Fluctuating light influences acclimation in Arabidopsis independently of light intensity.
Abstract
The acclimation of plants to light has been studied extensively, yet little is known about the effect of dynamic fluctuations in light on plant phenotype and acclimatory responses. We mimicked natural fluctuations in light over a diurnal period to examine the effect on the photosynthetic processes and growth of Arabidopsis (Arabidopsis thaliana). High and low light intensities, delivered via a realistic dynamic fluctuating or square wave pattern, were used to grow and assess plants. Plants subjected to square wave light had thicker leaves and greater photosynthetic capacity compared with fluctuating light-grown plants. This, together with elevated levels of proteins associated with electron transport, indicates greater investment in leaf structural components and photosynthetic processes. In contrast, plants grown under fluctuating light had thinner leaves, lower leaf light absorption, but maintained similar photosynthetic rates per unit leaf area to square wave-grown plants. Despite high light use efficiency, plants grown under fluctuating light had a slow growth rate early in development, likely due to the fact that plants grown under fluctuating conditions were not able to fully utilize the light energy absorbed for carbon fixation. Diurnal leaf-level measurements revealed a negative feedback control of photosynthesis, resulting in a decrease in total diurnal carbon assimilated of at least 20%. These findings highlight that growing plants under square wave growth conditions ultimately fails to predict plant performance under realistic light regimes and stress the importance of considering fluctuations in incident light in future experiments that aim to infer plant productivity under natural conditions in the field.
In the natural environment, plants experience a range of light intensities and spectral properties due to changes in sun angle and cloud cover in addition to shading from overlapping leaves and neighboring plants. Therefore, leaves are subjected to spatial and temporal gradients in incident light, which has major consequences for photosynthetic carbon assimilation (Pearcy, 1990; Chazdon and Pearcy, 1991; Pearcy and Way, 2012). As light is the key resource for photosynthesis, plants acclimate to the light environment under which they are grown to maintain performance and fitness. Acclimation involves altering metabolic processes (including light harvesting and CO2 capture) brought about by a range of mechanisms, from adjustments to leaf morphology to changes in photosynthetic apparatus stoichiometry (Terashima et al., 2006; Athanasiou et al., 2010; Kono and Terashima, 2014), all of which impact on photosynthesis. The primary determinant of crop yield is the cumulative rate of photosynthesis over the growing season, which is regulated by the amount of light captured by the plant and the ability of the plant to efficiently use this energy to convert CO2 into biomass and harvestable yield (Sinclair and Muchow, 1999). Currently, considerable research efforts in plant biology focus on improving performance, including the plant’s ability to cope with changing abiotic or biotic factors in order to increase or maintain crop biomass and yield, to support the rising demands for food and fuel (Ort et al., 2015). Many current studies employ transgenic approaches: plants are often grown in laboratory controlled conditions (Lefebvre et al., 2005; Simkin et al., 2015, 2017; Kono and Terashima, 2016), although with the ultimate aim to improve crops grown in the field (Rosenthal et al., 2011; Poorter et al., 2016). Light is one of the most dynamic environmental factors that directly impacts on plant performance; therefore, it is important to understand how plants acclimate to fluctuating light environments such as those experienced under field conditions (Lawson et al., 2012).
Plant acclimation to changes in irradiance can be categorized as (1) dynamic acclimation, which refers to a reversible biological process present within a given period of time (Walters and Horton, 1994; Yin and Johnson, 2000; Mullineaux et al., 2006; Okegawa et al., 2007; Athanasiou et al., 2010; Tikkanen et al., 2010; Alter et al., 2012; Suorsa et al., 2012; Yamori, 2016), or (2) developmental acclimation, which is defined as changes in morphology (e.g. leaf thickness and density) resulting from a given growth light environment and that are largely irreversible (Weston et al., 2000; Murchie et al., 2005), which is the focus of this study. The ability of plants to developmentally acclimate to a given light environment is particularly well demonstrated in leaves grown in sun and shade conditions, which differ in photosynthetic efficiency, biochemistry (e.g. Rubisco content and change in PSII and PSI ratio), anatomy (e.g. chloroplast size and distribution), and morphology (e.g. leaf mass area and thickness; Givnish, 1988; Walters and Horton, 1994; Weston et al., 2000; Bailey et al., 2001, 2004). Plants grown under high light intensity tend to develop thicker leaves than those grown under low light intensity (Evans and Poorter, 2001), which generally increases photosynthetic capacity per unit of area, improving the plant’s ability to utilize light for carbon fixation (Terashima et al., 2006). Increased photosynthetic capacity is often strongly correlated with the concentration of photosynthetic enzymes such as Rubisco, cytochrome f, H+-ATPase, and reaction centers (Foyer et al., 2012). Leaves acclimated to shade tend to have higher net photosynthetic rates at lower light levels and a lower light compensation point compared with sun leaves (Givnish, 1988). To compare plants/species with different leaf thicknesses, previous studies have used mass integrated photosynthesis as a proxy to assess the photosynthetic efficiency of plants with different volumes of photosynthetic tissues (Garnier et al., 1999; Evans and Poorter, 2001; Wright et al., 2001). Previous studies investigating developmental acclimation have focused primarily on the effect of light intensity, with less emphasis given to the effect of dynamic light during growth, like that experienced under a natural environment. Fluctuations in light could have a significant impact on acclimation processes during growth and need to be investigated alongside light intensity to assess the interaction between light regime and intensity (Lawson et al., 2012).
Acclimation is the result of a balance between the cost of increasing leaf photosynthetic capacity, which can be underutilized (Terashima et al., 2006; Oguchi et al., 2008), and the risk of photooxidative damage if the mechanisms to dissipate excess energy received by the plant are not sufficient (Li et al., 2009). Under natural environmental conditions, the random duration and intensity of fluctuating light from passing clouds or leaf movements (sun and shade flecks) result in incident light intensities below light saturation that reduce photosynthetic rates, while those intensities greater than saturated lead to excess excitation energy that can result in short potential stress periods and long-term damage to leaf photosynthesis (Baker, 2008). Therefore, plants employ mechanisms that enable them to deal with these changes in excitation pressure, including thermal dissipation of excitation energy. Such processes are termed nonphotochemical quenching (NPQ) and are associated mainly with changes in the xanthophyll cycle (Demmig-Adams and Adams, 1992; Müller et al., 2001) and protonation of PSII antenna proteins (Li et al., 2000, 2004), both of which are linked to the proton gradient across the thylakoid membrane. Large diversity in light acclimation exists between individuals and species (Murchie and Horton, 1997), partly due to the random nature of light fluctuations and species-specific responses.
To date, the majority of studies examining acclimation to fluctuating light conditions have been carried out on plants grown under constant intensities of light and swapped to a simple light pattern (consisting of one or more step changes in light intensity of different frequencies; Yin and Johnson, 2000; Tikkanen et al., 2010; Alter et al., 2012; Suorsa et al., 2012; Yamori, 2016). Under these light conditions, acclimation responses have often been monitored over a period of several days (Athanasiou et al., 2010; Alter et al., 2012). While this approach is powerful for studies on the mechanisms of dynamic light acclimation, it fails to recognize the importance of how plants developmentally acclimate to growth under fluctuating light intensities (Huxley, 1969), such as those found in the natural field environment (Frechilla et al., 2004). There are only a handful of studies that have examined the impact of real dynamic light environments on plant growth and performance, and as far as we are aware, none of these have used a controlled environment to examine the direct impact of light. For example, Yamori (2016) revealed the importance of unpredictable variations in environmental growth conditions (including light) that led to a reduction in photosynthesis because plants were unable to fully acclimate to the highly dynamic variation in light. However, in the study by Yamori (2016), plants were grown under natural environmental conditions that resulted in fluctuations in a number of environmental variables; therefore, the impact of light alone on the acclimation response could not be distinguished. Külheim et al. (2002) compared field-grown NPQ Arabidopsis (Arabidopsis thaliana) mutants with those grown in controlled environment chambers under constant or variable light intensity and demonstrated that NPQ is important for plant fitness in the field and under fluctuating environments reproduced in growth chambers. However, when the plants were grown under constant light conditions, no effect on plant performance was observed, emphasizing the influence of growth environment on plant fitness. Although the study by Külheim et al. (2002) was one of the first to examine the influence of dynamic and square wave growth light regimes on plant performance and growth, the dynamic light regime used in the controlled environment did not mimic that observed in the field.
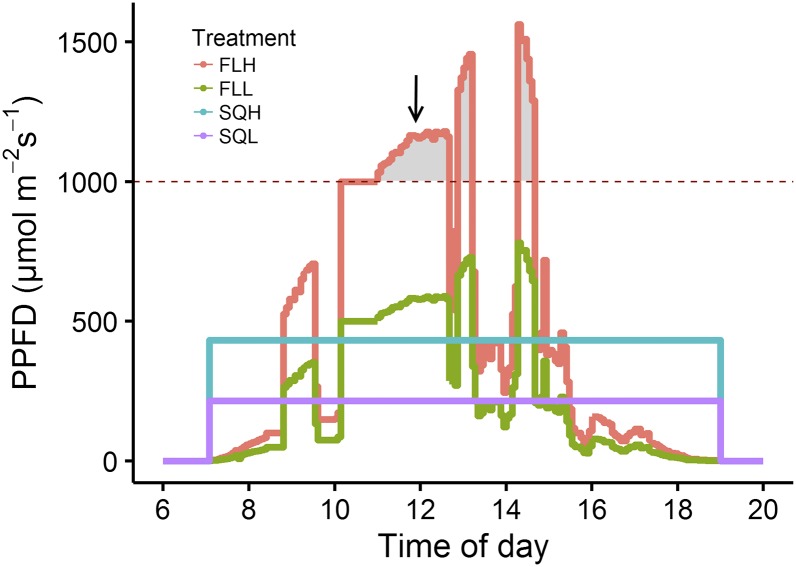
In order to fully understand how plants integrate fluctuations in incident light, and how this influences acclimation and modifies plant growth, there is a need to grow plants in a controlled but dynamic environment that mimics a light regime that would be experienced in the field. How plants perform under these conditions and the differences in responses with those grown in square wave light regimes are important, as models of steady-state photosynthesis tend to overestimate photosynthesis under fluctuating light regimes (Naumburg and Ellsworth, 2002). The importance of developmental acclimation for plant performance has been demonstrated in sun (high light) and shade (low light) leaves. However, it is not known if developmental acclimation to fluctuating light intensity exists and, if so, how it may influence plant performance under dynamic light conditions, such as those experienced in a natural environment, forcing us to rethink experimental growth conditions to draw conclusions on how plants will perform in the field. To address this, Arabidopsis plants were grown and measured under fluctuating and nonfluctuating (or square wave) light regimes at two different average intensities (high and low; Fig. 1), and the performance of these plants and their ability to convert light energy into biomass were evaluated.
