Abstract
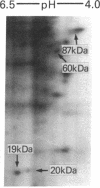
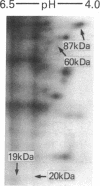
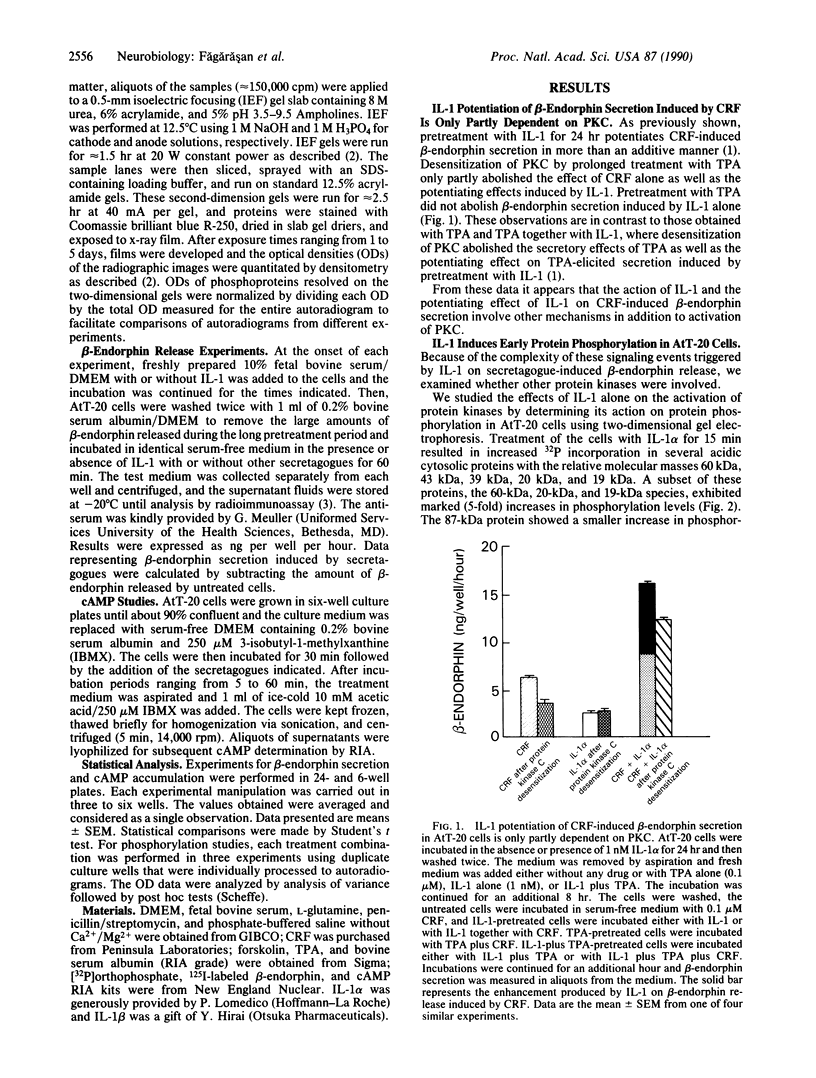
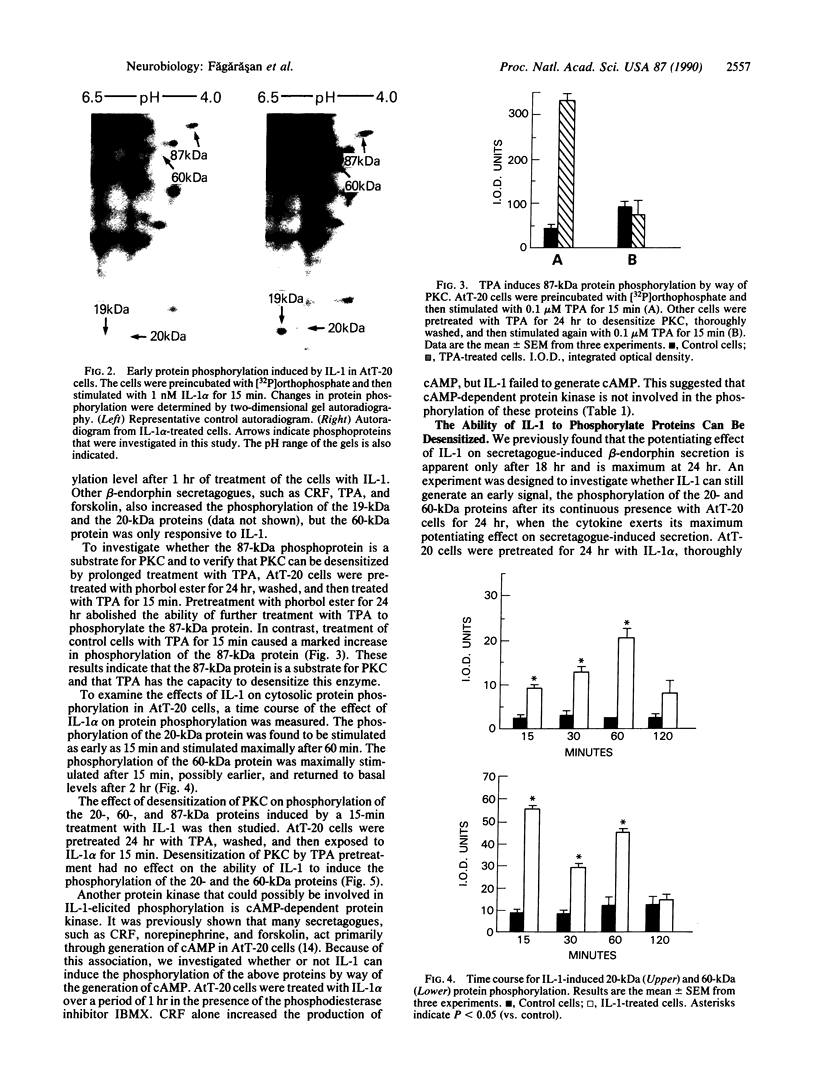
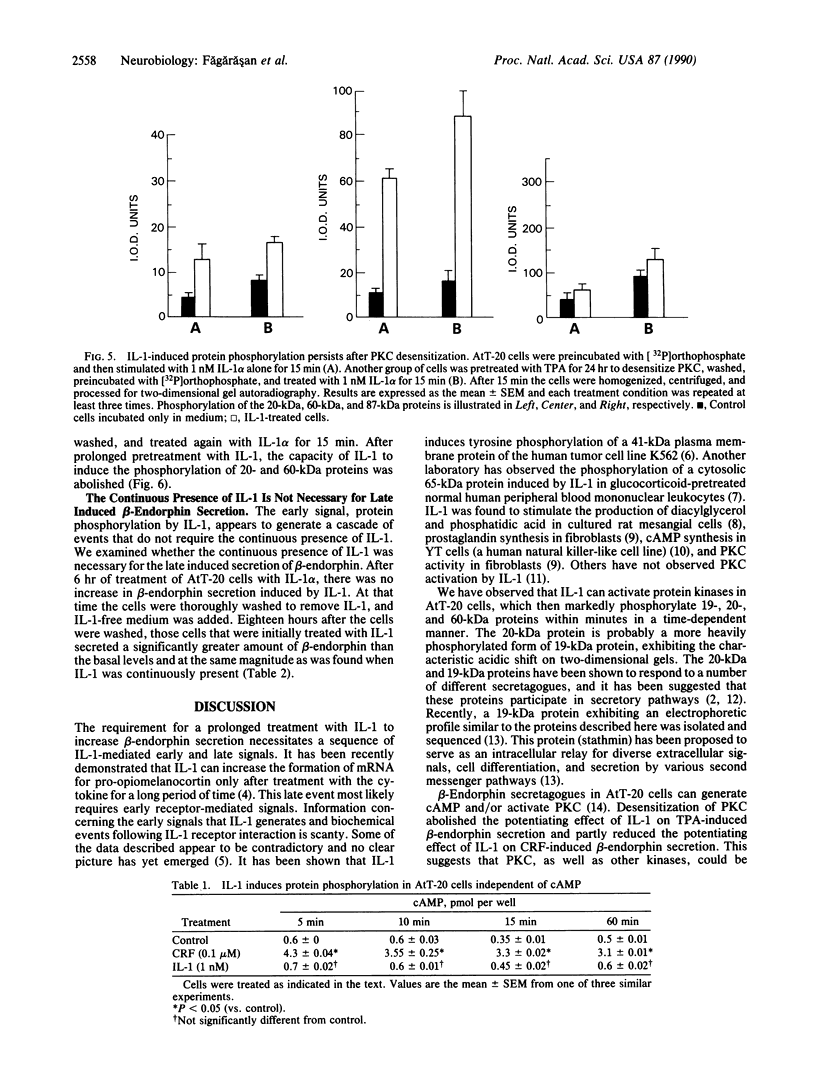
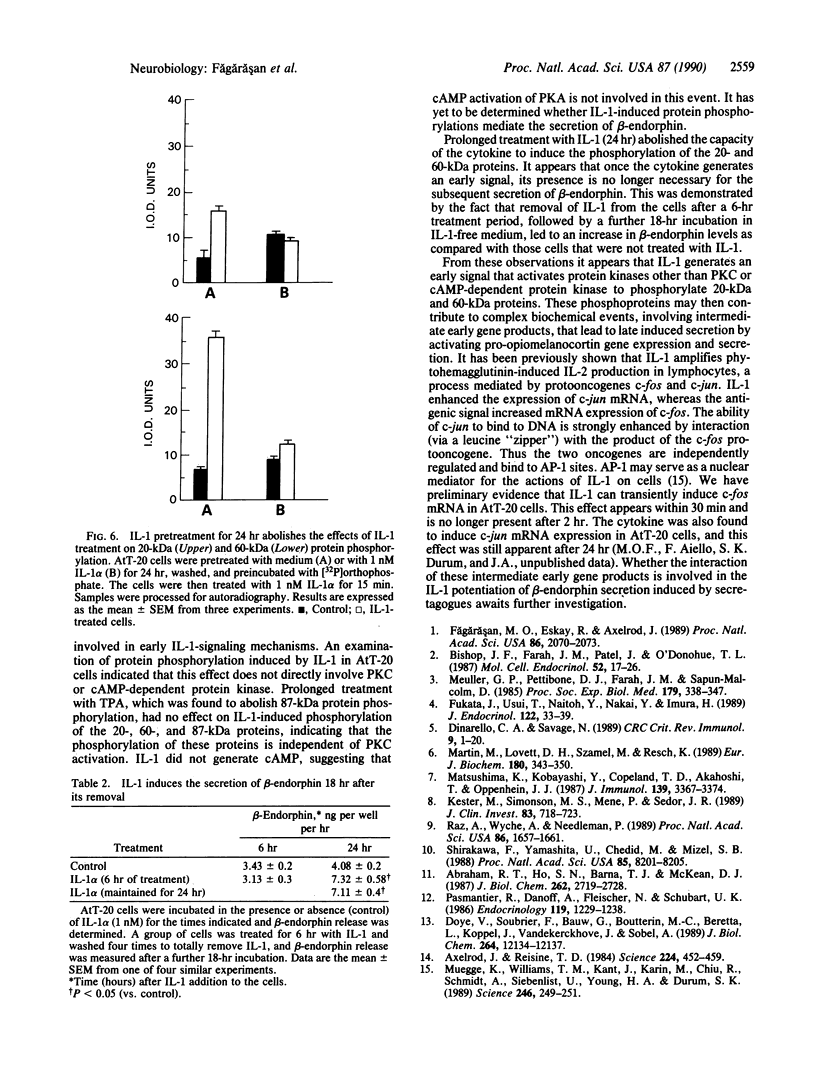
Previous work has shown that prolonged pretreatment of a mouse anterior pituitary cell line, AtT-20 cells, with the cytokine interleukin 1 (IL-1) stimulates beta-endorphin release and potentiates the secretion induced by many secretagogues. Desensitization of protein kinase C (PKC) by pretreatment with phorbol ester [phorbol 12-tetradecanoate 13-acetate (TPA)] for 8 hr abolished the secretion induced by TPA as well as the enhancement of TPA-induced beta-endorphin release produced by IL-1. Desensitization of PKC only partly abolished the potentiating effects of IL-1 on corticotropin-releasing factor-induced beta-endorphin secretion. In contrast, IL-1-induced beta-endorphin release was independent of PKC. We observed that treatment of AtT-20 cells with IL-1 markedly phosphorylated 19-, 20-, and 60-kDa proteins within minutes, presumably by early activation of protein kinases. Prolonged treatment with TPA, which was shown to desensitize an 87-kDa protein (a substrate for PKC), had no effect on IL-1-induced phosphorylation of 20-, 60-, and 