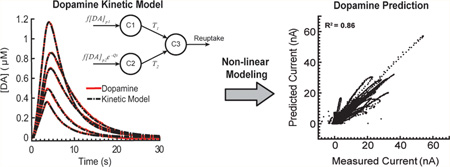
Abstract
Neurochemical changes evoked by electrical stimulation of the nervous system have been linked to both therapeutic and undesired effects of neuromodulation therapies used to treat obsessive-compulsive disorder, depression, epilepsy, Parkinson’s disease, stroke, hypertension, tinnitus, and many other indications. In fact, interest in better understanding the role of neurochemical signaling in neuromodulation therapies has been a focus of recent government- and industry-sponsored programs whose ultimate goal is to usher in an era of personalized medicine by creating neuromodulation therapies that respond to real-time changes in patient status. A key element to achieving these precision therapeutic interventions is the development of mathematical modeling approaches capable of describing the nonlinear transfer function between neuromodulation parameters and evoked neurochemical changes. Here, we propose two computational modeling frameworks, based on artificial neural networks (ANNs) and Volterra kernels, that can characterize the input/output transfer functions of stimulation-evoked neurochemical release. We evaluate the ability of these modeling frameworks to characterize subject-specific neurochemical kinetics by accurately describing stimulation-evoked dopamine release across rodent (R2 = 0.83 Volterra kernel, R2 = 0.86 ANN), swine (R2 = 0.90 Volterra kernel, R2 = 0.93 ANN), and non-human primate (R2 = 0.98 Volterra kernel, R2 = 0.96 ANN) models of brain stimulation. Ultimately, these models will not only improve understanding of neurochemical signaling in healthy and diseased brains but also facilitate the development of neuromodulation strategies capable of controlling neurochemical release via closed-loop strategies.
Keywords: Fast scan cyclic voltammetry, deep brain stimulation, dopamine, artificial neural network, Volterra kernels, machine learning, neurochemical sensing
Graphical Abstract
INTRODUCTION
Neurochemical signaling has been implicated in both therapeutic and undesired effects of neuromodulation therapies for a myriad of clinical indications. In fact, interest in better understanding the role of neurochemical signaling in neuromodulation therapies with regard to the intended therapeutic effects and unwanted side effects has been a focus of recent government and industry efforts, including the National Institutes of Health (NIH) Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative and Stimulating Peripheral Activity to Relieve Conditions (SPARC) Common Fund Program, the Defense Advanced Research Projects Agency (DARPA) Electrical Prescriptions (ElectRx) and Systems-Based Neurotechnology for Emerging Therapies (SUBNETS) Programs, and the GlaxoSmithKline Bioelectric Medicines Initiative. The fundamental goal of these programs is to bring forth an era of personalized neuromodulation therapies that are capable of detecting and responding to real-time changes in patient status. Achieving this goal requires characterization of the nonlinear relationships between the applied neuromodulation parameters and the evoked distal effects, such as changes in electrophysiologic activity and neurochemical signaling.
In general, the study of changes in neurochemical signaling evoked by electrical stimulation has been limited by the measurement and analysis techniques available. For example, noninvasive indirect measurement techniques such as positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) have poor spatial and temporal resolution,1,2 while invasive direct measurement techniques like dialysis alter the fundamental dynamics of local neurochemical release.3 Given the growing interest in and need for precision neuromodulation therapies, it is important to note that there is evidence suggesting that these low spatial or temporal resolution neurochemical sampling techniques may mischaracterize highly focal and nonhomogeneously evoked neurochemical release.4,5
Numerous studies have built upon existing low resolution neurochemical sampling techniques by taking advantage of lesion-based and pharmacological interventions to demonstrate that (1) electrical stimulation can elicit proximal and distal neurochemical changes and (2) changes in neurochemical signaling are related to symptom reduction or induction of side effect.6–8 Unfortunately, these approaches result in a binary measurement system with only “yes” or “no” resolution and provide very little information on the extent of neurochemical fluctuations associated with specific changes in the underlying physiology. Thus, measurement of neurochemical signaling with high spatial and temporal resolution is paramount to both elucidate the fundamental biological mechanisms of action underlying neuromodulation interventions and enable precision neurochemical control strategies (e.g., via closed-loop).
Fast-scan cyclic voltammetry (FSCV) is a neurochemical sensing technique capable of subsecond and sub-millimeter resolution.9,10 In contrast to dialysis techniques, FSCV creates minimal tissue disruption and can record neurochemical activity for up to several months.11 Combined, these characteristics make FSCV an excellent technique for informing the development of mathematical models and potentially as a feedback signal for neuromodulation therapies. In fact, FSCV data have already been utilized to develop mathematical models describing the relationship between synaptic neurochemical release and phasic changes in extracellular dopamine concentration.12–14 However, current mathematical modeling techniques aimed at characterizing the relationship between electrical stimulation parameters and the corresponding evoked changes in neurochemical signaling are limited. In fact, to our knowledge, no nonlinear modeling techniques have been presented in the literature.15
It is well-known that activation of nervous tissue near the stimulating electrode is subject to a number of variable factors such as distance from the electrode to the nervous tissue, orientation of the nerve/neuron with respect to the electrode, degree of myelination, tissue conductivity, and several others.16–18 Similarly, the ability to measure neurochemical release will depend upon several patient-specific factors such as diffusion properties, reuptake rates, stimulation and measurement targets, and disease processes.5,19,20 Consequently, a model of the input/output relationship between electrical stimulation parameters and evoked neurochemical changes must be able to incorporate these unknown and inherently nonlinear variables on a subject-by-subject basis. Additionally, model complexity must allow for real-time analysis, architectural flexibility, and cross-platform portability in order to facilitate the development and implementation of closed-loop control strategies and embedding in implantable neuromodulation devices.
Artificial neural networks (ANNs) and time-series approaches utilizing Volterra kernels are two common methods with varying computational complexity capable of modeling the input/output transfer functions in physiological systems with inherently nonlinear dynamics and several unknown parameters.21,22 Here, we evaluate the suitability of both ANN and Volterra kernels for modeling the input/output transfer functions between stimulation parameters and evoked changes in striatal dopaminergic signaling in rodent, swine, and nonhuman primate (NHP) models of electrical stimulation. In particular, Volterra kernels are able to characterize neurochemical dynamics of varying order (i.e., short-term and longterm effects of previous stimuli) and thus are uniquely suited for describing the dependence of neurochemical responses on previous stimuli sequences. Additionally, training of Volterra kernels is flexible, making it easily applicable to other neurotransmitters, anatomical targets (i.e., stimulation and neurochemical sensing locations), and animal models with little or no modification.23 In contrast the ANNs are uniquely suited for learning input/output relationships described by compartmental models of neurochemical release.12,24 This simplifies model training and improves model performance by decreasing the number of output parameters modeled by the network. Although using compartmental models of striatal dopamine release as a feature extraction method for the ANN modeling approach offers benefits, it also prevents the model from being extended to situations where the neurochemical release is less well understood and modeling techniques are not well established. Development of these mathematical characterization approaches will enable the development of a neurochemical patch-clamp system capable of studying neurochemical signaling evoked by neurostimulation therapies. In turn, this characterization will lead to the development of novel techniques for controlling changes in neurochemistry via modulation of electrical stimulation of nervous tissue.
RESULTS AND DISCUSSION
In these experiments, the focus was to develop mathematical modeling frameworks that can be used to characterize neurochemical signaling for multiple neurochemical analytes across different animal models, regardless of stimulation location, electrode/tissue geometry, and stimulation parameters. To this end, both the ANN and Volterra kernel models were selected as general, flexible models that could easily be extended to meet the requirements of a particular neurochemical stimulation and recording paradigm. These models were trained on neurochemical data collected using FSCV and evoked by electrical stimulation in rodent, swine, and NHP animal models (Figure 1).
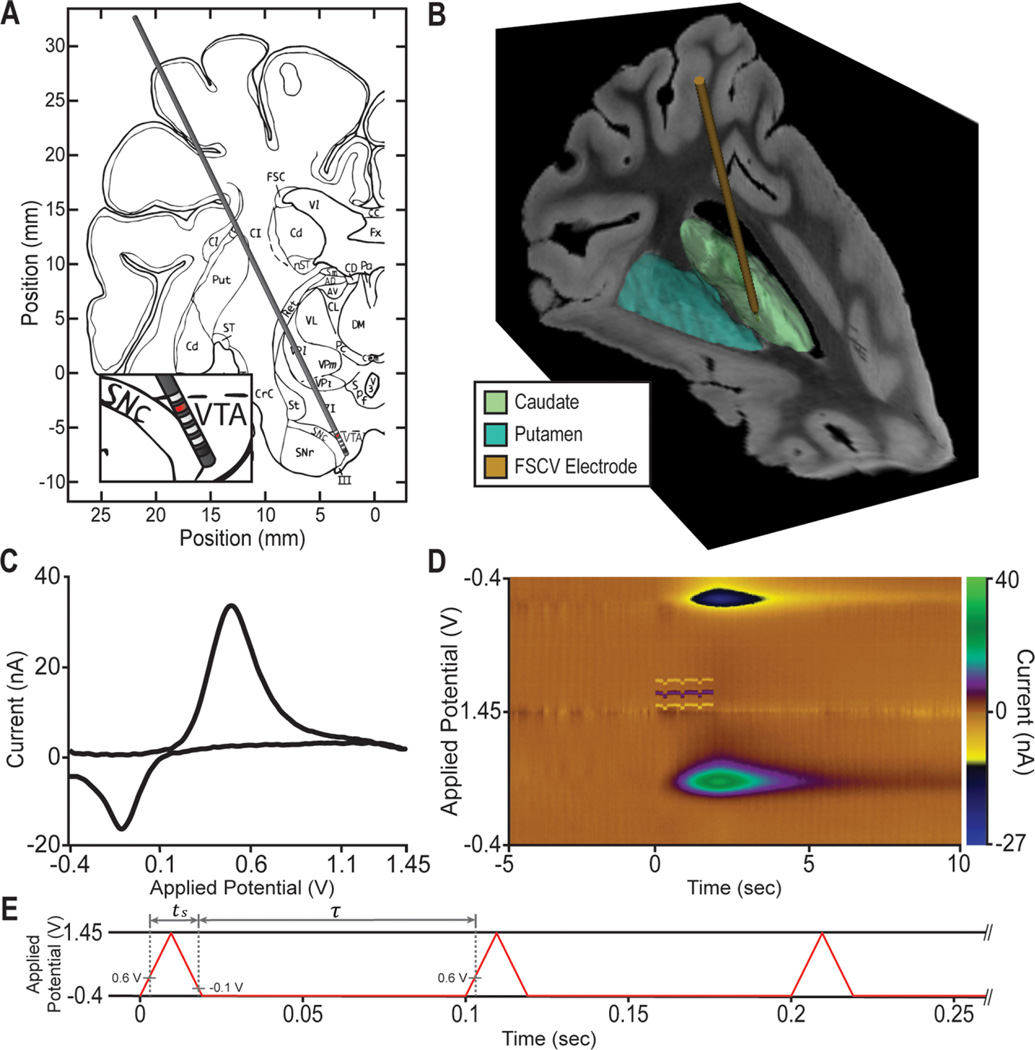
Figure 1.
