Mitochondrial transporter ATM3-dependent cytosolic Fe-S cluster assembly is important for the development of lateral roots, root apical meristem, and shoot apical meristem in rice.
Abstract
The mitochondrial ATP-binding cassette transporter ATM3 has been studied in Arabidopsis. Its function, however, is poorly understood in other model plant species. This study reports that the ATM3 is required for cytosolic iron-sulfur cluster assembly and is essential for meristem maintenance in rice (Oryza sativa). The loss of function of OsATM3 is lethal in rice at the four-leaf stage. In the osatm3 T-DNA insertion mutant, the fourth leaf fails to develop and the lateral roots are short. Cytosolic iron-sulfur protein activities were significantly reduced in both osatm3 and RNA interference transgenic lines. The expression profiles of many iron metabolism genes were altered in the osatm3 and RNA interference lines. Glutathione metabolism was impaired and reactive oxygen species, particularly superoxide, accumulated in osatm3. Promoter-β-glucuronidase staining of the transgenic line indicated that OsATM3 is highly expressed in lateral root primordia, root tip meristem zones, and shoot apical meristem regions. The average cell size was significantly greater in osatm3 than in the wild type. Massive cell death occurred in the osatm3 root tip meristem zone. Quantitative RT-PCR revealed transcriptional reprogramming of the genes in the osatm3 and RNAi lines involved in DNA repair and cell cycle arrest. Our results suggest that the mitochondrial ATM3 is essential for iron homeostasis in rice.
Iron-sulfur (Fe-S) proteins are important for photosynthesis, respiration, metabolism, and genome integrity (Balk and Pilon, 2011). The maturation of Fe-S proteins requires the assembly of Fe-S clusters, which takes place in mitochondria, chloroplasts, and cytosol of plant cells, respectively. A number of Fe-S cluster assembly proteins have been identified in Arabidopsis (Arabidopsis thaliana; Balk and Pilon, 2011; Balk and Schaedler, 2014). Fe-S clusters can be assembled in mitochondria by the Iron-Sulfur Cluster machinery, whereas they can be assembled in cytosol by the cytosolic iron-sulfur assembly (CIA) machinery. ATP-binding cassette (ABC) transporters are present in mitochondria. Many of these are half-transporters of the B subfamily. ABC subfamily B member 7 (ABCB7) in animals (Pondarré et al., 2006), Atm1 in yeast (Kispal et al., 1999), and ABC transporter of mitochondrion 3 (ATM3; systematic name: ABCB25) in plants (Bernard et al., 2009) are required for cytosolic Fe-S cluster biosynthesis. In yeast, Atm1 depletion leads to defective Fe-S protein maturation in the cytosol and abnormal iron accumulation in mitochondria (Kispal et al., 1997, 1999). In human cells, the knockdown of ABCB7 leads to decreased cytosolic Fe-S cluster biosynthesis and mitochondrial iron overload (Pondarré et al., 2006; Cavadini et al., 2007). Although complete knockout of ABCB7 is embryo-lethal in mammals, missense mutations in ABCB7 cause X-linked sideroblastic anemia and cerebellar ataxia (XLSA/A) in humans (Rouault and Tong, 2008).
In plants, the ATM3 gene has been studied in Arabidopsis (Kushnir et al., 2001; Kim et al., 2006; Chen et al., 2007; Bernard et al., 2009; Teschner et al., 2010). The atm3-1 mutant, also called sta1, demonstrates dwarfism and chlorosis, and has altered leaf- and nucleus morphologies (Kushnir et al., 2001). ATM3 may participate in the resistance to heavy metal toxicity. Plants overexpressing ATM3 have an enhanced resistance to cadmium (Kim et al., 2006). Three Atm1-like genes, ATM1, ATM2, and ATM3, are present in Arabidopsis. ATM3 resembles yeast Atm1 most closely in terms of Fe-S cluster biosynthesis (Bernard et al., 2009; Chen et al., 2007). The atm3 mutant plants display defects in root growth, chlorophyll content, and seedling establishment. Cytosolic Fe-S protein activities are significantly reduced in atm3 mutants whereas mitochondrial and plastid Fe-S proteins are unaffected (Bernard et al., 2009). Strikingly, the atm3 mutant plants do not display a dramatic iron homeostasis defect and do not accumulate iron in mitochondria. Molybdenum cofactor (Moco) is a prosthetic group in xanthine dehydrogenase (XDH) and aldehyde oxidase (AO). The activities of XDH and AO are significantly lower in atm3 mutants (Bernard et al., 2009). Cyclic pyranopterin monophosphate, the first intermediate in Moco biosynthesis, accumulates in atm3 mutant mitochondria. ATM3 may, therefore, be involved in Moco biosynthesis (Teschner et al., 2010). Because many nuclear Fe-S proteins are involved in DNA replication and repair, the CIA machinery is essential for genome integrity and stability (Paul and Lill, 2015). Several CIA components like AE7 and MMS19 are required for DNA repair in Arabidopsis (Luo et al., 2012; Han et al., 2015). A recent study showed that MMS19 is required for epigenetic regulation because it provides Fe-S clusters to DNA glycosylase ROS1. This enzyme prevents active genes from being silenced (Duan et al., 2015).
ATM3 transporter substrates have been studied. A functional study of an Atm1 ortholog of Novosphingobium aromaticivorans (NaAtm1) revealed that glutathione (GSH) derivatives serve as ATM3 transporter substrates. Because NaAtm1 mediates the export of metallated GSH complexes, it detoxifies heavy metals (Lee et al., 2014). Another study suggested that Atm1 exports GSH-coordinated [Fe-S] (Li and Cowan, 2015). When they are expressed in Lactococcus lactis, AtATM3 and ScAtm1 selectively transport either glutathione disulfide or glutathione trisulfide, but not reduced glutathione. ATM3 may export glutathione polysulfide, which may be a source of sulfide for the cytosolic Fe-S cluster assembly (Schaedler et al., 2014).
Putative Fe-S cluster assembly genes have been identified in rice (Oryza sativa). A few of these genes, including OsATM3, are highly responsive to abiotic stresses (Liang et al., 2014). Little is known about the function of OsATM3 in rice. In this work, an osatm3 T-DNA insertion mutant and RNA interference (RNAi) transgenic lines were characterized. The loss of function of OsATM3 is lethal in rice. Mutant seedlings do not survive to the four-leaf stage. The mitochondrial OsATM3 is essential for cytosolic Fe-S protein biogenesis and iron homeostasis. OsATM3 was highly expressed in lateral root primordia, root tip meristem zones, and shoot apical meristem (SAM) regions. Massive cell death was observed in the root tip meristem zone of the osatm3 mutant.
RESULTS
OsATM3 Knockout Is Lethal in Rice at the Four-Leaf Stage
BLASTP comparison indicated that a single ATM3 ortholog is present in the rice genome and encodes a half ABC transporter in mitochondria (locus: Os06g03770). It has 72% amino acid sequence identity with the AtATM3 of Arabidopsis, and 50% identity with ScAtm1 of yeast. This gene was named rice OsATM3 (Supplemental Fig. S1). Microscopy and transient expression of the OsATM3 protein in Arabidopsis protoplasts demonstrated that it targets mitochondria (Supplemental Fig. S2).