Figure 1.
Diurnal light regimes used for plant growth and leaf-level measurements of gas exchange. Areas under the curve represent the same average amount of light energy over the 12-h light regime for square wave and fluctuating treatments depending on the light intensity: SQH, FLH (mean = 460 µmol m−2 s−1), SQL, and FLL (mean = 230 µmol m−2 s−1). The arrow indicates the time point (12 pm) at which leaf discs were collected for protein and chlorophyll extraction. PPFD, Photosynthetic photon flux density.
RESULTS
Photoacclimation of Plants Grown under Different Light Regimes
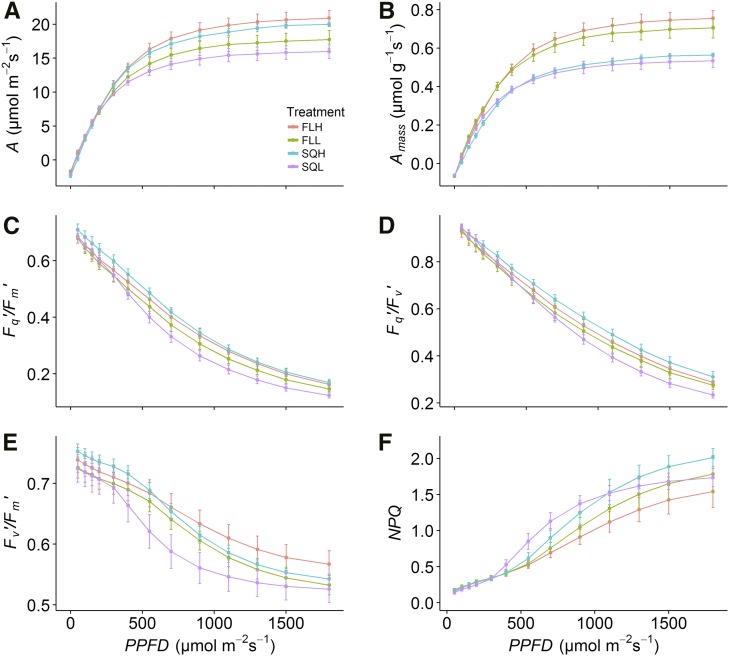
Light response curves in which net CO2 assimilation rate (A) was measured as a function of PPFD (A/Q curves; Fig. 2A) displayed similar A values at PPFD below 250 µmol m−2 s−1 in plants grown under all the different light regimes: square wave high light (SQH), square wave low light (SQL), fluctuating high light (FLH), and fluctuating low light (FLL). Measurements of A at PPFD above this value and light-saturated assimilation rates (Asat; Table I) were significantly greater in plants grown under high light intensity compared with those grown under low light, independently of the light regime (Fig. 2A).
Figure 2.
Photosynthesis as a function of light intensity (PPFD) of plants grown under the four light regimes SQH, FLH, SQL, and FLL. Parameters examined are A (A) Amass (B), Fq′/Fm′ (C), Fq′/Fv′ (D), Fv′/Fm′ (E), and NPQ (F). Error bars represent confidence intervals at 95% (n = 5).
Table I. Parameter values (means ± se) estimated from the response of assimilation to light intensity in plants grown under the four light regimes.
The light saturated rate of CO2 assimilation (Asat), quantum yield of photosynthesis (α), curvature parameter (θ), and light compensation point (Γ). Two values of dark respiration were estimated: the first from the model (Rd-model) and the second at the beginning of the diurnal period (Rd-diurnal). Letters represent the results of Tukey’s posthoc comparisons of group means.
| Treatment | Asat | Rd-model | Rd-diurnal | α | θ | Γ |
|---|---|---|---|---|---|---|
| FLH | 22.48 ± 0.2 a | 1.77 ± 0.03 b | 0.89 ± 0.22 a,b | 0.053 ± 0.0004 a | 0.78 ± 0.01 a | 33.77 ± 0.52 b |
| FLL | 18.78 ± 0.21 b | 1.59 ± 0.04 b | 0.41 ± 0.04 b | 0.054 ± 0.0007 a | 0.71 ± 0.01 a,b | 30.31 ± 0.57 b |
| SQH | 21.38 ± 0.07 a | 2.37 ± 0.05 a | 1.39 ± 0.17 a | 0.057 ± 0.0007 a | 0.78 ± 0.01 a | 42.87 ± 0.83 a |
| SQL | 17.38 ± 0.19 b | 1.95 ± 0.05 a,b | 1.03 ± 0.11 a,b | 0.062 ± 0.001 a | 0.63 ± 0.02 b | 33.02 ± 0.87 b |
Generally, photosynthesis is measured per unit of leaf area; however, this area also represents a volume of photosynthetic tissues that can differ among plants (e.g. different leaf thickness). To take into consideration photosynthesis per unit of leaf volume, we integrated A by mass of dry leaf (Amass). There was significantly greater Amass in plants grown under fluctuating light regimes compared with those grown under square wave light regimes (Fig. 2B) and, as expected, a tendency for plants grown under high-light regimes to have greater rates of Amass compared with plants grown under low-light regimes.
Dark respiration, derived from the A/Q curve (Rd-model), was significantly higher in plants grown under SQH, and there was a general tendency for higher respiration in plants grown in square wave light regimes compared with fluctuating regimes (Table I) in Rd-model as well as dark respiration measured during diurnals (Rd-diurnal). However it should be noted that Rd-diurnal measured at the start of the diurnal was lower than Rd-model determined from the A/Q analysis. Plants grown under SQH also had a significantly higher light compensation point compared with plants grown under fluctuating light regimes (Table I).
The large differences observed in the response of A to PPFD between plants grown under low and high light intensity were less significant for PSII operating efficiency (Fq′/Fm′; Fig. 2C). The response of Fq′/Fm′ to PPFD was driven mainly by changes in the PSII efficiency factor (Fq′/Fv′; Fig. 2D). Fq′/Fm′ also was affected, although to a lower extent, by the maximum efficiency of PSII (Fv′/Fm′), which was higher in plants grown under high PPFD (Fig. 2E), with low values illustrating greater NPQ. In general, plants grown under fluctuating regimes had higher Fv′/Fm′ compared with those grown under square wave, particularly when measured under high PPFD. Plants grown under SQL showed the lowest values in both quenching parameters: Fq′/Fv′ and Fv′/Fm′. NPQ increased more rapidly at low light intensity in plants grown under SQL compared with plants grown in the other lighting regimes, and in general, NPQ had a tendency to be lower in plants grown under fluctuating light (Fig. 2F).
Leaf Properties in Plants Acclimated to Different Light Regimes
Leaf absorbance (measured after 28 d of growth) was significantly different between plants grown in the different light regimes (P < 0.05), ranging from 0.88 (FLL) to 0.93 (SQH; Supplemental Fig. S1A). There was a small change in leaf absorbance with time, increasing by a maximum of 2% in all treatments between days 14 and 28 (data not shown). Plants grown under fluctuating light regimes had significantly lower absorbance values (P < 0.05) compared with those grown under square wave regimes, with a smaller but significant difference between high- and low-light treatments. The only difference in leaf reflectance was observed between the fluctuating treatments, with a higher value shown by FLL-grown plants (Supplemental Fig. S1B), while transmittance was generally higher in fluctuating light treatments compared with square wave-grown plants and in plants grown at lower light intensities (Supplemental Fig. S1C).
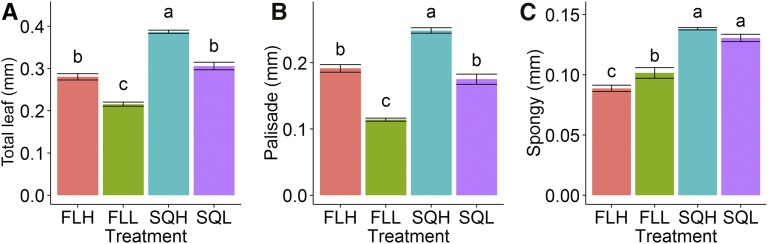
Differences in leaf thickness depended on both intensity and light regime (Fig. 3A), with significantly thinner leaves (P < 0.05) for plants grown under low light and fluctuating light compared with square wave-grown plants. A difference in leaf thickness was driven primarily by differences in the thickness of the mesophyll palisade layer in all treatments (Fig. 3B). The thickness of the palisade mesophyll layer was significantly (P < 0.05) higher in square wave-grown plants and in plants subjected to a higher intensity of light. The layer of spongy mesophyll cells was significantly thinner (P < 0.05) in plants grown under fluctuating light, while also being thinner in FLH compared with FLL (Fig. 3C). As a result of the increased leaf thickness in plants grown under square wave treatments, there was a tendency for a higher number of cells (as observed in Supplemental Fig. S2) with more circular cell shape in the palisade mesophyll compared with fluctuating treatments, measured by the length-width ratio (P = 0.06; Supplemental Table S1). Despite thicker leaves and a greater number of cells in square wave-grown plants, there was no significant difference in total protein content between treatments (Supplemental Fig. S3).
Figure 3.
Leaf anatomical properties including total leaf thickness (A), palisade layer thickness (B), and spongy layer thickness (C) of plants grown under the four light treatments SQH, FLH, SQL, and FLL. Data represent means ± se (n = 6). Letters represent the results of Tukey’s posthoc comparisons of group means.
The only significant differences observed in chlorophyll a/b ratio between plants grown under fluctuating or square wave light regimes was the lower ratio in FLL compared with SQL (Supplemental Table S2). Plants grown under SQL, FLL, and FLH had significantly lower total carotenoid-total chlorophyll ratio compared with plants grown under SQH (P < 0.05).
