Abstract
We performed genome-wide analyses to identify genomic loci that interact with sodium to influence blood pressure (BP) using single marker (one and two degree-of-freedom joint tests) and gene-based tests among 1,876 Chinese participants of the Genetic Epidemiology Network of Salt-Sensitivity (GenSalt) study. Among GenSalt participants, the average of three urine samples was used to estimate sodium excretion. Nine BP measurements were taken using a random-zero-sphygmomanometer. A total of 2.05 million SNPs were imputed using Affymetrix 6.0 genotype data and the Chinese Han of Beijing and Japanese of Tokyo HapMap reference panel. Promising findings (P <1.00×10−4) from GenSalt were evaluated for replication among 775 Chinese participants of the Multi-ethnic Study of Atherosclerosis (MESA). SNP and gene-based results were meta-analyzed across the GenSalt and MESA studies to determine genome-wide significance. The one degree-of-freedom tests identified interactions for UST rs13211840 on diastolic BP (P=3.13×10−9). The two degree-of-freedom tests additionally identified associations for CLGN rs2567241 (P=3.90×10−12) and LOC105369882 rs11104632 (P=4.51×10−8) with systolic BP. The CLGN variant rs2567241 was also associated with diastolic BP (P=3.11×10−22) and mean arterial pressure (P= 2.86×10−15). Genome-wide gene-based analysis identified MKNK1 (P=6.70×10−7), C2orf80 (P<1.00×10−12), EPHA6 (P=2.88×10−7), SCOC-AS1 (P=4.35×10−14), SCOC (P=6.46×10−11), CLGN (P=3.68×10−13), MGAT4D (P=4.73×10−11), ARHGAP42 (P=<1.00×10−12), CASP4 (P=1.31×10−8), and LINC01478 (P=6.75×10−10) that were associated with at least one BP phenotype. In summary, we identified 8 novel and 1 previously reported BP loci through the examination of SNP and gene-based interactions with sodium.
Keywords: Genome-wide, Gene-sodium interaction, Blood pressure, One degree-of-freedom test, Two degree-of-freedom joint test, Gene-based analysis
Introduction
High blood pressure (BP) is a major public health challenge worldwide due to its high prevalence and associated mortality and morbidity1, 2. As a complex trait, BP is influenced by both genetic and environmental factors as well as their interactions. Sodium intake has been demonstrated as an important environmental determinant of hypertension and varies greatly within and across populations3, 4. Numerous genome-wide association studies (GWAS) have identified robust associations between genomic loci and BP5–9. However, these findings explain only a small proportion of the estimated heritability of this complex phenotype10. Because the marginal effects of variants modified by environmental factors may be missed by traditional single-marker analyses, scientists postulate that additional genetic factors may be detected through the analysis of gene-environment interactions. Relatedly, an important interrelationship of sodium and genetic factors on BP has been demonstrated through the identifications of genes responsible for Mendelian forms of hypertension, with the vast majority of recognized genes exerting their effects via renal sodium handling11. Since genes may modify the effect of sodium on BP, examination of gene-dietary sodium intake interactions may help to identify novel genomic loci underlying BP regulation12. Furthermore, gene-based analyses, which test the joint contributions of SNPs with individually modest effects, may have higher power to identify BP loci13, 14. Although the examination of gene-sodium interactions in single marker and gene-based analyses could provide important insights into the molecular mechanisms underlying BP regulation, there has been a paucity of research in this area.
The objective of the current analysis was to identify novel genetic variants and genes influencing BP regulation by conducting genome-wide SNP-based and gene-based gene-sodium interaction analyses on BP among 1,876 Chinese participants of the Genetic Epidemiologic Network of Salt Sensitivity (GenSalt) study.
Methods
Study population
GenSalt is a family-based dietary intervention study designed to identify susceptibility genes that influence BP responses to environmental risk factors for hypertension in human populations. The study includes 1,906 Han Chinese participants aged 16 years and older from 633 families recruited from 6 study sites in rural, north China15. In the current study, genotypes, BP, and covariates at baseline were available for a total of 1,876 participants (98.4%).
Genotyping and Genotype Imputation
A total of 868,158 autosomal SNPs across the entire genome were genotyped using the Affymetrix 6.0 platform for each participant. Quality control excluded SNPs with Hardy-Weinberg Equilibrium P<1×10−6, missing rate >25% or MAF<1%. In addition, PLINK16, 17 and PedCheck18 were used to identify and correct any non-Mendelian inheritance errors. After quality control, a total of 820,017 genotyped SNPs remained for genotype imputation. Imputation of 2,416,663 SNPs from the HapMap release 22 build 36 CHB+JPT samples was conducted using MACH software19. After imputation, SNPs with r2<0.30, MAF<1%, or HWE P<1×10−6 were removed, and a total of 2,216,774 SNPs, with fractional values ranging from 0 to 2, remained for the analysis.