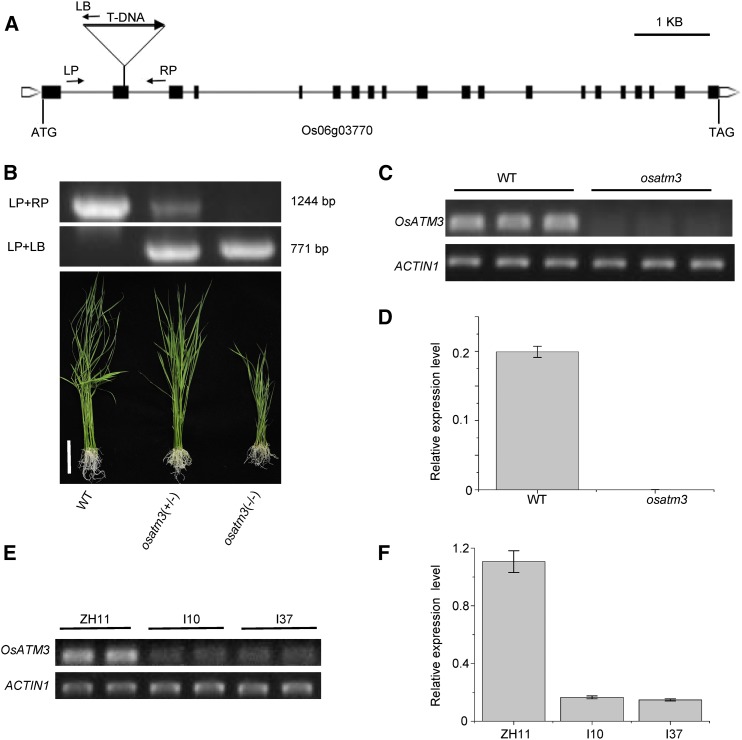
Three mutant lines were obtained with a T-DNA insertion at exon 2 (Fig. 1A), promoter (not shown), and 5′UTR (not shown), respectively. These were screened, and homozygous plants were identified. The mutant lines with insertions at promoter and 5′UTR, respectively, had no phenotype. The expression of the OsATM3 gene in homozygotes was not affected in these lines (not shown). In contrast, very few homozygotes were identified from the screening of the mutant line with an insertion at exon 2. Examination of the genotypes of 222 offspring seedlings germinated from heterozygote seeds revealed a 1:1.66:0.15 ratio of the wild type/heterozygotes/homozygotes. The number of homozygotes was far less than predicted. The results suggest that most of the homozygous mutants are either aborted or unable to germinate. The homozygous mutant seedlings showed a severe growth retardation phenotype (Fig. 1B). Therefore, the osatm3 mutant line with an insertion at exon 2 was used for subsequent analyses. Semiquantitative- and quantitative RT-PCR confirmed that the OsATM3 gene is completely knocked out in the osatm3 mutant (Fig. 1, C and D). RNAi transgenic lines were also generated. Semiquantitative- and quantitative RT-PCR confirmed that the expression of OsATM3 is significantly reduced in RNAi lines (Fig. 1, E and F).
Figure 1.
Identification of osatm3 T-DNA insertion mutant and RNAi transgenic lines. A, Structure of the OsATM3 gene (Os06g03770). Black boxes are exons; lines are introns. ATG and TAG are start and stop codons, respectively. The positions of the T-DNA insertion and the primers (LP, RP, and LB) are indicated. B, Identification of the heterozygote and homozygote of the mutant by genomic DNA PCR (top). Photographs of wild-type, heterozygote (osatm3+/−), and homozygote (osatm3−/−) seedlings are shown (bottom). Bar = 6 cm. C, Semiquantitative RT-PCR of OsATM3 transcripts in wild type and homozygous osatm3 mutant. ACTIN1 was used as a control. D, Quantitative RT-PCR of OsATM3 transcripts in the wild type and homozygous osatm3 mutant. ACTIN1 was used as a control. Error bars represent the sd of three biological replicates. E, Semiquantitative RT-PCR of OsATM3 transcripts in RNAi lines. ZH11 is the wild type; I10 and I37 are independent RNAi lines. F, Quantitative RT-PCR of OsATM3 in RNAi lines. ACTIN1 was used as a control.
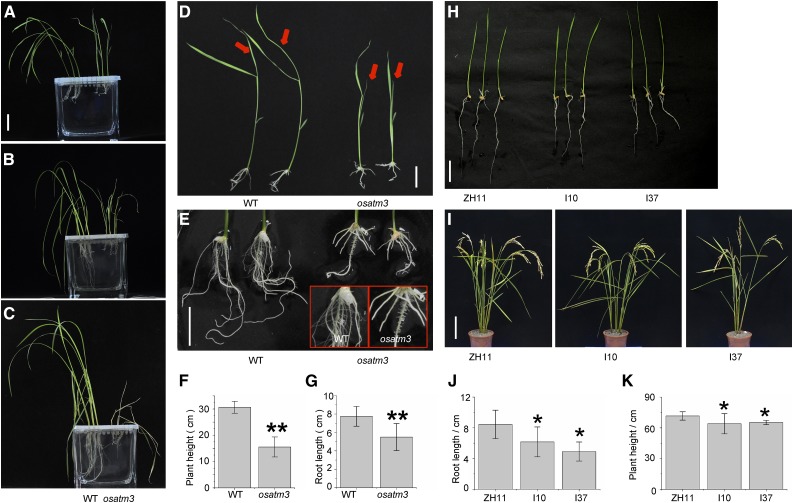
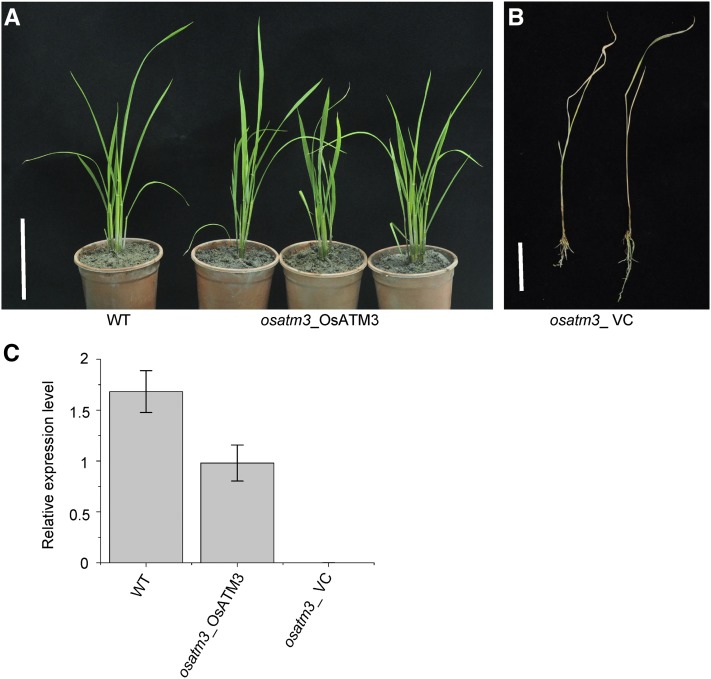
The osatm3 mutant exhibited dwarfism and its fourth leaf failed to develop (Fig. 2, A and D). Wild-type seedlings thrived after the four-leaf stage. In contrast, osatm3 seedlings did not survive this stage and died within two weeks (Fig. 2, B and C). The osatm3 seedlings had far fewer lateral roots than the wild type (Fig. 2E). Quantitation indicated that the average plant height and root length of osatm3 were significantly shorter than those of wild type (Fig. 2, F and G). The RNAi transgenic lines had slightly shorter shoots and roots than wild type (ZH11; Fig. 2, H–K). Recomplemented mutant lines were generated to confirm that these phenotypes were indeed caused by the insertional OsATM3 mutation. The complemented mutant lines survived the four-leaf stage and grew normally (Fig. 3). The complete reversal of the lethal phenotype in the complemented mutant lines indicated that the phenotypes in osatm3 are specifically caused by the loss of function of OsATM3. Overall, the results indicated that the knockout of OsATM3 is lethal in rice at the four-leaf stage.
Figure 2.
Phenotypes of osatm3 and RNAi lines. A, wild-type and osatm3 mutant seedlings were raised in hydroponic culture after germination and grown on 0.5× MS medium for 2 weeks (four-leaf stage). Bars = 3 cm in A to D. B, One week later, the mutant withered and its growth was completely retarded. C, Two weeks later, the mutant died, whereas the wild type continued to thrive. D, Comparison of wild-type and mutant seedlings after germination and growth on 0.5× MS medium for 2 weeks. Red arrows indicate the fourth leaves of the wild type and osatm3. E, Comparison of roots of the wild type and mutant after germination and grown on 0.5× MS medium for 2 weeks. Bar = 2 cm. The inset demonstrates lateral roots of the wild type and osatm3. F and G, Quantitation of plant height and root length of 2-week-old seedlings (n = 30). Main roots were measured. H, Seedlings of ZH11 and RNAi lines germinated in water for 10 d. Bar = 3 cm. ZH11 is the wild type; I10 and I37 are independent RNAi lines. I, ZH11 and RNAi line plants at the maturation stage. Bar = 15 cm. J, Quantitation of root length of seedlings germinated in water for 10 d (n = 30). Main roots were measured. K, Quantitation of height of plants grown in paddy field (n = 15). Error bars represent the sd. Significance analysis was performed in comparison with the wild type using Student’s t test. *P < 0.05, **P < 0.01. osatm3: Homozygous mutant.
Figure 3.
Complementation of osatm3 mutant with OsATM3. A, Comparison of the wild type and the complemented osatm3 mutant (osatm3_OsATM3). Bar = 15 cm. B, The osatm3 mutant transformed with empty vector (osatm3_ VC) died at the four-leaf stage. Bar = 3 cm. C, Quantitative RT-PCR of OsATM3 transcripts. (n = 3).