Representative experimental setup as conducted in the swine model of electrical stimulation. (A) A stimulating electrode was implanted in the substantia nigra pars compacta (SNc)/ventrotegmental area (VTA). (B) A carbon fiber microelectrode (CFM) was implanted in the anterior caudate. Electrode position shown is from coregistration of the preoperative targeting MRI with a 3D pig atlas (ref 47). (C) A representative cyclic voltammogram for dopamine obtained using fast scan cyclic voltammetry (FSCV). The voltammogram shows a dopamine oxidation peak at 0.6 V as well as a dopamine reduction peak at −0.2 V. (D) Pseudocolor representation of a FSCV recording showing dopamine release in response to a stimulus (4.75 V, 0.6 ms, 130 Hz, 260 pulses). (E) Triangular FSCV waveform repeated at 10 Hz that was used for recording extracellular changes in dopamine concentration evoked by deep brain stimulation.
Modulation and Neurochemical Recording of Stimulation-Evoked Dopamine Responses
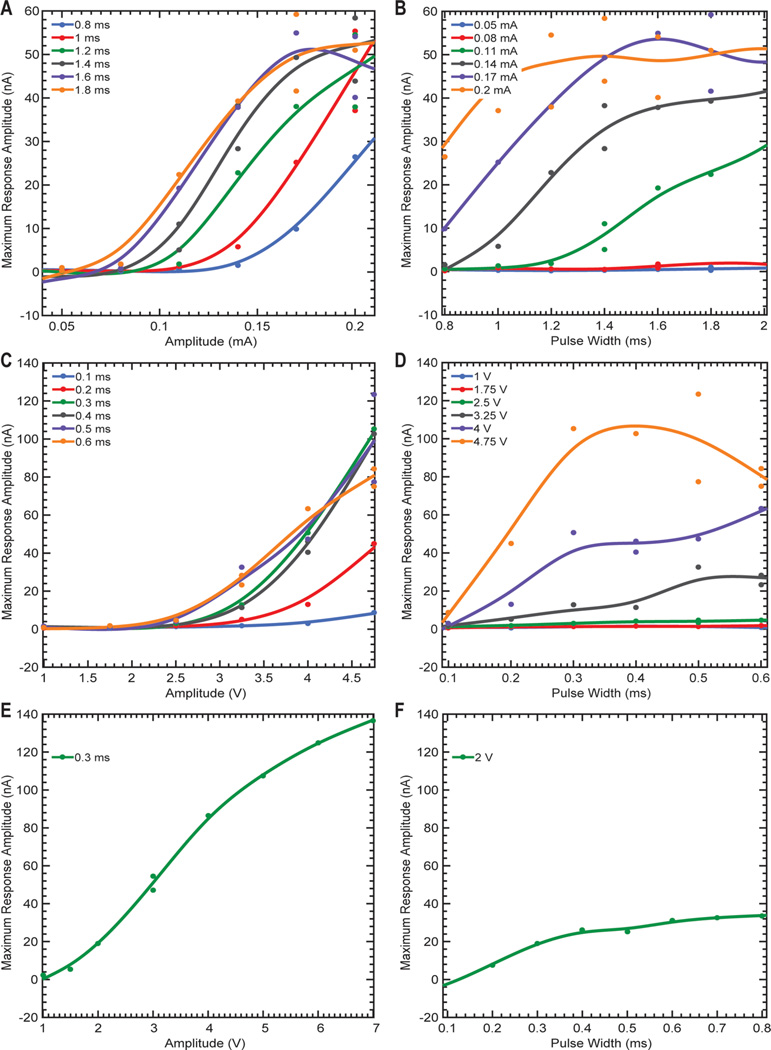
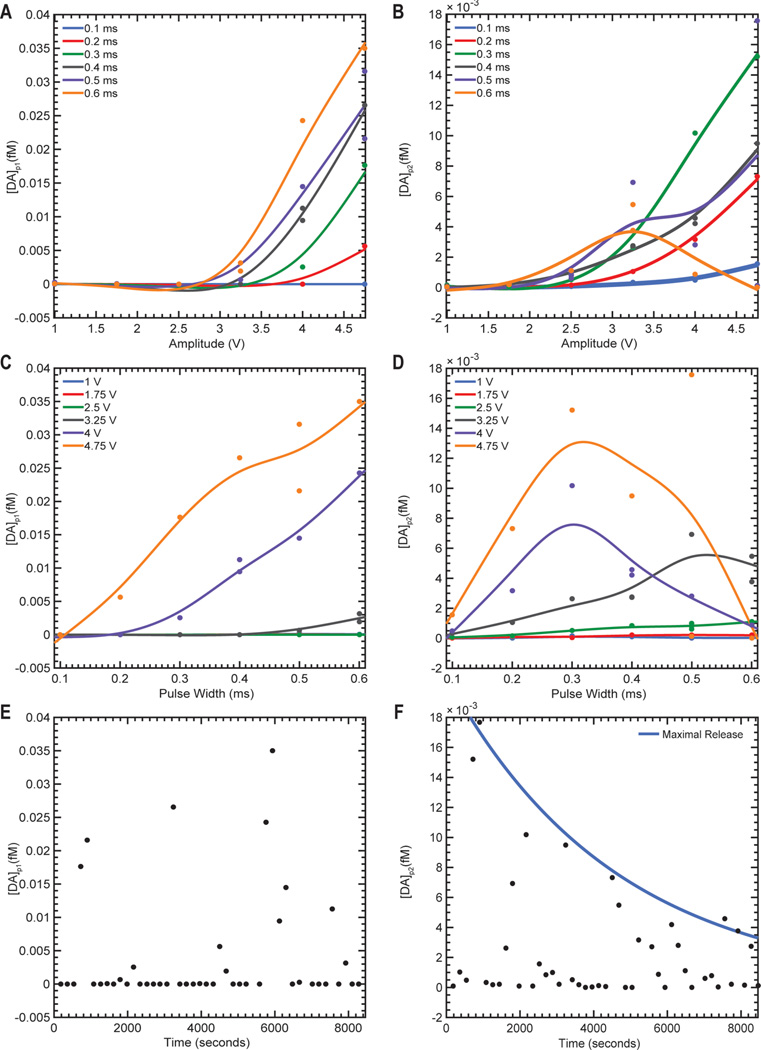
Dopaminergic signaling from the SNc/VTA, as well as dopaminergic axons in the MFB, have been shown to play important roles in both motivation and reward and have been implicated in depression and Parkinson’s disease.25–27 Our preliminary data showed maximal striatal dopamine release occurring at 60 Hz or greater for the rodent model,5 130 Hz for the swine model,28 and 120–130 Hz in the NHP model, which is an agreement with previously reported data.4 Thus, we chose to stimulate rodents, swine, and NHP models using 90, 130, and 130 Hz frequencies, respectively. Stimulation of the MFB or SNc/VTA in 12 anesthetized rats (MFB), four anesthetized swine (SNc/VTA), and one anesthetized NHP (SNc/VTA) led to nonlinear increases in striatal dopamine release as a function of stimulation amplitude (Figure 2A,C,E). Similarly, these stimulation-evoked extracellular dopamine levels increased nonlinearly as a function of stimulation pulse width (Figure 2B,D,F). Therefore, it is possible to modulate striatal dopamine by modulating the stimulus intensity (e.g., pulsewidth and amplitude) applied to the SNc/VTA and MFB.
Figure 2.
Peak stimulation-evoked dopamine release response as a function of stimulation amplitude (A, C, E) and pulse width (B, D, F) in the rat, swine, and non-human primate (NHP) model of electrical stimulation, respectively. Experimentally recorded peak values are shown by single dots with color corresponding to amplitude or pulse width of the applied stimulation. To show stimulus amplitude and pulse width related trends in the data correspondingly colored smoothing splines were fit using data in Matlab (The Mathworks, Natick, MA). NHP data (E, F) at other pulse widths and amplitudes contain a limited number of data points and are not shown for clarity.
