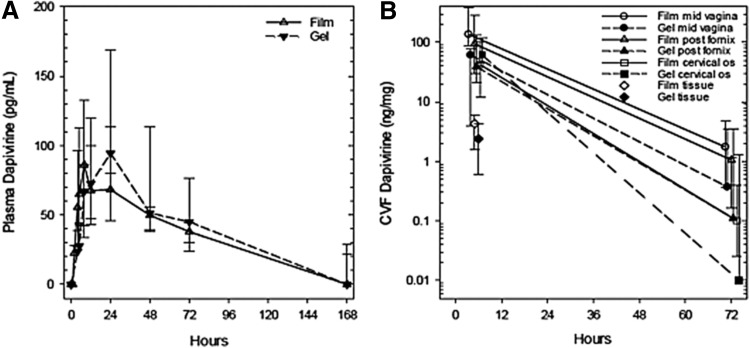
Following dosing of each product, plasma DPV concentrations rose to a peak between 12 and 24 h, then fell in log linear manner; at the last observation, 168 h, five of nine participants (two film, three gel) had detectable plasma DPV concentrations (Fig. 1A). Median [interquartile range (IQR)] plasma PK parameter estimates for the film product were: Cmax 91 (67–179) pg/mL, Tmax 12 (5–18) h, AUClast 7,952 (5,763–9,021) pg h/mL, and half-life 59 (51–82) h. Gel PK parameter estimates were: Cmax 132 (93–169) pg/mL, Tmax 24 (10–24) h, AUClast 7,832 (5,799–11,807) pg h/mL, and half-life 52 (42–64) h. There were no statistically significant differences between the film and gel products for any plasma PK parameter (Wilcoxon rank sum, all p-values >.05).
FIG. 1.
Dapivirine plasma and CVF concentration versus time for dapivirine film and gel. (A) Indicates plasma dapivirine concentration. Open symbols (solid line) are film, closed symbols (dashed lines) are gel. BLQ values are arbitrarily displayed as 0 pg/mL. (B) Indicates cervicovaginal fluid and cervical tissue homogenate dapivirine concentrations in log10 scale. Open symbols are film and closed are gel. Symbols represent mid vagina (round), posterior fornix (triangle), cervical os (square), and cervical tissue homogenate (diamond). Values are median with upper and lower quartiles. BLQ values are not shown; all medians at 168 h are BLQ, therefore that time is not shown. Nominal x-axis values are slightly offset to avoid overlap. BLQ, below limit of quantitation; CVF, cervicovaginal fluid.
CVF DPV concentrations declined from 5 through 168 h in log linear manner (Fig. 1B); at 168 h, median DPV concentration was BLQ for both products at all sites. Accordingly, because we didn't have reliable concentration estimates for the 168 h time point, half-life estimates were not made. There were no statistically significant differences between study products for DPV CVF concentrations at any sampling location (Table 1; all p > .05). Within participants, there were statistically significant differences among the three genital tract CVF sampling site concentrations for both film and gel (Table 1; Friedman test both p ≤ .02); in pairwise comparisons between sites, the general concentration trend was mid vagina >fornix >cervical os.
Table 1.
Pharmacokinetic Parameters of Dapivirine in Cervicovaginal Fluid and Cervical Tissue Homogenates After Application of Dapivirine Film Versus Gel
| Matrix-PK parameter | Film 5 h concentration ng/mg median (IQR) | Gel 5 h concentration ng/mg median (IQR) | Film 72 h concentration ng/mg median (IQR) | Gel 72 h concentration ng/mg median (IQR) | Film max half-life hours median (IQR) | Gel max half-life hours median (IQR) |
|---|---|---|---|---|---|---|
| CVF mid vagina | 136 (86–387)a | 61 (4–79) | 1.74 (0.37–4.75) | 0.37 (BLQ–3.52) | 11 (9–15) | 13 (4–18) |
| CVF fornix | 95 (30–287) | 39 (21–133) | 1.06 (0.17–1.18)b | 0.11 (BLQ–3.47) | 10 (7–12) | 12 (8–25) |
| CVF cervical os | 42 (12–103)c,d | 61 (46–117) | 0.10 (0.03–0.39)c,d | 0.01 (BLQ–1.28)c,d | 11 (8–13) | 5 (5–14) |
| Cervical tissue | 4.3 (1.6–6.0) | 2.4 (0.6–4.2) | BLQ (BLQ–BLQ) | BLQ (BLQ–0.39) | 6 (6–7) | 9 (7–19) |
Data for 168 h not shown as all medians were BLQ assay. One nanogram per milligram is ∼106 pg/mL (units of plasma DPV concentration in text).
p = .07 film versus gel (eight of nine film > gel).
p ≤ .05 fornix versus mid vagina.
p ≤ .05 cervical os versus fornix.
p ≤ .05 mid vagina versus cervical os.