Urinary Sodium and Covariate Measurement
Dietary sodium intake was measured from 24 hour urinary sodium level. During the three-day baseline examination, each GenSalt participant collected three urine samples, including one 24 hour urine sample and 2 overnight urine samples. Among a random sample of 238 participants, 24-hour urine samples were collected in two containers, one for day time and the other for overnight. Using this random sample, an equation was generated to estimate 24 hour urinary sodium level from the overnight urine sample. This equation was applied to the remaining overnight urine samples to estimate 24 hour urinary sodium levels among all study participants. The mean of one measured 24 hour urinary sodium level and two estimated 24 hour urinary sodium levels was used in the current analysis.
Demographic variables, including age, gender, and medication use were collected using standard questionnaires by trained study staff. Body weight and height were measured twice during the baseline examination with the participants in light indoor clothing without shoes. Body mass index (BMI) was calculated as kilograms per meters squared.
BP Measurement and Imputation
BP was measured three times at the same time each morning during the three-day baseline examination by trained and certified observers using a random zero sphygmomanometer according to a standard protocol. For participants taking anti-hypertensive medications, BP was imputed by adding 10 mm Hg to systolic BP (SBP) and 5 mm Hg to diastolic BP (DBP)20. After imputation, the mean of the 9 BP measures was used in the current analysis. Mean arterial pressure (MAP) was calculated as SBP/3+2DBP/3, and pulse pressure (PP) was calculated as SBP-DBP.
Replication Study and Genotype Imputation
We attempted to replicate promising GenSalt findings (P<1×10−4) among Chinese participants of the Multi-Ethnic Study of Atherosclerosis (MESA). MESA participants were recruited between July 2000 and August 2002 from 6 field centers around the US. Participants were aged 45–84 and free of cardiovascular diseases at the baseline examination. Phenotype and Affymetrix 6.0 genotype data from MESA participants were made publicly available through the NCBI’s database of Genotypes and Phenotypes (dbGaP) and were downloaded for the current analysis. Among 777 Chinese MESA participants, genotype, urinary sodium, BP, and all covariables at baseline were available for 775 (99.7%) participants. Similar to GenSalt, we imputed BP for participants taking antihypertensive medication by adding 10 and 5 mm Hg to SBP and DBP, respectively. Since sodium was measured from spot urine using baseline urine samples, 24-hour urine sodium was estimated using Tanaka’s equation21.
A total of 909,622 autosomal SNPs across the entire genome were genotyped using the Affymetrix 6.0 platform for each participant. We excluded SNPs with Hardy-Weinberg Equilibrium P<1×10−6, missing rate>5% and MAF<1%. Minimac software was used to perform targeted imputation of the 3 Mb region surrounding each identified SNP based on the ALL ancestry panel from the 1000G Phase I Integrated Release Version 3 Haplotypes, which contains haplotypes of 1,092 individuals of all ethnic background. After imputation, SNPs with r2<0.30, MAF<1%, or HWE P<1×10−6 were removed.
Statistical Analysis
Single Marker Analysis
To accommodate familial relationships in both GenSalt (discovery) and MESA (replication) studies, mixed effect models were used to examine SNP-sodium interactions on BP, after adjustment for age, gender, and BMI. Principal components analysis revealed population substructure in MESA (but not GenSalt). Therefore, ancestry was also accounted for in MESA by adjusting for the first three principal components. Both 1 degree of freedom (df) interaction and 2 df joint tests were explored22. Lead SNPs (defined as the most significant SNP at a locus) with interaction term P<1.0 ×10−4 (for the 1 df test) or joint P<1.0 ×10−4 (for the 2 df test) after genomic control in the discovery stage analyses were further evaluated for replication in Chinese MESA participants. For the 1 df interaction test, inverse-variance-weighted meta-analysis was conducted to combine results from GenSalt and MESA using METAL software23. For the 2 df joint test, meta-analysis was performed using methods described by Manning and colleagues24 which were implemented in METAL software23 with a patch source code provided by Manning and colleagues24. After ensuring the effect direction of interaction terms were consistent, SNPs with replication stage P<0.05 and meta-analysis P<5.0×10−8 were considered significant for the 1 df interaction test. SNPs with consistent effect directions in the main effect and interaction term, replication stage P<0.05, and meta-analysis P<5.0×10−8 were considered significant for the 2 df joint test.
Gene-based Analysis
SNPs within the 5 kilo bp flanking regions of a gene were first mapped to the gene according to physical position. SNPs within 5 kilo bp flanking regions of two genes were assigned to both genes. P values of both 1 df interaction and 2 df joint tests in single marker analyses were used to generate gene-based P values using the extended Simes procedure (GATES) method13. Genes with P<1.0×10−4 in the discovery stage analysis in GenSalt were further evaluated for replication among Chinese MESA participants. Among MESA participants, SNPs from promising genes were tested for SNP-sodium interactions using methods described in the above single marker analysis, and P values of these SNPs were again used to generate gene-based p values using the GATES method13. Fisher’s method was applied to combine gene-based p values across GenSalt and MESA25. Genes with replication stage P<0.05 and combined P<2.5×10−6 (correcting for approximately 20,000 genes across the genome: 0.05/20,000=2.5×10−6) were considered significant.