Parametric Characterization and Prediction of Stimulation-Evoked Neurochemical Release
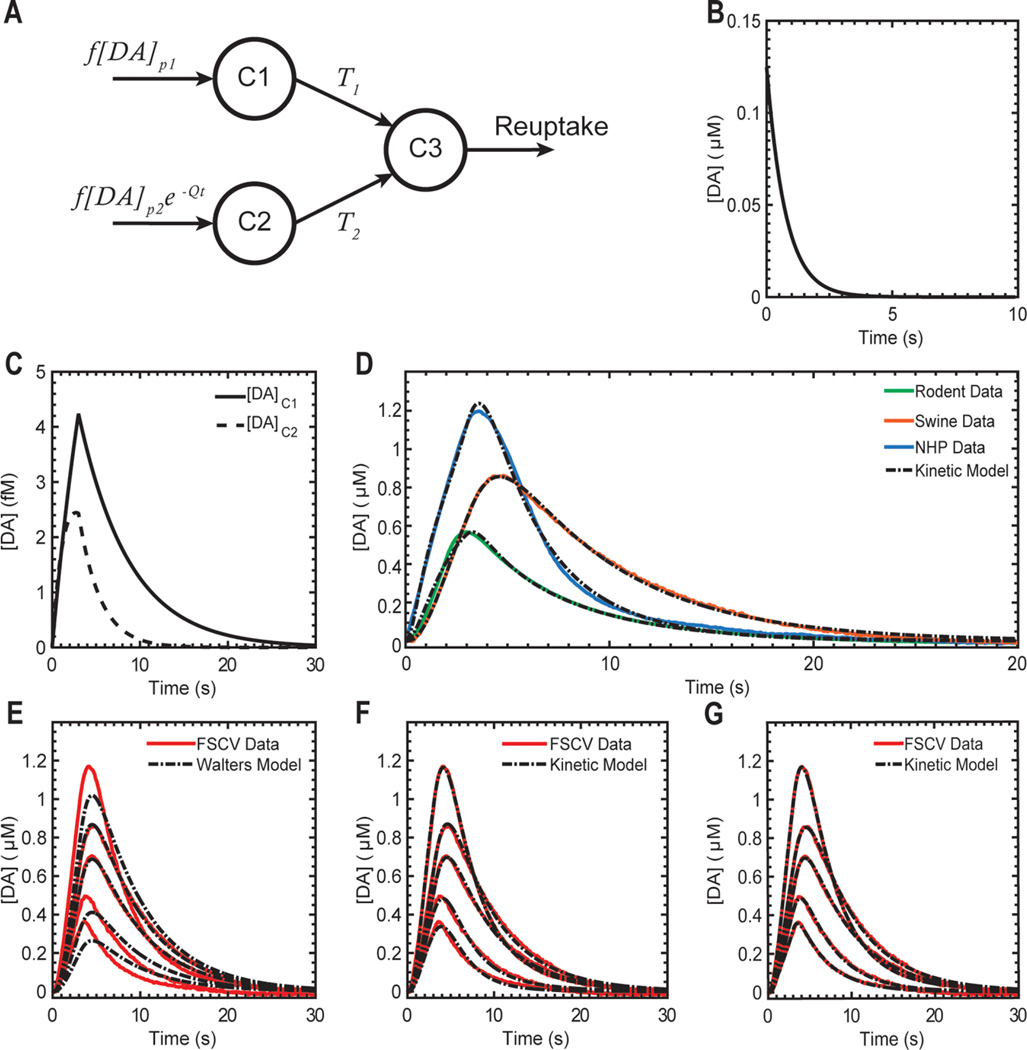
Stimulation-evoked dopamine release was modeled using parametric models of neurochemical kinetics (eqs 1–3, Figure 3A). These parametric mathematical models, trained on experimental in vivo neurochemical responses, accurately described the forward relationships between stimulus duration, amplitude, and pulse-width, and the corresponding stimulation-evoked extracellular dopamine responses for a wide range of stimulation parameters Table 1). Specifically, the compartmental model describing the kinetics of dopamine release (eqs 1–3) fit each experimental stimulation-evoked dopamine response with low nMSE in all three animal models (Figure 3D, Table 2). In order to decrease the number of free parameters needed to describe each stimulation-evoked dopamine response, model parameters were separated into subject-specific (Q, T1, T2, and reuptake parameters) and response-specific ([DA]p1 and [DA]p2) parameters. Subject-specific parameters described the rates of reuptake and transfer between model compartments. Response-specific parameters described individual components of release that result from the specific stimulation parameters applied. This approach allowed each stimulation-evoked dopamine release to be described by just two parameters, while only minimally decreasing the nMSE of the fit (Figure 3F,G, Table 2). When the compartmental model was applied to the recorded stimulation responses, both [DA]p1 and [DA]p2 increased nonlinearly with increases in amplitude (Figure 4A,C) and pulse width (Figure 4B,D). This is consistent with the expectation that stimulation-evoked dopamine increases as a function of stimulation amplitude and pulse width.
Figure 3.
Mathematical model of dopamine release evoked by electrical stimulation. (A) Compartment model used to describe the kinetics of dopamine (DA) release; C1, C2, and C3 are compartments 1, 2, and 3, respectively; f is the frequency of stimulation; [DA]p1 and [DA]p2 are DA concentration increases that occur in C1 and C2 with each pulse of stimulation; Q is the rate of attenuation of release into C2 with time; T1 and T2 are transfer rates between compartments; reuptake is a process that follows either linear or Michaelis-Menten kinetics depending on the animal model. (B) Impulse response function used to describe dopamine concentration [DA] at a carbon fiber microelectrode. (C) Dopamine concentration in C1 and C2 ([DA]C1 and [DA]C2) in response to a stimulus of (4 V, 0.5 ms, 130 Hz, 390 pulses) intensity in a swine model of electrical stimulation. (D) Fits of the kinetic model data to stimulation-evoked dopamine responses measured using fast scan cyclic voltammetry (FSCV) in rodent, swine, and non-human primate models of electrical stimulation. (E) Dopamine responses modeled using the kinetic model described by Walters et al.12,24 to five stimulation-evoked dopamine release responses measured over 2 h from a single subject. These modeled responses relied on subject-specific transfer and reuptake parameters. (F) Fit of our kinetic model to the same dopamine release responses using subject-specific parameters for R, T1, T2, and reuptake. (G) Fit of our kinetic model to the same responses using a combination of subject-specific and response-specific parameters for each stimulation-evoked response.
Table 1.
Range of Stimulation Parameters Used to Evoke Dopaminergic Responses
| frequency (Hz) |
pulse width (µs) |
amplitude | duration (s) |
|
|---|---|---|---|---|
| rodent | 60–100 | 0.1–2.0 | 0.01–0.80 mA | 1–2 |
| swine | 130 | 0.1–1.1 | 1.0–8.0 V | 2–3 |
| NHP | 130 | 0.1–0.8 | 1.0–7.0 V | 3 |
Table 2.
Performance of the Kinetic Model in Fitting Stimulation-Evoked Dopaminergic Responses Recorded from All Three Animal Modelsa
| nMSE |
||
|---|---|---|
| kinetic model (all data) |
kinetic model with subject-specific parameters (all data) |
|
| rodent | 0.18 (0.13) | 0.33 (0.14) |
| swine | 1.66 (0.63) | 2.06 (0.79) |
| NHP | 0.098 | 0.125 |
Errors reported correspond to fits obtained with and without using subject-specific reuptake and transfer rates for all responses in a data set. The normalized mean squared error (nMSE), standard deviation (STD), and correlation coefficient between experimental data and predicted responses are given for all three animal models.
Figure 4.
Result of fitting the kinetic model to a representative data set from a swine model of electrical stimulation. (A) The concentrations of dopamine (DA) released into compartment 1 of the kinetic model ([DA]p1), shown as a function of amplitude. (B) The concentrations of dopamine (DA) released into compartment 1 of the kinetic model ([DA]p2), shown as a function of amplitude. (C, D) The relationships between [DA]p1 and [DA]p2, respectively, and pulse width. To show stimulus amplitude and pulse width related trends in the data correspondingly colored smoothing splines were fit using data in Matlab (The Mathworks, Natick, MA). (E) The concentration [DA]p1 for each stimulation-evoked release shown as a function of time. (F) The concentration of [DA]p2 for each release shown as a function of time. There is a clear decrease in the maximal [DA]p2 that can be evoked over time. This relationship is shown by fitting a declining exponential to the data filtered with a 500 s window maximum filter.
The multicompartmental model for dopamine kinetics described here builds upon a restricted diffusion model recently proposed by Walters and colleagues,12,24 which relies on a single component of dopamine release equivalent to [DA]p1.12 When applied to our stimulation-evoked dopamine release data, the Walters et al. model was capable of fitting individual dopamine release responses (results not shown). However, the Walters model was unable to accurately characterize the initial dopamine kinetics and peak concentration in response to varying stimulation parameters, when subject-specific parameters where used (Figure 3E). In our multicompartment model, the maximum [DA]p1 value that can be evoked remained constant over the duration of each experimental session, while the maximum evocable value of [DA]p2 decreased exponentially (Figure 4F). Previous studies of dopamine release in the dorsal striatum suggest that there are multiple spatial domains exhibiting fast and slow dopamine release kinetics.5,20,29 Specifically, these studies suggest that there is a slow background domain with small pockets of fast release interspersed throughout the dorsal striatum.29 As such, fast scan cyclic voltammetry recordings may receive contributions from both fast and slow dopamine domains based on the specific anatomical location of the recording electrode. Thus, [DA]p1 may describe the magnitude of dopamine release from slow domains around the recording electrode, while [DA]p2 may describe the magnitude from fast domains around the recording electrode. However, additional research incorporating pharmacological interventions that differentially manipulate fast and slow domains is required to validate the physiological significance of these model parameters.29
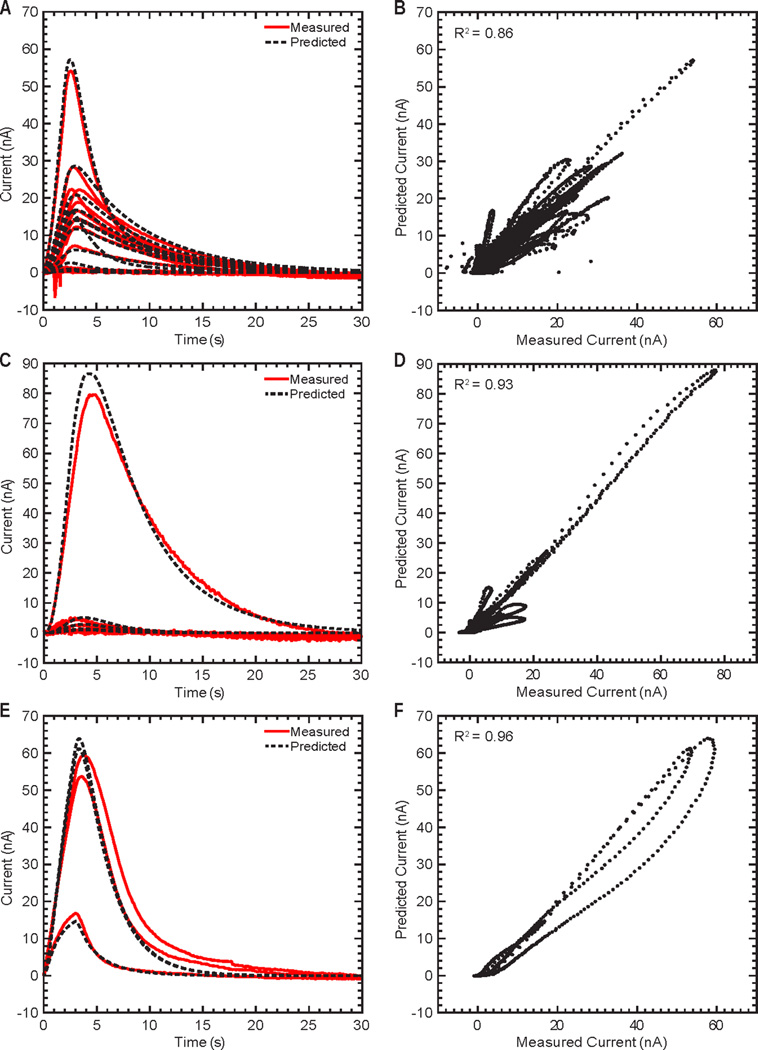
Predictive models trained on the subject-specific parameters of the compartmental model accurately characterized the relationship between stimulation parameters and evoked dopaminergic responses (Figure 2) for all stimulation-evoked neurochemical responses and for all stimuli. These predictors were based on ANNs due to their ability to learn the input/ output transfer functions without a priori knowledge of the nonlinear system dynamics and their excellent generalization capabilities.30,31 Using this approach, the 30 s of neurochemical responses that followed the onset of each stimulation event were modeled. The trained ANN models resulted in successful prediction of the kinetic changes in dopamine responses evoked by novel stimulation parameters (i.e., not used during training) across all three animal models (R2 = 0.86 for rodent, R2 = 0.93 for swine, R2 = 0.96 for NHP) (Figure 5, Table 3). The subject-specific model parameters remained constant during each experiment, while response-specific parameters were identified for each evoked response in a given experiment. This resulted in reduced ANN complexity and training time, as well as improved system performance as determined by low MSE values. A sensitivity analysis on the fitting performance revealed that the optimal predictive performance (i.e., lowest fitting MSE) for the rodent data was obtained when 9 ± 7 hidden neurons were used to train the model. For the swine and NHP data, optimal performance was observed when 14 ± 6 and 8 hidden neurons, respectively, were used.