BLQ, below limit of quantitation; CVF, cervicovaginal fluid; DPV, dapivirine; IQR, interquartile range.
Cervical tissue homogenate DPV concentrations were also not different comparing film to gel at 5 h (Table 1; Fig. 1B). At 72 h after dosing, only three of nine samples after gel and none of nine samples after film had DPV cervical tissue homogenate concentrations above the LLOQ—a fall of at least 1.5 log10 (gel) and 1.8 log10 (film) between 5 and 72 h. Comparing concentrations across biological matrices 5 h after study product dosing (where all matrices for all products and subjects are in the quantifiable range), CVF DPV concentrations (median across the three genital tract) were 1.5 log10 greater than tissue concentrations and 6 log10 greater than plasma concentrations. Plasma half-life was significantly greater than estimated maximum half-life for both CVF and cervical tissue, which were similar to each other.
No statistical comparisons are made between products or among matrices since these CVF and cervical tissue half-life estimates are very crudely based on too sparse data (two to three samples, 5 h concentrations very likely before peak concentration is achieved, uncertain time to LLOQ, 31% overall BLQ values requiring imputation).
Pharmacodynamics (HIV-1 p24 measurement)
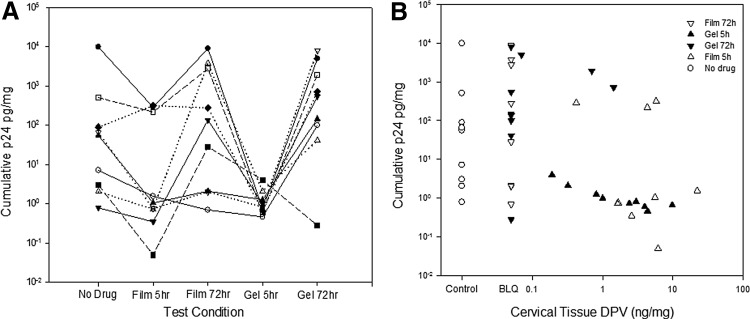
At least seven of nine participants' 5-h cumulative p24 antigen concentrations were lower than baseline and the 72-h values for the corresponding product (Fig. 2A). For cervical biopsies collected 5 h after film dosing, the cumulative p24 antigen production, median 1.0 pg/mg (IQR 0.5, 252), was significantly less than the cumulative p24 antigen 72 h after film dosing, 136 pg/mg (2, 3258; Wilcoxon rank sum test; p = .01), and trended toward being less than the no study product baseline, 55 pg/mg (3, 297; p = .07).
FIG. 2.
Cumulative HIV p24 antigen from ex vivo HIV challenge of cervical tissue explants after dosing with DPV film versus gel. (A) Indicates cumulative p24 antigen by false sequence categories for comparison. Each symbol-line pair is a unique research participant. (B) Indicates concentration–response relationship of cervical tissue homogenate dapivirine concentration (x-axis). No drug is open circle. Open triangles are film; closed triangles are gel. Upward pointing triangles are samples taken 5 h after dosing; downward pointing triangles are collected 72 h postdosing. DPV, dapivirine.
After gel dosing, 5- and 72-h cumulative p24 concentrations were 1.0 pg/mg (1.0, 1.5) and 543 pg/mg (71, 3437), respectively, with 5 h values trending toward statistical significance (p = .06) compared to 72 h values and were significantly lower than no study product baseline values (p = .04). Baseline values were not different than 72 h values for either product (p > .10). Neither was there any statistically significant difference in p24 production at either 5 or 72 h postdose when gel was compared to film.
Figure 2A, B demonstrates a highly variable 4 log10 spread of cumulative p24 concentrations from baseline cervical biopsy samples (no drug), as well as for cervical samples collected within 72 h of dosing, nearly all of which have BLQ DPV concentrations. The coefficient of variation for all baseline “no drug” biopsies was 262%. To increase the sample size of baseline “no drug” biopsies and achieve more robust coefficient of variation estimates, we combined these true baseline biopsies with postdrug biopsies having BLQ DPV concentrations, which resulted in 169% coefficient of variation.