Results
Characteristics of both GenSalt participants and Chinese MESA participants are shown in Table 1. MESA participants were older, had lower urinary sodium level and a higher proportion of hypertension. Participants in both studies had optimal BP levels.
Table 1.
Characteristics of GenSalt and Chinese MESA participants
| Variables | GenSalt (n=1876) | MESA (n=775) |
|---|---|---|
| Age, y, mean (SD) | 38.7 (9.5) | 62.4 (10.4) |
| Women, % | 47.2 | 50.8 |
| Hypertension, % | 9.5 | 40.8 |
| Hypertension medication, % | 4.4 | 29.0 |
| BMI, kg/m2, mean (SD) | 23.3 (3.2) | 24 (3.3) |
| 24-h urinary Na, gram, mean (SD) | 5.6 (1.5) | 3.5 (0.8) |
| Baseline SBP, mmHg, mean (SD) | 116.9 (14.2) | 127.4 (23.8) |
| Baseline DBP, mmHg, mean (SD) | 71.0 (9.7) | 74.8 (12.0) |
SD=standard deviation; SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI=body mass index;
In the discovery stage analyses of genome-wide gene-sodium interactions, a total of 141, 149, 150, and 165 independent loci (r2<0.3) had 1 df test P<1.0×10−4 for SBP, DBP, MAP, and PP, respectively. A total 145, 130, 131, and 207 independent loci were identified by the 2 df joint test for SBP, DBP, MAP and PP, respectively. We identified 454 loci using the 1 df interaction test. The 2 df test identified 701 loci, including 260 which were also identified by the 1 df test. In aggregate, we identified 895 loci in the discovery stage analysis. Genome-wide analysis results from both 1 df test and 2 df joint test are shown in supplementary Figures S1–S4.
Loci that reached genome-wide significance in the meta-analysis of GenSalt and MESA results from the 1 df interaction test are shown in Table 2. Variant rs13211840 at the novel UST locus interacted with sodium on DBP (GenSalt P=7.29×10−8, MESA P=1.19×10−2, and Meta-analysis P=3.13×10−9).
Table 2.
Results Achieving Genome-Wide Significance in the Meta-Analysis of Gene-Sodium Interactions Using the 1 Degree of Freedom Test.
| rsID | Chr | Position (Build 36) |
CA | CAF | Region | Genes | Studies | Beta | SE | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Diastolic blood pressure | ||||||||||
| rs13211840 | 6 | 149153883 | T | 0.985 | intronic | UST | GenSalt | −0.09 | 0.02 | 1.02E-07 |
| MESA | −0.06 | 0.02 | 1.19E-02 | |||||||
| Meta | −0.08 | 0.01 | 3.13E-09 | |||||||
Chr=chromosome; CA=coded allele; CAF=coded allele frequency; SE=standard error
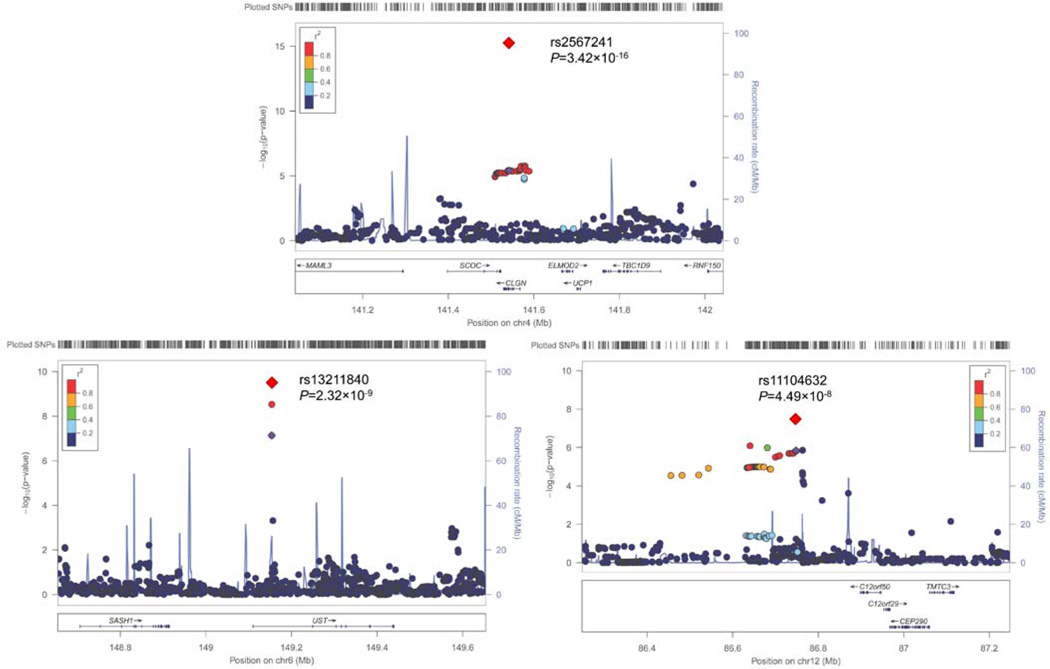