Figure 5.
Predictions of stimulation-evoked dopamine release for 30 s of data following each test stimulation using artificial neural networks coupled with the parametric model of dopamine kinetics shown in Figure 2. Measured and predicted dopamine release responses from representative rodent (A), swine (C), and non-human primate (E) subjects. Correlation between the measured and predicted dopamine release responses for all rodent (B), swine (D), and non-human primate (F) subjects. The dopamine oxidation current measured for the first 30 s following each test stimulation is shown by the black dots.
Table 3.
Performance of the Artificial Neural Network Predictors Evaluated on the Test Data Set (30 s Following Each Stimulus)a
| nMSE (STD) |
||
|---|---|---|
| train | test | |
| rodent | 0.5 (0.2) | 0.35 (0.33) |
| swine | 3.7 (1.5) | 1.8 (0.8) |
| NHP | 0.21 | 0.13 |
The normalized mean squared error (nMSE), standard deviation (STD), and correlation coefficient between experimental data and predicted responses are given for all three animal models.
Time-Series Characterization and Prediction of Stimulation-Evoked Neurochemical Release
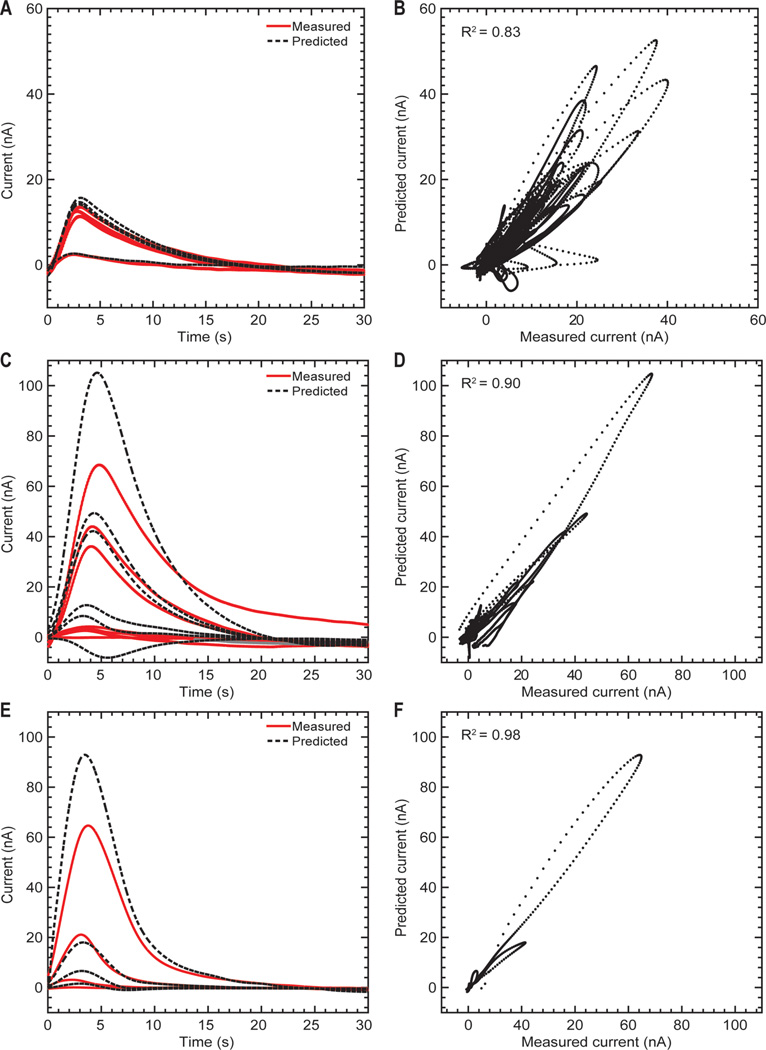
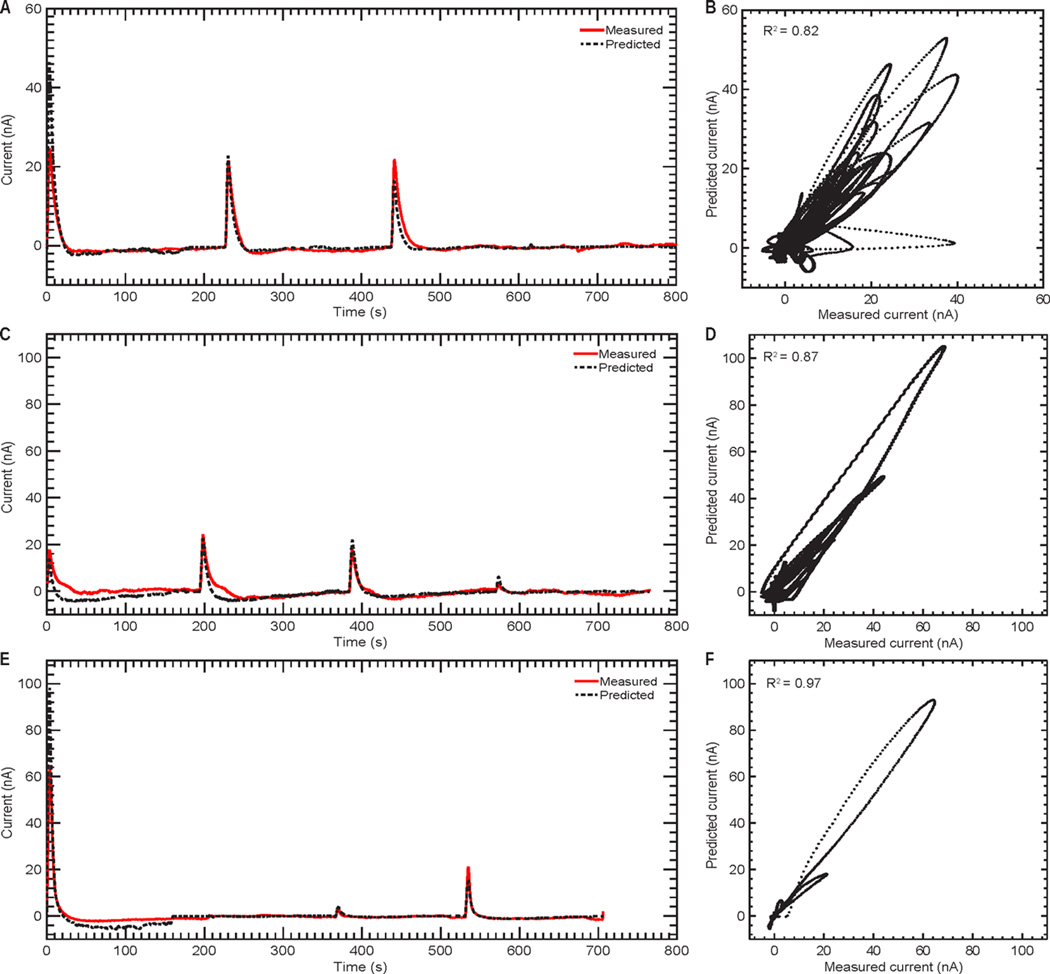
The Stone–Weierstrass theorem suggests that time series based on Volterra series can approximate a nonlinear system to any desired precision as long as the polynomial order is sufficiently large.32 Based on this theorem, Volterra kernels are capable of modeling the nonlinear dynamics between stimuli and the corresponding evoked dopamine responses as a function of time. Herein, time-series analysis with Volterra kernels successfully described the stimulation-evoked dopamine responses with high coefficients of determination (R2 = 0.83 for rodent, R2 = 0.90 for swine, R2 = 0.98 for NHP) under a wide range of stimulation parameters and across all three animal models (Figure 6, Table 4).
Figure 6.
Predictions of stimulation-evoked dopamine release for 30 s of data following each test stimulation using the second order Volterra kernel modeling approach. Measured and predicted dopamine release responses from representative rodent (A), swine (C), and non-human primate (E) subjects. Correlation between the measured and predicted dopamine release responses for all rodent (B), swine (D), and non-human primate (F) subjects. The dopamine oxidation current measured for the first 30 s following each test stimulation is shown by the black dots.
Table 4.
Performance of the Second-Order Volterra Kernel and ANN Predictorsa
| Volterra kernels |
ANN predictors |
||
|---|---|---|---|
| time series | 30 s post-stimulus | 30 s post-stimulus | |
| rodent | 227.2 (75.6) | 3.5 (1.8) | 0.35 (0.33) |
| swine | 2239.6 (109.3) | 12.8 (5.8) | 1.8 (0.8) |
| NHP | 4345.3 | 1.5 | 0.13 |
Performance is given by the normalized mean squared error and standard deviation (in parentheses) for all three animal models.
The Volterra predictor of stimulation-evoked dopamine release presented here is based on a sequence of stimuli, rather than on the parameters from one individual stimulus. This is crucial for understanding the dependence of dopamine release on previous stimuli and stimuli sequences. The trade-off was the exponential growth of the unknown model parameters, which could lead to overfitting of the data. The use of second and third order Volterra kernels greatly reduced the dopamine response prediction error (Table 5). However, third-order kernels resulted in overfitting of the test data. For the test data, the second-order kernel offered the best predictive performance (nMSE = 5.7). The number of model parameters also grew exponentially as a function of kernel order, resulting in 1 + L unknown parameters for the first order model, 1 + L + LK for the second-order model, and 1 + L + LK + LKK for the third-order model (eq 8). Ridge regression showed the lowest nMSE when a second-order kernel with K = 10 basis functions and a regularization value λ = 0.13 were used (eq 10). An unbiased estimate of the model parameters required a large amount of input/output data. Unfortunately, the experimental data collected for each subject was limited to approximately 2000 stimuli within a period of 5 h. With a time resolution of 100 ms and a history dependence of 180 to 300 s, the number of free model parameters with a second order approximation corresponded to more than one million. Therefore, it is paramount to carefully select specific kernels and kernel order in order to fit the problem at hand while minimizing the number of parameters required.