DPV tissue concentration was inversely correlated with cumulative p24 values, demonstrating a rough concentration–response relationship (Spearman correlation coefficient = −0.483, p = .003). With log-log transformation of values, an inverse linear relationship between DPV concentration and p24 production was seen (β = −0.82 [0.26 standard error], p = .004) with participant (β = −0.12 [0.05], p = .03), but not the product arm (β = 0.79 [0.47], p = .11) as significant covariates. The data failed to fit any nonlinear inhibitory pharmacodynamic models (no statistically significant parameter estimates; data not shown).
Discussion
Our single-dose comparison study indicated that both DPV film and gel were well tolerated by study participants, with no SAEs reported after single doses of either product and there were no differences in AEs between products. There were also no statistically significant PK differences between the two formulations in plasma, tissue, or CVF, except for a statistical trend toward a twofold greater DPV mid-vaginal CVF concentration with film compared to gel. There were, however, differences in DPV concentrations among anatomic sites at similar sampling times, generally highest at the mid-vaginal position, especially compared to the cervical os, for both formulations.
We had anticipated greater local concentrations for the dehydrated film at early times compared to the semisolid and larger gel volume. We sampled specific anatomic locations within the lower female reproductive tract to understand intraluminal drug distribution differences over time and how these concentrations aligned with prior studies of simulated HIV exposures (highest at the cervical os and posterior fornix). Five hours after product dosing, when drug was readily detectable in all participants' samples, DPV concentrations were far greater in CVF compared with either cervical tissue (32-fold greater) or plasma (106-fold greater). Plasma DPV half-life (>50 h) was at least several times longer than the cervical tissue DPV half-life (<10 h, a significant overestimate).
These anatomic differences are very similar to two other DPV studies (FAME 02 and MTN-013), where the samples were analyzed in the same laboratory and paired samples from the same women are available for plasma, cervical tissue, and CVF.21,22 FAME 02 compared daily use of the same DPV film and gel products as this study, although for a total of 7 consecutive days. In their analysis, Bunge et al.22, found that 2 to 4 h after the final dose, DPV concentrations in cervicovaginal lavage (CVL) samples were 4 log10 greater than in plasma and were the same as in cervical tissue. Median DPV plasma concentrations (220–310 pg/mL) drawn 2 to 4 h after the final dose were higher than our values by twofold, but samples were collected after seven daily doses, which allow for DPV accumulation in plasma due to a long DPV half-life relative to the daily dosing interval.
Median DPV tissue concentration following daily dosing for 7 days was the same as what we detected after a single dose (2–7 ng/mg)—no accumulation between our single and their seven daily doses is also consistent with a half-life in tissue that is much shorter compared to plasma.
The anatomical differences we showed—vaginal greater than cervical os CVF DPV concentration—are consistent with FAME 02, which showed greater DPV concentrations and antiviral effect (ex vivo explants) in vaginal tissue compared to cervical tissue. Median CVL concentrations were 2 to 5 ng/mg, which is ∼20 to ∼60-fold lower than our 5 h CVF concentrations, but the dilutional effect of the 10 mL lavage volume on drug in 100–300 μL resident CVF volume largely accounts for this difference. No accumulation would be expected in CVF since the products were identical and repeated dosing should have no effect in CVF concentrations. So, accounting for the multiple dose accumulation of DPV in plasma and the dilutional effect of lavage fluid on CVF, the DPV PK findings are very similar to what was observed in this study.
Unlike our single dose study, FAME 02 noted a statistically significant greater tissue DPV concentration after gel dosing compared to film dosing. The FAME 02 report suggested that the tissue DPV concentrations after gel dosing were increased, compared to film, likely due to residual gel adherent to the tissue biopsies; sample handling, therefore, might have differed between studies.
In addition, in our FAME 02B study, a gynecologist administered all film doses, whereas in FAME 02, research participants self-administered film doses, some of whom had difficulty. The concentration differences we noted might also be attributed to speculum-assisted placement in mid vagina (5 h samples) or study-related sampling (72 h). Finally, FAME 02 was a much larger study and had greater statistical power to detect differences than FAME 02B; however, FAME 02B actually trended in the other direction (film greater than gel tissue DPV concentration). Taken together, these differences support the finding that film and gel likely achieved similar tissue DPV concentrations.