87-kDa proteins, indicating that the phosphorylation of these proteins does not involve PKC. IL-1 does not generate cAMP in AtT-20 cells, suggesting that a cAMP-dependent protein kinase is also not involved. Prolonged treatment with IL-1 abolishes the capacity of cytokine to induce the phosphorylation of 20- and 60-kDa proteins. The presence of IL-1 was required initially only for a short time to induce late secretion in AtT-20 cells. These observations indicate that once IL-1 generates an early signal, its presence is no longer necessary for the subsequent secretion of beta-endorphin.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. T., Ho S. N., Barna T. J., McKean D. J. Transmembrane signaling during interleukin 1-dependent T cell activation. Interactions of signal 1- and signal 2-type mediators with the phosphoinositide-dependent signal transduction mechanism. J Biol Chem. 1987 Feb 25;262(6):2719–2728. [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Bishop J. F., Farah J. M., Patel J., O'Donohue T. L. Activation of distinct second messenger systems in anterior pituitary corticotrophic tumor cells alters the phosphorylation states of both shared and distinct cytosolic proteins. Mol Cell Endocrinol. 1987 Jul;52(1-2):17–26. doi: 10.1016/0303-7207(87)90092-x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Savage N. Interleukin-1 and its receptor. Crit Rev Immunol. 1989;9(1):1–20. [PubMed] [Google Scholar]