Table 5.
Performance of the Volterra Kernel Models Evaluated on the Test Data Set for Different Kernel Ordersa
| first-order Volterra kernel |
second-order Volterra kernel |
third-order Volterra kernel |
||||
|---|---|---|---|---|---|---|
| train | test | train | test | train | test | |
| rodent | 1.6 (1.3) | 4.6 (6.1) | 0.3 (0.2) | 2.0 (3.1) | 0.2 (0.1) | 3.1 (3.7) |
| swine | 133.5 (220.3) | 13.1 (19.7) | 12.4 (17.7) | 5.7 (7.8) | 2.9 (2.6) | 6.0 (8.5) |
| NHP | 2.3 | 1.5 | 0.9 | 1.5 | 0.8 | 1.8 |
The normalized mean squared error (nMSE) and standard deviation (STD) between experimental data and predicted responses are given for all three animal models.
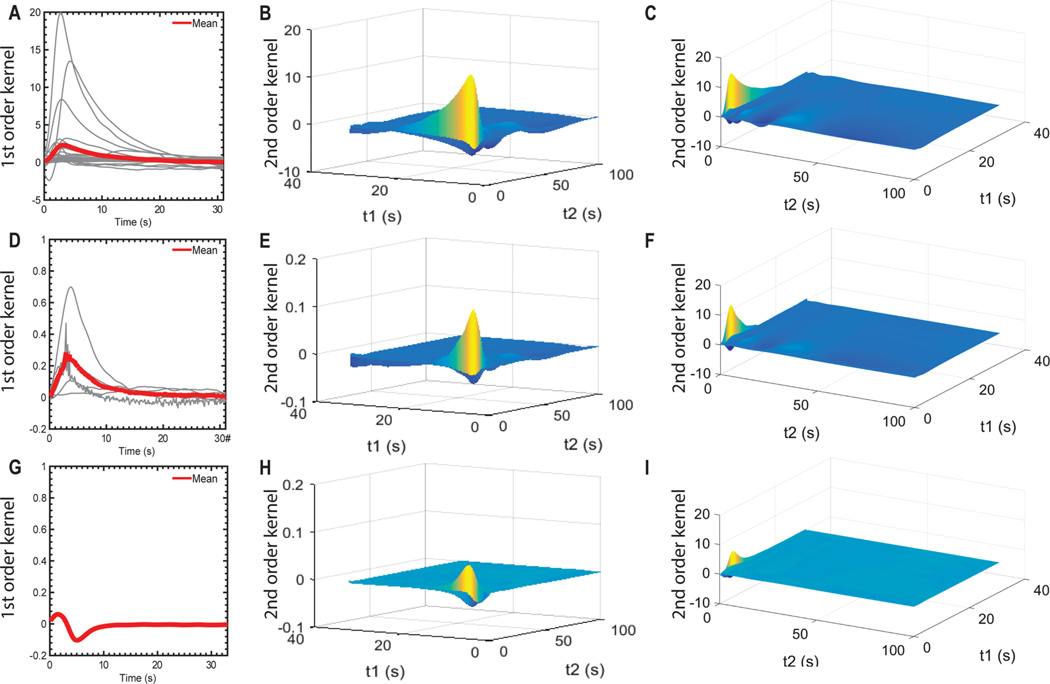
Appropriate basis functions were identified using principal component analysis and Ridge regression regularization. This prevented excessive growth of the free parameters and allowed characterization of the kinetics of dopamine release for single and paired stimuli, while reducing the probability of overfitting the training data. The basis functions of the first and second order kernels were chosen using the first 4 ± 3 principal components of the experimental data (i.e., FSCV signals measured) and the first 10 principal components of exponential decay functions (eq 11), which resulted in a balance between improved fit performance and reduced computational cost. The use of these 10 basis functions reduced model complexity for the second and higher order kernels without compromising model performance. In this model, the first order kernel and the first dimension of the second order kernel represent the kinetics of dopamine release (Figure 7). The kernels identified for all three animal models showed a decaying exponential behavior.
Figure 7.
Volterra kernels trained using experimental stimulation-evoked dopamine release responses. First-order Volterra kernels created for rodent (A), swine (D), and non-human primate (G) data. Subject-specific kernels are shown using gray lines. The average kernel behavior is shown using a red line. Second order Volterra kernels created for rodent (B,C), swine (E,F), and nonhuman primate (H,I) data. For clarity, the average kernel is shown from two complementary viewing angles.
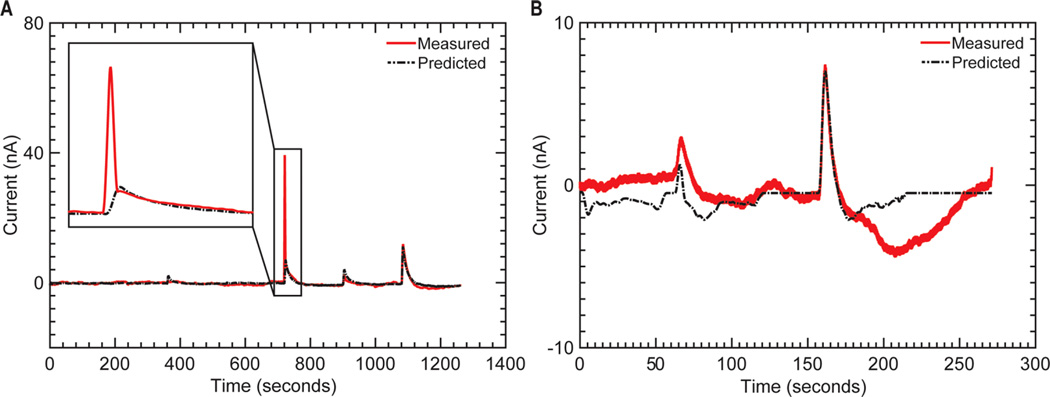
The predictive ability of the second-order Volterra model evaluated on the 30 s of data following each stimulus onset, as well as the complete time-series (including time between stimuli), is shown in Figures 6 and 8, respectively. Considerable variation in performance was observed across data sets (Table 4). Despite the ability to predict the responses to trains of stimuli, the nMSE for prediction of the complete time series was greater than the error calculated from predicting the 30 s of data immediately following each stimulation event. The Volterra model showed the largest errors when predicting swine responses (Table 4) because the measured FSCV background currents defining the baseline for all measurements showed significant shifts during the course of an experiment (Figure 9B). In contrast, the Volterra model exhibited the lowest error when predicting the NHP data due to a particularly stable baseline for this data set.
Figure 8.
Predictions of stimulation-evoked dopamine release using the second order Volterra kernel modeling approach. (A) Representative example of measured (red lines) and predicted (dotted black lines) stimulation-evoked dopamine release responses in (A) rodent, (C) swine, and (E) non-human primate models of electrical stimulation. Correlation between the measured and predicted dopamine release responses for all rodent (B), swine (D), and non-human primate (F) subjects. The dopamine oxidation current measured during the entire time-series is shown using black dots.
Figure 9.
Example of disturbance effects on the prediction (black dotted line) of experimentally measured stimulation-evoked dopamine release responses (red line) using Volterra kernels in one swine subject. (A) Model prediction is not affected despite the presence of a stimulation artifact observed in the recording. (B) Model prediction is negatively affected by fluctuations in baseline.
Changes in baseline, also known as background current, can be the result of faradaic currents caused by unrelated neurochemical events such as changes in ion concentration, release of other electroactive neurochemicals, pH changes, biofouling, and dissolution of the recording electrode.10,33–35 These fluctuations in the measured current cannot be described by the relationship between stimulation parameters and evoked responses and, therefore, negatively affected model performance. To improve characterization of the phasic dopaminergic signals, baseline was subtracted from the experimental data by applying a detrending algorithm prior to model training. However, rapid baseline fluctuations negatively affected the measurement of phasic responses to stimuli, which decreased model performance and increased prediction errors (Figure 9B). Prediction errors were further increased by the presence of stimulus artifacts in the FSCV recordings (Figure 9A). Interleaving stimulation with the FSCV waveform minimized the effect of stimulus artifacts.36
Comparison of Parametric and Time-Series Approaches for Prediction of Stimulation-Evoked Neurochemical Release
Volterra kernels were able to predict neurochemical release evoked by stimulation by taking into account previous stimulation-evoked responses. The ability of Volterra kernels to characterize and describe the dependence of neurochemical responses on previous stimuli sequences offers a significant advantage for designing algorithms for optimal characterization and ultimately control of dopamine release. Additionally, the flexibility of the Volterra kernel approach makes it applicable to other neurotransmitters, anatomical targets for stimulation and neurochemical sensing, and animal models with little or no modification. However, Volterra kernels were sensitive to noise and baseline shifts and did not adapt to changing environmental factors in real time. In contrast, models based on ANNs were less sensitive to noise and baseline shifts and could easily be adapted by adjusting the ANN weights based on novel data in real-time. Additionally, the parametric approach used for extracting ANN features greatly reduced the number of parameters required to characterize stimulation-evoked experimental data, thereby reducing computational complexity and making the development of an embedded implantable control system a real possibility. On the other hand, the compartmental model used to describe striatal dopamine release may require adjustments in order to model other neurochemicals or brain regions or even to reflect changes in release, diffusion, and reuptake mechanisms during disease progression. The ANN approach is not dependent on the history of previous stimulation. In contrast, Volterra series approaches relies on historical data. Therefore, the ANN greatly benefits from randomly selected data sets as a way to learn the relationship between inputs and outputs without learning the effects of hysteresis. Conversely, the Volterra series approach inherently requires contiguously selected data. In order to take full advantage of both modeling techniques and allow the best comparison of the two models, different test data from the same experiments were used to test each modeling technique. However, a comparison of performance on the same test data was completed by retraining the ANN model using the training and test data as used for the Volterra kernel approach (Table 6). Although this provides a direct comparison of the two modeling approaches on the same test data set, the training of the ANN model is suboptimal due to the potential of biasing the network with sequential training and test data. The general frameworks described herein were applied to measurements of neurochemical signaling but could also be be applicable to the analysis of electrophysiological activity.23,37–41 Overall, both ANN and Volterra kernel-based approaches successfully modeled the nonlinear and time-variant relationships between stimulation parameters and evoked dopamine release responses for all three animal models. Thus, it is likely that optimal characterization of stimulation-evoked dopamine responses may require a combination of parametric and time-series approaches and that optimal architecture configuration will depend on the desired application.
