Abstract
Virgin olive oil (VOO) constitutes the main source of fat in the Mediterranean diet. VOO is rich in oleic acid, displaying health-promoting properties, but also contains minor bioactive components, especially phenolic compounds. Hydroxytyrosol (HT), the main polyphenol of olive oil, has been reported to be the most bioactive component. This review aims to compile the results of clinical, animal and cell culture studies evaluating the effects of HT on the features of Metabolic Syndrome (MetS) (body weight/adiposity, dyslipidemia, hypertension, and hyperglycemia/insulin resistance) and associated complications (oxidative stress and inflammation). HT was able to improve the lipid profile, glycaemia, and insulin sensitivity, and counteract oxidative and inflammatory processes. Experimental studies identified multiple molecular targets for HT conferring its beneficial effect on health in spite of its low bioavailability. However, rodent experiments and clinical trials with pure HT at biologically relevant concentrations are still lacking. Moreover, the roles of intestine and its gut microbiota have not been elucidated.
Keywords: olive oil, oleuropein, hydroxytyrosol, tyrosol, body weight, dyslipidemia, hyperglycemia, hypertension, oxidative stress, inflammation
1. Introduction
Metabolic syndrome (MetS), a cluster of several interrelated cardiovascular risk factors (hyperglycemia, hypertension, dyslipidemia, insulin resistance and central adiposity) [1] lead to an increased prevalence of cardiovascular diseases (CVD) and type 2 diabetes mellitus (T2DM). A report published in 2015 claimed that the mortality for T2DM is around five millions persons per year, and it is expected that 23.6 million will die of CVD in the world by 2030 [2]. Olive oil, a natural juice from olive, is the primary source of fat in the Mediterranean diet, which is associated with a lower incidence of CVD mortality [3] and contains minor components, especially phenolic compounds, which are recognized as health beneficial components [4]. Hydroxytyrosol (HT), a non-flavonoid polyphenolic compound derived from oleuropein (OLE) and, notably present in olive and olive oil, could be involved in lower incidence of CVD and T2DM in the Mediterranean region, despite a high intake of fat as olive oil. HT could be an efficient bioactive phenolic candidate, owing to its protective action of health towards inflammation and oxidative stress. Despite its well-described actions, the low plasmatic concentrations of HT after 25 mL extra-virgin olive oil (EVOO) consumption, ranging from 50 to 160 nM [5,6] questioned the assessment of its bioactivity. In addition, among the exponential increase of published studies on HT, few are related to its effects on MetS key components and the associated complications. Herein, this review discusses the effects of HT on MetS key components and the molecular mechanisms exerting its health protective effects.
2. Mediterranean Diet and Olive Oil as Primary and Secondary Preventive Nutritional Strategies
The Mediterranean diet pattern is characterized by a high consumptions of fruits, vegetables, beans, nuts, unrefined grains, and fish; a lower intake of meats and full-fat dairy products; and a daily consumption of olive oil as the mostly used fat in culinary practices. Fatty acid consumption, characterized by a higher rate of monounsaturated (MUFAs) and polyunsaturated (PUFAs) fatty acids consumption than saturated fatty acid (SFAs), is central in the consumption of VOO in the Mediterranean population countries. Indeed, Greece, Italy and Spain are characterized by a higher consumption of MUFAs compared to SFAs, whereas, in the US, the ratio MUFA/SFA is ~1 [7,8].
Lifestyle interventions using a Mediterranean-type diet reported inverse associations between a good adherence to this pattern and the risk of CVD [9] or T2DM [10] and showed a reduction in the incidence of key components of MetS including obesity [11,12,13], hypertension [14,15,16], glucose tolerance [17], dyslipidemia [18] and insulin resistance [19]. Olive oil, the main MUFAs in the Mediterranean diet, has been widely identified as the initiator of these health benefits with increasing consumption of virgin olive oil (VOO) enhancing lipid profile, reducing blood pressure and endothelial dysfunction, improving inflammatory and prothrombotic environment through reduced Low Density Lipoprotein (LDL) oxidizability [20]. Recently, the Prevencion con Dieta mediterránea study (PREDIMED) [21] showed that patients with a Mediterranean diet supplemented with extra-virgin olive oil (EVOO) or nuts had a lower incidence of T2DM and a reduced associated-mortality. EVOO provided in the Mediterranean diet was beneficial for blood pressure, glycemia, dyslipidemia, oxidative stress and inflammation [22,23]. Interestingly, the EUROLIVE (Effect of Olive Oils on Oxidative Damage in European Populations) study highlighted inverse relationships between the total cholesterol/High Density Lipoprotein (HDL)-cholesterol ratio or oxidative stress markers and the phenolic content of the olive oil [24]. The cardio-protective actions of olive oil components have been reported [25,26,27] and the non-saponifiable minor bioactive compounds [28,29,30] such as phenolic compounds including HT and its precursor OLE, rather than oleic acid, were reported responsible for the protective properties [31].
3. Olive Oil Composition and Polyphenolic Fraction
The olive oil composition can be divided into two fractions, saponifiable and unsaponifiable fractions. The saponifiable fraction of olive oil corresponds to the total amount of SFAs, MUFAs and PUFAs. Olive oil is characterized by a high content of MUFAs, whereas the concentrations of SFAs and PUFAs range from 8% to 26% and from 3% to 22%, respectively. Oleic acid is present up to 83% in virgin olive oil [32].
The unsaponifiable fraction contains more than 200 compounds; among them, phenolic compounds account for 3% of the total oil composition [33]. This fraction contributes to the specific characteristics of olive oil, such as aroma, taste, color and oxidative stability [34]. The most abundant fraction of the unsaponifiable fraction is hydrocarbons (squalene, β-carotene and lutein). Other compounds are phytosterols, triterpenic compounds in the form of dialcohols or acids and fat-soluble phenols including tocopherol and tocotrienols. The most polar fraction consists in phenolic compounds, which can be classified into several groups: phenolic acids (caffeic, ferulic, gallic, gentisic, o-coumaric, p-coumaric, p-hydroxybenzoic and protocatehuic acids), phenolic alcohols 3,4-dihydroxyphenylethanol named HT and tyrosol (Tyr), phenolic secoiridoids, hydroxyl-isocromans (formed by the reaction between HT and benzaldehyde or vanillin), flavonoids and lignans [35]. In olive fruit, phenolic compounds are present as glycosylated forms. Oleuropein aglycone is a phenolic secoiridoid liberated from the glucoside form, OLE, upon the action of a β-glucosidase during olive ripening. In olive oil, oleuropein aglycone is degraded into elenolic acid, the secoiridoid moiety, and HT, the phenolic moiety.
4. Virgin Olive Oil Phenols Concentration
Not less than 30 polyphenols have been identified in olive oil and considerable variations have been noted in the concentrations of these phenolic compounds. Phenolic concentration in EVOO ranges from 50 to 800 mg/kg [36] with a mean of 230 mg/kg, whereas in refined olive oil it is much lower [37].
HT and their corresponding secoiridoid derivatives constitute around 90% of the total phenolic content of VOO [38]. Olive oil phenol concentration depends of olive variety [39], agricultural environment and practices [39], the maturity stages of the fruit [39], storage conditions and processing [40,41].
5. Bioavailability of Hydroxytyrosol and Metabolism
A consensus indicated that HT does not exert any cytotoxic effect on cells [42], or animals [43,44]. In 2000, Visioli et al. [45] showed that olive oil phenols, especially HT and Tyr, are dose-dependently absorbed in humans after ingestion and excreted in urine. However, the levels of free HT and Tyr in urine were lower compared with their glucuronide metabolites. In addition to glucuronides, other metabolites of HT were found in plasma and urine such as the methylated or sulfated forms [46]. It has been reported that there is a significant absorption (40%–95%) of HT [45,46,47,48] indicating that human intestine absorbs a major part of ingested VOO phenolic compounds. Moreover, HT is more assimilated when given as an olive oil compared to an aqueous solution [49], and its absorption was greater when ingested in its natural form present in extra-virgin olive oil rather than added in refined olive oil or incorporated into a yoghurt, as shown by its urinary recoveries being 44%, 23% and 5.8%, respectively [50]. Such results suggested that the olive oil matrix could act as a protective factor preventing the degradation of phenolic compounds in the gastrointestinal tract. The range levels of circulating metabolites of OLE was 10–60 µg post-ingestion of a 50 mL high-phenol-containing VOO [51,52].
Consumption of VOO in the Mediterranean countries is expected to be around 30–50 g/day [53] leading to an intake of 200 µg of phenolic compounds. Taking into account that the absorption rate of phenols is in the range of 40%–95%, it results an amount of 4–9 mg/day of olive oil phenols. Note that, in the gastrointestinal tract, HT and Tyr result from the degradation of aglycones of OLE and its monophenolic form ligstrosides. Its degradation is incomplete and OLE can be readily absorbed across the intestine [54] by possible implication of glucose transporter [55]. Besides, glucuronidation of HT was previously reported in intestinal Caco-2 and in HepG2 cells [56,57,58].