Cytosolic Fe-S Cluster Assembly Is Impaired in osatm3 Mutant and RNAi Lines
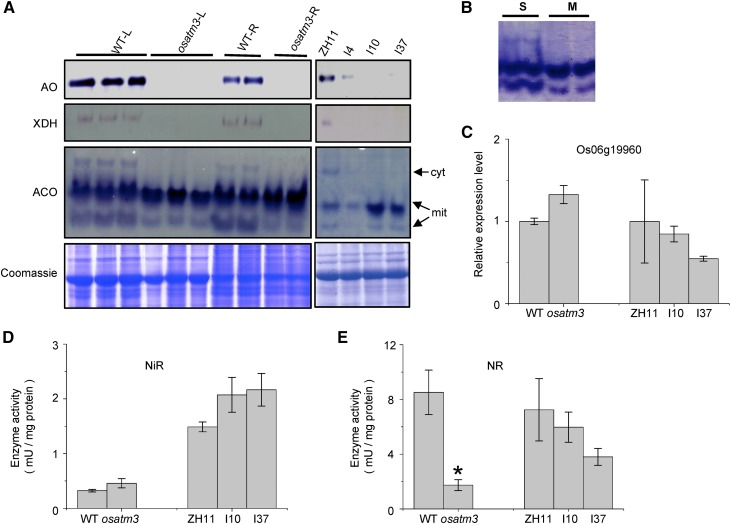
ATM3-like proteins are required for cytosolic Fe-S cluster biosynthesis in various species (Kispal et al., 1999; Pondarré et al., 2006; Bernard et al., 2009). To determine whether OsATM3 is involved in the cytosolic Fe-S cluster assembly of rice, the enzyme activities of three cytosolic Fe-S proteins, aldehyde oxidase (AO), xanthine dehydrogenase (XDH), and aconitase (ACO), were analyzed. AO and XDH contain [2Fe-2S] clusters and Moco cofactors, whereas ACO contains a [4Fe-4S] cluster (Bernard et al., 2009). The activities of AO and XDH were significantly reduced in osatm3 and RNAi lines relative to the wild type (Fig. 4A). Plant ACO consists of one isoform in the cytosol and two in the mitochondria (Fig. 4B). The activity of cytosolic ACO was significantly reduced in the osatm3 and RNAi lines whereas those of the mitochondrial isoforms were unaffected (Fig. 4A). The quantitative RT-PCR data indicated that the cytosolic ACO gene expresses normally in osatm3 and RNAi lines (Fig. 4C). Analysis of other Fe-S and Moco proteins like nitrite reductase (NiR) in chloroplasts and nitrate reductase (NR) in the cytosol indicated that Fe-S protein activities in the cytosol decreased whereas those in other organelles remain unaffected in the osatm3 and RNAi lines (Fig. 4, D and E). These results indicate that OsATM3 is required for cytosolic Fe-S cluster assembly in rice.
Figure 4.
Fe-S cluster biosynthesis is impaired in osatm3 and RNAi lines. A, In-gel activity assays for AO, XDH, and ACO. Coomassie blue staining of SDS-PAGE indicated equal loading. ZH11 represents the wild type; I4, I10, and I37 are independent RNAi lines. B, Identification of mitochondrial ACO isoforms by in-gel activity assay. C, Quantitative RT-PCR analysis of Os06g19960 (predicted to encode a cytosolic aconitase in rice; n = 3). D, Activity assay for NiR in leaf (n = 3). E, Activity assay for NR in leaf (n = 3). Significance analysis was performed in comparison with the wild type using Student’s t test. *P < 0.05. Cyt, Cytosolic ACO isoform; L, leaf; M, protein extract from wild-type mitochondrial fraction; mit, mitochondrial isoform; R, root; S, protein extract from whole wild-type seedlings.
Iron Metabolism Is Impaired in osatm3 Mutant and RNAi Lines
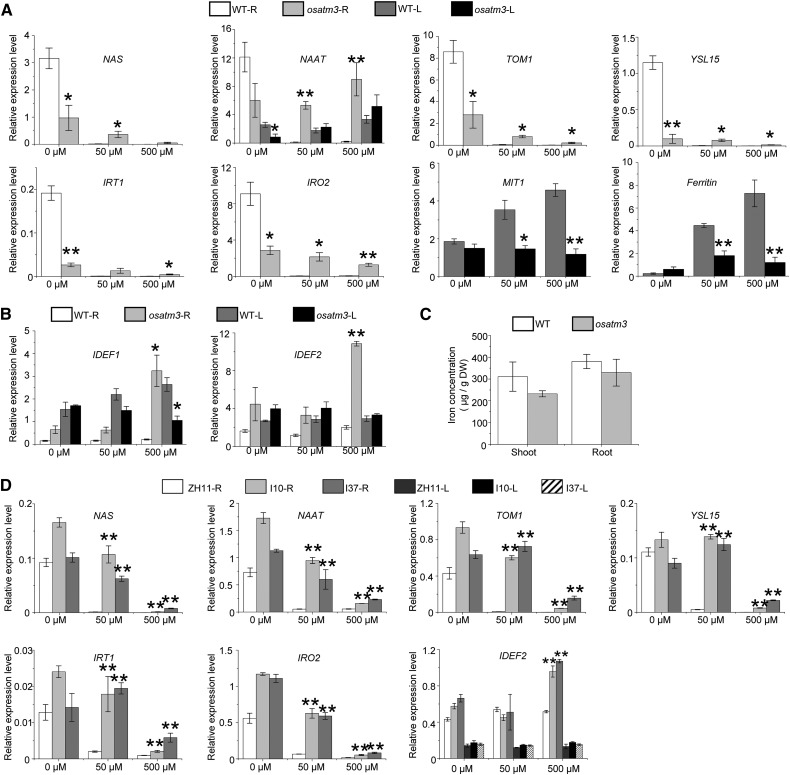
Several rice genes involved in iron metabolism are induced by iron deficiency (Kobayashi and Nishizawa, 2012). In roots, NAS, NAAT, and TOM1 are responsible for the biosynthesis and export of iron chelators whereas YSL15, IRT1, and IRO2 encode iron transporters and transcriptional regulators. The iron metabolism genes were expressed at minimum levels under normal (50 µm Fe) and excess (500 µm Fe) iron concentrations. These genes were strongly induced under iron deficiency (0 μm Fe) in the wild type (Fig. 5A). In contrast, they were constitutively expressed at low levels in the osatm3 regardless of external iron conditions (Fig. 5A). MIT1 encodes a mitochondrial iron transporter and ferritin encodes the iron storage protein in chloroplasts. In rice, they are repressed by iron deficiencies and induced by excesses of iron (Kobayashi and Nishizawa, 2012). The expression of MIT1 and ferritin was up-regulated by high iron concentrations in the wild type. In contrast, they were constitutively expressed at low levels in osatm3 (Fig. 5A). IDEF1 and IDEF2 encode transcriptional regulators for iron metabolism and are constitutively expressed in the wild type. In osatm3, excess iron strongly induced both IDEF1 and IDEF2 (Fig. 5B). Relative to the wild type, the iron concentration was not significantly altered in osatm3 (Fig. 5C). Constitutive expression of several iron metabolism genes was also found in RNAi lines (Fig. 5D). In summary, the expression profiles of several iron metabolism genes were altered in both the osatm3 and the RNAi lines.
Figure 5.
Expression profiles of iron metabolism genes are altered in osatm3 and RNAi lines. A, Quantitative RT-PCR analysis of iron metabolism genes in osatm3 that are regulated by iron concentrations in the wild type (n = 3). Iron concentrations in hydroponic culture are indicated: 0, 50, and 500 µm. B, Quantitative RT-PCR of transcriptional regulator genes, IDEF1 and IDEF2, which are constitutively expressed in the wild type (n = 3). C, Quantitation of iron concentrations in rice seedlings grown on 0.5× MS medium for 2 weeks (n = 3). D, Quantitative RT-PCR of iron metabolism genes in RNAi lines (n = 3). Significance analysis was performed in comparison with wild type using Student’s t test. *P < 0.05, **P < 0.01. L: leaf; R: root.