Table 6.
Performance of the Second-Order Volterra Kernel and ANN Predictors When Applied to the Same Test Dataa
| Volterra kernels, 30 s post-stimulus |
ANN predictors, 30 s post-stimulus |
|
|---|---|---|
| rodent | 3.5 (1.8) | 0.97 (0.59) |
| swine | 12.8 (5.8) | 2.4 (3.7) |
| NHP | 1.5 | 0.37 |
Please note that although this provides a direct comparison of the two modeling approaches, the resulting performance of the ANN model is suboptimal due to the sequential selection of training data. Performance is given by the normalized mean squared error and standard deviation (in parentheses) for all three animal models.
CONCLUSIONS
In this paper, we present two novel mathematical frameworks for characterizing in vivo neurochemical dynamics evoked by electrical stimulation. This mathematical characterization was achieved by mapping the forward relationship between stimulation amplitude and pulse width, and the instantaneous changes in extracellular dopamine concentration evoked by electrical stimulation in rodent, swine, and non-human primate brains. The frameworks presented herein include mathematical models that enable novel preclinical studies where control of neurochemical responses can be used to study the specific roles of dopamine, adenosine, serotonin, and other important neurotransmitters during normal physiologic and pathologic brain function. Although striatal dopaminergic signaling in a healthy brain was the focus of the experiments presented herein, both mathematical models, based on ANNs and Volterra kernels, can be extended to other neurochemical transmitters, sensing and stimulation paradigms, anatomical targets, and different neurologic disorders.
In addition to characterizing neurochemical responses, both modeling frameworks accurately predicted stimulation-evoked dopamine release in response to novel stimuli not used experimentally (Figures 5 and 6), which is paramount for enabling precise control of extracellular neurotransmitter concentration. In this way, these mathematical models will facilitate novel studies into the role of extracellular dopamine and other neurotransmitters in neurologic health and disease, as well as leading to the development of precision therapeutic interventions.
METHODS
Animal Subjects
Stimulation-evoked dopaminergic responses were studied in 12 female Sprague-Dawley rats weighing 250–350 g, four male young adult pigs age 8–12 months weighing 25–35 kg, and one male non-human primate rhesus macaque three years of age. Rodents were kept in social housing, while swine and the NHP remained in individual areas. All animals were kept on a 12-h light/dark cycle at a constant temperature (21 °C) and humidity (45%) and were fed once daily, with ad libitum access to water. All animal studies were performed in vivo with approval from the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) and following National Institutes of Health (NIH) guidelines for animal research.
Surgical Procedure
Rodents were sedated prior to surgery with intraperitoneal urethane (1.5 g/kg in a 0.26 g/mL). Swine were sedated with intramuscular Telazol (6 mg/kg) and xylazine (2 mg/kg), which was followed by intubation and inhalation anesthesia,42 and the NHP was sedated with intramuscular ketamine (10 mg/kg) and xylazine (0.5 mg/kg), followed by intubation and inhalation anesthesia. Anesthesia was maintained for both swine and NHP studies using 1.5–3% isoflurane during electrode implantation and 1.5–2% isoflurane during the rest of the experiment. Analgesia was maintained with intramuscular buprenorphine for both rodents and pigs (0.06 mg/kg rodents, 0.03 mg/kg in swine) and intramuscular buprenorphine slow release (SR) for NHP (0.06 mg/kg). For swine and NHP, SpO2 (100), heart rate (70–110 bpm for swine and 100–130 bpm for NHP), blood pressure (110/80 mmHg for swine and 98 ± 17/54 ± 13 mmHg for NHP), and core body temperature (36–38 °C) were continuously monitored to ensure proper anesthesia depth and to determine overall animal welfare. Additionally, vecuronium (3 mg intravenous bolus, followed by a 17 mg/h intravenous drip) was administered to swine during neurochemical recording to prevent motion artifacts induced by electrical stimulation.
During the surgical procedure, anesthetized swine and NHP were secured in a custom-made stereotactic frame.28,43 Rodents were placed in a Kopf stereotactic system (David Kopf Instruments, Tujunga, CA). A scalp incision (1.5–2.0 cm for rodents, 5–7 cm for swine and NHP) was made to expose the skull, and three trephine burr holes (approximately 3 and 7 mm in diameter for rodent and large animals, respectively) were drilled to allow implantation of the stimulating, neurochemical sensing, and reference electrodes (Figure 1A,B).
Stereotactic Targeting and Electrode Implantation
To implant the electrodes in rodents, the stereotactic coordinates of the medial forebrain bundle (MFB) and striatum with respect to bregma were obtained according to a rat brain atlas.44 A dual manipulator stereotactic system (David Kopf Instruments, Tujunga, CA) was used to insert a bipolar stimulating electrode (stainless steel, 250 µm diameter, 20 mm long polymide-coated shaft, PlasticsOne, Roanoke, VA) into the rodent MFB. The same stereotactic system was used to implant a custom-made carbon fiber microelectrode (CFM) into the rat striatum for neurochemical sensing via fast-scan cyclic voltammetry. The stimulating and FSCV electrode coordinates are given in Table 7. A silver/silver-chloride FSCV reference electrode was implanted into the contralateral hemisphere.
Table 7.
Stereotactic Coordinates for Fast Scan Cyclic Voltammetry (FSCV) and Stimulating Electrodesa
| AP (mm) | ML (mm) | DV (mm) | |
|---|---|---|---|
| stimulation | −4.6 | +1.2 | −8.7 |
| FSCV | +1.2 | +2.0 | −5.5 |
Coordinates are given in mm from bregma in the anteroposterior (AP), mediolateral (ML), and dorsoventral (DV) directions.
For both the swine and NHP models, preoperative 3T MRI was used to identify the location of the striatal and ventrotegmental area/substantia nigra pars compacta (VTA/SNc) targets. The stereotactic targets and electrode trajectories were defined using the COMPASS stereotactic targeting platform (COMPASS International Innovations, Rochester, MN) and anatomical brain atlases (Figure 1A,B).45–47 A six-contact stimulating electrode (0.625 mm in diameter and 0.5 mm height, separated by 0.5 mm) (NuMed, Hopkinton, NY) was implanted into the VTA/SNc region. Electrode insertion was individually controlled for the stimulating and FSCV electrodes using a computer-driven microdrive (Alpha Omega Co., Alpharetta, GA). The lead of the stimulating electrode was secured to the skull using bone screws and retaining plate. In contrast, the FSCV electrode was held at its target location via the stereotactic head frame.
Deep Brain Stimulation for Characterization of Dopaminergic Response
Subsequent to stereotactic targeting and electrode implantation, the target region (MFB for rats and SNc/VTA for swine and NHP) was stimulated using a comprehensive range of stimulation parameters (Table 1). Simultaneously, the magnitudes and temporal patterns of dopamine release evoked by stimulation were measured. The minimum and maximum stimulation amplitudes leading to threshold and saturation dopamine responses when the pulse width was held constant at 0.2 ms were identified. Then, pulse width values were identified that lead to threshold and saturation dopamine responses when amplitude was held at the previously identified maximum value. Each combination of stimulation parameters was presented in random order to identify system dynamics while eliminating the effects of hysteresis. Each stimulation event was followed by a dopamine stabilization period of 3–5 min.
Neurochemical Recording of Stimulation-Evoked Dopamine Responses
Stimulation-evoked dopamine responses were measured within the ipsilateral striatum using FSCV.10,48–50 A triangular FSCV waveform was created by ramping the microelectrode potential at 400 V/s from a resting value of −0.4 V to a maximum of 1.5 V, before ramping the potential back to the resting value (Figure 1E). This waveform was repeated at 10 Hz with the CFM held at the resting potential between scans. The magnitude of the dopamine oxidation current, typically detected at +0.6 V on the triangular FSCV waveform (Figure 1C,D), was measured and converted to dopamine concentration using postoperative in vitro calibration of each CFM.51 Calibration was performed by placing each CFM in a flowing stream of Tris buffer solution to which multiple 5 s boluses of a sodium chloride dopamine solution (i.e., 0.1 µM, 0.5 µM, 1.0 µM) were injected via an electronic loop injector (FIAlab Instruments, Seattle, WA) at 6 mL/min. FSCV was performed to identify the relationships between injected dopamine concentrations and the corresponding oxidation currents measured by the CFM.
Parametric Characterization and Prediction of Stimulation-Evoked Neurochemical Release
In vivo stimulation-evoked striatal dopamine responses measured by FSCV were characterized using parametric models of release and reuptake (Figure 3A) based on biological kinetics and compartmental models.12,13,24 This mathematical model consisted of three coupled differential equations that describe the concentration of stimulation-evoked dopamine, [DA], across three compartments C1, C2, and C3 ([DA]C1, [DA]C2, and [DA]C3, respectively). Compartment C3 represents the dopamine concentration at the FSCV electrode, and all three compartments are abstract mathematical constructs used to model dopamine release and transfer processes and are not associated with any anatomical structures (Figure 3A). In this model, dopamine released into compartment C1 ([DA]C1) is described by eq 1 and occurs during stimulation at a rate proportional to the stimulation frequency (f) and a concentration increase of [DA]p1 per pulse of stimulation. Dopamine transfer from C1 to C3 is described by a rate constant T1. Dopamine concentration in compartment 2 ([DA]C2) is described using similar processes of release and transfer as those used for compartment C1 (eq 1) plus an additional exponential decay at a rate Q. Dopamine transfer from compartment C2 to compartment C3 is defined by rate T2.