Significant findings from the meta-analysis of results from the 2 df joint test are presented in Table 3. Consistent with findings from the 1 df test, the novel UST locus variants were again identified for DBP (rs13211840: GenSalt P=4.41×10−7, MESA P=1.52×10−2, and Meta-analysis P=7.02×10−9). In addition, variant at the novel LOC105369882 loci was associated with SBP (rs11104632: GenSalt P=1.46×10−6, MESA P=2.16×10−2, and Meta-analysis P=4.51×10−8); and novel CLGN variant rs2567241 was associated with SBP (GenSalt P=1.50×10−6, MESA P=4.34×10−5, and Meta-analysis P=3.90×10−12), DBP (GenSalt P=3.85×10−6, MESA P=3.36×10−10, and Meta-analysis P=3.11×10−22), and MAP (GenSalt P=2.01×10−7, MESA P=1.49×10−6, and Meta-analysis P=2.86×10−15). Regional association plots for the 3 novel loci (CLGN, UST, and LOC105369882) with genome-wide significance in the meta-analyses are presented in Figure 1. Although some loci (e.g., rs2567241) achieved genome-wide significance for more than two interactions, only one regional association plot is shown for each loci using the most significant interaction.
Table 3.
Results Achieving Genome-Wide Significance in the Meta-Analysis of Gene-Sodium Interactions Using the 2 Degree of Freedom Joint Test.
| rsID | Chr | Position (Build 36) |
CA | CAF | region | Nearest Gene |
Studies | Main Effect | Interaction Effect | Joint P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | P | Beta | SE | P | |||||||||
| Systolic blood pressure | ||||||||||||||
| rs2567241 | 4 | 141542612 | C | 0.990 | exonic | CLGN | GenSalt | 3.02 | 2.10 | 1.49E-01 | −0.10 | 0.02 | 2.04E-05 | 2.24E-06 |
| MESA | 11.33 | 4.62 | 1.82E-02 | −0.05 | 0.13 | 7.06E-01 | 4.34E-05 | |||||||
| Meta | −0.09 | 0.02 | 3.90E-12 | |||||||||||
| rs11104632 | 12 | 86747816 | G | 0.982 | intergenic | LOC105 | GenSalt | 4.07 | 1.46 | 5.40E-03 | 0.08 | 0.02 | 2.48E-04 | 2.19E-06 |
| 369882 | MESA | 6.79 | 2.98 | 2.74E-02 | 0.08 | 0.10 | 4.36E-01 | 2.16E-02 | ||||||
| Meta | 0.09 | 0.02 | 4.51E-08 | |||||||||||
| Diastolic blood pressure | ||||||||||||||
| rs2567241 | 4 | 141542612 | C | 0.990 | exonic | CLGN | GenSalt | 2.37 | 1.50 | 1.13E-01 | −0.07 | 0.01 | 8.16E-07 | 5.47E-06 |
| MESA | 7.34 | 2.98 | 1.77E-02 | −0.04 | 0.08 | 6.46E-01 | 3.36E-10 | |||||||
| Meta | −0.06 | 0.01 | 3.11E-22 | |||||||||||
| rs13211840 | 6 | 149153883 | T | 0.985 | intronic | UST | GenSalt | 1.42 | 1.42 | 3.20E-01 | −0.09 | 0.02 | 7.29E-08 | 6.52E-07 |
| MESA | 6.45 | 3.11 | 4.39E-02 | −0.06 | 0.02 | 1.19E-02 | 1.52E-02 | |||||||
| Meta | −0.08 | 0.01 | 7.02E-09 | |||||||||||
| Mean arterial pressure | ||||||||||||||
| rs2567241 | 4 | 141542612 | C | 0.990 | exonic | CLGN | GenSalt | 2.59 | 1.62 | 1.10E-01 | −0.08 | 0.01 | 1.45E-07 | 3.01E-07 |
| MESA | 8.51 | 3.40 | 1.58E-02 | −0.04 | 0.09 | 7.05E-01 | 1.49E-06 | |||||||
| Meta | −0.07 | 0.01 | 2.86E-15 | |||||||||||
Chr=chromosome; CA=coded allele; CAF=coded allele frequency; SE=standard error
Figure 1.