GSH Metabolism Is Impaired in osatm3 Mutant and RNAi Lines
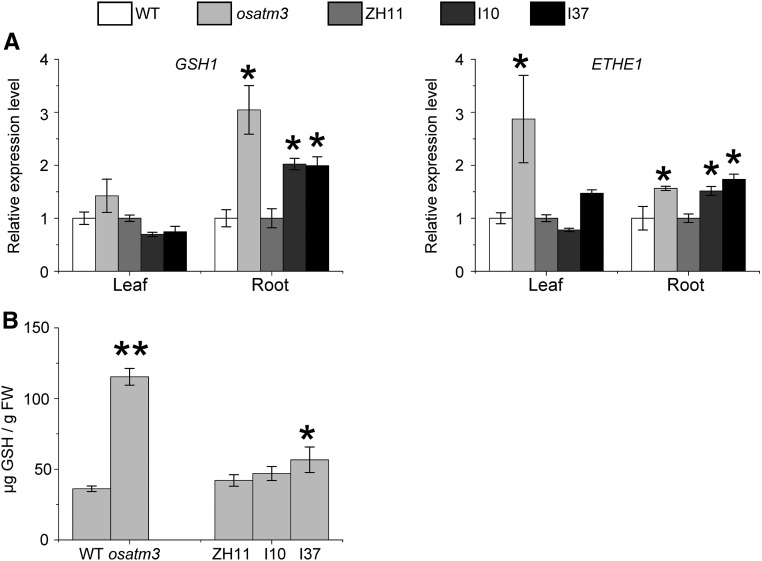
GSH persulfide-containing complexes may be substrates for ATM3-like transporters (Lee et al., 2014; Schaedler et al., 2014; Srinivasan et al., 2014). Loss of function of OsATM3 may impair GSH metabolism in rice. The expression of GSH1 was analyzed. It encodes a Glu-Cys ligase that catalyzes the first step in glutathione synthesis (Rawlins et al., 1995; Wachter et al., 2005). GSH1 was up-regulated in osatm3 and RNAi roots (Fig. 6A), so GSH biosynthesis may have been enhanced. Glutathione disulfides accumulate in the mitochondria of the Arabidopsis mutant atatm3 (Schaedler et al., 2014). ETHE1 is mitochondrial sulfur dioxygenase that oxidizes persulfide and sulfide to thiosulfate and sulfate, respectively (Holdorf et al., 2012; Krüssel et al., 2014). The expression of ETHE1 was analyzed and found to be up-regulated in osatm3 and RNAi roots (Fig. 6A). Total GSH was enhanced in both osatm3 and one of the RNAi lines (Fig. 6B). Taken together, the results suggest that mitochondrial GSH- and persulfide metabolism are impaired in the osatm3 and RNAi lines.
Figure 6.
GSH metabolism is impaired in osatm3 and RNAi lines. A, Quantitative RT-PCR analysis of genes encoding Glu-Cys ligase (GSH1) and sulfur dioxygenase (ETHE1) in osatm3 and RNAi lines (n = 3). B, Total GSH quantitation of seedlings (n = 3). Significance analysis was performed in comparison with the wild type using Student’s t test. *P < 0.05, **P < 0.01.
Reactive Oxygen Species, Especially Superoxide Anions, Accumulate in osatm3 Mutant
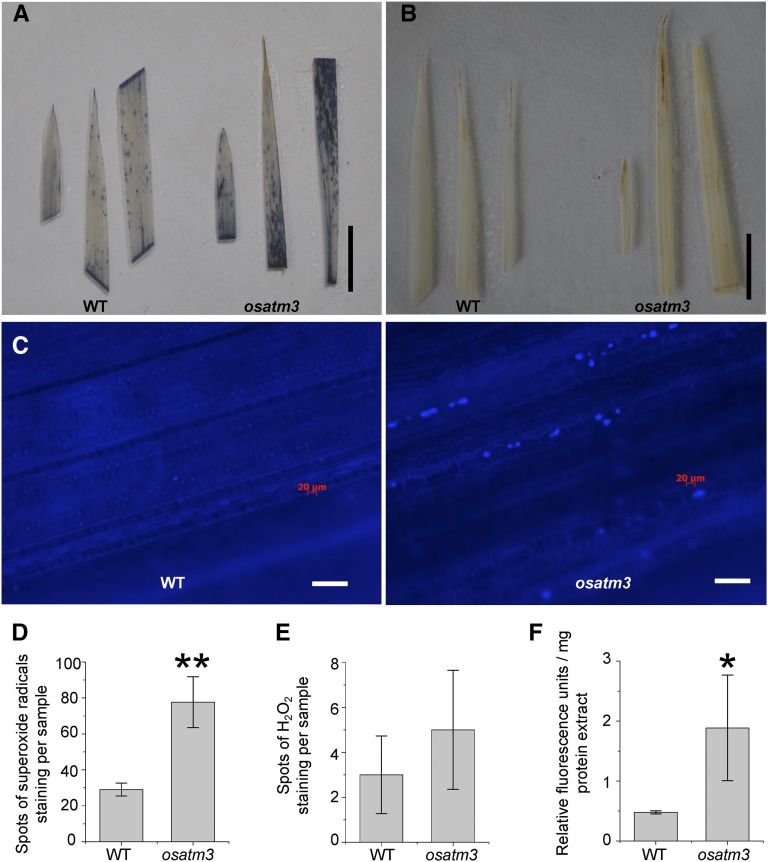
Mitochondrial ATM3-like transporters are often linked to oxidative stress because they transport substances like GSH persulfides and heavy metals. The loss of function of OsATM3 may cause oxidative stress in rice. The levels of reactive oxygen species (ROS) were analyzed in the osatm3 mutant. Significant amounts of superoxide anions but not peroxides accumulated in osatm3 leaves (Fig. 7, A, B, D, and E). Callose, a polysaccharide produced in response to biotic and abiotic stresses, deposited in osatm3 leaves (Fig. 7C). Total ROS in whole lysate increased nearly 4-fold in osatm3 relative to the wild type (Fig. 7F). Overall, these results suggest that there is significant oxidative stress in osatm3.
Figure 7.
ROS species accumulate in osatm3 mutant. A, Histochemical staining of superoxide radicals in leaves. Bar = 1 cm. B, Histochemical staining of H2O2 in leaves. Bar = 1 cm. C, Callose deposition in osatm3 mutant revealed by Aniline Blue staining. Bar = 60 μm. D and E, Quantitation of superoxide radical spots (D) and H2O2 spots (E); n = 4. F, Total ROS measurement in whole lysates using 2′,7′-dichlorofluorescein (n = 3). Significance analysis was performed in comparison with wild type using Student’s t test. *P < 0.05, **P < 0.01.
OsATM3 Is Highly Expressed in the Meristem
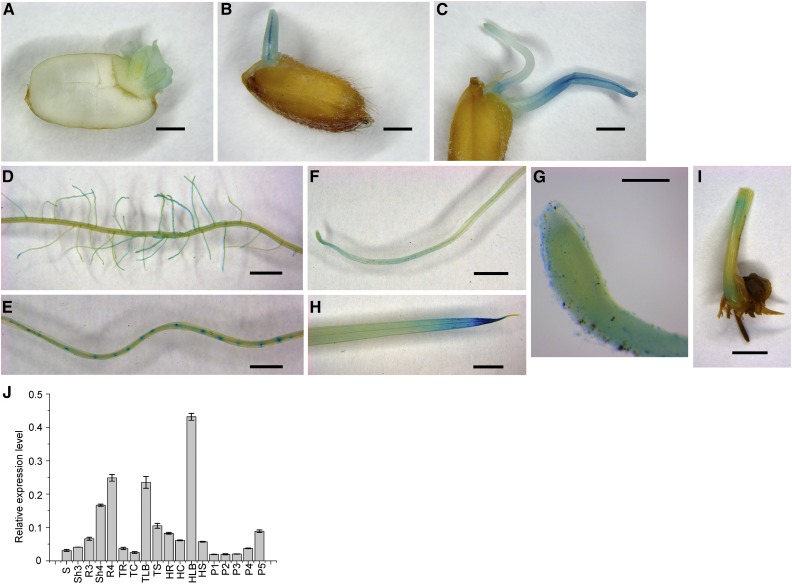
Spatiotemporal OsATM3 expression was investigated by generating pOsATM3:GUS transgenic rice and performing β-glucuronidase (GUS) staining on its seedlings. OsATM3 was initially expressed in the fresh embryo after 2 d germination (Fig. 8A). It was moderately expressed in the coleoptile 4 d after germination (Fig. 8B). OsATM3 was expressed mainly in the primary root tip and hypocotyl. Six d after germination, however, it was strongly expressed in the coleoptile (Fig. 8C).
Figure 8.