Note that D’Angelo et al. [43] have found that intravenous administration of HT led to a fast and extensive uptake of this molecule within 5 min after injection in several tissues such as skeletal muscle, heart, liver, lungs and kidney.
6. Hydroxytyrosol, Body Weight and Development of Adipose Tissue
Body weight is the main outcome to define obesity and body mass index increase is positively correlated with MetS. Only one clinical study assessed the effect of HT supplementation on body weight showing that 12-week supplementation of HT (9.67 mg/day) associated with OLE (51.1 mg/day) did not exert any effect on body weight in overweight men [59]. The absence of effect was confirmed in numerous rodent experiments [60,61,62,63,64], except for one study that showed a beneficial effect of HT (50 mg/kg/day × 17 weeks) supplementation against diet-induced obesity [65].
Whereas no in vivo studies experienced the impact of VOO phenolic compounds on adipose tissue development, in vitro, VOO phenolic compounds have been shown to influence adipocyte hyperplasia and hypertrophy through the expression of genes related to obesity. It was reported that HT (25 and 150 µM) reduced hyperplasia and hypertrophy by reducing triglycerides content by downregulating adipogenesis-related genes, peroxisome proliferator-activated receptors α and γ, peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α), lipoprotein lipase, hormone sensitive lipase, acetyl CoA carboxylase-1, carnitine palmitoyltransferase-1, CCAAT/enhancer binding protein α, and sterol regulatory element-binding transcription factor-1 transcription factors and downstream genes (glucose transporter-4, CD36 and fatty acid synthase) [66,67]. Taken together, these data suggested that HT might reduce the size of adipocytes and be beneficial for reducing the risk of obesity.
The processes of adipose hypertrophy and hyperplasia are associated with mitochondrial stress and dysfunction observed by a reduction of adenosine triphosphate (ATP) formation and a reduction of mitochondrial complex expression subunits. In a murine model of high fat diet (HFD)-induced obesity, it has been shown that HT (50 mg/kg/day × 17 weeks) could normalize mitochondrial complex subunit expression and mitochondrial fission marker dynamin-related protein-1 [65]. The high amount of subunit expression could be attributed to an enhancement of mitochondria quantity. Indeed, Hao et al. [68] have found that HT allows to enhance Mitochondrial transcription factor A (1 µM), Nuclear respiratory factors 1 and 2 (Nrf1 and Nrf2) (1 µM) mRNA, key activators of mitochondrial transcription and genome replication, thus increasing protein levels of complexes 1, 2, and 3 in adipocytes (0.1, 1 and 10 µM). The authors also found an increase of peroxisome proliferator-activated receptors α and γ (1 and 10 µM) and carnitine palmitoyltransferase I (1 and 10 µM) expressions, which are implicated in mitochondria biogenesis, suggesting a possible better oxidative status in adipocytes.
Furthermore, it seems that HT (1 µM) acts as a starving agent, since an increase in adenosine monophosphate kinase (AMPK), acetyl CoA carboxylase, hormone-sensitive lipase and lipase phosphorylation were reported in adipocytes [68].
7. Hydroxytyrosol and Lipid Metabolism
The prospective EUROLIVE study demonstrated that olive oils with different levels of polyphenols led to a reduction in LDL-c and TG [24] in a dose-dependent manner. The absence of body weight gain suggested that HT could possess a lipolytic function, especially in adipose tissue. Whereas clinical trials were not undertaken, some experimental studies, performed in rodent and murine models (0.03% × 8 weeks and 50 mg/kg/day × 17 weeks) [63,65] or adipocytes (150 µM and 1 µM) [66,68] reported that HT attenuates TGs accumulation in adipocytes, blood, liver and skeletal muscles (50 mg/kg/day × 17 weeks and 25 µM) [65,69]; glycerol release (75 µM) [69]; and lowers serum cholesterol in HFD-rats (10 mg/kg/day × 5 weeks) [70], and LDL and HDL-c levels (50 mg/kg/day × 17 weeks) [65] and plasma cholesterol in control rats (0.03% × 8 weeks) [63]. Moreover, HT treatment inhibited epididymal and perirenal fat formation and limited liver weight gain (50 mg/kg/day × 17 weeks) [65]. On the other hand, it has been demonstrated in a db/db model of mice, that HT (10 mg/kg/day × 8 weeks) increased the activity of mitochondrial complex, and lipolysis fatty acid oxidation-related genes [65]. In contrast, Acin et al. [71] reported that HT (10 mg/kg/day × 10 weeks) had deleterious effects with increasing plasma cholesterol, very low density lipoprotein-cholesterol, and LDL-c and reducing ApoA-1 resulting in an increased atheroma plaque formation.
In vitro, HT was reported to increase oxygen consumption, suggesting a higher oxidative rate to produce ATP [65], proteins implicated in mitochondria biogenesis, mitochondria mass and size [68]. AMPK was decreased during chronic stress situation, thus reducing glycolysis and fatty acid oxidation. Moreover, Cao et al. [65] have reported, in obese mice, that HT supplementation (50 mg/kg/day × 17 weeks) leads to a reduction in SREBP-1c level, a well-known regulator of fatty acid and cholesterol synthesis in liver.
8. Hydroxytyrosol, Glucose Homeostasis and Insulin-Resistance
The strength of olive oil to reduce the incidence of all the glucose-associated disorders is no longer to be demonstrated. Moreover, the enhancement of glucose tolerance was shown to be dependent of the concentration of polyphenols and olive oil [72]. Clinical trials regarding the impact of HT on carbohydrate metabolism are still lacking but experiments in rodent models of MetS are available and suggested that HT is able to reduce plasmatic glucose concentration (50 mg/kg/day × 17 weeks, 20 mg/kg × 2 months and 0.04% × 8 weeks) [65,73,74] and insulin secretion (50 µg/mL) [73] leading to a decrease of insulin-resistance [65,74]. Moreover, Pirozzi et al. [70] found that HT (10 mg/kg/day × 5 weeks) enhances glucose tolerance and increases insulin sensitivity leading to a decrease of homeostatic model assessment-insulin resistance. Interestingly, in a db/db model of mice, Cao et al. [65] have reported that HT given at 10 mg/kg/day for 8 weeks decreases fasting glucose level.
9. Hydroxytyrosol and Hypertension
Clinical trials have demonstrated that olive oil is more efficient than any other oil at reducing blood pressure [75,76,77,78]. It has been hypothesized that the effect of olive oil on blood pressure was not only mediated through its MUFAs content but also through its polyphenol content. Indeed, some studies mentioned that the polyphenols of olive oil were responsible of the anti-hypertensive effect of olive oils, as demonstrated in hypercholesterolemic [79] or pre-hypertensive subjects [80] after consuming polyphenols enriched olive oil. Ruiz-Gutierrez et al. [81] reported a reduction of both systolic (SBP) and diastolic (DBP) blood pressures after an olive oil-rich diet but not after a high-oleic-acid sunflower diet. In this sense, clinical trials proved that consumption of OLE was able to reduce SBP and DBP after consumption of OLE in both pre-hypertensive subjects (136 mg/day + 6 mg/day HT × 6 weeks) [82] and hypertensive rats (30 mg/day × 5 weeks) [83]. Given the fact that OLE is degraded into HT, the question arose if the blood pressure lowering effect was due to OLE or HT. Lopez-Villodres et al. [84] found that HT supplementation (10 mg/kg/day × 2 months) increased in diabetic rats the levels of nitrites and nitrates, potent donors of NO acting as vasorelaxing agent. In addition, Storniolo et al. [85] demonstrated that HT (10 µM) counteracted hyperglycemia-induced endothelin-1 expression, a well-known hypertensive agent, in a more extend than oleic acid.
10. Associated Complications: Oxidative Stress, Inflammation and Cardiovascular Dysfunction
10.1. Antioxidative Properties
Oxidative stress is a central physiologic process playing an important role in the maintenance of intracellular homeostasis. However, despite intracellular protective mechanisms, including superoxide dismustase (SOD), Catalase (Cat) and reduced glutathione, excess reactive oxygen species (ROS) is detrimental to cellular physiology. Obesity and T2DM are characterized by an excessive amount of ROS overwhelming intracellular defenses and leading to reinforce MetS associated complications. Polyphenols have been used as nutraceutical antioxidant for several years since an increased amount of fruits and vegetables were linked to the reduction of oxidative pathologies.