In MTN-013, a 28-day DPV ring comparison study, the average daily dose was 0.16 mg based on the 25 mg DPV ring content as manufactured and 82% DPV dose retained in the rings, on average, after 28 days intravaginally.21 This is roughly 13% of the 1.25 mg film and gel dose in FAME 02 and FAME 02B.
The mean (SD) steady-state (or 28 day) concentrations in MTN-013 were: plasma, 175 (45) pg/mL, which exceeds our single-dose median peak plasma DPV concentrations and falls below the plasma concentrations reported in FAME 02, indicating both accumulation of plasma DPV with time, as well as more efficient drug delivery per administered dose with the sustained DPV release from the ring; cervical tissue, 0.6 (0.9) ng/mg, which falls below our single-dose 5-h (and FAME 02's steady-state 2–4 h sampled) postdose cervical tissue concentrations, although proportionally higher than expected accounting for the lower average daily ring dose compared to film and gel and does not account for trough concentrations in the FAME studies which were not measured; CVF, 5.7 (18.7) ng/mg, which is lower by 8- to 15-fold compared to our values, also consistent with formulation dose differences.
Steady-state CVF DPV concentrations are 5 log10 greater than plasma and 1 log10 greater than cervical tissue, which are highly consistent with FAME 02 and FAME 02B given steady-state release of DPV from the ring and fluctuating concentrations with the film and gel.
A cervical tissue DPV concentration-ex vivo explant challenge response relationship was demonstrated when controlling for participant as a covariate; in addition, participant specific differences in p24 production were identified. Product arm, however, was not statistically significant in this model, consistent with our simpler nonparametric comparisons. However, these data failed to fit traditional pharmacodynamic models, which we hoped would provide useful IC50 concentrations. IC50 concentrations are useful in evaluating the comparability and appropriate scaling of ex vivo IC50 contrasted to in vivo IC50, which is essential to evaluate the clinical predictive value of such ex vivo tests.
Lack of success with pharmacodynamic model fitting likely occurred due to too few cervical tissue concentrations above the LLOQ at 72 h (only one-third for gel and none for film) at which point the antiviral effect was consistent with predose baselines; in addition, the explant assay has a high degree of intra- and interparticipant variability. This was not, apparently, a limitation for the simpler linear model.
For reference, the MTN-013 DPV vaginal ring and FAME 02 DPV film and gel also demonstrated a statistically significant linear relationship with a similar log-log transform of the variables. The consistent explant assay concentration–response seen in MTN-013, FAME 02, and FAME 02B indicates a clear dapivirine antiviral effect. There may also be a vehicle barrier effect contributing to this antiviral effect (as noted for some microbicide vehicles), but this cannot be determined for this product without a vehicle only control for comparison.33
In summary, we found no significant PK or PD differences between the DPV film and gel products. The PK findings were consistent with those found with 1 week of daily dosing (FAME 02), allowing for evidence of DPV accumulation in plasma, and dilution of CVL compared to CVF. Comparing both FAME studies (film and gel) with a 28-day DPV ring study (MTN-013), plasma and cervical tissue DPV concentrations were similar, accounting for steady-state versus intermittent dosing differences.
In addition, we demonstrated a linear concentration–response relationship using ex vivo explant challenge when controlling for participant as covariate, despite a large degree of assay variability. Especially in combination with the data from the companion FAME 02 study, which reported a high degree of acceptability and tolerability of the film product over 1 week of daily dosing, the film product may provide a more portable alternative to a DPV gel for women interested in periodic PrEP or who wish to avoid an indwelling DPV vaginal ring. Effectiveness of any vaginal formulation of DPV awaits the outcome of randomized controlled clinical trials.