Impact of Growth Light on Photosynthetic Capacity
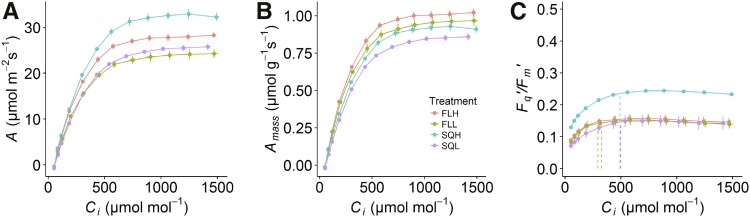
Assimilation rate measured as a function of intercellular [CO2] (Ci) was higher in plants grown under SQH (Fig. 4A) and generally greater in plants grown under high light intensity regimes. The light- and CO2-saturated rate of A was highest in plants grown under square wave regimes compared with plants grown under fluctuating light regimes irrespective of light intensity, with SQH-grown plants more than 15% higher than plants in all other growth treatments. In contrast, the light- and CO2-saturated rate of Amass was significantly higher in plants grown under fluctuating light regimes compared with square wave light regimes (Fig. 4B). Nevertheless, the differences in Amass between fluctuating and square wave light regimes (Fig. 4B) were smaller than those observed in the A/Q curves (Fig. 2B). The maximum rate of carboxylation by Rubisco (Vcmax) and the maximum electron transport rate (Jmax) for ribulose 1,5-bisphosphate (RuBP) regeneration (Fig. 4A) were highest in plants grown under square wave conditions and those grown under high light intensities (Table II). Estimates of mesophyll conductance (gm) using the constant J method ranged from 0.154 to 0.927 mol m−2 s−1; however, the only significant difference was the greater values measured in the SQH plants (Table II).
Figure 4.
Photosynthesis as a function of Ci of plants grown under the four light treatments SQH, FLH, SQL, and FLL. Parameters examined are A (A), Amass (B), and Fq′/Fm′ (C). Data represent means ± se (n = 6).
Table II. Photosynthetic parameters (means ± se) estimated from the response of A to Ci of plants grown under the four light regimes (SQH, SQL, FLH, and FLL).
Letters represent the results of Tukey’s posthoc comparisons of group means.
Fq′/Fm′ was significantly higher in plants grown under the SQH regime compared with those grown under the other light regimes at all CO2 concentrations measured (Fig. 4C). Plants grown under the other three light regimes (FLH, FLL, and SQL) showed no significant difference at high CO2, but the Ci where the switch between the Rubisco- and RuBP regeneration-limited A occurs (Cic) was significantly higher in plants grown under square wave light regimes compared with fluctuating light conditions (Fig. 4C).
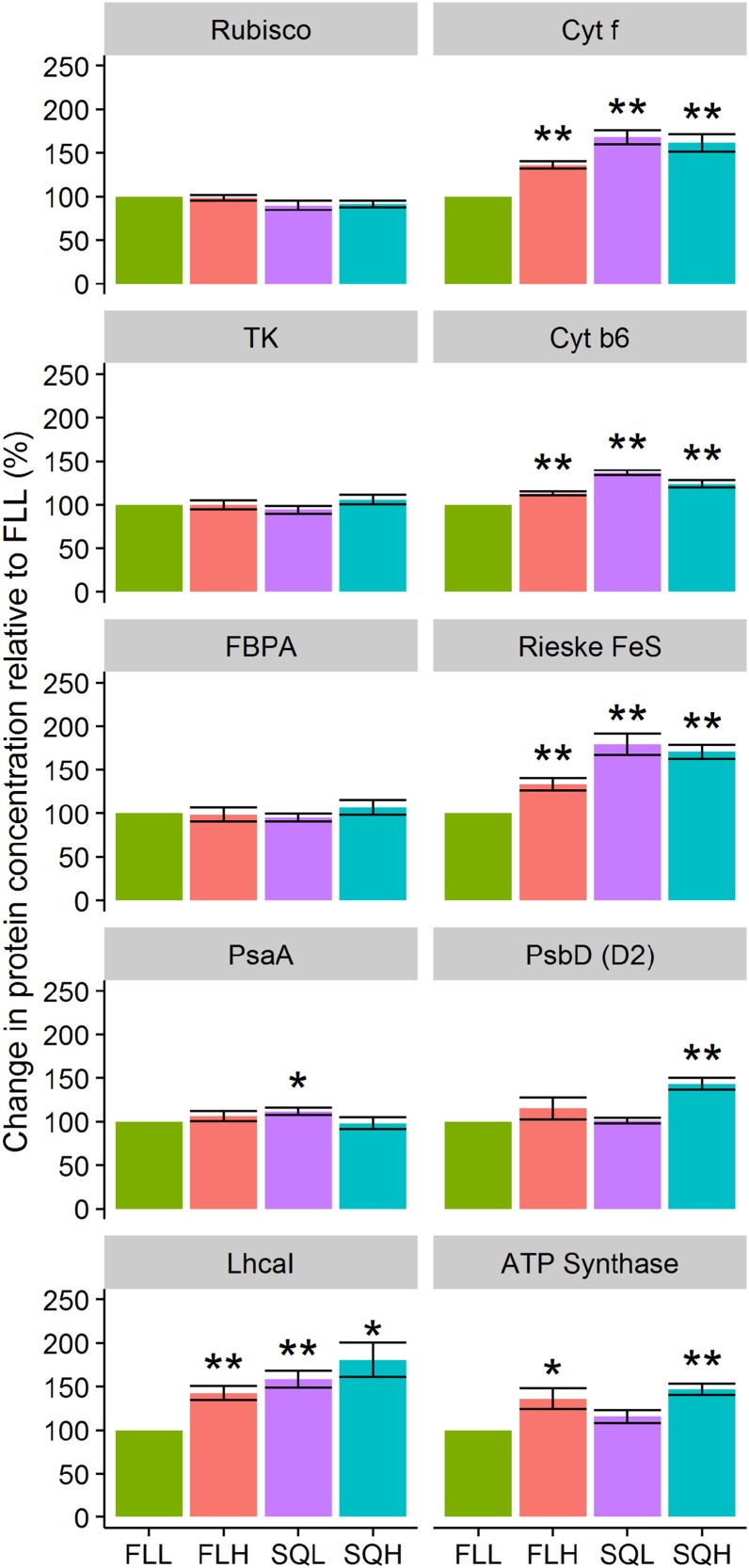
Although significant differences in Vcmax were found between high- and low-light treatments, there was no significant difference in Rubisco content or the contents of the Calvin-Benson cycle proteins Fru-1,6-bisP aldolase (FBPA) or transketolase (TK) between light treatments (Fig. 5). Furthermore, compared with FLL-grown plants, we observed a small but significant increase in protein levels of the PSI protein PsaA in SQL-grown plants. Interestingly, we did observe a significant increase in the level of three key proteins of the cytochrome b6f complex, Cyt f, Cyt b6, and Rieske FeS, in plants grown under SQL compared with FLL as well as for the PSI type I chlorophyll a/b-binding protein (Lhca1), matching the observed differences in Jmax (Table II). A similar tendency for these proteins was found between high-light treatments with higher protein levels in SQH-grown plants compared with FLH. A significant increase in protein level was observed in FLH-grown plants compared with FLL plants for Lhca1, proteins of the cytochrome b6f complex, and ATP synthase. The level of PsbD (D2), which forms the reaction center of PSII, was higher under high-light treatments but only significantly between SQL- and SQH-grown plants.
Figure 5.
Percentage change in protein concentration relative to FLL treatment determined from four replicate immunoblot analyses of leaves grown under the four light treatments SQH, FLH, SQL, and FLL. Rubisco and the Calvin-Benson cycle proteins TK and FBPA were probed along with the electron transport cytochrome b6f complex proteins Cyt f, Cyt b6, and Rieske FeS, the PSI Lhca1 and PsaA proteins, the PSII PsbD/D2 proteins, and the ATP synthase δ-subunit. Treatments were statistically analyzed against FLL-grown plants using a one-sample Student’s t test (*, P < 0.05 and **, P < 0.01).
Diurnal Leaf-Level Responses of Gas Exchange and Chlorophyll Fluorescence
Measurements under Diurnal High-Light Fluctuating Conditions
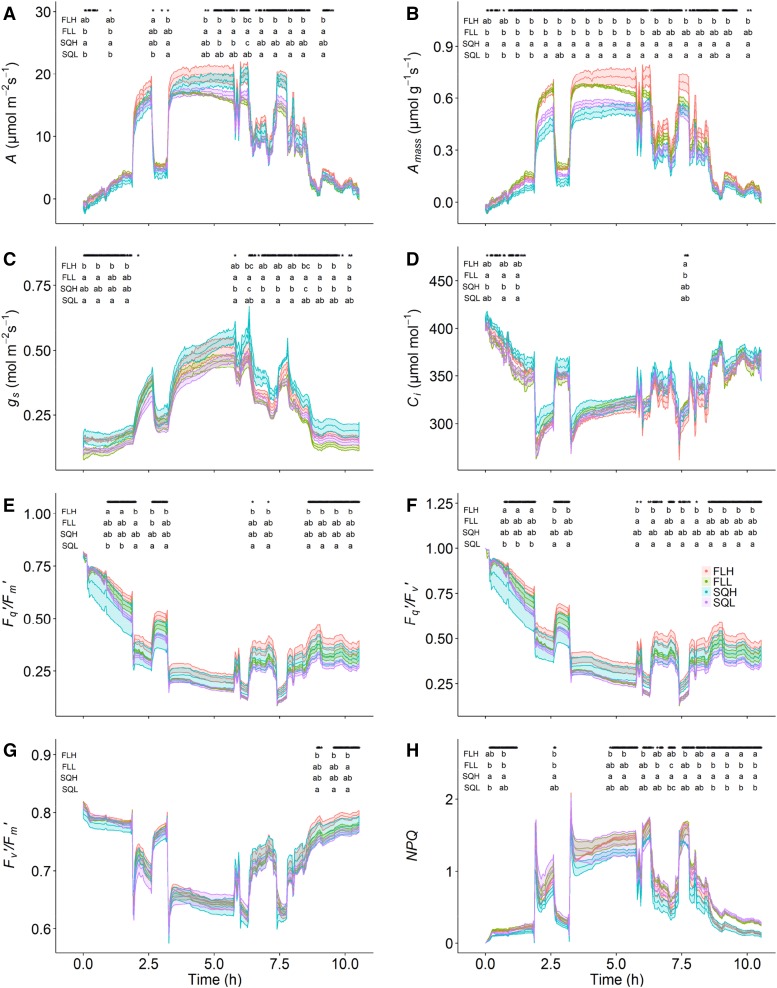
To determine the impact of acclimation to different growth light regimes on operational rates of photosynthesis (A), plants were measured under a diurnal fluctuating high light regime (DFhigh). Infrared gas-exchange measurements of A, Ci, and stomatal conductance to water vapor (gs) were recorded every 2 min along with the chlorophyll fluorescence parameters Fq′/Fm′, Fv′/Fm′, and Fq′/Fv′ in plants from all experimental growth conditions. In general, plants grown under fluctuating conditions had the greatest net photosynthetic rates on an area (A) basis through the majority of the diurnal period; however, these differences were only significant at specific light periods (indicated by letters in Fig. 6A). Photosynthesis measured on a mass integrated (Amass) basis was highest in plants grown under fluctuating light compared with square wave-grown plants; however, differences were only significant (for all light levels during the diurnal) in high-light-grown plants (Fig. 6B). This matched with a higher Fq′/Fm′ compared with plants grown under square wave conditions irrespective of light intensity. Despite the generally lower photosynthetic rates, gs in plants grown under the SQH regime was significantly higher than in plants grown under low-light conditions (Fig. 6C), particularly at the beginning and the end of the diurnal period. Despite the differences in A and gs, no differences in Ci were observed between the treatments for most of the DFhigh period (Fig. 6D).