Tissue-specific expression of OsATM3. A to I, pOsATM3:GUS expression analysis in germinating seeds and 2-week-old transgenic rice seedlings. A, Seed germinated for 2 d. B, Seed germinated for 4 d. C, Seed germinated for 6 d. D, Lateral roots. E, Root maturation zone. The densely stained spots are lateral root primordia. F, Root elongation zone. G, Root tip. H, Leaf blade. I, Basal region of shoot. Bars = 1 mm (A–E), 2 mm (F, H, and I), and 200 μm (G), respectively. J, Spatiotemporal expression analysis of OsATM3 in wild-type rice by quantitative RT-PCR (n = 3). HC, HLB, HR, and HS, Culm, leaf blade, root, and sheath at the heading stage; P1 to P5, panicles at various developmental stages as measured by the panicle length; P1, 5 cm; P2, 10 cm; P3, 18 cm; P4, 23 cm; P5, mature; R3, shoot and root at the three-leaf stage; S, seed; Sh3 and R3, shoot and root at the three-leaf stage; Sh4 and R4, shoot and root at the four-leaf stage; TC, TLB, TR, and TS, culm, leaf blade, root, and sheath at the tillering stage.
Many lateral roots developed along the main root at the maturation zone. In rice seedlings at the four-leaf stage, OsATM3 was highly expressed in the lateral roots but not the main root (Fig. 8D). OsATM3 was also highly expressed in lateral root primordia (Fig. 8E). The results suggest that OsATM3 may be required for lateral root development. OsATM3 was also highly expressed in the root tip elongation- and meristem zones (Fig. 8, F and G). OsATM3 expression was generally low in the shoot. It was, however, highly expressed in the leaf blade tip (Fig. 8H) and the basal region of the shoot (Fig. 8I), which contain leaf primordia and SAMs. The meristem consists of cells that can divide persistently and periodically and drive tissue growth and differentiation. A high expression of OsATM3 in the root- and shoot meristems suggests that it may be important for cell division.
Next, the spatiotemporal expression of rice OsATM3 was investigated using quantitative RT-PCR (Fig. 8J). In seeds, this gene was expressed at a minimum level. The expression of OsATM3 was induced at germination and gradually increased in both the shoot and the root. OsATM3 expression was high at the four-leaf stage and was higher in the root than the shoot. At the tillering stage, OsATM3 expression was minimal in the root and the culm but high in the leaf blade. In the panicles, the expression of OsATM3 was enhanced 4-fold from the P3- to the P5 stage. Therefore, OsATM3 may be required for seed maturation. In summary, both the GUS staining and the qRT-PCR data indicate that OsATM3 participates in early seedling development (especially the root) and that tissue-specific OsATM3 expression is highly regulated in rice.
Root Tip Meristem Cell Death in osatm3 Mutant
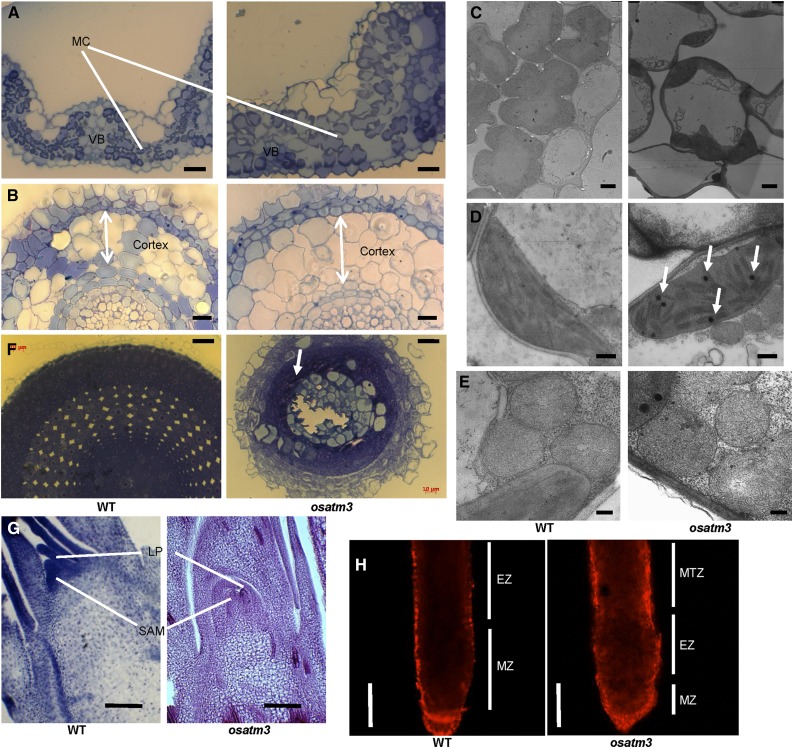
It is yet unknown why the osatm3 seedlings died at the four-leaf stage. In the attempt to determine the cause, osatm3 mutant tissues were dissected at the four-leaf stage and observed with light and transmission electron microscopy (TEM). The average mesophyll cell sizes in the leaf blade cross sections were 11 (±1.8) µm in the wild type and 22 (±4.9) µm in osatm3 (Fig. 9A). The average cortex parenchyma cell sizes in the root cross sections were 25 (±3.2) µm in the wild type and 31 (±3.5) µm in osatm3 (Fig. 9B). TEM imaging revealed that osatm3 mesophyll cells lost their typical morphology and were significantly larger than those in the wild type (Fig. 9C). The cell enlargement in osatm3 suggests that cell division is limited there. TEM also revealed that the morphology and ultrastructure of chloroplasts (Fig. 9D) and mitochondria (Fig. 9E) seem normal but several osmiophilic globules were present in osatm3 chloroplasts.
Figure 9.
Microscopic imaging of osatm3. A, Cross section of leaf blades of wild type (left) and osatm3 mutant (right). Bars = 20 µm. Eight cells were counted. B, Cross section of roots. Arrows indicate cortex thickness. Bars = 20 μm. Eight cells were counted. C, Mesophyll cells under TEM. Bars = 2 μm. D, Chloroplasts under TEM. Arrows indicate osmiophilic globules. Bars = 500 nm. E, Mitochondria under TEM. Bars = 200 nm. F, Cross section of root apex. The arrow indicates dead cells. Bars = 20 µm. G, Longitudinal section of shoot apex. Bars = 80 µm. H, PI staining of root. Bars = 200 µm. EZ, Elongation zone; MC, mesophyll cells; MTZ, maturation zone; MZ, meristem zone; VB, vascular bundles.
OsATM3 is highly expressed in the root tip (Fig. 8, F and G) and the basal shoot region (Fig. 8I). These tissues contain the apical root and shoot meristems, respectively. We dissected these locations and observed them under the microscope. The wild-type root apex cross section included cells with large, aligned central nuclei. These are root apical meristem (RAM) cells (Fig. 9F). In contrast, cross-section imaging revealed many dead cells and cell debris in the root apex of the osatm3 mutant. These results suggest that the RAM is nonfunctional in osatm3 and that the death of these cells might be a cause of death in osatm3 seedlings. The longitudinal section of shoot apex demonstrated that both the leaf primordia (LP) and SAM are significantly shorter in osatm3 than those in the wild type (Fig. 9G). There was no evidence of cell death in SAM. Propidium iodine (PI) is a fluorescent nucleic acid stain. It reveals damaged and dead cells whereas intact cells are impermeable to it. The roots were examined using PI staining. In contrast to the wild type, the osatm3 root was heavily stained by PI (Fig. 9H). Therefore, cell damage and cell death occurred in osatm3. PI staining also revealed that the root meristem- and elongation zones in osatm3 collapsed.