Regional Association Plots for Loci Achieving Genome-Wide Significance in the Meta-Analysis of both 1 degree of freedom (df) Interaction Test (a,b) and 2 df Joint Test (c) Results. For the SNPs associated with multiple phenotypes, the results for the most significant phenotype are shown. The index SNPs are shown in purple diamond; the correlation (r2) of each of the surrounding SNPs to the index SNP are shown on a scale from minimal (blue) to maximal (red), The final P values for the index SNPs are shown and also indicated by the red diamonds. The genes in the 1 Mb regions around the index SNPs (500 kb on each side) are indicated at the bottom, and recombination rates are shown in light blue line. The regional plots are drawn using LocusZoom online software.
Discovery stage genome-wide gene-based analysis results are shown in supplementary Figures S5–S8. A total of 134 promising genes were identified and tested for replication among MESA participants. Genome-wide significant findings from the combined analysis of GenSalt and MESA participants (including both the 1 df interaction test and 2 df joint test) are shown in Table 4. Gene-based analysis for the 1 df interaction test identified one CASP4 gene for MAP phenotype. Gene-based analysis of the 2 df joint test identified 9 genes at 6 loci, including MKNK1 at 1p33, C2orf80 at 2q34, EPHA6 at 3q11.2, SCOC-AS1, SCOC, CLGN, and MGAT4D at 4q31.1, ARHGAP42 at 11q22.1, and LINC01478 at 18q12.3. Locus 4q31.1 was also identified in the single-marker analysis.
Table 4.
Gene-based Analysis Results for Gene-Sodium Interaction Analysis
| Gene | Chr | Start (Build 36) |
Gene Length (bp) |
GenSalt P | MESA P | Meta P |
|---|---|---|---|---|---|---|
| 1 DF Test | ||||||
| Mean Arterial Pressure | ||||||
| CASP4 | 11 | 104318803 | 25733 | 4.23E-08 | 4.80E-02 | 4.27E-08 |
| 2 DF Joint Test | ||||||
| Systolic Blood Pressure | ||||||
| MKNK1 | 1 | 46795665 | 46889 | 4.20E-06 | 4.08E-02 | 2.80E-06 |
| SCOC | 4 | 141484064 | 39097 | 9.40E-05 | 4.70E-05 | 9.00E-08 |
| CLGN | 4 | 141529056 | 39210 | 3.40E-06 | 1.30E-04 | 1.00E-08 |
| MGAT4D | 4 | 141583978 | 55004 | 2.30E-06 | 2.57E-03 | 1.20E-07 |
| LINC01478 | 18 | 40157397 | 208264 | 1.50E-08 | 6.51E-03 | 2.30E-09 |
| Diastolic Blood Pressure | ||||||
| SCOC | 4 | 141484064 | 39097 | 7.47E-05 | 2.50E-11 | 6.40E-14 |
| SCOC-AS1 | 4 | 141424329 | 89668 | 9.95E-05 | 1.95E-11 | 6.77E-14 |
| CLGN | 4 | 141529056 | 39210 | 1.38E-05 | 1.09E-09 | 4.94E-13 |
| MGAT4D | 4 | 141583978 | 55004 | 6.74E-05 | 3.79E-08 | 7.07E-11 |
| Mean Arterial Pressure | ||||||
| SCOC-AS1 | 4 | 141424329 | 89668 | 1.57E-05 | 1.51E-07 | 6.58E-11 |
| SCOC | 4 | 141484064 | 39097 | 1.16E-05 | 1.83E-07 | 5.92E-11 |
| CLGN | 4 | 141529056 | 39210 | 1.08E-06 | 4.69E-06 | 1.37E-10 |
| MGAT4D | 4 | 141583978 | 55004 | 1.36E-06 | 1.10E-04 | 3.53E-09 |
| Pulse Pressure | ||||||
| C2orf80 | 2 | 208738315 | 24704 | 6.38E-07 | 1.03E-12 | <4.72E-24 |
| EPHA6 | 3 | 98641126 | 429464 | 1.11E-05 | 9.85E-03 | 1.86E-06 |
| ARHGAP42 | 11 | 100063616 | 303251 | 2.64E-07 | 4.72E-24 | <4.72E-24 |
DF=degree of freedom;
Discussion
In the first genome-wide gene-sodium interaction analysis conducted in the Chinese population, we identified consistent interactions of UST rs13211840 at 6q25.1 with sodium intake on the DBP phenotype using both 1 df and 2 df interaction tests. The 2 df test also revealed associations of novel LOC105369882 rs11104632 variant at 12q21.32 with SBP, while the novel CLGN rs2567241 variant was associated with SBP, DBP and MAP in the 2 df test. Furthermore, genome-wide gene-based analysis of both 1 df and 2 df interaction tests identified MKNK1 at 1p33, C2orf80 at 2q34, EPHA6 at 3q11.2, SCOC-AS1, SCOC, CLGN, and MGAT4D at 4q31.1, ARHGAP42 at 11q22.1, and LINC01478 at 18q12.3 that were associated with at least one BP phenotype.