Figure 6.
Diurnal measurements of gas exchange of A (A), Amass (B), gs (C), Ci (D), and the chlorophyll fluorescence parameters Fq′/Fm′ (E), Fq′/Fv′ (F), Fv′/Fm′ (G), and NPQ (H) estimated under DFhigh in the four light regimes SQH, FLH, SQL, and FLL. Data represent means ± se. Stars above the curves denote significant differences between the light regimes using a one-way ANOVA with unequal variance (n = 5). Letters represent the results of Tukey’s posthoc comparisons of group means.
During these measurements, it was noted that, after approximately 4 h into the light of the DFhigh period and under saturating light conditions, the plants grown under low-light regimes (FLL and SQL) started to display a decrease in A that was not correlated with a decrease in gs, in contrast to plants grown under high-light regimes, which maintained a high level of A throughout the diurnal period. The decrease in A observed in plants grown under low-light regimes continued through the day, and during periods of saturating light intensities (greater than 1,000 µmol m−2 s−1) at ∼6 and 8 h into the light period, more pronounced decreases in A were detected compared with plants grown under high-light regimes (P < 0.05). The kinetics of Amass did not change, but in general, Amass was significantly higher in plants grown under fluctuating light regimes (similar to the A/Q analysis; Fig. 2; P < 0.05; +50% Amass) compared with plants grown under square wave light regimes over the majority of the diurnal period (Fig. 6B).
At periods of low light intensity (less than 300 µmol m−2 s−1), Fq′/Fm′ displayed significantly higher values in plants grown under the FLH regime compared with the other growing conditions (Fig. 6E). In all treatments, Fq′/Fm′ decreased through the DFhigh period, with significantly lower values at the end of the diurnal compared with the beginning even under comparable PPFD. This difference in Fq′/Fm′ was driven mostly by changes in Fq′/Fv′, which mirrored Fq′/Fm′ through the DFhigh period (Fig. 6F). No differences in Fv′/Fm′ were observed until the end of the diurnal period, with the highest values observed in the FLH-grown plants (Fig. 6G). On the other hand, measurements of NPQ showed significant differences between FLH- and SQH-grown plants (Fig. 6H) during most of the DFhigh period. At the end of the DFhigh period, significantly lower NPQ was observed in plants grown under high light intensity compared with low-light growing conditions.
Measurements under Diurnal Low-Light Fluctuating Conditions
To further investigate the interaction of light intensity and fluctuating pattern on plant dynamic responses, plants grown under the different treatments were measured under the same fluctuating pattern but applied at the lower light intensity (DFlow). For long periods of the DFlow, plants grown under high-light regimes (FLH and SQH) showed significantly higher A compared with those grown under low-light regimes (P < 0.05), with the highest values of A recorded in plants grown under the FLH regime (Supplemental Fig. S4A). However, this difference in A between growing conditions was apparent only at PPFD above 300 µmol m−2 s−1. In contrast to the observations made for A, a significant difference in Amass was observed between the fluctuating and square wave light treatments, with the highest values observed in FLH-grown plants, approximately 50% higher than in plants grown under SQH (Supplemental Fig. S4B).
Plants grown under high light intensity (FLH and SQH) also displayed significantly higher gs during long periods of DFlow compared with plants grown under low light intensity (FLL and SQL; Supplemental Fig. S4C). During periods of higher light intensity (greater than 500 µmol m−2 s−1), the gs of SQH-grown plants was generally higher than in the other treatments. Similar to the results of plants measured under DFhigh, Ci was not significantly different between treatments (Supplemental Fig. S4D).
As observed under DFhigh, Fq′/Fm′ decreased significantly through the DFlow period. Fq′/Fm′ (Supplemental Fig. S4E) and Fq′/Fv′ (Supplemental Fig. S4F) were significantly higher in FLH-grown plants over the entire DFlow period. Fv′/Fm′ showed significantly higher values in plants grown under high-light regimes (FLH and SQH) compared with low-light conditions through the entire diurnal period (Supplemental Fig. S4G). As predicted, plants grown under low light intensity showed a significantly higher NPQ compared with high-light-grown plants, with the lowest values observed in SQH-grown plants (Supplemental Fig. S4H). In comparison with DFhigh measurements, DFlow measurements showed significantly higher Fv′/Fm′ in high-light-grown plants.
Comparison of Measured Diurnal Photosynthesis with Predicted Values from A/Q Analysis
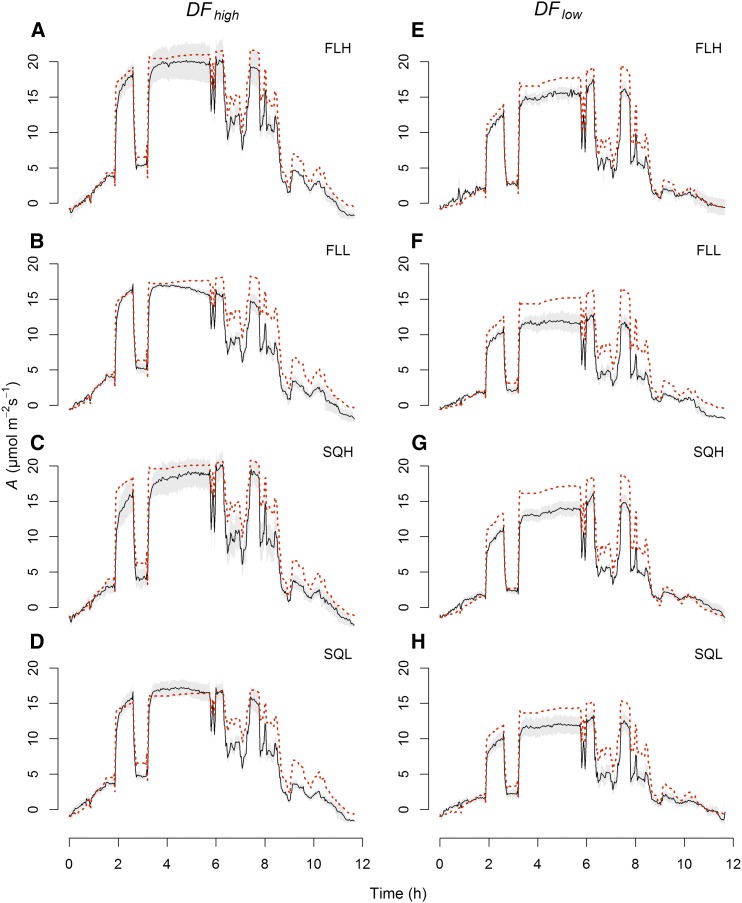
To reveal the potential limitation of A during the diurnal period, A was predicted from the A/Q response curves assuming no gs limitation and a maximized activation of the biochemistry associated with photosynthesis. During the initial 4 to 6 h of DFhigh (Fig. 7, A–D), all plants irrespective of their growing conditions reached the predicted A. However, after this period, there was a general tendency for measured A to be lower than that predicted from the model A response. The difference between expected and observed A values integrated over the diurnal period was 18.8% for FLH-grown plants but more than 22% in all other treatments.
Figure 7.
Diurnal measurements of observed A (black lines) and predicted net CO2 assimilation modeled from the A/Q responses (see Eq. 3; red dashed lines) of the four light regimes SQH, FLH, SQL, and FLL over diurnal periods of DFhigh (A–D) and DFlow (E–H; n = 5). Gray shading represents confidence intervals at 95%.
Surprisingly, none of the plants measured under DFlow reached the predicted A values at any point over the diurnal regimes (Fig. 7, E–H). The lowest integrated differences between predicted and measured A values were observed for plants grown under high-light regimes (less than 26.4%), with the lowest values for FLH-grown plants (19.8%). Differences of greater than 30% were observed in plants grown under low-light regimes. In general, measurements under DFlow regimes showed a larger difference between predicted and observed A values but were able to maintain levels of A throughout the diurnal period compared with measurements under DFhigh, which showed a continuous increase in the divergence between observed and predicted A.
Influence of Growth Light Regimes on Plant Development
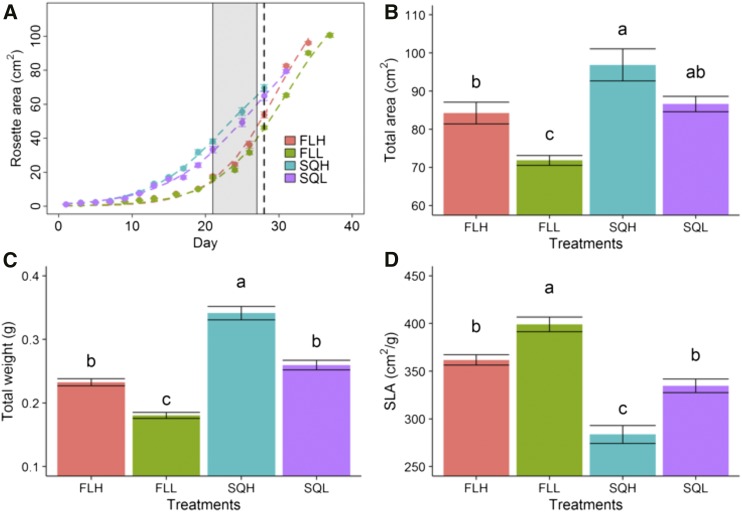
The increase in rosette area as a function of time was modeled using a sigmoidal curve (Fig. 8A; Supplemental Table S3) and revealed a higher initial growth rate in plants grown under square wave light regimes compared with those grown under fluctuating light, commencing on day 10 until day 28 (Fig. 8A). After this period of time, plants grown under fluctuating light regimes caught up with plants grown under square wave light regimes. It is interesting that the plants grown under square wave light regimes flowered ∼6 d before those grown under fluctuating light regimes, irrespective of the light intensity (Fig. 8A).