- Doye V., Soubrier F., Bauw G., Boutterin M. C., Beretta L., Koppel J., Vandekerckhove J., Sobel A. A single cDNA encodes two isoforms of stathmin, a developmentally regulated neuron-enriched phosphoprotein. J Biol Chem. 1989 Jul 25;264(21):12134–12137. [PubMed] [Google Scholar]
- Fukata J., Usui T., Naitoh Y., Nakai Y., Imura H. Effects of recombinant human interleukin-1 alpha, -1 beta, 2 and 6 on ACTH synthesis and release in the mouse pituitary tumour cell line AtT-20. J Endocrinol. 1989 Jul;122(1):33–39. doi: 10.1677/joe.0.1220033. [DOI] [PubMed] [Google Scholar]
- Făgăraşan M. O., Eskay R., Axelrod J. Interleukin 1 potentiates the secretion of beta-endorphin induced by secretagogues in a mouse pituitary cell line (AtT-20). Proc Natl Acad Sci U S A. 1989 Mar;86(6):2070–2073. doi: 10.1073/pnas.86.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester M., Simonson M. S., Mené P., Sedor J. R. Interleukin-1 generates transmembrane signals from phospholipids through novel pathways in cultured rat mesangial cells. J Clin Invest. 1989 Feb;83(2):718–723. doi: 10.1172/JCI113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Lovett D. H., Szamel M., Resch K. Characterization of the interleukin-1-induced tyrosine phosphorylation of a 41-kDa plasma membrane protein of the human tumor cell line K 562. Eur J Biochem. 1989 Mar 15;180(2):343–350. doi: 10.1111/j.1432-1033.1989.tb14654.x. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Kobayashi Y., Copeland T. D., Akahoshi T., Oppenheim J. J. Phosphorylation of a cytosolic 65-kDa protein induced by interleukin 1 in glucocorticoid pretreated normal human peripheral blood mononuclear leukocytes. J Immunol. 1987 Nov 15;139(10):3367–3374. [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Mueller G. P., Pettibone D. J., Farah J. M., Jr, Sapun-Malcolm D. Glucocorticoid inhibition of immunoreactive beta-endorphin release from the anterior lobe of the rat pituitary: in vitro and in vivo studies. Proc Soc Exp Biol Med. 1985 Jul;179(3):338–347. doi: 10.3181/00379727-179-42106. [DOI] [PubMed] [Google Scholar]
- Pasmantier R., Danoff A., Fleischer N., Schubart U. K. P19, a hormonally regulated phosphoprotein of peptide hormone-producing cells: secretagogue-induced phosphorylation in AtT-20 mouse pituitary tumor cells and in rat and hamster insulinoma cells. Endocrinology. 1986 Sep;119(3):1229–1238. doi: 10.1210/endo-119-3-1229. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]