Transcriptional Reprogramming of Genes Involved in DNA Repair and Cell Cycle Arrest in osatm3 Mutant and RNAi Lines
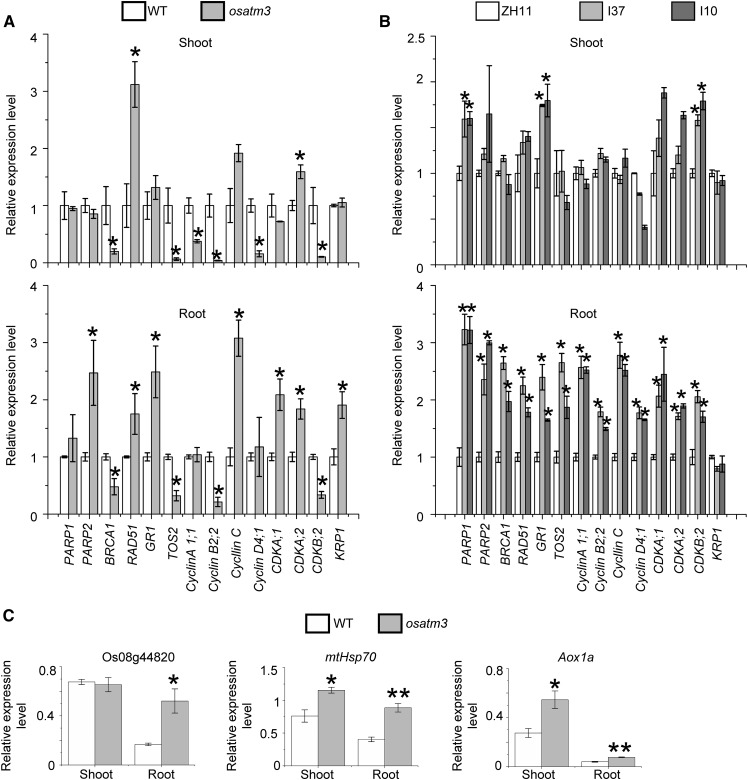
ATM3 and the CIA pathway proteins like AE7 are required for the maturation of the nuclear Fe-S proteins involved in DNA duplication and repair. In Arabidopsis, DNA repair genes (PARP1, PARP2, BRCA1, RAD51, TOS2, and GR1) are up-regulated when DNA is damaged (Luo et al., 2012). The effect of loss of function of OsATM3 on DNA repair was analyzed using the expression of the aforementioned genes. PARP2, RAD51, and TOS2 were up-regulated in osatm3 root, whereas BRCA1 and GR1 were down-regulated (Fig. 10A). Therefore, osatm3 responds abnormally to DNA damage.
Figure 10.
Quantitative RT-PCR analysis of genes involved in DNA repair and cell cycle control in osatm3 and RNA lines. A, Expression analysis of genes involved in DNA repair and cell cycle arrest in osatm3 seedlings (n = 3). PARP1, PARP2, BRCA1, RAD51, TOS2, and GR1 are involved in DNA repair. The other genes are involved in cell cycle arrest. B, Expression analysis of genes involved in DNA repair and cell cycle arrest in RNAi lines (n = 3). C, Expression analysis of genes involved in the PCD (apoptosis) of osatm3 seedlings (n = 3). Significance analysis was performed in comparison with the wild type using Student’s t test. *P < 0.05, **P < 0.01.
Cell division may be limited in osatm3 because its cells were larger than those in the wild type (Fig. 9). Cell cycle control genes were also analyzed. Various cyclins and cyclin-dependent kinases (CDK) are required for G1-S and G2-M phase transitions whereas KIP-related protein is a CDK inhibitor (Inzé and De Veylder, 2006; Komaki and Sugimoto, 2012). Several cyclin- and CDK genes were down-regulated whereas KIP-related protein was up-regulated (Fig. 10A). Thus, there may be a cell cycle arrest in osatm3 cells. Several other genes like CDKA;1 and CDKA;2 were up-regulated in osatm3 possibly in response to RAM damage. These genes are crucial for root stem cells and meristems (Nowack et al., 2012). Several genes involved in DNA repair and cell cycle control were also up-regulated in RNAi lines (Fig. 10B).
ROS and other stressors may induce programmed cell death (PCD; apoptosis) in plants. The NAC transcription factor, known as Os08g44820 in rice, is involved in oxidative stress response (De Clercq et al., 2013). Mitochondrial heat shock protein 70 is up-regulated in salt-induced PCD of rice (Qi et al., 2011). Mitochondrial alternative oxidase 1a is induced by oxidative stress (Li et al., 2013). Upon analysis, all three genes were found to be up-regulated in osatm3 (Fig. 10C).
DISCUSSION
The function of mitochondrial ATM3 is poorly understood in plant species other than Arabidopsis. In this study, our results demonstrate that ATM3 is essential for iron homeostasis in rice. Several iron metabolism genes that are regulated by iron concentrations in wild-type rice were constitutively expressed in the osatm3 and RNAi lines (Fig. 5), although this alteration is not as dramatic as the constitutive up-regulation found in yeast and mouse mutants (Kispal et al., 1999; Pondarré et al., 2006). These findings suggest that OsATM3, or the signal exported by it, may participate in the transcriptional regulation of some of these genes. MIT1, which encodes the mitochondrial iron importer, and ferritin, which encodes the iron storage protein in chloroplasts, were down-regulated in osatm3 (Fig. 5A). Therefore, iron accumulation is improbable in the mitochondria and chloroplasts of osatm3. The results suggest that certain functional aspects of ATM3 in plants are indeed different from the yeast and mouse homologs.
The spatial and temporal expression of OsATM3 was strictly regulated in rice. It was highly up-regulated from the three- to four-leaf stage in both the shoot and the root (Fig. 8J). In rice, the four-leaf stage represents a critical transition from the juvenile- to the adult phase. The first three leaves of the rice seedling are differentiated from the embryo whereas all subsequent leaves develop from the SAM (Poethig, 1990; Asai et al., 2002). Failure of the fourth- and subsequent leaves to develop in osatm3 may be explained by the dysfunction of SAM, which, in turn, may be caused by the loss of function of OsATM3. OsATM3 was highly expressed in the lateral roots and their primordia (Fig. 8, D and E). Therefore, OsATM3 is essential for the initiation and development of lateral roots in rice. In fact, the osatm3 mutant has a remarkable lateral root development phenotype (Fig. 2E). OsATM3 was also highly expressed in the root tip meristem zone (Fig. 8, F and G) wherein the RAM is located. RAM cells divide to generate new roots. The loss of function of OsATM3 may limit RAM cell division. This inhibition may cause massive meristem zone cell death (Fig. 9F) and the collapse of the root tip architecture in osatm3 (Fig. 9H). The severe abnormality identified in the root of osatm3 impairs its nutrient uptake function and may kill it altogether. The expression of several genes involved in DNA repair and cell cycle arrest was significantly altered in both osatm3 and RNAi lines. The changes in the gene expression, however, were different between osatm3 and RNAi lines, probably because that the insertional mutation is so severe and has secondary effects in osatm3 mutant.
ABCB7, the ATM3 homolog in mammals, is required for Fe-S protein biogenesis in the cytosol and the nuclei (Rouault and Tong, 2008; Stehling et al., 2014). Gene mutations affecting a transmembrane domain in the ABCB7 transporter cause XLSA/A in humans (Beilschmidt and Puccio, 2014; Stehling et al., 2014). A component of the cytosolic Fe-S cluster assembly (CIA) such as MMS19 participates in DNA repair and genomic integrity (Stehling et al., 2012). Because ABCB7 acts upstream of the CIA pathway, a defect in ABCB7 would probably impair the functionality of the MMS19 and the CIA pathway. Therefore, an investigation into the defects in DNA repair in XLSA/A patients might be useful.
A previous study showed that OsATM3 is an essential Fe-S cluster assembly gene involved in abiotic stress tolerance in rice (Liang et al., 2014). Its expression, especially in roots, is up-regulated in response to excess iron, oxidative stress, and heavy metals. These stressors severely inhibit root growth (Liang et al., 2014). This study revealed that OsATM3 is important in lateral root development and root tip growth (Figs. 2, 8, and 9). The resistance of rice to abiotic stress might be increased by enhancing OsATM3-mediated root development.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The osatm3 T-DNA insertion mutants were obtained from the Crop Biotech Institute, Kyung Hee University, Republic of Korea (Jeon et al., 2000; Jeong et al., 2006). The mutant nomenclature is Oryza sativa var Japonica ‘Dongjin’. Homozygotes were screened by sowing the seeds in soil under natural conditions then determining seedling genotypes by genomic DNA PCR using primers designed from the RiceGE database of the Salk Institute (http://signal.salk.edu/cgi-bin/RiceGE). For this study, the mutant line with a T-DNA insertion at exon 2 was used. Its homozygous seedling is lethal at the four-leaf stage. Seeds harvested from the heterozygous mutant were germinated and grown for 2 weeks (four-leaf stage) on 0.5× Murashige and Skoog (MS) medium containing 3% Suc and 0.8% agar. All rice seedlings were grown at 25 to 28°C on 13-h-light/11-h-dark cycles. They were then transferred to hydroponic solution as described in Zhang et al. (2012). Homozygous mutant seedlings were screened and used for all subsequent analyses.
Plasmid Construction and Rice Transformation
To analyze the OsATM3 promoter, a 2167-bp promoter region upstream of the start codon was amplified by PCR from wild-type genomic DNA using primers designed from NCBI/Primer-BLAST (Supplemental Table S1). The amplicon was subcloned upstream of uidA in pCAMBIA1391z digested with HindIII and EcoRI. To construct the RNAi vector, two 378-bp fragments of the OsATM3 coding sequence region were subcloned downstream of the Ubi-1 promoter in pTCK303 with digestion sites at KpnI/BamHI and SpeI/SacI, respectively.