Acknowledgments
The authors thank the participants who volunteered for this study. The authors also thank the staff of the Johns Hopkins University Drug Development Unit, Clinical Pharmacology Analytical Laboratory and Charlene Dezzutti, PhD, University of Pittsburgh and Magee-Womens Research Institute. This study was funded, in part, by the National Institutes of Health, Division of AIDS, Integrated Pre-Clinical/Clinical Program for HIV Topical Microbicides (U19 AI082639). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIAID. This project has also been funded, in part, with federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200800014C.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS/WHO: Fact Sheet: 2014 Global Statistics. World Health Organization, Geneva, 2014 [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. : Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. : Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. : Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434 [DOI] [PubMed] [Google Scholar]
- 6.Choopanya K, Martin M, Suntharasamai P, et al. : Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083–2090 [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. : Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Ramjee G, Richardson BA, et al. : Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015;372:509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USAID: FACTS 001 Trial: Questions and Answers. Available at www.usaid.gov/sites/default/files/documents/1864/FACTS-001.pdf, accessed November30, 2015
- 10.Minnis AM, Gandham S, Richardson BA, et al. : Adherence and acceptability in MTN 001: A randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav 2013;17:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts ST, Heffron R, Ngure K, et al. : Preferences for daily or intermittent pre-exposure prophylaxis regimens and ability to anticipate sex among HIV uninfected members of Kenyan HIV serodiscordant couples. AIDS Behav 2014;18:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack N, Evens EM, Tolley EE, et al. : The importance of choice in the rollout of ARV-based prevention to user groups in Kenya and South Africa: A qualitative study. J Int AIDS Soc 2014;17(Suppl 2):19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunawardana M, Remedios-Chan M, Miller CS, et al. : Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 2015;59:3913–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Herrewege Y, Michiels J, Van Roey J, et al. : In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother 2004;48:337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jespers VA, Van Roey JM, Beets GI, Buve AM: Dose-ranging phase 1 study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J Acquir Immune Defic Syndr 2007;44:154–158 [DOI] [PubMed] [Google Scholar]
- 16.Romano J, Variano B, Coplan P, et al. : Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses 2009;25:483–488 [DOI] [PubMed] [Google Scholar]
- 17.Nel AM, Coplan P, Smythe SC, et al. : Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res Hum Retroviruses 2010;26:1181–1190 [DOI] [PubMed] [Google Scholar]
- 18.Nel AM, Coplan P, van de Wijgert JH, et al. : Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS 2009;23:1531–1538 [DOI] [PubMed] [Google Scholar]
- 19.Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW: Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. Hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr 2010;55:161–169 [DOI] [PubMed] [Google Scholar]
- 20.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N: A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 2014;28:1479–1487 [DOI] [PubMed] [Google Scholar]
- 21.Chen BA, Panther L, Marzinke MA, et al. : Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: A double-blind randomized trial. J Acquir Immune Defic Syndr 2015;70:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunge KE, Dezzutti CS, Rohan LC, et al. : A phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of a novel dapivirine vaginal film. J Acquir Immune Defic Syndr 2015;71:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cranston RD, Hoesley C, Carballo-Dieguez A, et al. : A randomized male tolerance study of dapivirine gel following multiple topical penile exposures (MTN 012/IPM 010). AIDS Res Hum Retroviruses 2014;30:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashuba AD, Gengiah TN, Werner L, et al. : Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial: Importance of adherence for microbicide effectiveness. J Acquir Immune Defic Syndr 2015;69:264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Straten A, Stadler J, Montgomery E, et al. : Women's experiences with oral and vaginal pre-exposure prophylaxis: The VOICE-C qualitative study in Johannesburg, South Africa. PLoS One 2014;9:e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coggins C, Elias CJ, Atisook R, et al. : Women's preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS 1998;12:1389–1391 [DOI] [PubMed] [Google Scholar]
- 27.Raymond E, Alvarado G, Ledesma L, et al. : Acceptability of two spermicides in five countries. Contraception 1999;60:45–50 [DOI] [PubMed] [Google Scholar]
- 28.Akil A, Parniak MA, Dezzuitti CS, et al. : Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res 2011;1:209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louissaint NA, Cao YJ, Skipper PL, et al. : Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013;29:1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DAIDS: Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Addendum 1: Female Genital Grading Table for Use in Microbicide Studies. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS, 2007 [Google Scholar]
- 31.DAIDS: Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. 2014 February 9, 2015. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS, 2014 [Google Scholar]
- 32.Seserko LA, Emory JF, Hendrix CW, Marzinke MA: The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis 2013;5:2771–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyva FJ, Fuchs EJ, Bakshi RP, et al. : Simultaneous evaluation of safety, acceptability, peri-coital kinetics, and ex vivo pharmacodynamics comparing four rectal microbicide vehicle candidates. AIDS Res Hum Retroviruses 2015;31:1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]