Figure 8.
Growth analysis of plants grown under the four light regimes SQH, FLH, SQL, and FLL. A, Kinetics of the increase in rosette area, with each point representing a mean of 10 plants. The gray area represents the period during which gas-exchange measurements were taken. The dotted line indicates the time of harvest for all treatments. The last point of each curve was measured upon the appearance of the first inflorescence. B to D, Total leaf area of each plant (B), total aboveground dry mass (C), and specific leaf area (SLA; D). Data represent means ± se (n = 8–10). Letters represent the results of Tukey’s posthoc comparisons of group means.
Plants grown under square wave light regimes (SQH and SQL) had significantly greater total leaf areas at 28 d of growth compared with plants grown under fluctuating light regimes (FLH and FLL; Fig. 8B). In general, high-light-grown plants had a higher total leaf area, and plants grown under fluctuating light regimes were significantly higher than square wave-grown plants. Plants grown under square wave light regimes had greater total leaf mass than those grown in fluctuating light regimes (Fig. 8C). Specific leaf area was significantly lower in plants grown under square wave light regimes and under high light intensity (Fig. 8D), resulting mainly from a change in leaf thickness (Supplemental Fig. S2; thinner leaves for plants grown under fluctuating light intensity regimes).
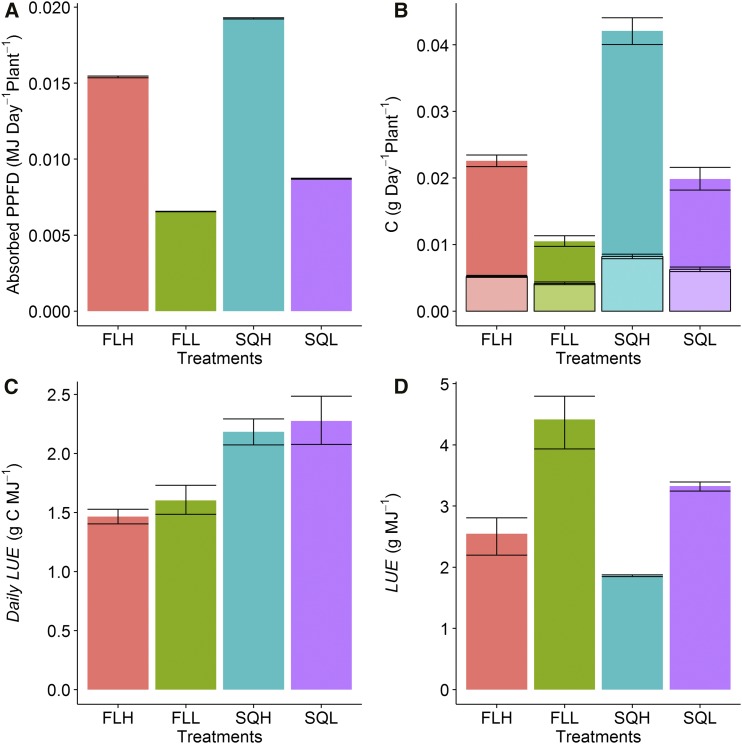
The differences in rosette area and leaf absorbance described previously influenced the total average light absorbed by the plants grown under the different light regimes, with a significantly higher amount of light absorbed in plants grown under square wave light regimes compared with plants grown under fluctuating light regimes (Fig. 9A). The predicted A and Rd-model (from the A/Q curves) integrated over the course of a 24-h period revealed a significantly higher integrated carbon assimilation in plants grown under square wave light regimes and higher light intensities (Fig. 9B). It also should be noted that the integrated daily carbon gain (Fig. 9B) is determined from the integrated daily net photosynthetic rate minus respiratory losses in the dark, which can represent a cost between 20% to 40% of total daily carbon gain (Fig. 9B). Overall, the amount of carbon lost to respiration in the dark was higher in square wave-grown plants, although this represented a smaller proportion of the total carbon gain over 24 h compared with fluctuating light-grown plants, irrespective of light intensity. Daily light use efficiency (LUE), the ratio of the daily integrated carbon assimilation and absorbed light, describes how efficiently the plants convert the light absorbed into biomass (Fig. 9C). Daily LUE was significantly higher in plants grown under square wave light regimes independently of the light intensity. Long-term LUE calculated over 28 d of growth gave a different picture, with a significantly higher LUE in plants grown under low light intensity as well as in plants grown under fluctuating light intensity (Fig. 9D). The long-term LUE is the sum of the daily LUE and, therefore, includes the variation through time as well as the heterogeneity between and within leaves.
Figure 9.
Total daily absorbed light (A), net carbon (“C”) gain (darker colors) and carbon loss by dark respiration (lighter colors; B), modeled daily LUE (C), and overall long-term LUE (D) of plants grown under the four light treatments SQH, FLH, SQL, and FLL. Error bars represent 95% confidence intervals (n = 8).
DISCUSSION
Most of our knowledge regarding photoacclimation during development in Arabidopsis has been gained from growing plants under high or low square wave light regimes in a controlled environment (Yin and Johnson, 2000; Tikkanen et al., 2010; Alter et al., 2012; Suorsa et al., 2012; Yamori, 2016) or focused on plants grown in glasshouses with natural fluctuations in light intensity but with uncontrolled and often unreproducible environmental conditions (Külheim et al., 2002; Athanasiou et al., 2010). The aim of the approach taken here was to mimic natural fluctuations in light intensity in a controlled manner, to enable the study of the light acclimation response of Arabidopsis in order to further our understanding of how plants operate in a realistic field environment. As a first step toward understanding how fluctuating light intensities influence photosynthesis and the development of Arabidopsis, we examined the effect of the growth light regimes on photoacclimation by comparing the phenotypes and performance of plants grown under fluctuating and square wave light regimes.
Acclimation Effects on Photosynthetic Rates and Capacity
One of the most common approaches to assess light acclimation is to measure photosynthesis as a function of light (a light response curve [A/Q]; Retkute et al., 2015). Analysis of A/Q response curves revealed higher Asat values in plants grown under high light, irrespective of whether this was delivered in a square or fluctuating light regime, suggesting minimal limitation of photosynthetic rates by Rubisco and demonstrating that plants acclimate to the average light intensity (Chabot et al., 1979; Watling et al., 1997) rather than a maximum or minimum light value. Photosynthetic capacity also has been reported to depend on the pattern of switching between high and low light intensity (Yin and Johnson, 2000; Retkute et al., 2015). Higher Asat values observed in high-light-grown plants are often related to the amount of photosynthetic components, including Rubisco, cytochrome f, H+-ATPase, and reaction centers (Bailey et al., 2001). Although Rubisco content (on a leaf area basis) did not change between treatments, the difference in leaf thickness and cell number suggests a greater Rubisco content per cell in plants grown under fluctuating light (although this does not necessarily correlate with Rubisco activity). This higher Rubisco concentration per cell in thinner leaves enabled plants grown under fluctuating light to achieve similar Asat values to square wave-grown plants on a leaf area basis and a higher Asat value on a mass basis. Terashima et al. (2006) demonstrated that thicker leaves are often associated with acclimation to sun (or high light) but have less Rubisco content per cell compared to shade (or low light) conditions and consequently operate with a higher CO2 concentration at the site of carboxylation, enabling higher rates of photosynthesis (measured on an area basis). These observations are supported by our A/Ci analysis of SQH-grown plants, which suggested switching from Rubisco to RuBP limitation at a higher Ci concentration and a higher apparent gm. Compared with plants grown under square wave conditions, those grown under fluctuating light were more limited by RuBP regeneration, as illustrated by the lower Jmax values estimated from A/Ci response curves. However, plants grown under fluctuating light will not necessarily benefit from an increase in Jmax, as under ambient conditions, [CO2] will be more limiting than regeneration of RuBP under periods of high light such as those encountered under the fluctuating regimes (Pearcy, 2007). Additionally, higher Jmax values and the higher Fq′/Fm′ at saturating light and high [CO2] in plants grown under SQH conditions suggest higher potential electron transport rates than in plants grown under low or fluctuating light treatments. The higher content of Lhca1, PsbD, and electron transport proteins (Cyt f, Cyt b6, Rieske FeS, and ATP synthase) in square wave-grown plants also would facilitate greater light absorption and an enhanced capacity to process light. All of these observations together suggest that SQH-grown plants have the ability and resources to invest in greater capacity for photosynthesis on an area basis, even if the potential to fully utilize this investment is not realized on a day-to-day basis (as shown in the diurnal responses). It is interesting that increases in the abundance of proteins associated with electron transport processes were not accompanied by similar increases in Calvin cycle proteins. The fact that improvements to photosynthesis (and plant growth) have been achieved by manipulating components of the Calvin cycle emphasizes that any limitation by electron transport rate ultimately depends on growth environment and measurement conditions (Lefebvre et al., 2005; Zhu et al., 2010; Rosenthal et al., 2011; Simkin et al., 2015, 2017).