Novel UST, LOC105369882 variants rs13211840 and rs11104632, respectively, showed significant interactions with sodium on BP in the current analysis. The UST signal was consistent across the 1 df and 2 df interaction tests, while the LOC105369882 locus was identified only in the 2 df test. Although the potential role of UST in BP is unclear, other genes at this locus represent interesting BP candidates. For example, the SASH1 gene has been associated with thyroid function26 and suggestively associated with responses to rate control therapy among patients with atrial fibrillation27. A more recent study indicated that the SASH1 gene may interact with smoking on SBP28. While the role of LOC105369882 in BP is unclear, another gene at this locus, CEP290, is potentially relevant to BP regulation29, with mutations of this gene shown to associate with many disease phenotypes characterized by renal impairment30, 31. Considering the important roles of the thyroid in blood pressure regulation and kidney in sodium filtration, both loci warrant further studies to identify the true causal variant(s).
Association of CLGN at 4q28.3-q31.1 with blood pressure phenotypes was identified consistently in the single marker and gene-based analyses. In single marker analysis, three genome-wide significant exonic missense mutations were identified at the CLGN locus, including lead CLGN SNP rs2567241 along with two other SNPs, rs358304 of CLGN1 and rs2175563 of nearby SCOC. Although the physiologic relevance of the CLGN1 and SCOC genes with BP regulation is unclear, future studies are warranted to determine the potential functional relevance of these promising single-marker findings. In gene-based analysis, CLGN1 represented one of a cluster of genes including SCOC-AS1, SCOC and MGAT4D which interacted with sodium to influence BP. It is unlikely that they are all etiologically relevant to BP regulation. Future works are warranted to delineate the causal genes involved in BP regulation at this locus.
Genome-wide gene-based analysis also identified novel genes MKNK1 at 1p33, C2orf80 at 2q34, EPHA6 at 3q11.2, CASP4 at 11q22.3, and LINC01478 at 18q12.3, as well as previously reported BP gene ARHGAP42 at 11q22.15, 6. MNK1 encodes a Ser/Thr protein kinase that interacts with ERK1 and p38 mitogen-activated protein kinase (MAPK)32, a pathway that is involved in BP regulation through norepinephrine and angiotensin II33. Despite its potential biological relevance, our study provides the first evidence that the MKNK1 gene is involved in BP regulation in human populations. The CASP4 gene encodes a protein in the cysteine-aspartic acid protease family and plays an import role in inflammation and innate immunity through activation of caspase-134. Pathophysiological studies showed that overexpression of the CASP4 gene was involved in the loss of proximal tubules and renal injury in nephropathic cystinosis patients35. Considering kidney’s key roles in sodium filtration and reabsorption, future studies of CASP4 genes are warranted to identify the causal variant(s).. Previous studies have identified associations of the EPHA6 gene with obesity-related traits36. The EPHA6 locus also includes the ARL6 gene, which encodes ADP-ribosylation factor-like 6. Mutations of the ARL6 gene cause Bardet-Beidl syndrome37, a heterogeneous disorder which increases the risk of hypertension and diabetes38. Gene-based analysis also revealed novel associations of C2orf80 at 2q34, and LINC01478 at 18q12.3. However, the mechanisms linking these genes to blood pressure regulation are unclear. In aggregate, the identification of these 6 novel loci in gene-based analyses highlights the power and potential utility of gene-based analysis in genomic study.
Our study has several strengths. First, stringent quality control methods were used in measuring genotype, phenotype and covariate data for both the discovery and replication stage samples. This can reduce phenotype measurement error and increase statistical power in identifying both single SNPs and genes underlying BP regulation. Second, since we limited our analysis exclusively to Chinese participants, population stratification should be minimized. Finally, a total of 9 BPs were measured using a random zero sphygmomanometer at the same time during the 3 day baseline examination in the discovery stage. This can greatly reduce measurement error of BP, and increase statistical power to detect genetic variants for BP. Certain limitations should also be acknowledged. The MESA replication study has a smaller sample size than that of GenSalt, potentially limiting our ability to replicate and identify important gene-sodium interactions. In addition, 24 hour urinary sodium was estimated from spot urine among the Chinese MESA participants using Tanaka’s equation. Although Tanaka’s equation has been widely validated39–41, there might be some measurement error that could reduce statistical power to replicate genetic loci identified in GenSalt participants, increasing false negative findings. MESA had a higher proportion of participants taking antihypertensive medication. We imputed BP by adding 10 and 5 mm Hg to SBP and DBP, respectively. Although this approach is widely applied to genetic studies of BP6, 7, 9, any resulting inaccuracy may dilute associations between genetic factors and BP. Furthermore, additional factors such as age and urbanization level differ between the GenSalt and MESA samples. If these factors influence gene-sodium interactions on BP, our power to detect (or replicate) SNP-BP associations could again be reduced. Although the SNPs identified in GenSalt have low MAFs, the findings were verified through replication in MESA participants. Still, future replication studies with larger sample size, more homogeneous populations and better measurement of urinary sodium levels are warranted.