O. sativa var Japonica ‘Zhonghua 11’ was transformed with Agrobacterium tumefaciens strain EHA105 according to a previously published method (Hiei et al., 1997). All transgenic plants were generated on media containing 25 mg·L−1 hygromycin B then transferred to 0.5× MS medium (supplemented with 1.5% Suc and 0.8% agar) for rooting. The regenerated plants were grown on soil under natural conditions. The T2 or T3 generation of the homozygous transgenic lines was screened using PCR (Supplemental Table S1).
To complement the osatm3 mutant, the OsATM3 ORF sequence was subcloned into pCAMBIA2301 at the EcoRI and AdeI digestion sites and driven by its native promoter (a 2-kb region upstream of the start codon). An empty vector was used as a negative control. The construct was used to transform callus induced from the seeds of heterozygous mutants via A. tumefaciens-mediated transformation. There were not enough homozygous seeds available for callus induction. G418 disulfate salt (Sigma-Aldrich) was used as a selection marker. Positive plants with a homozygous mutant background were screened from the T1 or T2 generation by PCR.
Subcellular Localization of OsATM3
The full-length coding region (2,199 bp without stop codon) or the signal peptide region (294 bp) of OsATM3 was amplified by PCR (Supplemental Table S1). The PCR amplicon was digested with SalI and SacI and fused to a p-35S-GFP vector driven by a CaMV 35S promoter. The resulting constructs were used for transformation and transient expression in Arabidopsis protoplasts via polyethylene glycol-mediated transformation as described in Yoo et al. (2007). Mt-rk was used as a mitochondrial marker. It has a yeast (Saccharomyces cerevisiae) cytochrome oxidase IV mitochondrial targeting sequence fused with the red fluorescent protein mCherry. The transformed protoplasts were viewed under a fluorescence microscope (Axio Imager A2; Carl Zeiss).
Semiquantitative RT-PCR and Quantitative RT-PCR
Total RNA was extracted from rice tissues using Plant RNA Kit (Omega) following the manufacturer’s instructions. The extracted RNA was reverse-transcribed into cDNA using a reverse transcription kit including a genomic DNA eraser (TaKaRa). Semiquantitative RT-PCR was performed in 25 or 28 cycles for ACTIN1 and other genes and 35 cycles for OsATM3. ACTIN1 (Os03g50890) was used as the internal control. Quantitative RT-PCR was conducted in 40 cycles on a Light Cycler 480II (Roche) according to the manufacturer’s manual (SYBR Premix Ex Taq; TaKaRa). Primer sequences are shown in Supplemental Table S1.
Enzyme Activity Assays
Proteins were extracted by grinding leaf or rot tissues with equal volumes of extraction buffer (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 1% [v/v] Triton X-100, 1 mm DTT, Roche Protease Inhibitor Cocktail, and 1 mm PMSF), followed by centrifugation at 12,000 rpm, 4°C for 30 min. Protein was quantified using Protein Assay Kit II (Bio-Rad). For the ACO, AO, and XDH activity assays, 50 to 100 µg protein was mixed with loading buffer (20 mm Tris-HCl 8.0, 80% [v/v] glycerol, and 0.1% [w/v] Bromophenol Blue) and then separated on native PAGE gel. The details of in-gel activity assays for ACO, AO, and XDH were described previously (Koshiba et al., 1996; Bernard et al., 2009). NR activity was measured based on nitrite production. NiR activity was assayed as described by Takahashi et al. (2001). Two-hundred microliters of protein extract was mixed with 800 µL of a solution containing 100 mm P buffer at pH 7.5, 75 mm KNO3, and 0.5 mg·mL−1 NADH incubated at 25°C for 30 min. Immediately after incubation, 1 mL of 1% (w/v) sulfanilamide dissolved in 3 m HCl and 1 mL of 0.02% (w/v) N-(1-naphthyl)ethylenediamine dihydrochloride were added to the mixture that was then left to stand 15 min after which its optical density was measured in a spectrophotometer at 540 nm.
Mitochondrial Fractionation
Mitochondria-enriched fractions were prepared from hydroponically cultured rice seedlings following the method of Sweetlove et al. (2007). Approximately 10 g rice seedlings were homogenized on ice with 50 mL extraction buffer containing 0.3 m Suc, 25 mm Na4P2O7, 2 mm EDTA, 10 mm KH2PO4, 1% (w/v) PVP-40, 1% (w/v) BSA, and 20 mm ascorbic acid at pH 7.5. The extract was filtered through Miracloth (Merck Millipore). The filtrate was centrifuged at 1,100g for 5 min at 4°C. The resulting supernatant was centrifuged at 18,000g for 20 min at 4°C. Pellets were collected as mitochondria-enriched fractions.
Iron Quantification and GSH Measurement
Iron concentrations in rice tissues were quantified by inductively coupled plasma mass spectroscopy (7700 series; Agilent Technologies) as described by Liang et al. (2014). The GSH content was measured as described by Kim et al. (2006) but with modifications. Whole plants were ground in 5% sulfosalicylic acid at 4°C then centrifuged twice at 14,000g for 15 min at 4°C. The supernatant was diluted in 0.1 m P buffer (pH 7.4), of which 2.48 mL of the diluted supernatant was mixed with 20 μL of 10 mm 5′,5′-dithiobis (2-nitrobenzoic acid) and incubated for 5 min. Absorbance was measured at 412 nm. The GSH content was calculated using a GSH standard curve.
Detection of ROS
ROS were detected by histochemical staining and fluorospectrophotometry. Superoxide anion was stained with NBT. Hydrogen peroxide was stained with diaminobenzidine tetrahydrochloride. Leaf fragments of equal size were selected and stained to enumerate NBT and diaminobenzidine tetrahydrochloride spots. Statistical analyses were applied to the spot counts. To determine total ROS, whole plants were ground in liquid nitrogen, dissolved in 10 mm Tris-HCl (pH 7.2), and centrifuged at 12,000g for 20 min at 4°C. The supernatant was mixed with esterified 2′,7′-dichlorofluorescein and its fluorescence monitored in an LS 55 Fluorescence Spectrometer (Perkin-Elmer). Callose deposition was visualized by Aniline Blue staining (Wang and Liu, 2006) and observed under a fluorescence microscope (Axio Imager A2; Carl Zeiss).
β-GUS Staining
T2 or T3 generation plants were used for GUS staining as described elsewhere, but with modifications (Jefferson et al., 1987). Tissues were sampled and incubated in 50 mm sodium P buffer (pH 7.0) containing 1 mm X-gluc, 1 mm EDTA, 0.05% (v/v) Triton X-100, 0.1 mm potassium ferrocyanide, and 0.1 mm potassium ferricyanide at 37°C overnight, then transferred into 70% (w/v) ethanol to remove the chlorophyll. Stained tissues were imaged with a M165C stereomicroscope (Leica).
Sections and Microscopy Observation
Leaves or roots from 2-week-old rice seedlings were fixed in 4% paraformaldehyde (Sigma-Aldrich) and 2% glutaraldehyde (Sigma-Aldrich) in 0.1 m PBS (pH 7.2) and postfixed in 2% (w/v) osmium tetroxide in 0.1 M PBS overnight. The fixed specimens were embedded in SPI-Pon 812 (SPI Supplies), and used to make ultrathin (80 nm) or semithin (2 µm) sections with an Ultracut E ultramicrotome (Leica). TEM was performed following the manufacturer’s manual. Images were photographed using a JEM-1010 Electron Microscope (JEOL) at 15 kV. Basal region of the 2-week-old rice seedling was fixed in 70% ethanol, 5% acetic acid, and 3.7% formaldehyde for 24 h, and dehydrated through a graded ethanol series. After vitrification by dimethylbenzene, samples were embedded in paraffin and sectioned on a RM2255 microtome (Leica). The 8- to 10-µm-thick sections were attached to adhesion slides (positively charged) stained with Toluidine Blue or sarranine and viewed with an Axio Imager A2 (Carl Zeiss). Root tips were stained with 5 mg/L propidium iodide dissolved in sterile water for 20 min, treated with chloral hydrate, and observed under an LSM-510 confocal laser scanning microscope (Carl Zeiss).
Statistics
Significance was determined using Student’s t test in the software SPSS (IBM). P < 0.05 was considered significant.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Amino acid sequence alignment of ScAtm1p, AtATM3, and OsATM3.