Diurnal Responses of Plants Acclimated to Different Growth Light Regimes
In order to examine the impact of developmental acclimation to growth irradiance, the ability of the plants to operate in fluctuating light environments was assessed by gas exchange and chlorophyll fluorescence under DFhigh and DFlow light regimes in plants from all growth treatments (Fig. 6; Supplemental Fig. S4). In general, plants grown under fluctuating light regimes had higher photosynthetic rates and photosynthetic efficiency than their square wave-grown counterparts, which was particularly evident when measured under the DFlow lighting regimes. The significantly higher Fq′/Fm′ along with higher Fq′/Fv′ illustrates that the great PSII operating efficiency in these plants was due to an ability to utilize the products of linear electron transport (Baker, 2008). The greater capacity to utilize light for processes downstream of PSII in the fluctuating light plants was not accompanied by a significantly higher gs or greater Ci, indicating that greater CO2 flux from the atmosphere to inside the leaf could not account for these differences. These observations also suggest that plants grown in fluctuating light may have greater water use efficiency (Lawson and Blatt, 2014; McAusland et al., 2016), which is worthy of further investigation.
Alter et al. (2012) suggested that plants subjected to rapid fluctuations in light (20 s) responded by enhancing mechanisms for energy dissipation and photoprotection, presumably because they are unable to quickly utilize the additional light energy for carbon assimilation provided in this form. When measured under fluctuating light regimes (DFhigh and DFlow), the differences in dissipation of excess absorbed energy (NPQ) between plants grown under fluctuating and square wave regimes illustrated differences in photoprotective strategies and developmental acclimation (particularly when measured under DFlow). As expected, irrespective of the regime, plants grown under low light exhibited a greater NPQ over most of the diurnal period compared with those grown under high-light conditions, as these plants were acclimated to a lower level of energy utilization (Demmig-Adams and Adams, 1992). Despite slightly higher A during DFlow and DFhigh, plants grown under FLH regimes also displayed higher NPQ than those grown under SQH regimes, suggesting that FLH plants have greater capacity to tolerate the high-light stress associated with these conditions. The temporal response of NPQ through the diurnal period was in contradiction with the observations from the A/Q curves (which illustrated reduced NPQ in plants grown under fluctuating light conditions), revealing a more complex nature of the regulation of excess energy dissipation than the one observed in steady state. Furthermore, there is a temporal component of the NPQ response that is not observed during an A/Q curve, illustrated by the difference in NPQ at the start and end of the diurnal period when light intensities are similar. A possible explanation for this increase in NPQ toward the end of the light period is the development of photoinhibition following exposure to high light levels toward the middle of the photoperiod. This is also supported by the fact that smaller differences in NPQ between the start and end of the photoperiod are evident when measured under the low-light (DFlow) regime.
During these diurnal measurements, we also noted that, when measured under DFhigh, all plants displayed a decrease in A after 4 h into the diurnal period, despite the fact that gs increased over the same period and Ci was not limiting. The decrease in Fq′/Fv′ along with A suggests that this was due mainly to a decrease in sink capacity for the end products of electron transport, namely ATP and NADPH (Murchie and Lawson, 2013). To our knowledge, this is the first time that both gas-exchange and chlorophyll fluorescence parameters have been assessed simultaneously over a full 12-h diurnal period with such frequency. The combination of these results suggests that there is a process that slows down Calvin cycle activity later in the diurnal period, which, for example, could be sugar accumulation in the leaf applying a feedback control on photosynthesis through changes in photosynthetic gene expression (Paul and Foyer, 2001; Paul and Pellny, 2003). An alternative explanation has been proposed by Yamori (2016), who stated that, under fluctuating light, the electron transport system accumulates excess reducing power, which cannot be dissipated as heat and may cause a strong reducing burst, eventually leading to photoinhibition of PSI or PSII and a decrease in CO2 assimilation. The higher level of A sustained in plants grown under FLH over a longer period of time compared with plants grown in the other light regimes suggests plasticity in such processes that could involve one or both of these mechanisms in the acclimation of plants to fluctuating light; however, this requires further investigation. This plasticity of response theoretically could be used as a potential screen to identify plants with maintained photosynthetic efficiency over the entire diurnal period. Such sustained photosynthetic rates could, according to our model predictions, increase total diurnal carbon assimilation by at least 20% (Fig. 7).
We compared measured leaf-level gas-exchange values with predicted values of assimilation rate (determined from A/Q analyses) measured under DFhigh and DFlow conditions to examine the effect of fluctuating light on photosynthetic processes over the diurnal period. It is interesting and unexpected that none of the plants measured under DFlow were able to achieve the predicted A irrespective of their growth light regimes. One possible explanation for this is that predicted A is based on the A/Q response curves that are conducted under conditions maximizing processes such as Rubisco activation (Ernstsen et al., 1997; Carmo-Silva and Salvucci, 2013) and ensuring no stomatal limitation of A (Parsons et al., 1998). For example, the A/Q response curves were initiated by stabilizing a leaf in a cuvette for 30 to 60 min at saturating light to ensure that gs and A were maximal, after which light was decreased rapidly and A was recorded when a new steady-state value was reached (1–3 min). The short delay between each measurement was not long enough for gs and the activation of Rubisco to reach the new steady state; consequently, each measurement was recorded when the conditions were most favorable for photosynthesis. During the diurnal period, Ci values did not indicate a gs limitation of A, but the slow increase in light and the rapid fluctuations could prevent full activation of Rubisco and may be a possible explanation for the differences observed (Carmo-Silva and Salvucci, 2013). These findings suggest that photosynthetic acclimation does not include the activation of enzymes or the activation of photosynthetic processes, as all the plants showed the same behavior when measured under fluctuating low light irrespective of growth condition. Although a typical A/Q response curve is a useful tool for characterizing photoacclimation, it may not truly reflect how plants behave in the natural field environment. As A/Q response curves represent a maximum A (at all light levels), users should be cautious when employing A/Q curves for predicting A under natural fluctuating light conditions.
Impact of Light Regime on Growth
The amount of light received during the day acts as both an acclimatory signal, to which plants respond by adapting their morphology and physiology to optimize photosynthetic carbon gain, and as a signal to increase tolerance to light intensity and avoid photooxidative stress (Niinemets, 2007, 2010; Pearcy, 2007). To assess the impact of light regime on plant growth efficiency, we determined daily LUE on mature leaves (between 21 and 28 d old) and long-term LUE over the entire growth period (28 d), allowing us to examine instantaneous values of plant performance to convert absorbed light into carbon as well as the long-term integrated LUE values.
Under both light intensities, LUE was higher in plants grown under fluctuating light regimes, suggesting a specific adaptation to maximize the light utilized for carbon fixation, facilitated by their improved light-saturated rate of photosynthesis (on a mass basis) and lower cost of maintenance (illustrated by lower respiration rates). Plants grown under square wave regimes (and high light intensity) absorbed more light and had a greater daily carbon gain and a greater biomass compared with fluctuating light- and low-light-grown plants, despite having lower LUE. The lower LUE in square wave-grown plants could be the result of greater investment in cells, metabolic components, and leaf structure relative to the carbon gained by this investment (Weraduwage et al., 2015). Compared with low-light-grown plants, the lower LUE in plants grown under high light could be the result of an increase in the energy dissipated through processes such as NPQ associated with the higher growth light intensity, reducing the amount of carbon fixed relative to the amount of light absorbed (Porcar-Castell et al., 2012). For example, the lower specific leaf area and higher Rd-model in plants grown under square high light often is associated with an extra cost in growth and maintenance of the leaf, decreasing LUE (Pearcy, 2007; Oguchi et al., 2008). In general, plants grown under square wave light regimes had higher photosynthetic capacity on an area basis, but this was not sufficient enough to fully utilize the absorbed light for carbon fixation, resulting in a reduction in LUE. This suggests that, under fluctuating (high) light, plants balance acclimation between the increase in photosynthetic capacity and the increase in dissipation of energy through alternative processes (Givnish, 1988).
At the leaf level, daily LUE represents the efficiency of the plant to convert the incident light into carbon over a 24-h diurnal period (Medlyn, 1998). For example, a decrease in daily LUE can be explained during periods of high light intensity that may occur in a fluctuating environment by the fact that plants cannot utilize all the available light for carbon fixation. This is illustrated in Figure 1, which shows the light intensity that saturates photosynthesis in FLH-grown plants (dotted line); the shaded areas show the proportion of light that is higher than saturation for photosynthesis, and light above this intensity will not drive additional carbon fixation in these plants. This theoretically decreases the average growth light intensity that FLH plants were grown in. Daily LUE was lowest in plants grown under fluctuating light regimes, due to a lower absorbance and smaller rosette area. These plants also displayed low gm that could limit CO2 diffusion, and therefore A, but may be compensated for by an increase in Asat per leaf mass. In plants grown under fluctuating light, the greater investment in photosynthetic capacity by area along with the greater proportion of daily respiration in the dark induced an extra cost to growth, which could explain the slow development of these leaves at early stages (Pearcy, 2007). After the initial period of slow growth, the rosette area of plants grown under fluctuating light regimes increased rapidly as the light absorbed by these plants was converted more efficiently into biomass, as illustrated by the increased long-term LUE. These results seem contradictory, but one explanation could be that the partitioning of the carbon fixed at different growth stages was not the same between treatments, with generally more carbon invested in processes other than growth (such as photoprotection) early in development in plants grown under fluctuating light compared with square wave light regimes.
CONCLUSION
This study has revealed two major insights into the impact of fluctuating light on plant acclimation as well as evaluated some of the current methodologies often used to assess photoacclimation. Plants grown under fluctuating light showed a previously undescribed phenotype, exhibiting thinner leaves, with lower light absorption compared with square wave-grown plants yet similar photosynthetic rates per unit of leaf area and greater values when considered on a leaf mass basis. The fluctuating light-grown phenotype enabled these plants to perform more effectively in dynamic environments than square wave-grown plants, with greater rates of photosynthesis along with lower gs. These plants also had higher photosynthetic efficiency, generally due to a greater ability to process light energy downstream of PSII, despite a reduction in investment in electron transport chain protein and leaf structure. The lower level of the cytochrome b6f complex in the fluctuating treatments was unexpected, as this is known to regulate the balance between photochemical quenching and NPQ, which would be necessary under rapidly fluctuating light. We observed that, although A/Q analyses were useful to describe photoacclimation, characterizing the difference in photosynthetic capacity, these types of analyses failed to accurately predict assimilation rates over the diurnal period, overestimating these by up to 38% particularly under low light. This suggests a light-driven activation of photosynthesis that was not fully induced when measured under low fluctuating light. Our unique data set, describing diurnal gas exchange and chlorophyll fluorescence during a 12-h photoperiod of fluctuating light, also revealed a negative feedback on photosynthesis that resulted in an ∼20% decrease of the predicted total daily carbon assimilated.