Supplementary Material
Perspectives.
In the first genome-wide gene-sodium interaction analyses of BP in a Chinese population, which included both single marker and gene-based analyses, we identified 9 novel loci that interacted with dietary sodium intake on BP phenotypes. In addition to the 1 novel locus identified through the traditional 1 df single marker interaction analysis (UST rs13211840), another two loci were documented using the 2 df test (CLGN rs2567241, and LOC105369882 rs11104632). Gene-based analysis provided consistent support for the CLGN gene (plus several other genes clustered at the CLGN locus), and identified an additional 5 novel genes, including MKNK1, C2orf80, EPHA6, CASP4, and LINC01478. In comparison to the previous large scale GWAS meta-analyses of BP conducted in East Asians, the current study was able to robustly identify several novel loci with a relatively small sample size. Such findings highlight the importance of examining gene-sodium interactions and conducting gene-based analysis to identify genomic mechanisms influencing BP. Further, these findings contribute to understanding the mechanisms of BP regulation. Sequencing along with functional studies are needed to help delineate causal variants underlying the strong signals identified here.
Novelty and Significance.
What Is New?
By examining genome-wide gene-sodium interactions on BP through both single makers based and gene-based analyses in a Chinese population, with replication in Chinese participants of the MESA study, we identified 3 novel loci from single marker analysis that interacted with dietary sodium intake on BP phenotypes.
At the CLGN locus, 3 missense variants rs2567241, rs358304 and rs2175563 were identified for SBP, DBP, and MAP phenotypes.
Genome-wide gene-based analysis additionally identified 5 novel BP loci.
What is Relevant?
Our findings highlighted the utility of examining gene-environment (sodium) interactions and conducting gene-based analyses to identify genes and variants that influence blood pressure.
Summary
Only a small proportion of the genetic factors influencing blood pressure have been found. Scientists postulate that additional genetic factors may be identified through the analysis of gene-environment interactions. In addition, gene-based analysis methods may also increase power to detect important genetic determinants of BP. Very few studies have explored the two approaches. We examined the interactions between dietary sodium intake and genetic factors on blood pressure using data from 1,906 Chinese participants of the GenSalt study. To avoid false positive results, we evaluated findings from GenSalt among 775 Chinese participants of the MESA study. We robustly identified 3 novel genetic loci that interacted with dietary sodium intake on blood pressure in single-marker analyses. In gene-based analyses, we additionally identified 5 novel genes that were associated with blood pressure phenotypes. These novel loci were successfully replicated among the Chinese participants of MESA. In aggregate, our findings highlighted the utility of examining gene-environment interactions and conducting gene-based analyses to identify genes and variants that influence blood pressure.
Acknowledgments
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and CTSA UL1-RR-024156. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, California, USA) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) using the Affymetric Genome-Wide Human SNP Array 6.0.
Sources of Funding
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. Dr. Bazzano was supported by a career development award (K08HL091108) from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global burden of disease and risk factors. Washington (DC): World Bank; 2006. [PubMed] [Google Scholar]
- 3.Cutler JA, Follmann D, Allender PS. Randomized trials of sodium reduction: An overview. Am J Clin Nutr. 1997;65:643S–651S. doi: 10.1093/ajcn/65.2.643S. [DOI] [PubMed] [Google Scholar]
- 4.Johnson C, Raj TS, Trudeau L, Bacon SL, Padwal R, Webster J, Campbell N. The science of salt: A systematic review of clinical salt studies 2013 to 2014. J Clin Hypertens (Greenwich) 2015;17:401–411. doi: 10.1111/jch.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wain LV, Verwoert GC, O'Reilly PF, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehret GB, Caulfield MJ. Genes for blood pressure: An opportunity to understand hypertension. European heart journal. 2013;34:951–961. doi: 10.1093/eurheartj/ehs455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly TN, He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens. 2012;30:861–873. doi: 10.1097/HJH.0b013e3283524949. [DOI] [PubMed] [Google Scholar]
- 12.He J, Kelly TN, Zhao Q, et al. Genome-wide association study identifies 8 novel loci associated with blood pressure responses to interventions in han chinese. Circ Cardiovasc Genet. 2013;6:598–607. doi: 10.1161/CIRCGENETICS.113.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li MX, Gui HS, Kwan JS, Sham PC. Gates: A rapid and powerful gene-based association test using extended simes procedure. Am J Hum Genet. 2011;88:283–293. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, Hayward NK, Montgomery GW, Visscher PM, Martin NG, Macgregor S, Investigators A. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group GCR. Gensalt: Rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–646. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percell S Plink (v1.07) [Google Scholar]