The parameter [DA]p1 describes a component of dopamine release that remains constant during stimulation (eq 1). Similarly, the parameter [DA]p2 describes an exponentially decreasing component found in dopamine release (eq 2). The dopamine concentration measured by FSCV is represented by [DA]C3, which depends on dopamine reuptake and transfer from C1 and C2. A volume correction for compartment C3 is defined by VC3 and was set as 106 µm3 as described in previous literature.12,24
Equation 3a describes the dopamine concentration measured by FSCV in the NHP subject. Here, dopamine reuptake was modeled as a continuous process that obeys Michaelis-Menten kinetics.14 In contrast, eq 3b describes the dopamine concentration measured by FSCV in the rodent and swine subjects. In this case, dopamine reuptake was modeled as a first order kinetic process with a rate R. This approximates Michaelis-Menten kinetics when [DA]C3 ≪ Km with , where Vmax defines the maximum reuptake rate and Km represents the substrate concentration at which the reaction rate is half of Vmax. Changes in extracellular dopamine concentration ([DA]C3) evoked during stimulation were determined by solving the following system of differential equations:
| (1) |
| (2) |
| (3a) |
| (3b) |
At the end of stimulation, the stimuli-dependent release described in eqs 1 and 2 disappears, and the equations become
| (4) |
| (5) |
while eq 3 remains unchanged. Additionally, the measured dopamine signal depends on the temporal response of dopamine measurement via FSCV, which is primarily affected by adsorption/deadsorption of dopamine at the electrode interface and needs to be accounted for by using impulse response function. In particular, the kinetics of adsorption/deadsorption of dopamine and dopamine-o-quinone are described in eq 652,53
| (6) |
where DAsoln and DOQsoln correspond to dopamine and dopamine-o-quinone dissolved in solution, DAads and DOQads correspond to dopamine and dopamine-o-quinone adsorbed at the carbon fiber microelectrode, k1 and k2 describe the adsorption rate constants, and k−1 and k−2 describe the deadsorption rate constants. Fa(m) represents the impulse response function for measurement of dopamine at the CFM (Figure 3C) and is given by53
| (7) |
where m is the FSCV scan number, τ is the time during the FSCV scan over which dopamine is adsorbed, and Ts is the time over which dopamine is being oxidized. The parameters τ and Ts were determined from the FSCV repetition and scan rates, respectively (Figure 1E). The deadsorption rate constants k−1 and k−2 were determined in vitro by averaging the least-squares fits of three CFMs to three 0.5 µM bolus injections of dopamine delivered over 2 s each. Taking into account the temporal response of dopamine measurement via FSCV, the measured dopamine oxidation current was calculated by convolving the response measured at the CFM (Fa(m)) with the extracellular dopamine concentration ([DA]C3)
| (8) |
where y(t) describes the dopamine oxidation current measured with FSCV at the CFM.
The model parameters describing the stimulation-evoked dopamine responses for each subject were identified by performing a nonlinear least-squares fit of the kinetic model to a series of intrasubject experimental responses (i.e., global fit). Herein, kinetic model parameter values obtained using a global fit are referred to as subject-specific values, while parameter values that differ across individual responses are referred to as response-specific values. Global parameter fit was repeated using 20 different initial conditions to prevent getting caught in local minima. Initial parameters were selected according to previous experience and values reported in the literature.12,24 Subject-specific values for T2 and Q were calculated by performing a second global fit using the previously calculated values for T1 and reuptake (R or Vmax and Km), while the response-specific values, [DA]p1 and [DA]p2, were calculated by fitting each individual dopamine response using the subject-specific parameters for T1, T2, Q, and reuptake.
Following mathematical parametrization, the nonlinear and time-varying relationships between stimulation-evoked dopamine kinetics and stimulation parameters were characterized for each experimental data set using a multilayer feed-forward artificial neural network (ANN) in order to predict stimulation-evoked responses for novel stimuli not used for model training. This ANN model, or system model, was implemented using Matlab (The Mathworks, Natick, MA) via a double layer feed forward ANN with sigmoidal and linear transfer functions in the hidden and output layers, respectively. The system model consisted of three inputs (pulse amplitude, pulse width, and time since the beginning of stimulation), H hidden neurons, and two outputs (changes in dopamine concentration [DA]p1 and [DA]p2). The network weights and biases were initialized using random values prior to training the system model using the Levenberg-Marquardt backpropagation algorithm. A sensitivity analysis was performed for each experimental data set to optimize the number of neurons in the hidden layer from 5 to 20 hidden neurons. Additionally, ten ANNs were trained using random initial weights and biases, and the ANN with the lowest validation mean square error was selected.
Time-Series Characterization and Prediction of Stimulation-Evoked Neurochemical Release
In addition to the parametric characterization and prediction described in the previous section, a time series approach based on Volterra kernels was used to characterize the dopaminergic responses evoked by electrical stimulation, y(t):
| (9) |
where x(t) describes electrical stimuli at time t and hi describes the Volterra kernels of order i that approximate the kinetics of dopamine release. The Volterra kernels were approximated using a weighted sum of finite length basis functions
| (10a) |
| (10b) |
| (10c) |
| (10d) |
| (10e) |
| (10f) |
| (10g) |
| (10h) |
where Bl is the lth basis of the h1 kernel defined by the first L principal components selected from normalized dopamine release data, bl represents the unknown parameters in the first-order Volterra model h1, M describes the dopamine release evoked by the current stimulus, N describes the combined effect of previous stimuli on the current stimulation-evoked dopamine response for the second and higher order Volterra models, K represents the number of principal components selected for the previous stimuli, T1 - T2 and T2 - T3 describe the time between the current and previous stimuli, and Ck is the kth basis function and determines the dependence of dopamine release on previous stimuli separated by time td. Ck is defined by a principal component expansion of decaying exponentials of the form
| (11) |
A principal component analysis (PCA) of the stimulation-evoked dopamine response was performed for the first order kernel to determine the number of optimal principal components (i.e., principal components whose eigenvalues were greater than 1% of the largest eigenvalue), and thus the number of basis functions, L. The length of these basis functions for the first-order kernel was defined as the minimum time between adjacent stimuli. In contrast, the length of the basis for the second and higher order kernels was set to 100 s. A sample data set from each animal model was analyzed to determine the appropriate Volterra kernel order that did not result in overfitting. The unknown coefficients of the basis functions, W, were estimated using least-squares optimization via Ridge regression54 as follows:
| (12) |
| (13) |
where Y is the measured stimulation-evoked dopamine, A is defined by the linear convolution of the input stimuli and the model basis functions, n represents the length of the time series, p defines the number of unknown parameters (basis weights, bl,kl,ck,… plus a bias term, h0), λ is the ridge regularization parameter; and I represents the identity matrix. When λ is zero, eq 13 becomes the solution of the least-squares optimization. Prior to fitting the stimulation-evoked responses using Volterra kernels, experimental data were detrended using a moving average filter with a 400 s time window, which allowed retention of phasic responses throughout the time series. Once the free parameters W were estimated from the detrended FSCV data, eq 12 predicted dopamine responses evoked by novel stimuli.
Training and Evaluation of the Dopaminergic Response Predictors
Both Volterra and ANN predictor models were trained and tested on experimental stimulation-evoked dopamine responses recorded using FSCV data from rodent, swine, and NHP models of electrical stimulation. The experimental data from each subject was divided into smaller subsets encompassing 70%, 15%, and 15% of the data for training, validation, and testing, respectively. For the Volterra kernel model, the first 70% of the measured FSCV data was used for training and the subsequent 15% of the data was used to evaluate model performance. The remaining 15% of the data were ignored to allow a direct comparison of the performance between the parametric and time-series approaches on the same amount of data. Hyperparameters of the Volterra kernel model (e.g., the number of basis functions, type of regression (lasso vs ridge), and regularization parameters) were optimized to improve model performance on the validation data (one data set from each species). The optimal Volterra kernel order was selected based on an F-test and considering computational cost necessary to lower validation error. This analysis was performed on a restricted data set due to the computational time involved.
For the parametric ANN model, the data for each category (i.e., training, validation, and test) was selected at random. Validation data was used to prevent overfitting and estimate generalization error, while test data was used only for evaluating model performance. Network complexity was controlled by early stopping once the smallest prediction error with respect to the validation data set was obtained.55 As an additional way to compare the ANN and Volterra kernel approaches, both were evaluated on the same sequentially acquired test data.
Model performance was evaluated for each model using their respective test data sets. For the Volterra kernel model, the predictive performance was analyzed for the entire testing data set as well as for a shorter response period of 30 s following each stimulus to allow for direct comparison with the parametric model. Finally, the performance of both parametric and time-series predictors was determined by calculating the mean squared error (MSE) between the dopamine responses predicted by the model in response to novel stimuli, and the corresponding responses measured experimentally in vivo—normalized by the product of the mean of experimental and predicted responses (nMSE).
Acknowledgments
The authors gratefully acknowledge Bruce Knudsen, Andrea McConico, Jill L. Anderson, and Michael Marsh for their assistance with animal care and surgery, Drs. Grant W. Mallory and Jan T. Hachmann for their assistance with swine and rodent surgery and data collection, Drs. Paul H. Min and Erika Ross for their assistance with NHP surgery and data collection, Kendyl Greimann, Dr. Aldo Mendez, and Dr. Peter Grahn for their assistance with data collection, Brian S. Paek, Evan Nicolai, and Megan L. Settel for their assistance with electrode manufacturing, Dr. Su youne Chang, Dr. Charles Blaha, and Innyong Kim for their guidance in performing FSCV, Drs. Allan Bieber, Leonardo Espin, and Joshua Jacobs, as well as Elizabeth Mosier, for reviewing the manuscript, and Joshua B. Boesche, Christopher Kimble, Diane Eaker, Kenneth R. Kressin, Sidney V. Whitlock, Malcolm Mcintosh, and their colleagues of the Mayo Clinic Division of Engineering for their help in interfacing with WINCS Harmoni and WINCSware.
Funding
This work was supported by the National Institutes of Health, NINDS (R01 NS084975 award to J.L.L. and R01 NS75013 and R01 NS70872 awards to K.H.L.), and The Grainger Foundation.
Footnotes
Author Contributions
J.K.T. contributed to experimental design, data collection, data analysis, algorithm development, and manuscript preparation. A.Y. contributed to data analysis, algorithm development, and manuscript preparation. H.O.P. contributed to data analysis, algorithm development, and manuscript preparation. J.J.B. contributed to data analysis, algorithm development, and manuscript preparation. K.H.L. contributed to experimental design. J.L.L. contributed to experimental design, data collection, data analysis, algorithm development, and manuscript preparation.
The authors declare no competing financial interest.
The authors of this manuscript would like to encourage other researchers attempting to replicate or take advantage of these approaches to contact us, James Trevathan (trevathan.james@mayo.edu) or J. Luis Lujan, Ph.D. (lujan.luis@mayo.edu), for specific support in the form of code or answers to questions.