Our findings illustrate the impact of growing plants in dynamic light regimes, similar to that experienced in the natural environment, on the phenotype and physiology of Arabidopsis and provide a first step toward understanding how fluctuating light intensities influence plant function and growth. More importantly, they emphasize that growing plants under laboratory conditions and square wave illumination does not represent plant development under a natural environment, with significant variation in leaf anatomy, biochemistry, and performance, underestimating LUE by 30%. Our approach of growing plants under dynamic LED light regimes provides a compromise between a controlled regulated environment and natural conditions.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana ecotype Columbia) plants were grown in peat-based compost (Levingtons F2S; Everris) in a controlled environment with growth conditions maintained at a relative humidity of 55% to 65%, air temperature of 21°C to 22°C, and a CO2 concentration of 400 µmol mol−1. Fluctuating light growth conditions were provided by a Heliospectra LED light source, with the light regime recreated from natural variations in light intensity recorded during a relatively clear day in July at the University of Essex (Fig. 1) and the assumption of a constant spectral distribution. The average light intensity was 460 µmol m−2 s−1 for high-light conditions and 230 µmol m−2 s−1 for low-light conditions. Plants were maintained under well-watered conditions, with position under the growth light source moved daily at random to take into account any heterogeneity in spectral quality and quantity. All gas-exchange, chlorophyll fluorescence, and absorption measurements were taken on the youngest fully expanded leaf of 21- to 27-d-old plants.
Growth Analysis
Rosette area, taken as the area (cm2) of the visible rosette of the plant, was measured from when each plant was sown and placed under the lights (day 0) until the appearance of inflorescence (days 28–37). Total leaf area (cm2), total leaf dry weight (g), and specific leaf area (cm2 g−1) were measured on all treatments at the same time once the first inflorescence had appeared on any treatment (SQH plants exhibited the first inflorescence after 28 d). All growth analysis measurements are means of eight to 10 plants.
Transmittance and Reflectance
Leaf absorbance was measured using a Skye Instruments light meter and an Ulbricht integrating sphere (built at the University of Essex). Ten measurements of transmittance and reflectance were made per treatment, using the youngest fully expanded leaf on each plant after 14, 21, and 28 d of growth. The transmittance and reflectance for each leaf was used to calculate absorbance, with the mean absorbance for each treatment being determined from the 10 combined measurements.
Analysis of Photosynthetic Pigments
For pigment analysis, leaf discs (1 cm2) were taken from attached leaves 5 h into the light treatment (Fig. 1) without dark adaptation, frozen in liquid nitrogen, and kept at −80°C until extraction. Pigments were extracted as described by Matsubara et al. (2005) and were separated by ultra-performance liquid chromatography as described by Zapata et al. (2000), with chlorophyll a, chlorophyll b, and total carotenoid content identified via their absorption spectra and retention times.
Leaf Cross-Section Analysis
Most recent fully expanded leaves were collected from plants after 28 d of growth. One-millimeter-wide strips were cut from the center of the leaf, extending from the midvein to the edge of the leaf. Samples were preserved in 5% glutaraldehyde and refrigerated for a minimum of 24 h. An ethanol series (20%, 40%, 80%, and 100%) was then performed, leaving samples in each concentration for 15 min, and then 24 h at 100% to clear the leaves. The samples were then placed in LR White acrylic resin (Sigma-Aldrich), refrigerated again for 24 h, embedded in capsules, and then placed in an oven at 60°C for a further 24 h to harden. For light microscopy, 0.5-μm sections were cut from the samples using a Reichert-Jung Ultracut microtome (Ametek) and were fixed, stained, and viewed using a method described previously (López-Juez et al., 1998).
Leaf Gas Exchange
All photosynthetic gas-exchange (A and gs) and chlorophyll fluorescence parameters were recorded using a Li-Cor 6400XT portable gas-exchange system with a 6400-40 fluorometer head unit connected to a Li-Cor 610 portable dew point generator (Li-Cor) to maintain a leaf-to-air water vapor pressure deficit of 1 ± 0.2 kPa. Unless stated otherwise, all measurements were taken with Li-Cor cuvette conditions maintained at a CO2 concentration of 400 µmol mol−1 and leaf temperature of 25°C. The youngest fully expanded leaf was used for all measurements.
Photosynthetic Measurements
The response of A to Ci (A/Ci response curves) was measured at a saturating light intensity of 1,500 μmol m−2 s−1. Leaves were initially stabilized for a minimum of 10 to 15 min at an ambient CO2 concentration of 400 µmol mol−1; upon reaching a stable signal, a measurement was taken before ambient CO2 was decreased to 250, 150, 100, and 50 µmol mol−1 before returning to the initial value of 400 µmol mol−1, and then was increased to 550, 700, 900, 1,100, 1,300, 1,500, and 1,750 µmol mol−1. Recordings were taken at each new CO2 level when A had reached a new steady state (∼1–3 min).
The response of A to PPFD (A/Q response curves) was measured under the same cuvette conditions as the A/Ci curves mentioned above. Leaves were initially stabilized at irradiance above saturation at 1,800 μmol m−2 s−1 and a measurement was recorded, at which point PPFD was decreased in 13 steps (1,500, 1,300, 1,100, 900, 700, 550, 400, 300, 200, 150, 100, 50, and 0 μmol m−2 s−1), with a new recording being taken at each new light level once A had reached a new steady state (∼1–3 min) and before gs changed to the new light levels.
Diurnal Measurements
Leaves were initially placed in the cuvette in darkness, with A and gs allowed to stabilize under the controlled cuvette conditions for a minimum of 15 to 30 min. After readings of A and gs were stable for at least 5 min, the automatic 12-h light program (fluctuating high and low light) was started, with measurements of A, gs, and chlorophyll fluorescence parameters recorded every 2 min. On each parameter derived from the diurnal measurement, a one-way ANOVA with light treatment as a factor and corrected for unequal variance (White’s adjustment) was applied on each recorded time. When significant differences were observed, a Tukey’s posthoc test using a White-corrected covariance matrix was used to compare the different light treatments. The ANOVA was performed using the R statistical software (version 3.2.4).
Protein Extraction and Western Blotting
Four leaf discs (1 cm diameter) were collected from four plants per treatment at 12 pm (Fig. 1), immediately plunged into liquid nitrogen, and stored at −80°C. Protein was extracted in extraction buffer (50 mm HEPES, pH 8.2, 5 mm MgCl2, 1 mm EDTA, 10% glycerol, 0.1% Triton X-100, 2 mm benzamidine, 2 mm aminocaproic acid, 0.5 mm phenylmethanesulfonyl fluoride, and 10 mm DTT), and the insoluble material was removed by centrifugation at 14,000g for 10 min (4°C) and protein quantification was determined (Harrison et al., 1998). Samples were loaded on a leaf area basis, separated using 12% (w/v) SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed using antibodies raised against the Rubisco small subunit (Foyer et al., 1993). In addition to the aforementioned antibody, samples were probed using antibodies raised against TK (Henkes et al., 2001), the cytochrome b6f complex proteins Cyt f (PetA [AS08306]), Cyt b6 (PetB [AS03034]), and Rieske FeS (PetC [AS08330]), the PSI Lhca1 (AS01005) and PsaA (AS06172) proteins, and the PSII PsbD/D2 (AS06146) protein, all purchased from Agrisera (via Newmarket Scientific). FBPA antibodies were raised against a peptide from a conserved region of the protein [C]-ASIGLENTEANRQAYR-amide (Cambridge Research Biochemicals; Simkin et al., 2015). Proteins were detected using horseradish peroxidase conjugated to the secondary antibody and ECL chemiluminescence detection reagent (Amersham). Protein content was determined as a percentage of protein levels in plants grown in FLL and quantified using a Fusion FX Vilber Lourmat imager (Peqlab).
Estimating Photosynthetic Capacities and Limitations
The switch between the Rubisco- and RuBP regeneration-limited A is difficult to estimate and is a source of uncertainty in the estimation of the photosynthetic capacities of the leaf (Bernacchi et al., 2013). Therefore, the fluorescence data collected during the A/Ci curves were used to calculate Fq′/Fm′, which reaches a plateau when A is RuBP regeneration limited (Long and Bernacchi, 2003). An exponential model was fitted (Eq. 1) on the Fq′/Fm′-Ci curves to determine Ci where the switch between the Rubisco- and RuBP regeneration-limited A occurs (Cic) when 95% of the variation of Fq′/Fm′ was reached:
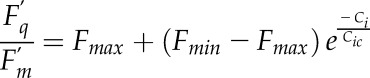 |
(1) |
where Fmin and Fmax represent the minimum and maximum values, respectively, of Fq′/Fm′, and Cic represents the Ci where Fq′/Fm′ reached 63% of the variation. The value representing Fq′/Fm′ at 95% was determined to be equal to 3 times that of the value at 63%.
The photosynthetic capacities (Vcmax and Jmax) and Rday were estimated using the method described by Sharkey et al. (2007). Estimations of the limiting factor were based on Cic described previously. Every observation below this point was considered as Rubisco limited.
gm
gm was estimated using the constant J method described by Harley et al. (1992). This method made the assumption of a constant gm when Ci is changing under saturating light. Values of A were selected based on the Cic described previously and used to derive J:
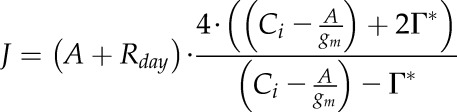 |
(2) |
Where Γ* is the CO2 compensation point in the absence of Rday corrected for the leaf temperature following Walker et al. (2013). As J is constant above Cic, the best gm corresponds to the value that minimizes the variance 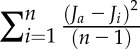 , where Ja is the average value of J and Ji is the value for J for each calculated Cj.
, where Ja is the average value of J and Ji is the value for J for each calculated Cj.
Modeling A
A as a function of light intensity (PPFD) was modeled using a nonrectangular hyperbola:
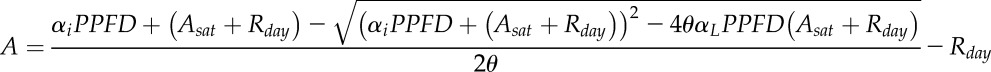 |
(3) |
where αi is the quantum yield of photosynthesis, Asat is the maximum A at saturating light, Rday is the day respiration, and θ is the curvature parameter. This equation was used to simulate the maximum diurnal variations of A in the absence of stomatal limitation under different light intensity conditions.