- 18.O'Connell JR, Weeks DE. Pedcheck: A program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. Mach: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension. 2002;40:7–12. doi: 10.1161/01.hyp.0000022693.11752.e9. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 22.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 23.Willer CJ, Li Y, Abecasis GR. Metal: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning AK, LaValley M, Liu CT, Rice K, An P, Liu Y, Miljkovic I, Rasmussen-Torvik L, Harris TB, Province MA, Borecki IB, Florez JC, Meigs JB, Cupples LA, Dupuis J. Meta-analysis of gene-environment interaction: Joint estimation of snp and snp × environment regression coefficients. Genet Epidemiol. 2011;35:11–18. doi: 10.1002/gepi.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Wiley: 2009. [Google Scholar]
- 26.Porcu E, Medici M, Pistis G, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9:e1003266. doi: 10.1371/journal.pgen.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolek MJ, Edwards TL, Muhammad R, Balouch A, Shoemaker MB, Blair MA, Kor KC, Takahashi A, Kubo M, Roden DM, Tanaka T, Darbar D. A genome-wide association study to identify genomic modulators of rate control therapy in patients with atrial fibrillation. Am J Cardiol. 2014;114:593–600. doi: 10.1016/j.amjcard.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basson J, Sung YJ, de Las Fuentes L, Schwander K, Cupples LA, Rao DC. Influence of smoking status and intensity on discovery of blood pressure loci through gene-smoking interactions. Genet Epidemiol. 2015;39:480–488. doi: 10.1002/gepi.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drivas TG, Bennett J. Cep290 and the primary cilium. Adv Exp Med Biol. 2014;801:519–525. doi: 10.1007/978-1-4614-3209-8_66. [DOI] [PubMed] [Google Scholar]
- 30.Ghaffari SR, Rafati M, Ghaffari G, Morra M, Tekin M. Familial intellectual disability in an iranian family with a novel truncating mutation in cep290. Clin Genet. 2014;86:387–390. doi: 10.1111/cge.12296. [DOI] [PubMed] [Google Scholar]
- 31.Coppieters F, Lefever S, Leroy BP, De Baere E. Cep290, a gene with many faces: Mutation overview and presentation of cep290base. Hum Mutat. 2010;31:1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 32.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases mnk1 and mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu D, Yang H, Raizada MK. Angiotensin ii regulation of neuromodulation: Downstream signaling mechanism from activation of mitogen-activated protein kinase. J Cell Biol. 1996;135:1609–1617. doi: 10.1083/jcb.135.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sollberger G, Strittmatter GE, Kistowska M, French LE, Beer HD. Caspase-4 is required for activation of inflammasomes. J Immunol. 2012;188:1992–2000. doi: 10.4049/jimmunol.1101620. [DOI] [PubMed] [Google Scholar]
- 35.Sansanwal P, Kambham N, Sarwal MM. Caspase-4 may play a role in loss of proximal tubules and renal injury in nephropathic cystinosis. Pediatr Nephrol. 2010;25:105–109. doi: 10.1007/s00467-009-1289-4. [DOI] [PubMed] [Google Scholar]
- 36.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, Butte NF. Novel genetic loci identified for the pathophysiology of childhood obesity in the hispanic population. PLoS One. 2012;7:e51954. doi: 10.1371/journal.pone.0051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereiro I, Valverde D, Piñeiro-Gallego T, Baiget M, Borrego S, Ayuso C, Searby C, Nishimura D. New mutations in bbs genes in small consanguineous families with bardet-biedl syndrome: Detection of candidate regions by homozygosity mapping. Mol Vis. 2010;16:137–143. [PMC free article] [PubMed] [Google Scholar]
- 38.Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, McManamon PJ, O'Leary E, Pryse-Phillips W. The cardinal manifestations of bardet-biedl syndrome, a form of laurence-moon-biedl syndrome. N Engl J Med. 1989;321:1002–1009. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- 39.Cogswell ME, Wang CY, Chen TC, Pfeiffer CM, Elliott P, Gillespie CD, Carriquiry AL, Sempos CT, Liu K, Perrine CG, Swanson CA, Caldwell KL, Loria CM. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am J Clin Nutr. 2013;98:1502–1513. doi: 10.3945/ajcn.113.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji C, Miller MA, Venezia A, Strazzullo P, Cappuccio FP. Comparisons of spot vs 24-h urine samples for estimating population salt intake: Validation study in two independent samples of adults in britain and italy. Nutr Metab Cardiovasc Dis. 2014;24:140–147. doi: 10.1016/j.numecd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Imai E, Yasuda Y, Horio M, Shibata K, Kato S, Mizutani Y, Imai J, Hayashi M, Kamiya H, Oiso Y, Murohara T, Maruyama S, Matsuo S. Validation of the equations for estimating daily sodium excretion from spot urine in patients with chronic kidney disease. Clin Exp Nephrol. 2011;15:861–867. doi: 10.1007/s10157-011-0523-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.