Supplemental Figure S2. Subcellular localization of OsATM3 in Arabidopsis protoplasts OsATM3 and OsATM3SP indicate the full-length protein and the signal peptide (98 amino acid residues from the N terminus as predicted by TargetP v. 1.1, http://www.cbs.dtu.dk/services/TargetP/), respectively.
Supplemental Figure S3. Semiquantitative RT-PCR analysis of iron metabolism genes in osatm3.
Supplemental Figure S4. Semiquantitative RT-PCR analysis of iron metabolism genes in RNAi lines.
Supplemental Figure S5. Semiquantitative RT-PCR analysis of GSH1 and ETHE1 in osatm3 and RNAi plants.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Peiwei Liu, Meihuan Wang, Kuaifei Xia, Lu Qin, and Hui Mo for technical assistance and Rufang Deng for transmission electron microscopy.
Footnotes
This work was funded by the 100 Talents Program award, Chinese Academy of Sciences, and by a grant from the Guangdong Science and Technology Department of China (no. 2015B020231009).
References
- Asai K, Satoh N, Sasaki H, Satoh H, Nagato Y (2002) A rice heterochronic mutant, mori1, is defective in the juvenile-adult phase change. Development 129: 265–273 [DOI] [PubMed] [Google Scholar]
- Balk J, Pilon M (2011) Ancient and essential: the assembly of iron-sulfur clusters in plants. Trends Plant Sci 16: 218–226 [DOI] [PubMed] [Google Scholar]
- Balk J, Schaedler TA (2014) Iron cofactor assembly in plants. Annu Rev Plant Biol 65: 125–153 [DOI] [PubMed] [Google Scholar]
- Beilschmidt LK, Puccio HM (2014) Mammalian Fe-S cluster biogenesis and its implication in disease. Biochimie 100: 48–60 [DOI] [PubMed] [Google Scholar]
- Bernard DG, Cheng Y, Zhao Y, Balk J (2009) An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron-sulfur proteins in Arabidopsis. Plant Physiol 151: 590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadini P, Biasiotto G, Poli M, Levi S, Verardi R, Zanella I, Derosas M, Ingrassia R, Corrado M, Arosio P (2007) RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood 109: 3552–3559 [DOI] [PubMed] [Google Scholar]
- Chen S, Sánchez-Fernández R, Lyver ER, Dancis A, Rea PA (2007) Functional characterization of AtATM1, AtATM2, and AtATM3, a subfamily of Arabidopsis half-molecule ATP-binding cassette transporters implicated in iron homeostasis. J Biol Chem 282: 21561–21571 [DOI] [PubMed] [Google Scholar]
- De Clercq I, Vermeirssen V, Van Aken O, Vandepoele K, Murcha MW, Law SR, Inzé A, Ng S, Ivanova A, Rombaut D, et al. (2013) The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 25: 3472–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan CG, Wang X, Tang K, Zhang H, Mangrauthia SK, Lei M, Hsu CC, Hou YJ, Wang C, Li Y, Tao WA, Zhu JK (2015) MET18 connects the cytosolic iron-sulfur cluster assembly pathway to active DNA demethylation in Arabidopsis. PLoS Genet 11: e1005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YF, Huang HW, Li L, Cai T, Chen S, He XJ (2015) The cytosolic iron-sulfur cluster assembly protein MMS19 regulates transcriptional gene silencing, DNA repair, and flowering time in Arabidopsis. PLoS One 10: e0129137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Komari T, Kubo T (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35: 205–218 [PubMed] [Google Scholar]
- Holdorf MM, Owen HA, Lieber SR, Yuan L, Adams N, Dabney-Smith C, Makaroff CA (2012) Arabidopsis ETHE1 encodes a sulfur dioxygenase that is essential for embryo and endosperm development. Plant Physiol 160: 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D, De Veylder L (2006) Cell cycle regulation in plant development. Annu Rev Genet 40: 77–105 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, et al. (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22: 561–570 [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Park S, Kang HG, Park GG, Kim SR, Sim J, Kim YO, Kim MK, Kim SR, et al. (2006) Generation of a flanking sequence-tag database for activation-tagging lines in Japonica rice. Plant J 45: 123–132 [DOI] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Kushnir S, Noh EW, Martinoia E, Lee Y (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140: 922–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G, Csere P, Guiard B, Lill R (1997) The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346–350 [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63: 131–152 [DOI] [PubMed] [Google Scholar]
- Komaki S, Sugimoto K (2012) Control of the plant cell cycle by developmental and environmental cues. Plant Cell Physiol 53: 953–964 [DOI] [PubMed] [Google Scholar]
- Koshiba T, Saito E, Ono N, Yamamoto N, Sato M (1996) Purification and properties of flavin- and molybdenum-containing aldehyde oxidase from coleoptiles of maize. Plant Physiol 110: 781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüssel L, Junemann J, Wirtz M, Birke H, Thornton JD, Browning LW, Poschet G, Hell R, Balk J, Braun HP, Hildebrandt TM (2014) The mitochondrial sulfur dioxygenase ETHYLMALONIC ENCEPHALOPATHY PROTEIN1 is required for amino acid catabolism during carbohydrate starvation and embryo development in Arabidopsis. Plant Physiol 165: 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir S, Babiychuk E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G, Lill R, Van Montagu M (2001) A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yang JG, Zhitnitsky D, Lewinson O, Rees DC (2014) Structural basis for heavy metal detoxification by an Atm1-type ABC exporter. Science 343: 1133–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Liang DD, Li J, Duan YB, Li H, Yang YC, Qin RY, Li L, Wei PC, Yang JB (2013) Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice ALTERNATIVE OXIDASE 1 genes. Plant Cell Environ 36: 775–788 [DOI] [PubMed] [Google Scholar]
- Li J, Cowan JA (2015) Glutathione-coordinated [2Fe-2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport. Chem Commun (Camb) 51: 2253–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Qin L, Liu P, Wang M, Ye H (2014) Genes for iron-sulphur cluster assembly are targets of abiotic stress in rice, Oryza sativa. Plant Cell Environ 37: 780–794 [DOI] [PubMed] [Google Scholar]
- Luo D, Bernard DG, Balk J, Hai H, Cui X (2012) The DUF59 family gene AE7 acts in the cytosolic iron-sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. Plant Cell 24: 4135–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack MK, Harashima H, Dissmeyer N, Zhao X, Bouyer D, Weimer AK, De Winter F, Yang F, Schnittger A (2012) Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev Cell 22: 1030–1040 [DOI] [PubMed] [Google Scholar]
- Paul VD, Lill R (2015) Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim Biophys Acta 1853: 1528–1539 [DOI] [PubMed] [Google Scholar]
- Poethig RS. (1990) Phase change and the regulation of shoot morphogenesis in plants. Science 250: 923–930 [DOI] [PubMed] [Google Scholar]
- Pondarré C, Antiochos BB, Campagna DR, Clarke SL, Greer EL, Deck KM, McDonald A, Han AP, Medlock A, Kutok JL, et al. (2006) The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron-sulfur cluster biogenesis. Hum Mol Genet 15: 953–964 [DOI] [PubMed] [Google Scholar]
- Qi Y, Wang H, Zou Y, Liu C, Liu Y, Wang Y, Zhang W (2011) Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett 585: 231–239 [DOI] [PubMed] [Google Scholar]
- Rawlins MR, Leaver CJ, May MJ (1995) Characterisation of an Arabidopsis thaliana cDNA encoding glutathione synthetase. FEBS Lett 376: 81–86 [DOI] [PubMed] [Google Scholar]
- Rouault TA, Tong WH (2008) Iron-sulfur cluster biogenesis and human disease. Trends Genet 24: 398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedler TA, Thornton JD, Kruse I, Schwarzländer M, Meyer AJ, van Veen HW, Balk J (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J Biol Chem 289: 23264–23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Pierik AJ, Lill R (2014) Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1. Science 343: 1137–1140 [DOI] [PubMed] [Google Scholar]
- Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJ, Pierik AJ, Wohlschlegel JA, Lill R (2012) MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337: 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling O, Wilbrecht C, Lill R (2014) Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie 100: 61–77 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Taylor NL, Leaver CJ (2007) Isolation of intact, functional mitochondria from the model plant Arabidopsis thaliana. Methods Mol Biol 372: 125–136 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sasaki Y, Ida S, Morikawa H (2001) Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant Physiol 126: 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschner J, Lachmann N, Schulze J, Geisler M, Selbach K, Santamaria-Araujo J, Balk J, Mendel RR, Bittner F (2010) A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis. Plant Cell 22: 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41: 15–30 [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Z (2006) Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18: 350–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu YH, Yi HY, Gong JM (2012) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72: 400–410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.