Determination of Mass Integrated A
A was converted to a mass integrated measurement using leaf mass area (LMA) measured after 28 d of growth:
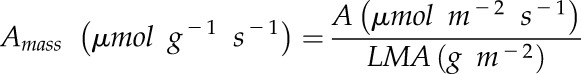 |
(4) |
Daily LUE
Daily LUE was calculated as the ratio between the predicted daily-integrated photosynthesis (g) and the daily-absorbed light intensity (MJ), which represents an instantaneous estimate of LUE. The daily-integrated photosynthesis was predicted using the response of A to light intensity. For each light intensity during the day, the corresponding photosynthesis was calculated and integrated over time. The integrated photosynthesis in µmol m−2 s−1 was converted into g using the molecular mass of C (12 g mol−1). Light intensity in µmol m−2 s−1 was converted into J using a conversion factor (0.16) described in the manual of the Li-Cor 6400 (red + blue light source).
LUE
LUE was calculated as the ratio between leaf dry mass (g) and absorbed light intensity (MJ). The absorbed light was calculated by taking into consideration the increase in area of the rosette through time. The rosette area for each day of growth was predicted using a sigmoidal model adjusted on the observed data (model and parameters are described in Fig. 8A and Supplemental Table S3).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Optical properties including absorbance, transmittance, and reflectance of leaves grown under the four light treatments.
Supplemental Figure S2. Cross sections of leaves grown under the four light treatments.
Supplemental Figure S3. Immunoblot analysis of leaves grown under the four light treatments.
Supplemental Figure S4. Diurnal measurements of gas exchange estimated under fluctuating low light (DFlow) in all four light treatments.
Supplemental Table S1. Cell size and shape from leaf tissues of plants grown under the four light treatments.
Supplemental Table S2. Chlorophyll a/b ratio and total carotenoid-total chlorophyll ratio of plants grown under the four light treatments.
Supplemental Table S3. Parameters describing the increase in area of the rosette as a function of time using a sigmoidal model.
Supplementary Material
Glossary
- NPQ
nonphotochemical quenching
- PPFD
photon flux density
- SQH
square wave high light
- SQL
square wave low light
- FLH
fluctuating high light
- FLL
fluctuating low light
- RuBP
ribulose 1,5-bisphosphate
- LUE
light use efficiency
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (grant no. BB/1001187_1 to T.L.) and by the Natural Environment Research Council (Ph.D. studentship grant no. Env-East DTP E14EE to J.S.A.M.).
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Alter P, Dreissen A, Luo FL, Matsubara S (2012) Acclimatory responses of Arabidopsis to fluctuating light environment: comparison of different sunfleck regimes and accessions. Photosynth Res 113: 221–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou K, Dyson BC, Webster RE, Johnson GN (2010) Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol 152: 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S, Horton P, Walters RG (2004) Acclimation of Arabidopsis thaliana to the light environment: the relationship between photosynthetic function and chloroplast composition. Planta 218: 793–802 [DOI] [PubMed] [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213: 794–801 [DOI] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Bagley JE, Serbin SP, Ruiz-Vera UM, Rosenthal DM, Vanloocke A (2013) Modelling C3 photosynthesis from the chloroplast to the ecosystem. Plant Cell Environ 36: 1641–1657 [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Salvucci ME (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol 161: 1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot BF, Jurik TW, Chabot JF (1979) Influence of instantaneous and integrated light-flux density on leaf anatomy and photosynthesis. Am J Bot 66: 940–945 [Google Scholar]
- Chazdon RL, Pearcy RW (1991) The importance of sunflecks for forest understory plants. Bioscience 41: 760–766 [Google Scholar]
- Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- Ernstsen J, Woodrow IE, Mott KA (1997) Responses of Rubisco activation and deactivation rates to variations in growth-light conditions. Photosynth Res 52: 117–125 [Google Scholar]
- Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24: 755–767 [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Foyer FH, Nurmi A, Dulieu H, Parry MAJ (1993) Analysis of two Rubisco-deficient tobacco mutants, h7 and sp25: evidence for the production of Rubisco large subunits in the sp25 mutant that form clusters and are inactive. J Exp Bot 44: 1445–1452 [Google Scholar]
- Frechilla S, Talbott LD, Zeiger E (2004) The blue light-specific response of Vicia faba stomata acclimates to growth environment. Plant Cell Physiol 45: 1709–1714 [DOI] [PubMed] [Google Scholar]
- Garnier E, Salager JL, Laurent G, Sonié L (1999) Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol 143: 119–129 [Google Scholar]
- Givnish TJ. (1988) Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol 15: 63–92 [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EP, Willingham NM, Lloyd JC, Raines CA (1998) Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 204: 27–36 [Google Scholar]
- Henkes S, Sonnewald U, Badur R, Flachmann R, Stitt M (2001) A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 13: 535–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley PA. (1969) The effect of fluctuating light intensity on plant growth. J Appl Ecol 6: 273–276 [Google Scholar]
- Kono M, Terashima I (2014) Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J Photochem Photobiol B 137: 89–99 [DOI] [PubMed] [Google Scholar]
- Kono M, Terashima I (2016) Elucidation of photoprotective mechanisms of PSI against fluctuating light photoinhibition. Plant Cell Physiol 57: 1405–1414 [DOI] [PubMed] [Google Scholar]
- Külheim C, Agren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotech 23: 215–220 [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M (2005) Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60: 239–260 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54: 2393–2401 [DOI] [PubMed] [Google Scholar]
- López-Juez E, Jarvis RP, Takeuchi A, Page AM, Chory J (1998) New Arabidopsis cue mutants suggest a close connection between plastid- and phytochrome regulation of nuclear gene expression. Plant Physiol 118: 803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, Bassi R, Osmond B (2005) Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may ‘lock-in’ lutein-based photoprotection during acclimation to strong light. J Exp Bot 56: 461–468 [DOI] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol 211: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlyn BE. (1998) Physiological basis of the light use efficiency model. Tree Physiol 18: 167–176 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol 141: 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Horton P (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20: 438–448 [Google Scholar]
- Murchie EH, Hubbart S, Peng S, Horton P (2005) Acclimation of photosynthesis to high irradiance in rice: gene expression and interactions with leaf development. J Exp Bot 56: 449–460 [DOI] [PubMed] [Google Scholar]
- Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64: 3983–3998 [DOI] [PubMed] [Google Scholar]
- Naumburg E, Ellsworth DS (2002) Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species. Tree Physiol 22: 393–401 [DOI] [PubMed] [Google Scholar]
- Niinemets U. (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30: 1052–1071 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25: 693–714 [Google Scholar]
- Oguchi R, Hikosaka K, Hiura T, Hirose T (2008) Costs and benefits of photosynthetic light acclimation by tree seedlings in response to gap formation. Oecologia 155: 665–675 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Long TA, Iwano M, Takayama S, Kobayashi Y, Covert SF, Shikanai T (2007) A balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I. Plant Cell Physiol 48: 1462–1471 [DOI] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, et al. (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112: 8529–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R, Weyers JDB, Lawson T, Godber IM (1998) Rapid and straightforward estimates of photosynthetic characteristics using a portable gas exchange system. Photosynthetica 34: 265–279 [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Pearcy R. (2007) Responses of plants to heterogeneous light environments. In Valladares F, Pugnaire F, eds, Functional Plant Ecology, Ed 2 CRC Press, Boca Raton, FL, pp 213–246 [Google Scholar]
- Pearcy RW. (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41: 421–453 [Google Scholar]
- Pearcy RW, Way DA (2012) Two decades of sunfleck research: looking back to move forward. Tree Physiol 32: 1059–1061 [DOI] [PubMed] [Google Scholar]
- Poorter H, Fiorani F, Pieruschka R, Wojciechowski T, van der Putten WH, Kleyer M, Schurr U, Postma J (2016) Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol 212: 838–855 [DOI] [PubMed] [Google Scholar]
- Porcar-Castell A, Garcia-Plazaola JI, Nichol CJ, Kolari P, Olascoaga B, Kuusinen N, Fernández-Marín B, Pulkkinen M, Juurola E, Nikinmaa E (2012) Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 170: 313–323 [DOI] [PubMed] [Google Scholar]
- Retkute R, Smith-Unna SE, Smith RW, Burgess AJ, Jensen OE, Johnson GN, Preston SP, Murchie EH (2015) Exploiting heterogeneous environments: does photosynthetic acclimation optimize carbon gain in fluctuating light? J Exp Bot 66: 2437–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR (2011) Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol 11: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Lopez-Calcagno PE, Davey PA, Headland LR, Lawson T, Timm S, Bauwe H, Raines CA (2017) Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase H-protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol J http://dx.doi.org/10.1111/pbi.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, McAusland L, Headland LR, Lawson T, Raines CA (2015) Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J Exp Bot 66: 4075–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Muchow RC (1999) Radiation use efficiency. Adv Agron 65: 215–265 [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57: 343–354 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Kangasjärvi S, Aro EM (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB (2013) Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ 36: 2108–2119 [DOI] [PubMed] [Google Scholar]
- Walters R, Horton P (1994) Acclimation of Arabidopsis thaliana to the light environment: changes in composition of the photosynthetic apparatus. Planta 195: 248–256 [DOI] [PubMed] [Google Scholar]
- Watling J, Ball M, Woodrow I (1997) The utilization of lightflecks for growth in four Australian rainforest species. Funct Ecol 11: 231–239 [Google Scholar]
- Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci 6: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston E, Thorogood K, Vinti G, López-Juez E (2000) Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 211: 807–815 [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M (2001) Strategy-shifts in leaf physiology, structure and nutrient content between species of high and low rainfall, and high and low nutrient habitats. Funct Ecol 15: 423–434 [Google Scholar]
- Yamori W. (2016) Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J Plant Res 129: 379–395 [DOI] [PubMed] [Google Scholar]
- Yin ZH, Johnson GN (2000) Photosynthetic acclimation of higher plants to growth in fluctuating light environments. Photosynth Res 63: 97–107 [DOI] [PubMed] [Google Scholar]
- Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton, a new HPLC method using a reversed phase C8 column and phridine-containing mobile phases. Mar Ecol Prog Ser 195: 29–45 [Google Scholar]
- Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.