REFERENCES
- 1.Brownell A-L, Jenkins BG, Elmaleh DR, Deacon TW, Spealman RD, Isacson O. Combined PET/MRS brain studies show dynamic and long-term physiological changes in primate model of Parkinson disease. Nat. Med. 1998;4:1308–1312. doi: 10.1038/3300. [DOI] [PubMed] [Google Scholar]
- 2.Moses WW. Fundamental Limits of Spatial Resolution in PET. Nucl. Instrum. Methods Phys. Res., Sect. A. 2011;648(Suppl. 1):S236–S240. doi: 10.1016/j.nima.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaquins-Gerstl A, Michael AC. A review of the effects of FSCV and microdialysis measurements on dopamine release in the surrounding tissue. Analyst. 2015;140:3696–3708. doi: 10.1039/c4an02065k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min H-K, Ross EK, Jo HJ, Cho S, Settell ML, Jeong H, Duffy PS, Chang S-Y, Bennet KE, Blaha CD, Lee KH. Dopamine Release in the Nonhuman Primate Caudate and Putamen Depends upon Site of Stimulation in the Subthalamic Nucleus. J. Neurosci. 2016;36:6022–6029. doi: 10.1523/JNEUROSCI.0403-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor IM, Ilitchev AI, Michael AC. Restricted Diffusion of Dopamine in the Rat Dorsal Striatum. ACS Chem. Neurosci. 2013;4:870–878. doi: 10.1021/cn400078n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Cai LX, Lelyveld VS, Hai A, Jasanoff A. Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science. 2014;344:533–535. doi: 10.1126/science.1249380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel VD, Lee DE, Alexoff DL, Dewey SL, Schiffer WK. Imaging dopamine release with Positron Emission Tomography (PET) and 11C-raclopride in freely moving animals. NeuroImage. 2008;41:1051–1066. doi: 10.1016/j.neuroimage.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal. Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 10.Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 11.Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PEM. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters SH, Taylor IM, Shu Z, Michael AC. A Novel Restricted Diffusion Model of Evoked Dopamine. ACS Chem. Neurosci. 2014;5:776–783. doi: 10.1021/cn5000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wightman RM, Amatorh C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- 15.Behrend CE, Cassim SM, Pallone MJ, Daubenspeck JA, Hartov A, Roberts DW, Leiter JC. Toward feedback controlled deep brain stimulation: Dynamics of glutamate release in the subthalamic nucleus in rats. J. Neurosci. Methods. 2009;180:278–289. doi: 10.1016/j.jneumeth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Howell B, Huynh B, Grill WM. Design and in vivo evaluation of more efficient and selective deep brain stimulation electrodes. J. Neural Eng. 2015;12:046030. doi: 10.1088/1741-2560/12/4/046030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntyre CC, Miocinovic S, Butson CR. Computational analysis of deep brain stimulation. Expert Rev. Med. Devices. 2007;4:615–622. doi: 10.1586/17434440.4.5.615. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre CC, Butson CR, Maks CB, Noecker AM. 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2006. EMBS ’06. Piscataway, NJ: IEEE; 2006. Optimizing Deep Brain Stimulation Parameter Selection with Detailed Models of the Electrode-Tissue Interface; pp. 893–895. [DOI] [PubMed] [Google Scholar]
- 19.Garris PA, Walker QD, Wightman RM. Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 1997;753:225–234. doi: 10.1016/s0006-8993(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 20.Taylor IM, Nesbitt KM, Walters SH, Varner EL, Shu Z, Bartlow KM, Jaquins-Gerstl AS, Michael AC. Kinetic diversity of dopamine transmission in the dorsal striatum. J. Neurochem. 2015;133:522–531. doi: 10.1111/jnc.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chon KH, Holstein-Rathlou NH, Marsh DJ, Marmarelis VZ. Comparative nonlinear modeling of renal autoregulation in rats: Volterra approach versus artificial neural networks. IEEE Trans. Neural Netw. 1998;9:430–435. doi: 10.1109/72.668884. [DOI] [PubMed] [Google Scholar]
- 22.Marmarelis VZ, Zhao X. Volterra models and three-layer perceptrons. IEEE Trans. Neural Netw. 1997;8:1421–1433. doi: 10.1109/72.641465. [DOI] [PubMed] [Google Scholar]
- 23.Zanos TP, Courellis SH, Berger TW, Hampson RE, Deadwyler SA, Marmarelis VZ. Nonlinear modeling of causal interrelationships in neuronal ensembles. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2008;16:336–352. doi: 10.1109/TNSRE.2008.926716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters SH, Robbins EM, Michael AC. Modeling the Kinetic Diversity of Dopamine in the Dorsal Striatum. ACS Chem. Neurosci. 2015;6:1468. doi: 10.1021/acschemneuro.5b00128. [DOI] [PubMed] [Google Scholar]
- 25.Gálvez JF, Keser Z, Mwangi B, Ghouse AA, Fenoy AJ, Schulz PE, Sanches M, Quevedo J, Selvaraj S, Gajwani P, Zunta-Soares G, Hasan KM, Soares JC. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: A review of published data. Prog. Neuro- Psychopharmacol. Biol. Psychiatry. 2015;58:59–70. doi: 10.1016/j.pnpbp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Howe MW, Dombeck DA. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016;535:505–510. doi: 10.1038/nature18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan H, Sarre S, Ebinger G, Michotte Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J. Neurosci. Methods. 2005;144:35–45. doi: 10.1016/j.jneumeth.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Shon Y-M, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, Bennet K, Blaha CD, Chang S-Y. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci. Lett. 2010;475:136–140. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitch Taylor I, Jaquins-Gerstl A, Sesack SR, Michael AC. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J. Neurochem. 2012;122:283–294. doi: 10.1111/j.1471-4159.2012.07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida JS. Predictive non-linear modeling of complex data by artificial neural networks. Curr. Opin. Biotechnol. 2002;13:72–76. doi: 10.1016/s0958-1669(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Eddy Patuwo B, Y Hu M. Forecasting with artificial neural networks:: The state of the art. Int. J. Forecast. 1998;14:35–62. [Google Scholar]
- 32.Stone M. The Generalized Weierstrass Approximation Theorem. Math. Mag. 1948;21:237–254. [Google Scholar]
- 33.Kume-Kick J, Rice ME. Dependence of dopamine calibration factors on media Ca2+ and Mg2+ at carbon-fiber microelectrodes used with fast-scan cyclic voltammetry. J. Neurosci. Methods. 1998;84:55–62. doi: 10.1016/s0165-0270(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 34.Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Anal. Chem. 2011;83:6658–6666. doi: 10.1021/ac2011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takmakov P, Zachek MK, Keithley RB, Bucher ES, McCarty GS, Wightman RM. Characterization of Local pH Changes in Brain Using Fast-Scan Cyclic Voltammetry with Carbon Microelectrodes. Anal. Chem. 2010;82:9892–9900. doi: 10.1021/ac102399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KH, Lujan JL, Trevathan JK, Ross EK, Bartoletta JJ, Park HO, Paek S, Nicolai E, Min PH, Kimble CJ, Blaha CD, Bennet KE. WINCS Harmoni: Closed-loop dynamic neurochemical control of therapeutic interventions. Sci. Rep. 2017 doi: 10.1038/srep46675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lujan JL, Crago PE. Automated optimal coordination of multiple-degree-of-freedom neuromuscular actions in feedforward neuroprostheses. IEEE Trans. Biomed. Eng. 2009;56:179–187. doi: 10.1109/TBME.2008.2002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pienkowski M, Shaw G, Eggermont JJ. Wiener-Volterra Characterization of Neurons in Primary Auditory Cortex Using Poisson-Distributed Impulse Train Inputs. J. Neurophysiol. 2009;101:3031–3041. doi: 10.1152/jn.91242.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song D, Robinson BS, Granacki JJ, Berger TW. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Piscataway, NJ: IEEE; 2014. Implementing spiking neuron model and spike-timing- dependent plasticity with generalized Laguerre-Volterra models; pp. 714–717. [DOI] [PubMed] [Google Scholar]
- 40.Yamins DLK, DiCarlo JJ. Using goal-driven deep learning models to understand sensory cortex. Nat. Neurosci. 2016;19:356–365. doi: 10.1038/nn.4244. [DOI] [PubMed] [Google Scholar]
- 41.Yamins DLK, Hong H, Cadieu CF, Solomon EA, Seibert D, DiCarlo JJ. Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc. Natl. Acad. Sci. U.S.A. 2014;111:8619–8624. doi: 10.1073/pnas.1403112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min H-K, Hwang S-C, Marsh MP, Kim I, Knight E, Striemer B, Felmlee JP, Welker KM, Blaha CD, Chang S-Y, Bennet KE, Lee KH. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. NeuroImage. 2012;63:1408–1420. doi: 10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min H-K, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, Marsh MP, Striemer B, Felmlee JP, Lujan JL, Goerss S, Duffy PS, Blaha CD, Chang S-Y, Bennet KE. Subthalamic Nucleus Deep Brain Stimulation Induces Motor Network BOLD Activation: Use of a High Precision MRI Guided Stereotactic System for Nonhuman Primates. Brain Stimulat. 2014;7:603–607. doi: 10.1016/j.brs.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain atlas in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 45.Félix B, Léger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP, et al. Stereotaxic atlas of the pig brain. Brain Res. Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 46.Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, Collins DL. An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space) NeuroImage. 2011;55:1435–1442. doi: 10.1016/j.neuroimage.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Saikali S, Meurice P, Sauleau P, Eliat P-A, Bellaud P, Randuineau G, Verin M, Malbert C-H. A three- dimensional digital segmented and deformable brain atlas of the domestic pig. J. Neurosci. Methods. 2010;192:102–109. doi: 10.1016/j.jneumeth.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 48.Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of micro- dialysis and fast-scan cyclic voltammetry. Neurosci. Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- 49.Chang S-Y, Jay T, Munoz J, Kim I, Lee KH. Wireless fast-scan cyclic voltammetry measurement of histamine using WINCS – a proof-of-principle study. Analyst. 2012;137:2158–2165. doi: 10.1039/c2an16038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J. Neurochem. 1997;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- 51.Griessenauer CJ, Chang S-Y, Tye SJ, Kimble CJ, Bennet KE, Garris PA, Lee KH. Wincs-Based Wireless Electrochemical Monitoring of Serotonin (5-Ht) Using Fast-Scan Cyclic Voltammetry: Proof of Principle. J. Neurosurg. 2010;113:656–665. doi: 10.3171/2010.3.JNS091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond Adsorption and Desorption of Dopamine at Carbon-Fiber Microelectrodes. Anal. Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 53.Venton BJ, Troyer KP, Wightman RM. Response Times of Carbon Fiber Microelectrodes to Dynamic Changes in Catecholamine Concentration. Anal. Chem. 2002;74:539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- 54.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd. New York, NY: Springer Science & Business Media; 2009. [Google Scholar]
- 55.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd. New York, NY: Corr. Springer; 2011. [Google Scholar]