10.1.1. Hydroxytyrosol and LDL Oxidizability
Oxidation of the lipid part of LDL leads to a change in the lipoprotein conformation by which LDL is better able to enter into monocytes/macrophage of the arterial wall and develop the atherosclerotic process. Human studies suggested that olive oil protects LDL against oxidation as indicated by decreased LDL oxidizability [86,87] and this strong effect prevails on linoleate-rich particles [88]. It has been well demonstrated that phenolic compounds, especially HT, are protective against LDL oxidation. Based on this protective effect, the European Food Safety Authority claimed that 5 mg of HT (as free and derived forms) should be consumed daily. To prove that HT is efficient, its supplementation (45–50 mg/day × 3 weeks) in sunflower oil was shown to reduce oxLDL [89], suggesting that HT could prevent CVD. These results in clinical trials were corroborated by animal experiments [74,90]. Increase of lag-time [91,92] is the main outcome of reduction in LDL oxidizability and this change could be attributed to an increase in oleic acid [88] or HT [38,60,93] rate in LDL. Such mechanism occurs rapidly and increases with phenolic compounds in olive oil [94].
Mateos et al. [95] reported that consumption of polyphenol-rich VOO leads to a reduction of the expression of pro-atherogenic genes such as CD40 antigen ligand and oxLDL receptor-1 when compared with the refined olive oil, which was depleted in polyphenols [87]. Another mechanism that can be implicated in the protection of LDL by olive oil could be the enhancement of arylesterase plasma activity, an enzyme presents on HDL surface, suggested to contribute to the antioxidant protection conferred by HDL on LDL oxidation [96,97]. However, the scavenging properties of HT cannot be excluded in the protection of LDL oxidizability (10 µM) [98,99]. In fact, Briante et al. [100] reported that HT protects, in vitro, LDL from oxidation at a concentration >18 µg/mg of LDL.
10.1.2. Hydroxytyrosol and Mitochondria
There is evidence that mitochondrial dysfunction in MetS is associated with T2DM [101,102]. Genetic factors, oxidative stress, mitochondrial biogenesis and aging may affect mitochondrial function, leading to insulin resistance. Fewer and smaller-sized mitochondria have been found in skeletal muscles of insulin-resistance, obese, or T2DM subjects and are linked with a lower mitochondrial oxidative capacity [103]. The decreased mitochondrial oxidative capacity is associated with the reduction in expression of mitochondrial genome [104]. To counteract such effect and enhance oxidative metabolism, HT was supposed to be a good candidate. Although no clinical trial investigated the effect of HT supplementation, studies in obese- and diabetic-rendered rats and in doxorubicin-induced cardiotoxicity rats revealed that HT (0.5, 10 and 50 mg/kg/day) is able to increase mitochondrial function through an enhancement of mitochondrial complex subunit expression [65,105] and activity [105,106]. Enhanced mitochondrial activity was associated with an increase of uncoupling protein-2 protein expression (100 µM) [107]. All the animal experiments supported the impact of HT in the protection of mitochondria from oxidative damages, which operates a shift towards a more efficient oxidative metabolism. Furthermore, it has been demonstrated that HT increases the mitochondrial deoxyribonucleic acid content (1 µM and 10 and 50 mg/kg/day) [68,108], the mitochondria function and membrane potential (0.1 and 10 µg/mL) [109] and density (1 µM) [68]. Mitochondrial biogenesis and respiration were stimulated by PGC-1α by strongly inducing its gene expression. Rodent and culture cell experiments reported also that HT was able to increase PGC-1α and Nrf2 expression (0.1, 1 and 10 µM and 100 µM) [68,107] and AMPK, an upstream regulator of PGC-1α [68,107]. Note that an increase in maximal oxygen consumption was found (1 and 10 µM) [68].
10.1.3. Hydroxytyrosol and Antioxidant Protein Expression
There is a recognized link between oxidative stress and key components of MetS. Besides LDL oxidation, other oxidative markers also showed improvements. HT was reported to prevent the increase of protein carbonyl levels and lipid peroxidation markers, and to normalize liver glutathione level, liver glutathione S-transferase, and total SOD activity in obese mice (10 and 50 mg/kg/day) [65]. In cell culture, it was also demonstrated that HT increases Cu/SOD expression (100 µM) [107]; normalizes glutathione concentrations; increases glutathione peroxidase, glutathione reductase and glutathione-S-transferase protein expression (0.5 to 10 µM) [110]; increases CAT activity (50 µM) [111]; and reduces the reduced glutathione/oxidized glutathione (known as GSH:GSSG ratio) (1 and 5 µM) in presence of hydrogen peroxide, suggesting a reduction in the oxidative status [63]. The antioxidative capacities are not limited to the expression of type 2 detoxifying proteins as SOD, Cat, and glutathione peroxidase. Indeed, it exists adaptive systems as those implying heme oxygenase-1, the expression of which is regulated by Nrf2. The positive impact of HT on Nrf2 nuclear translocation was shown and associated to phosphoinositide/protein kinase B and extracellular signal-regulated kinase pathway (0.5 to 10 µM) [110] and also AMPK/forkhead box 3a (50 µM) [111].
Moreover, it has been found that HT (20 µg/day) for four weeks was able to reduce plasma hydroperoxide concentrations, normalize plasma malondialdehyde and conjugated dienes, and increase plasma antioxidant capacity in a rat model of HFD-induced obesity [64].
10.1.4. Hydroxytyrosol and Superoxide (O2•−) Scavenging Properties
In an acute model of oxidative stress driven in rat aortas, Rietjens et al. [98] showed that HT acts as a scavenging agent. Since, numerous studies were published and confirmed that HT protect against ROS production in human vascular endothelial cells, erythrocytes and renal epithelial tubular cells (5 to 80 µM) [111,112,113], displays scavenger activity for peroxynitrous acid (5 µM to 1 mM) [114,115] and has a protective role on deoxyribonucleic acid damages associated to peroxinitrous acid (0.05 to 1 mM) [114]. Moreover, it can inhibit superoxide anion burst from macrophages but not from neutrophils where it can only scavenge hydrogen peroxide (1 to 50 µM) [116]. It also protects erythrocytes from hemolysis (50 to 200 µM) [117], endothelial cells from monocytes adhesion (0.5 to 2.5 µM) [118] and hepatocytes (10 to 40 µM) [119], and protects from lipid peroxidation in rat livers (5 to 60 µM) [120] as well as from lipid oxidation (10 µM) [91].
10.2. Hydroxytyrosol and Inflammation
It is well known that the pathophysiology of MetS causes chronic inflammation. HT has been reported to possess significant anti-inflammatory capacity. In fact, in clinical trial, HT (25 mg/day × one week) led to a reduction of plasma CRP and isoprostane levels, but did not exert any effect on other inflammatory markers as interleukin-6, monocytes chemoattractant protein-1 and tumor necrosis factor-α (TNF-α) [121]. In rodent experiments, it was demonstrated that HT reduces TNF-α, IL-6 and cyclooxygenase-2 expression in liver (50 mg/kg/day × 17 weeks) [65], increases the anti-inflammatory IL-10 expression (12.5 µg/mL and 10 mg/kg/day × 10 days) [122,123], reduces inducible nitric oxide synthase (iNOS) expression (12.5 µg/mL and 5 mg/kg/day × 30 days) [122,124,125] and cyclooxygenase-2 expression [125]. Considering that leptin, a well-known protein acting on satiety and having inflammatory property, HT was shown to lower leptin level in mice thus suggesting that could act as a starving and anti-inflammatory agent (0.03% × 8 weeks and 50 mg/kg/day × 17 weeks) [63,65] and attenuate TNF-α and IL-1β expression in animal model [125,126]. In vitro, HT has been reported to increase adiponectin expression and secretion in the presence of TNF-α (1 to 20 µM) [127], reduce iNOS, cyclooxygenase-2 and TNF-α expressions (25 to 100 µM) [128], reduce nuclear factor-κB (NF-κB) binding activity (50 and 100 µM) [129] and, interestingly, increase iκBα expression, an inhibitor of NF-κB binding activity [129]. Moreover, a reduction of metalloproteinase-9 activity and secretion (1 and 10 µM) [130] and a reduction of prostaglandin E2 secretion and expression were found (1 and 10 µM) [130].
10.3. Hydroxytyrosol and Atherosclerosis
Phenolic compounds in VOO were shown to improve endothelial dysfunction and reduce oxidative stress plasma parameters, both playing a key role in the development of atherosclerosis [131,132]. Moreover, VOO phenolic compounds could counteract inflammation, which is an important trigger in the development of atherosclerosis though the expression of adhesion molecules. In a clinical trial enrolling healthy volunteers, it has been demonstrated that sunflower oil supplemented with HT decreased vascular cell adhesion protein (VCAM-1) plasmatic concentration (45–50 mg/kg/day × 3 weeks) [89] and monocyte chemoattractant protein-1 and interleukin-8 receptor expression (366 mg/kg/day × 3 weeks) [87]. Such results were confirmed in rodent models, where HT reduced platelet aggregation, VCAM-1 and IL-1β expressions (0.5 to 10 mg/kg/day × 2 months) [84] and TNF-α expression (0.04%) [74]. Interestingly, Gonzalez et al. [60] reported that HT (4 mg/kg/day × 2 months) reduced the size of atherosclerotic lesions in rabbits fed with a high fat and high cholesterol diet.
