To the Editor
Opioid-use disorder has reached epidemic proportions, with high attendant costs in terms of increases in overdoses and infectious diseases and in economic costs.1 Despite the demonstrated efficacy of maintaining abstinence by treating patients with opioid agonists, patients can remain on clinic waiting lists for months, during which time they are at risk of premature death.2 The use of interim treatment with buprenorphine without formal counseling while patients remain on waiting lists may mitigate this risk during delays in treatment.3
In a randomized pilot study (ClinicalTrials.gov number, NCT02360007), we evaluated the efficacy of an interim regimen of buprenorphine for reducing illicit opioid use among 50 persons on waiting lists for entry into treatment for opioid abuse. (The protocol is available with the full text of this letter at NEJM.org.) Participants had used opioids for a mean (±SE) of 7.2±6.1 years, 78% had used intravenous opioids, and 30% had previously overdosed, with an average of 3.6 overdoses each. (Participant characteristics at baseline, including a history of drug use, are listed in the Supplementary Appendix, available at NEJM.org.) While remaining on the waiting list, 25 participants were randomly assigned to receive interim treatment with buprenorphine and 25 participants were not assigned to receive this treatment. Participants in the treatment group visited the clinic every 2 weeks to provide urine samples for toxicologic screening and to ingest buprenorphine under the observation of the staff. The remaining doses were provided through a computerized dispenser that permitted buprenorphine administration at home to reduce the risk of nonadherence. The device used in the study was the Med-O-Wheel Secure dispenser (Addoz), a portable, disk-shaped device that makes each day's dose available during a preprogrammed 3-hour window. Participants in the treatment group also received daily calls to assess drug use, craving, and withdrawal by means of an interactive voice-response telephone system as well as system-generated random callbacks. Participants in the control group remained on the waiting list of their local clinic and did not receive these services.
At 4, 8, and 12 weeks, all participants completed assessments that included the provision of urine specimens that were collected under staff observation and the completion of a structured clinical interview based on the Addiction Severity Index (ASI),4 which addresses problem severity in seven areas commonly affected in substance abusers: drug use, alcohol use, employment, legal issues, family and social issues, psychiatric issues, and medical issues. (Patient scores are available in the Supplementary Appendix.) The primary outcome was the percentage of specimens with negative results for illicit opioids obtained at assessments at 4, 8, and 12 weeks; missing urine specimens were considered to be positive. Secondary outcomes included intravenous drug use, ASI scores, adherence to the treatment regimen, and patient satisfaction.
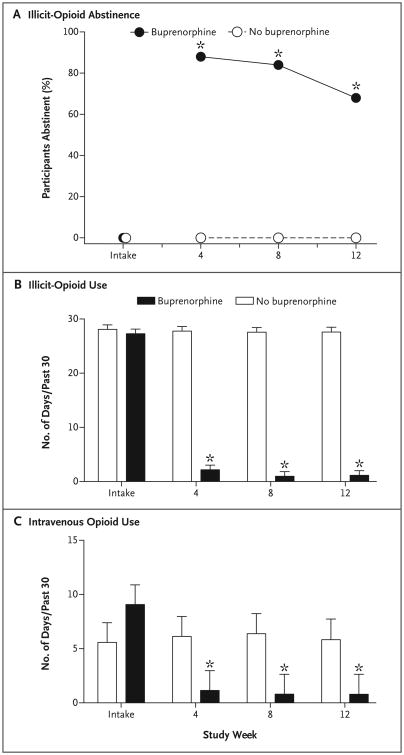
Participants assigned to receive interim treatment with buprenorphine submitted a higher percentage of specimens that were negative for illicit opioids than those in the control group at 4 weeks (88% vs. 0%), 8 weeks (84% vs. 0%), and 12 weeks (68% vs. 0%), which was the primary outcome (P<0.001 for all comparisons) (Fig. 1). These participants also had greater reductions in the frequency of use of any intravenous drug (P<0.001) and in scores on the drug (P<0.001) and psychiatric (P = 0.02) subscales of the ASI. Adherence to the regimens for buprenorphine administration (99%), daily monitoring calls (96%), and random callbacks (96%) was high, as were ratings of treatment satisfaction (4.6±0.7 on a 5-point scale).
Figure 1. Abstinence from Illicit Opioids and Intravenous Opioids over 12 Weeks with Interim Buprenorphine.
Panel A shows the effects of interim buprenorphine on abstinence from illicit opioid use over 12 weeks. Data points represent the percentage of participants who submitted urine specimens with negative results for illicit opioids at intake and at assessments every 4 weeks thereafter. Panel B shows the effects of interim buprenorphine on the self-reported frequency of the use of illicit opioids, and Panel C shows the effects of interim buprenorphine on the use of intravenous opioids. The y axis for intravenous opioid use is presented on a smaller scale to allow for more detailed inspection of the data. Asterisks represent observations at assessments at 4, 8, and 12 weeks that were significantly different between groups (P<0.001). T bars represent standard errors.
Among patients on a waiting list to receive comprehensive treatment, interim dosing with buprenorphine, paired with technology-assisted components intended to support adherence, was associated with a statistically significant reduction in the use of illicit opioids and intravenous drugs as compared with remaining on the waiting list alone over 12 weeks. These results suggest that interim buprenorphine dosing could reduce drug-related risks and consequences when comprehensive treatment is unavailable. Interim treatment with buprenorphine may be suitable for patients in rural areas where there are limited treatment options.5 Further trials with larger sample sizes and longer durations are needed to replicate these preliminary findings.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (R34DA037385, to Dr. Sigmon) and (T32DA007242, to Dr. Higgins) and the National Institute of General Medical Sciences (P20GM103644, to Dr. Higgins).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Stacey C. Sigmon, University of Vermont, Burlington, VT
Taylor A. Ochalek, University of Vermont, Burlington, VT
Andrew C. Meyer, University of Vermont, Burlington, VT
Bryce Hruska, University of Vermont, Burlington, VT
Sarah H. Heil, University of Vermont, Burlington, VT
Gary J. Badger, University of Vermont, Burlington, VT
Gail Rose, University of Vermont, Burlington, VT
John R. Brooklyn, University of Vermont, Burlington, VT
Robert P. Schwartz, Friends Research Institute, Baltimore, MD
Brent A. Moore, Yale University School of Medicine, New Haven, CT
Stephen T. Higgins, University of Vermont, Burlington, VT
References
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 2.Peles E, Schreiber S, Adelson M. Opiate-dependent patients on a waiting list for methadone maintenance treatment are at high risk for mortality until treatment entry. J Addict Med. 2013;7:177–82. doi: 10.1097/ADM.0b013e318287cfc9. [DOI] [PubMed] [Google Scholar]
- 3.Sigmon SC, C Meyer A, Hruska B, et al. Bridging waitlist delays with interim buprenorphine treatment: initial feasibility. Addict Behav. 2015;51:136–42. doi: 10.1016/j.addbeh.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–23. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiol Drug Saf. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.