Several in vitro studies confirmed the anti-inflammatory capacity of HT by reducing VCAM-1, intercellular adhesion protein expressions (0.5 to 75 µM) [22,118,133]. Molecular mechanisms leading to this reduction probably involved the reduction of NF-κB activation (0.5 to 75 µM) [22,23]. Despite the great interest surrounding HT as a nutraceutical, Acin et al. [71] reported that HT supplementation (10 mg/kg/day) for 10 weeks led to an increase in atherosclerotic plaque, in monocyte activation and a reduction in ApoA-I from HDL in ApoE-deficient mice. However, these mice did not develop obesity, low-grade inflammation and oxidative stress, thus explaining this deleterious effect because HT could act as an oxidant.
10.4. Hydroxytyrosol and Vascular Dysfunction
Nitric oxide (NO•) plays a pivotal role in endothelial function and its decreased bioavailability is correlated with altered vascular tone. Lopez-Villodres et al. [84] found that in streptozotocin-induced model of diabetes, HT supplementation (10 mg/kg/day × 2 months) increased the level of nitrates and nitrites. HT was reported to be ineffective on eNOS expression and activity (i.e., phosphorylation on its Ser1177) in absence of oxidative stress in HUVECs (0.1 to 100 µM) [134]. In an endothelial cell culture model of hyperglycemia, Storniolo et al. [85] showed that HT (10 µM) increases NO• production, which is correlated to an increase of endothelial nitric oxide synthase phosphorylation (P-eNOS)/endothelial nitric oxide synthase (eNOS) ratio. When stimulated by acetylcholine, an activator of eNOS/NO• signaling pathway, HT increased NO• production, more than oleic acid; this increase was linked to higher intracellular calcium concentration. Rietjens et al. [98] evidenced that HT (10 µM) enhances endothelium-dependent relaxation in addition to increase P-eNOS/eNOS ratio. This enhancement was associated with a cGMP increase, a downstream molecule of eNOS acting on smooth muscle cells relaxation. In a vascular endothelial cell culture model, Zrelli et al. [135] found that HT (50 µM) increases P-eNOS resulting in increasing NO• production, associated to the decreased of NF-κB and iκBα phosphorylation in the presence of TNF-α. These results suggest that eNOS could decrease inflammatory response, and thereafter, decrease thrombus formation. Furthermore, it was demonstrated that HT reduced iNOS expression (25 to 100 µM) [128], known for its inflammatory, and oxidative properties in monocytes.
10.5. Hydroxytyrosol and Cardiac Dysfunction
All of the cardiovascular risk factors of MetS are associated with increased risk of heart failure. HT was revealed as cardiac protective after olive oil consumption. Bayram et al. [136] have found that female SAMP8 mice fed with a Western diet enriched with a high-polyphenol content (mainly tyrosol (20.8 mg/kg oil) and hydroxytyrosol (18.9 mg/kg oil)) had lower TBARS levels in cardiac muscle. Alterations of cardiac function were correlated with cardiac remodeling leading to blood pressure increase thus raising CVD. Mnafgui et al. [137] showed that a HT supplementation (2 and 5 mg/kg/day × 1 week) in a rodent CVD model leads to a reduction of heart weight and heart weight/body weight ratio. These morphological changes were followed by the reductions of SBP, DBP and mean arterial blood pressure, heart rate and ST segment elevation. Moreover, these authors [137] found an increase of lactate dehydrogenase and creatine kinase protein expressions showing an enhancement of glucose consumption, probably producing higher ATP. Granados-Principal et al. [106] found in cardiac tissue that HT (0.5 mg/kg, 5 days/week × 6 weeks) increases expression of mitochondria complexes 1, 2 and 3 and reduces specific markers of oxidative damages to proteins. These HT beneficial effects on cardiac function are probably due to its antioxidative properties (0.1 and 10 µg/mL) [109], since HT was not found in rat heart (1, 10 and 100 mg/kg) [138].
11. Functional Applications in Food Processing
Nowadays, several antioxidants are used to reduce food oxidation and thus extend shelf life. Adding 100 mg/kg HT in frankfurters was effective against lipid oxidation during storage, to a greater extent than a mix of butylated hydroxyanisole/butylated hydroxytoluene [139]. Nieto et al. [140] also showed a reduced lipid oxidation in sausages with HT (50 ppm). Thus HT added in fat used could help to maintain the nutritional and sensorial qualities of processed food products.
12. Limitations of Experimental Studies
Ex vivo and in vitro studies with HT are well documented, but question the extrapolation to human relevancy. In fact, high concentrations were usually used in cell models [22,111,135], certainly due to the oxidation of HT [141]. HT has been tested as a sole molecule, and not with other antioxidant compounds (phenolic compounds, tocopherols, carotenoids and vitamin C) that occur in human context. Moreover, the influence of food matrix on the bioactivity of HT is avoided in most of experimental studies.
13. Conclusions
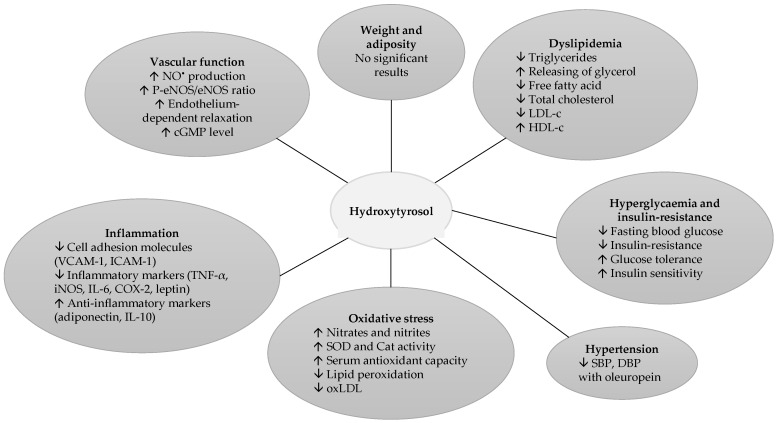
To conclude, the beneficial effects of HT were extensively studied in rodent experiments and clinical trials evidencing the role of HT in the reduction of MetS and its associated complications, which are briefly presented in Figure 1. Both experimental and clinical studies demonstrated that HT reduced oxidative stress and inflammation, thus altering positively MetS key components. However, contradictory results for obesity were reported, probably resulting from differences in the study design, administered doses and type of animals. Moreover, actually, no experiment assesses the impact of pure HT on blood pressure in normotensive or hypertensive subjects. In this sense, larger and more experimental studies and clinical trials are needed.
Figure 1.
Effect of hydroxytyrosol on metabolic syndrome-associated complications and metabolic syndrome. ↑: Increase in; ↓: Decrease in. LDL-c: Low-Density Lipoprotein-cholesterol; HDL-c: High Density Lipoprotein-cholesterol; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; SOD: Super Oxide Dismutase; Cat: Catalase; oxLDL: oxidized Low-Density Lipoprotein; VCAM-1: Vascular Cell Adhesion Molecule-1; ICAM-1: Intercellular Adhesion Molecule-1; TNF-α: Tumor Necrosis Factor alpha; iNOS: inducible Nitric Oxide Synthase; IL-6: Interleukin-6; COX-2: Cyclooxygenase-2; IL-10: Interleukin-10; NO•: Nitric Oxide; P-eNOS/eNOS: Phosphorylated Endothelial Nitric Oxide Synthase/endothelial Nitric Oxide Synthase ratio; cGMP: cyclic Guanosine Monophosphate.
The role of dietary polyphenols in human health depends largely on their bioavailability and, because HT bioavailability is reduced, with systemic concentration ranging in the nanomolar levels, it could be expected that HT exerts its effects directly in the gastrointestinal tract before being absorbed. Indeed, the concentration of phenolic compounds may reach the millimolar range. In this sense, modulation of gut microbiota could be the most remarkable local effect exerted by HT. Recently, another component of the Mediterranean diet, resveratrol, has been shown to modulate positively gut microbiota enhancing glucose tolerance in a mice model of obesity [142] and also through a duodenal Sirt-1 pathway into the hypothalamus [143] enhancing hypothalamic insulin-resistance. Sirt-1 is an energy sensitizer upregulating antioxidative and anti-inflammatory gene expression and improving mitochondrial biogenesis through PGC-1α. Direct effects of HT on duodenal Sirt-1 pathway cannot be excluded.
Acknowledgments
The authors declare that they have received no grants in support of their research work and no funds for covering the costs to publish in open access.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPK | Adenosine monophosphate kinase |
| ATP | Adenosine triphosphate |
| Cat | Catalase |
| CVD | Cardiovascular diseases |
| eNOS | Endothelial nitric oxide synthase |
| EVOO | Extra-virgin olive oil |
| HDL | High-density lipoprotein |
| HFD | High Fat Diet |
| IL | interleukin |
| iNOS | Inducible nitric oxide synthase |
| LDL | Low density lipoprotein |
| MUFA | Monounsaturated fatty acid |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor 2 |
| OLE | Oleuropein |
| P-eNOS | Phosphorylated endothelial nitric oxide synthase |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PUFA | Polyunsaturated fatty acid |
| ROS | Reactive oxygen species |
| SFA | Saturated fatty acid |
| SOD | Superoxide dismustase |
| TBARS | Thiobarbituric acid reactive substances |
| TNF-α | Tumor necrosis factor-alpha |
| VCAM-1 | Vascular adhesion molecule-1 |
| VOO | Virgin olive oil |
Author Contributions
All authors analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.-C., James W.P.T., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Laslett L.J., Alagona P., Clark B.A., Drozda J.P., Saldivar F., Wilson S.R., Poe C., Hart M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012;60:S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Estruch R., Ros E., Salas-Salvadó J., Covas M.-I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., et al. Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 4.Covas M.-I., Ruiz-Gutiérrez V., de la Torre R., Kafatos A., Lamuela-Raventós R.M., Osada J., Owen R.W., Visioli F. Minor Components of Olive Oil: Evidence to Date of Health Benefits in Humans. Nutr. Rev. 2006;64:S20–S30. doi: 10.1111/j.1753-4887.2006.tb00260.x. [DOI] [Google Scholar]
- 5.Miro-Casas E., Covas M.-I., Farre M., Fito M., Ortuño J., Weinbrenner T., Roset P., de la Torre R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003;49:945–952. doi: 10.1373/49.6.945. [DOI] [PubMed] [Google Scholar]
- 6.Weinbrenner T., Fitó M., de la Torre R., Saez G.T., Rijken P., Tormos C., Coolen S., Albaladejo M.F., Abanades S., Schroder H., et al. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr. 2004;134:2314–2321. doi: 10.1093/jn/134.9.2314. [DOI] [PubMed] [Google Scholar]
- 7.Freisling H., Fahey M.T., Moskal A., Ocké M.C., Ferrari P., Jenab M., Norat T., Naska A., Welch A.A., Navarro C., et al. Region-specific nutrient intake patterns exhibit a geographical gradient within and between European countries. J. Nutr. 2010;140:1280–1286. doi: 10.3945/jn.110.121152. [DOI] [PubMed] [Google Scholar]
- 8.Harika R.K., Eilander A., Alssema M., Osendarp S.J.M., Zock P.L. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann. Nutr. Metab. 2013;63:229–238. doi: 10.1159/000355437. [DOI] [PubMed] [Google Scholar]
- 9.Sofi F., Macchi C., Abbate R., Gensini G.F., Casini A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17:2769–2782. doi: 10.1017/S1368980013003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagiotakos D.B., Pitsavos C.H., Chrysohoou C., Skoumas J., Papadimitriou L., Stefanadis C., Toutouzas P.K. Status and management of hypertension in Greece: Role of the adoption of a Mediterranean diet: The Attica study. J. Hypertens. 2003;21:1483–1489. doi: 10.1097/00004872-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro B., Toietta G., Maggio R., Arciello M., Tarocchi M., Galli A., Balsano C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014;15:18508–18524. doi: 10.3390/ijms151018508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown L., Poudyal H., Panchal S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015;16:914–941. doi: 10.1111/obr.12313. [DOI] [PubMed] [Google Scholar]
- 13.Covas M.-I., de la Torre R., Fitó M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015;113(Suppl. S2):S19–S28. doi: 10.1017/S0007114515000136. [DOI] [PubMed] [Google Scholar]
- 14.Buckland G., Bach A., Serra-Majem L. Obesity and the Mediterranean diet: A systematic review of observational and intervention studies. Obes. Rev. 2008;9:582–593. doi: 10.1111/j.1467-789X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 15.Núñez-Córdoba J.M., Valencia-Serrano F., Toledo E., Alonso A., Martínez-González M.A. The Mediterranean diet and incidence of hypertension: The Seguimiento Universidad de Navarra (SUN) Study. Am. J. Epidemiol. 2009;169:339–346. doi: 10.1093/aje/kwn335. [DOI] [PubMed] [Google Scholar]
- 16.Toledo E., Hu F.B., Estruch R., Buil-Cosiales P., Corella D., Salas-Salvadó J., Covas M.I., Arós F., Gómez-Gracia E., Fiol M., et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013;11:207. doi: 10.1186/1741-7015-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito K., Marfella R., Ciotola M., Di Palo C., Giugliano F., Giugliano G., D’Armiento M., D’Andrea F., Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 18.Mekki K., Bouzidi-bekada N., Kaddous A., Bouchenak M. Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010;1:110–115. doi: 10.1039/c0fo00032a. [DOI] [PubMed] [Google Scholar]
- 19.Georgoulis M., Kontogianni M.D., Yiannakouris N. Mediterranean diet and diabetes: Prevention and treatment. Nutrients. 2014;6:1406–1423. doi: 10.3390/nu6041406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Miranda J., Pérez-Jiménez F., Ros E., De Caterina R., Badimón L., Covas M.I., Escrich E., Ordovás J.M., Soriguer F., Abiá R., et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010;20:284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Salas-Salvadó J., Martinez-González M.Á., Bulló M., Ros E. The role of diet in the prevention of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011;21(Suppl. S2):B32–B48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Carluccio M.A., Siculella L., Ancora M.A., Massaro M., Scoditti E., Storelli C., Visioli F., Distante A., de Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 23.Salas-Salvadó J., Garcia-Arellano A., Estruch R., Marquez-Sandoval F., Corella D., Fiol M., Gómez-Gracia E., Viñoles E., Arós F., Herrera C., et al. Investigators Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008;62:651–659. doi: 10.1038/sj.ejcn.1602762. [DOI] [PubMed] [Google Scholar]
- 24.Covas M.-I., Nyyssönen K., Poulsen H.E., Kaikkonen J., Zunft H.-J.F., Kiesewetter H., Gaddi A., de la Torre R., Mursu J., Bäumler H., et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 25.Panagiotakos D.B., Pitsavos C., Polychronopoulos E., Chrysohoou C., Zampelas A., Trichopoulou A. Can a Mediterranean diet moderate the development and clinical progression of coronary heart disease? A systematic review. Med. Sci. Monit. 2004;10:RA193–RA198. [PubMed] [Google Scholar]
- 26.Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 27.Babio N., Toledo E., Estruch R., Ros E., Martínez-González M.A., Castañer O., Bulló M., Corella D., Arós F., Gómez-Gracia E., et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. Can. Med. Assoc. J. 2014;186:E649–E657. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Jiménez F., Ruano J., Perez-Martinez P., Lopez-Segura F., Lopez-Miranda J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007;51:1199–1208. doi: 10.1002/mnfr.200600273. [DOI] [PubMed] [Google Scholar]
- 29.Cicerale S., Conlan X.A., Sinclair A.J., Keast R.S.J. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009;49:218–236. doi: 10.1080/10408390701856223. [DOI] [PubMed] [Google Scholar]
- 30.Visioli F., Bernardini E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011;17:786–804. doi: 10.2174/138161211795428885. [DOI] [PubMed] [Google Scholar]
- 31.Rafehi H., Ververis K., Karagiannis T.C. Mechanisms of action of phenolic compounds in olive. J. Diet. Suppl. 2012;9:96–109. doi: 10.3109/19390211.2012.682644. [DOI] [PubMed] [Google Scholar]
- 32.Hodson L., Fielding B.A. Stearoyl-CoA desaturase: Rogue or innocent bystander? Prog. Lipid Res. 2013;52:15–42. doi: 10.1016/j.plipres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 33.De la Lastra Romero C.A. An up-date of olive oil and bioactive constituents in health: Molecular mechanisms and clinical implications. Curr. Pharm. Des. 2011;17:752–753. doi: 10.2174/138161211795428894. [DOI] [PubMed] [Google Scholar]
- 34.Frankel E., Bakhouche A., Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. J. Agric. Food Chem. 2013;61:5179–5188. doi: 10.1021/jf400806z. [DOI] [PubMed] [Google Scholar]
- 35.Montserrat-de la Paz S., Bermudez B., Cardelo M.P., Lopez S., Abia R., Muriana F.J.G. Olive oil and postprandial hyperlipidemia: Implications for atherosclerosis and metabolic syndrome. Food Funct. 2016;7:4734–4744. doi: 10.1039/C6FO01422D. [DOI] [PubMed] [Google Scholar]
- 36.Vissers M.N., Zock P.L., Katan M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004;58:955–965. doi: 10.1038/sj.ejcn.1601917. [DOI] [PubMed] [Google Scholar]
- 37.Owen R.W., Mier W., Giacosa A., Hull W.E., Spiegelhalder B., Bartsch H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, Lignansand squalene. Food Chem. Toxicol. 2000;38:647–659. doi: 10.1016/S0278-6915(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 38.De la Torre-Carbot K., Chávez-Servín J.L., Jaúregui O., Castellote A.I., Lamuela-Raventós R.M., Fitó M., Covas M.-I., Muñoz-Aguayo D., López-Sabater M.C. Presence of virgin olive oil phenolic metabolites in human low density lipoprotein fraction: Determination by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Chim. Acta. 2007;583:402–410. doi: 10.1016/j.aca.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Esti M., Cinquanta L., La Notte E. Phenolic Compounds in Different Olive Varieties. J. Agric. Food Chem. 1998;46:32–35. doi: 10.1021/jf970391+. [DOI] [PubMed] [Google Scholar]
- 40.Okogeri O., Tasioula-Margari M. Changes occurring in phenolic compounds and alpha-tocopherol of virgin olive oil during storage. J. Agric. Food Chem. 2002;50:1077–1080. doi: 10.1021/jf010895e. [DOI] [PubMed] [Google Scholar]
- 41.Kalua C.M., Bedgood D.R., Bishop A.G., Prenzler P.D. Changes in volatile and phenolic compounds with malaxation time and temperature during virgin olive oil production. J. Agric. Food Chem. 2006;54:7641–7651. doi: 10.1021/jf061122z. [DOI] [PubMed] [Google Scholar]
- 42.Auñon-Calles D., Giordano E., Bohnenberger S., Visioli F. Hydroxytyrosol is not genotoxic in vitro. Pharmacol. Res. 2013;74:87–93. doi: 10.1016/j.phrs.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 43.D’Angelo S., Manna C., Migliardi V., Mazzoni O., Morrica P., Capasso G., Pontoni G., Galletti P., Zappia V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001;29:1492–1498. [PubMed] [Google Scholar]
- 44.Auñon-Calles D., Canut L., Visioli F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013;55:498–504. doi: 10.1016/j.fct.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Visioli F., Galli C., Bornet F., Mattei A., Patelli R., Galli G., Caruso D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000;468:159–160. doi: 10.1016/S0014-5793(00)01216-3. [DOI] [PubMed] [Google Scholar]
- 46.Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002;13:636–644. doi: 10.1016/S0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 47.Visioli F., Caruso D., Plasmati E., Patelli R., Mulinacci N., Romani A., Galli G., Galli C. Hydroxytyrosol, as a component of olive mill waste water, is dose dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001;34:301–305. doi: 10.1080/10715760100300271. [DOI] [PubMed] [Google Scholar]
- 48.Vissers M.N., Zock P.L., Roodenburg A.J.C., Leenen R., Katan M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002;132:409–417. doi: 10.1093/jn/132.3.409. [DOI] [PubMed] [Google Scholar]
- 49.Tuck K.L., Freeman M.P., Hayball P.J., Stretch G.L., Stupans I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001;131:1993–1996. doi: 10.1093/jn/131.7.1993. [DOI] [PubMed] [Google Scholar]
- 50.Visioli F., Galli C., Grande S., Colonnelli K., Patelli C., Galli G., Caruso D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003;133:2612–2615. doi: 10.1093/jn/133.8.2612. [DOI] [PubMed] [Google Scholar]
- 51.García-Villalba R., Carrasco-Pancorbo A., Nevedomskaya E., Mayboroda O.A., Deelder A.M., Segura-Carretero A., Fernández-Gutiérrez A. Exploratory analysis of human urine by LC-ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010;398:463–475. doi: 10.1007/s00216-010-3899-x. [DOI] [PubMed] [Google Scholar]
- 52.García-Villalba R., Larrosa M., Possemiers S., Tomás-Barberán F.A., Espín J.C. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: Comparison between pre- and postmenopausal women. Eur. J. Nutr. 2014;53:1015–1027. doi: 10.1007/s00394-013-0604-9. [DOI] [PubMed] [Google Scholar]
- 53.Corona G., Spencer J.P.E., Dessì M.A. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health. 2009;25:285–293. doi: 10.1177/0748233709102951. [DOI] [PubMed] [Google Scholar]
- 54.Edgecombe S.C., Stretch G.L., Hayball P.J. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr. 2000;130:2996–3002. doi: 10.1093/jn/130.12.2996. [DOI] [PubMed] [Google Scholar]
- 55.Gee J.M., DuPont M.S., Rhodes M.J., Johnson I.T. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 1998;25:19–25. doi: 10.1016/S0891-5849(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 56.Spencer J.P., Chowrimootoo G., Choudhury R., Debnam E.S., Srai S.K., Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458:224–230. doi: 10.1016/S0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- 57.Manna C., Galletti P., Maisto G., Cucciolla V., D’Angelo S., Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000;470:341–344. doi: 10.1016/S0014-5793(00)01350-8. [DOI] [PubMed] [Google Scholar]
- 58.Mateos R., Goya L., Bravo L. Metabolism of the Olive Oil Phenols Hydroxytyrosol, Tyrosol, and Hydroxytyrosyl Acetate by Human Hepatoma HepG2 Cells. J. Agric. Food Chem. 2005;53:9897–9905. doi: 10.1021/jf051721q. [DOI] [PubMed] [Google Scholar]
- 59.De Bock M., Derraik J.G.B., Brennan C.M., Biggs J.B., Morgan P.E., Hodgkinson S.C., Hofman P.L., Cutfield W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE. 2013;8:e57622. doi: 10.1371/journal.pone.0057622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González-Santiago M., Martín-Bautista E., Carrero J.J., Fonollá J., Baró L., Bartolomé M.V., Gil-Loyzaga P., López-Huertas E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis. 2006;188:35–42. doi: 10.1016/j.atherosclerosis.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Jemai H., Fki I., Bouaziz M., Bouallagui Z., El Feki A., Isoda H., Sayadi S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008;56:2630–2636. doi: 10.1021/jf072589s. [DOI] [PubMed] [Google Scholar]
- 62.Jemai H., El Feki A., Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009;57:8798–8804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 63.Giordano E., Dávalos A., Visioli F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014;24:1144–1150. doi: 10.1016/j.numecd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Hmimed S., Belarbi M., Visioli F. Hydroxytyrosol augments the redox status of high fat diet-fed rats. PharmaNutrition. 2016;4:139–142. doi: 10.1016/j.phanu.2016.09.001. [DOI] [Google Scholar]
- 65.Cao K., Xu J., Zou X., Li Y., Chen C., Zheng A., Li H., Li H., Szeto I.M.-Y., Shi Y., et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014;67:396–407. doi: 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 66.Drira R., Chen S., Sakamoto K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011;89:708–716. doi: 10.1016/j.lfs.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Warnke I., Goralczyk R., Fuhrer E., Schwager J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011;8:30. doi: 10.1186/1743-7075-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hao J., Shen W., Yu G., Jia H., Li X., Feng Z., Wang Y., Weber P., Wertz K., Sharman E., et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010;21:634–644. doi: 10.1016/j.jnutbio.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 69.Drira R., Sakamoto K. Modulation of adipogenesis, lipolysis and glucose consumption in 3T3-L1 adipocytes and C2C12 myotubes by hydroxytyrosol acetate: A comparative study. Biochem. Biophys. Res. Commun. 2013;440:576–581. doi: 10.1016/j.bbrc.2013.09.106. [DOI] [PubMed] [Google Scholar]
- 70.Pirozzi C., Lama A., Simeoli R., Paciello O., Pagano T.B., Mollica M.P., Di Guida F., Russo R., Magliocca S., Canani R.B., et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016;30:108–115. doi: 10.1016/j.jnutbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Acín S., Navarro M.A., Arbonés-Mainar J.M., Guillén N., Sarría A.J., Carnicer R., Surra J.C., Orman I., Segovia J.C., de la Torre R., et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. J. Biochem. 2006;140:383–391. doi: 10.1093/jb/mvj166. [DOI] [PubMed] [Google Scholar]
- 72.Violi F., Loffredo L., Pignatelli P., Angelico F., Bartimoccia S., Nocella C., Cangemi R., Petruccioli A., Monticolo R., Pastori D., et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes. 2015;5:e172. doi: 10.1038/nutd.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamden K., Allouche N., Damak M., Elfeki A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009;180:421–432. doi: 10.1016/j.cbi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Tabernero M., Sarriá B., Largo C., Martínez-López S., Madrona A., Espartero J.L., Bravo L., Mateos R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014;5:1556–1563. doi: 10.1039/c3fo60677e. [DOI] [PubMed] [Google Scholar]
- 75.Ruiz-Gutiérrez V., Pérez-Espinosa A., Vázquez C.M., Santa-María C. Effects of dietary fats (fish, olive and high-oleic-acid sunflower oils) on lipid composition and antioxidant enzymes in rat liver. Br. J. Nutr. 1999;82:233–241. [PubMed] [Google Scholar]
- 76.Salas J., López Miranda J., Jansen S., Zambrana J.L., Castro P., Paniagua J.A., Blanco A., López Segura F., Jiménez Perepérez J.A., Pérez Jiménez F., et al. The diet rich in monounsaturated fat modifies in a beneficial way carbohydrate metabolism and arterial pressure. Med. Clínica. 1999;113:765–769. [PubMed] [Google Scholar]
- 77.Ferrara L.A., Raimondi A.S., d’Episcopo L., Guida L., Dello Russo A., Marotta T. Olive oil and reduced need for antihypertensive medications. Arch. Intern. Med. 2000;160:837–842. doi: 10.1001/archinte.160.6.837. [DOI] [PubMed] [Google Scholar]
- 78.Thomsen C., Storm H., Holst J.J., Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am. J. Clin. Nutr. 2003;77:605–611. doi: 10.1093/ajcn/77.3.605. [DOI] [PubMed] [Google Scholar]
- 79.Ruano J., Lopez-Miranda J., Fuentes F., Moreno J.A., Bellido C., Perez-Martinez P., Lozano A., Gómez P., Jiménez Y., Pérez Jiménez F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005;46:1864–1868. doi: 10.1016/j.jacc.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 80.Valls R.-M., Farràs M., Suárez M., Fernández-Castillejo S., Fitó M., Konstantinidou V., Fuentes F., López-Miranda J., Giralt M., Covas M.-I., et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015;167:30–35. doi: 10.1016/j.foodchem.2014.06.107. [DOI] [PubMed] [Google Scholar]
- 81.Ruíz-Gutiérrez V., Muriana F.J., Guerrero A., Cert A.M., Villar J. Plasma lipids, erythrocyte membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic acid from two different sources. J. Hypertens. 1996;14:1483–1490. doi: 10.1097/00004872-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 82.Lockyer S., Rowland I., Spencer J.P.E., Yaqoob P., Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero M., Toral M., Gómez-Guzmán M., Jiménez R., Galindo P., Sánchez M., Olivares M., Gálvez J., Duarte J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7:584–593. doi: 10.1039/C5FO01101A. [DOI] [PubMed] [Google Scholar]
- 84.López-Villodres J.A., Abdel-Karim M., De La Cruz J.P., Rodríguez-Pérez M.D., Reyes J.J., Guzmán-Moscoso R., Rodriguez-Gutierrez G., Fernández-Bolaños J., González-Correa J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016;37:94–100. doi: 10.1016/j.jnutbio.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Storniolo C.E., Roselló-Catafau J., Pintó X., Mitjavila M.T., Moreno J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014;2:971–977. doi: 10.1016/j.redox.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De la Torre-Carbot K., Chávez-Servín J.L., Jaúregui O., Castellote A.I., Lamuela-Raventós R.M., Nurmi T., Poulsen H.E., Gaddi A.V., Kaikkonen J., Zunft H.-F., et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010;140:501–508. doi: 10.3945/jn.109.112912. [DOI] [PubMed] [Google Scholar]
- 87.Castañer O., Covas M.-I., Khymenets O., Nyyssonen K., Konstantinidou V., Zunft H.-F., de la Torre R., Muñoz-Aguayo D., Vila J., Fitó M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012;95:1238–1244. doi: 10.3945/ajcn.111.029207. [DOI] [PubMed] [Google Scholar]
- 88.Reaven P., Parthasarathy S., Grasse B.J., Miller E., Steinberg D., Witztum J.L. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects. J. Clin. Investig. 1993;91:668–676. doi: 10.1172/JCI116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vázquez-Velasco M., Esperanza Díaz L., Lucas R., Gómez-Martínez S., Bastida S., Marcos A., Sánchez-Muniz F.J. Effects of hydroxytyrosol-enriched sunflower oil consumption on CVD risk factors. Br. J. Nutr. 2011;105:1448–1452. doi: 10.1017/S0007114510005015. [DOI] [PubMed] [Google Scholar]
- 90.Fki I., Sahnoun Z., Sayadi S. Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J. Agric. Food Chem. 2007;55:624–631. doi: 10.1021/jf0623586. [DOI] [PubMed] [Google Scholar]
- 91.Visioli F., Bellomo G., Montedoro G., Galli C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis. 1995;117:25–32. doi: 10.1016/0021-9150(95)05546-9. [DOI] [PubMed] [Google Scholar]
- 92.Fitó M., Covas M.I., Lamuela-Raventós R.M., Vila J., Torrents L., de la Torre C., Marrugat J. Protective effect of olive oil and its phenolic compounds against low density lipoprotein oxidation. Lipids. 2000;35:633–638. doi: 10.1007/s11745-000-0567-1. [DOI] [PubMed] [Google Scholar]
- 93.Covas M.-I., de la Torre K., Farré-Albaladejo M., Kaikkonen J., Fitó M., López-Sabater C., Pujadas-Bastardes M.A., Joglar J., Weinbrenner T., Lamuela-Raventós R.M., et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006;40:608–616. doi: 10.1016/j.freeradbiomed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 94.González-Santiago M., Fonollá J., Lopez-Huertas E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010;61:364–370. doi: 10.1016/j.phrs.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 95.Mateos R., Martínez-López S., Baeza Arévalo G., Amigo-Benavent M., Sarriá B., Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016;205:248–256. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Mackness M.I., Arrol S., Abbott C., Durrington P.N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993;104:129–135. doi: 10.1016/0021-9150(93)90183-U. [DOI] [PubMed] [Google Scholar]
- 97.Mackness M.I., Durrington P.N. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–253. doi: 10.1016/0021-9150(94)05524-M. [DOI] [PubMed] [Google Scholar]
- 98.Rietjens S.J., Bast A., de Vente J., Haenen G.R.M.M. The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NObullet response of isolated rat aorta. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1931–H1936. doi: 10.1152/ajpheart.00755.2006. [DOI] [PubMed] [Google Scholar]
- 99.Khymenets O., Fitó M., Touriño S., Muñoz-Aguayo D., Pujadas M., Torres J.L., Joglar J., Farré M., Covas M.-I., de la Torre R. Antioxidant activities of hydroxytyrosol main metabolites do not contribute to beneficial health effects after olive oil ingestion. Drug Metab. Dispos. 2010;38:1417–1421. doi: 10.1124/dmd.110.032821. [DOI] [PubMed] [Google Scholar]
- 100.Briante R., Febbraio F., Nucci R. Antioxidant/prooxidant effects of dietary non-flavonoid phenols on the Cu2+-induced oxidation of human low-density lipoprotein (LDL) Chem. Biodivers. 2004;1:1716–1729. doi: 10.1002/cbdv.200490130. [DOI] [PubMed] [Google Scholar]
- 101.Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D.L., DiPietro L., Cline G.W., Shulman G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stump C.S., Short K.R., Bigelow M.L., Schimke J.M., Nair K.S. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. USA. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ritz P., Berrut G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005;31:5S67–5S73. doi: 10.1016/S1262-3636(05)73654-5. [DOI] [PubMed] [Google Scholar]
- 104.Morino K., Petersen K.F., Dufour S., Befroy D., Frattini J., Shatzkes N., Neschen S., White M.F., Bilz S., Sono S., et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng A., Li H., Xu J., Cao K., Li H., Pu W., Yang Z., Peng Y., Long J., Liu J., et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015;113:1667–1676. doi: 10.1017/S0007114515000884. [DOI] [PubMed] [Google Scholar]
- 106.Granados-Principal S., El-Azem N., Pamplona R., Ramirez-Tortosa C., Pulido-Moran M., Vera-Ramirez L., Quiles J.L., Sanchez-Rovira P., Naudí A., Portero-Otin M., et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014;90:25–33. doi: 10.1016/j.bcp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Zhu L., Liu Z., Feng Z., Hao J., Shen W., Li X., Sun L., Sharman E., Wang Y., Wertz K., et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010;21:1089–1098. doi: 10.1016/j.jnutbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Zheng A., Li H., Cao K., Xu J., Zou X., Li Y., Chen C., Liu J., Feng Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015;26:190–199. doi: 10.1016/j.jnutbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Bali E.B., Ergin V., Rackova L., Bayraktar O., Küçükboyaci N., Karasu Ç. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: Comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med. 2014;80:984–992. doi: 10.1055/s-0034-1382881. [DOI] [PubMed] [Google Scholar]
- 110.Martín M.A., Ramos S., Granado-Serrano A.B., Rodríguez-Ramiro I., Trujillo M., Bravo L., Goya L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010;54:956–966. doi: 10.1002/mnfr.200900159. [DOI] [PubMed] [Google Scholar]
- 111.Zrelli H., Matsuoka M., Kitazaki S., Zarrouk M., Miyazaki H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011;660:275–282. doi: 10.1016/j.ejphar.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 112.Deiana M., Incani A., Rosa A., Atzeri A., Loru D., Cabboi B., Paola Melis M., Lucas R., Morales J.C., Assunta Dessì M. Hydroxytyrosol glucuronides protect renal tubular epithelial cells against H(2)O(2) induced oxidative damage. Chem. Biol. Interact. 2011;193:232–239. doi: 10.1016/j.cbi.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Paiva-Martins F., Silva A., Almeida V., Carvalheira M., Serra C., Rodrígues-Borges J.E., Fernandes J., Belo L., Santos-Silva A. Protective activity of hydroxytyrosol metabolites on erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2013;61:6636–6642. doi: 10.1021/jf4016202. [DOI] [PubMed] [Google Scholar]
- 114.Deiana M., Aruoma O.I., Bianchi M.L., Spencer J.P., Kaur H., Halliwell B., Aeschbach R., Banni S., Dessi M.A., Corongiu F.P. Inhibition of peroxynitrite dependent DNA base modification and tyrosine nitration by the extra virgin olive oil-derived antioxidant hydroxytyrosol. Free Radic. Biol. Med. 1999;26:762–769. doi: 10.1016/S0891-5849(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 115.De la Puerta R., Martínez Domínguez M.E., Ruíz-Gutíerrez V., Flavill J.A., Hoult J.R. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001;69:1213–1222. doi: 10.1016/S0024-3205(01)01218-8. [DOI] [PubMed] [Google Scholar]
- 116.O’Dowd Y., Driss F., Dang P.M.-C., Elbim C., Gougerot-Pocidalo M.-A., Pasquier C., El-Benna J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004;68:2003–2008. doi: 10.1016/j.bcp.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 117.Manna C., Galletti P., Cucciolla V., Montedoro G., Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J. Nutr. Biochem. 1999;10:159–165. doi: 10.1016/S0955-2863(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 118.Manna C., Napoli D., Cacciapuoti G., Porcelli M., Zappia V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem. 2009;57:3478–3482. doi: 10.1021/jf8037659. [DOI] [PubMed] [Google Scholar]
- 119.Goya L., Mateos R., Bravo L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Eur. J. Nutr. 2007;46:70–78. doi: 10.1007/s00394-006-0633-8. [DOI] [PubMed] [Google Scholar]
- 120.Gutierrez V.R., de la Puerta R., Catalá A. The effect of tyrosol, hydroxytyrosol and oleuropein on the non-enzymatic lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 2001;217:35–41. doi: 10.1023/A:1007219931090. [DOI] [PubMed] [Google Scholar]
- 121.Crespo M.C., Tomé-Carneiro J., Burgos-Ramos E., Loria Kohen V., Espinosa M.I., Herranz J., Visioli F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol. Res. 2015;95–96:132–137. doi: 10.1016/j.phrs.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 122.Takeda Y., Bui V.N., Iwasaki K., Kobayashi T., Ogawa H., Imai K. Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependent inflammatory response of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 2014;446:1225–1230. doi: 10.1016/j.bbrc.2014.03.094. [DOI] [PubMed] [Google Scholar]
- 123.Carito V., Ciafrè S., Tarani L., Ceccanti M., Natella F., Iannitelli A., Tirassa P., Chaldakov G.N., Ceccanti M., Boccardo C., et al. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. DellIstituto Super. Sanità. 2015;51:382–386. doi: 10.4415/ANN_15_04_21. [DOI] [PubMed] [Google Scholar]
- 124.Sánchez-Fidalgo S., Sánchez de Ibargüen L., Cárdeno A., Alarcón de la Lastra C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012;51:497–506. doi: 10.1007/s00394-011-0235-y. [DOI] [PubMed] [Google Scholar]
- 125.Silva S., Sepodes B., Rocha J., Direito R., Fernandes A., Brites D., Freitas M., Fernandes E., Bronze M.R., Figueira M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015;26:360–368. doi: 10.1016/j.jnutbio.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 126.Gong D., Geng C., Jiang L., Cao J., Yoshimura H., Zhong L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. PTR. 2009;23:646–650. doi: 10.1002/ptr.2686. [DOI] [PubMed] [Google Scholar]
- 127.Scoditti E., Massaro M., Carluccio M.A., Pellegrino M., Wabitsch M., Calabriso N., Storelli C., De Caterina R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE. 2015;10:e0128218. doi: 10.1371/journal.pone.0128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X., Cao J., Zhong L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn. Schmiedebergs Arch. Pharmacol. 2009;379:581–586. doi: 10.1007/s00210-009-0399-7. [DOI] [PubMed] [Google Scholar]
- 129.Zhang X., Cao J., Jiang L., Zhong L. Suppressive effects of hydroxytyrosol on oxidative stress and nuclear Factor-kappaB activation in THP-1 cells. Biol. Pharm. Bull. 2009;32:578–582. doi: 10.1248/bpb.32.578. [DOI] [PubMed] [Google Scholar]
- 130.Scoditti E., Nestola A., Massaro M., Calabriso N., Storelli C., De Caterina R., Carluccio M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis. 2014;232:17–24. doi: 10.1016/j.atherosclerosis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 131.Parkinson L., Cicerale S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Mol. Basel Switz. 2016;21:1734. doi: 10.3390/molecules21121734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rigacci S., Stefani M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016;17:843. doi: 10.3390/ijms17060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dell’Agli M., Fagnani R., Mitro N., Scurati S., Masciadri M., Mussoni L., Galli G.V., Bosisio E., Crestani M., De Fabiani E., et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 2006;54:3259–3264. doi: 10.1021/jf0529161. [DOI] [PubMed] [Google Scholar]
- 134.Schmitt C.A., Handler N., Heiss E.H., Erker T., Dirsch V.M. No evidence for modulation of endothelial nitric oxide synthase by the olive oil polyphenol hydroxytyrosol in human endothelial cells. Atherosclerosis. 2007;195:e58–e64. doi: 10.1016/j.atherosclerosis.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 135.Zrelli H., Wu C.W., Zghonda N., Shimizu H., Miyazaki H. Combined treatment of hydroxytyrosol with carbon monoxide-releasing molecule-2 prevents TNF α-induced vascular endothelial cell dysfunction through NO production with subsequent NFκB inactivation. BioMed Res. Int. 2013;2013:912431. doi: 10.1155/2013/912431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bayram B., Ozcelik B., Grimm S., Roeder T., Schrader C., Ernst I.M.A., Wagner A.E., Grune T., Frank J., Rimbach G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012;15:71–81. doi: 10.1089/rej.2011.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mnafgui K., Hajji R., Derbali F., Khlif I., Kraiem F., Ellefi H., Elfeki A., Allouche N., Gharsallah N. Protective Effect of Hydroxytyrosol Against Cardiac Remodeling After Isoproterenol-Induced Myocardial Infarction in Rat. Cardiovasc. Toxicol. 2016;16:147–155. doi: 10.1007/s12012-015-9323-1. [DOI] [PubMed] [Google Scholar]
- 138.López de las Hazas M.-C., Rubió L., Kotronoulas A., de la Torre R., Solà R., Motilva M.-J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015;59:1395–1399. doi: 10.1002/mnfr.201500048. [DOI] [PubMed] [Google Scholar]
- 139.Cofrades S., Salcedo Sandoval L., Delgado-Pando G., López-López I., Ruiz-Capillas C., Jiménez-Colmenero F. Antioxidant activity of hydroxytyrosol in frankfurters enriched with n-3 polyunsaturated fatty acids. Food Chem. 2011;129:429–436. doi: 10.1016/j.foodchem.2011.04.095. [DOI] [PubMed] [Google Scholar]
- 140.Nieto G., Martínez L., Ros G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017 doi: 10.1002/jsfa.8240. [DOI] [PubMed] [Google Scholar]
- 141.Long L.H., Hoi A., Halliwell B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys. 2010;501:162–169. doi: 10.1016/j.abb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 142.Sung M.M., Kim T.T., Denou E., Soltys C.-L.M., Hamza S.M., Byrne N.J., Masson G., Park H., Wishart D.S., Madsen K.L., et al. Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes. 2017;66:418–425. doi: 10.2337/db16-0680. [DOI] [PubMed] [Google Scholar]
- 143.Côté C.D., Rasmussen B.A., Duca F.A., Zadeh-Tahmasebi M., Baur J.A., Daljeet M., Breen D.M., Filippi B.M., Lam T.K.T. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat. Med. 2015;21:498–505. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]