Small molecule AA1 as a novel antagonist of ABA targets all ABA receptors of Arabidopsis.
Abstract
Abscisic acid (ABA), the most important stress-induced phytohormone, regulates seed dormancy, germination, plant senescence, and the abiotic stress response. ABA signaling is repressed by group A type 2C protein phosphatases (PP2Cs), and then ABA binds to its receptor of the ACTIN RESISTANCE1 (PYR1), PYR1-LIKE (PYL), and REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) family, which, in turn, inhibits PP2Cs and activates downstream ABA signaling. The agonist/antagonist of ABA receptors have the potential to reveal the ABA signaling machinery and to become lead compounds for agrochemicals; however, until now, no broad-spectrum antagonists of ABA receptors blocking all PYR/PYL-PP2C interactions have been identified. Here, using chemical genetics screenings, we identified ABA ANTAGONIST1 (AA1), the first broad-spectrum antagonist of ABA receptors in Arabidopsis (Arabidopsis thaliana). Physiological analyses revealed that AA1 is sufficiently active to block ABA signaling. AA1 interfered with all the PYR/PYL-HAB1 interactions, and the diminished PYR/PYL-HAB1 interactions, in turn, restored the activity of HAB1. AA1 binds to all 13 members. Molecular dockings, the non-AA1-bound PYL2 variant, and competitive binding assays demonstrated that AA1 enters into the ligand-binding pocket of PYL2. Using AA1, we tested the genetic relationships of ABA receptors with other core components of ABA signaling, demonstrating that AA1 is a powerful tool with which to sidestep this genetic redundancy of PYR/PYLs. In addition, the application of AA1 delays leaf senescence. Thus, our study developed an efficient broad-spectrum antagonist of ABA receptors and demonstrated that plant senescence can be chemically controlled through AA1, with a simple and easy-to-synthesize structure, allowing its availability and utility as a chemical probe synthesized in large quantities, indicating its potential application in agriculture.
The phytohormone abscisic acid (ABA) controls many vital plant physiological processes, such as seed development, seed dormancy, and germination, adaptive responses toward environmental stresses, such as drought and salinity, fruit ripening, and leaf senescence (Fujita et al., 2006; Cutler et al., 2010). The downstream factors of ABA signaling have been established, including group A type 2C protein phosphatases (PP2Cs), SNF1-related kinases2 (SnRK2s), and downstream effector proteins including transcription factors, such as ABI3, ABI4, and ABI5 (Zhu, 2002). In the absence of ABA, PP2Cs bind and physically block the activity of SnRK2s through dephosphorylation, keeping downstream effector proteins inactive. ABA receptors have been obscure for many years because of genetic redundancy. In 2009, ABA receptors, which are members of the START superfamily of ligand-binding proteins, were identified by chemical genetics and yeast two-hybrid screening (Ma et al., 2009; Park et al., 2009). The receptor family contains 14 members, defined as PYRABACTIN RESISTANCE1 (PYR1), PYR1-LIKE (PYL), or REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR). The binding of ABA to PYR/PYL/RCAR (PYR/PYL) induces physical interactions between PYR1/PYLs and PP2Cs, which, in turn, inhibit PP2C activity and, hence, relieve SnRK2 to activate downstream effectors to cause physiological responses to ABA (Fujii et al., 2009).
Structural studies highlighted a conserved gate-latch-lock mechanism underlying ABA perception and signal transduction (Melcher et al., 2009; Miyazono et al., 2009; Nishimura et al., 2009; Santiago et al., 2009; Yin et al., 2009). In Arabidopsis (Arabidopsis thaliana), PYR/PYLs are classified into two distinct categories: dimeric receptors (PYR1 and PYL1–PYL3) and monomeric receptors (PYL4–PYL12; Okamoto et al., 2013). The dimeric receptors interact with and inhibit PP2C activity in an ABA-dependent manner. The dimeric receptors possess an ABA-binding pocket, flanked by a gate and a latch loop, and the apo-form receptors are in an open conformation, permitting access of ABA to the binding pocket (Melcher et al., 2009). Upon ABA binding, the dimeric receptors close the gate, which, in turn, creates the interaction surfaces that permit the docking of PP2Cs onto the ABA-bound receptors. The ABA-induced receptor-PP2C interaction induces a new conformation change of the protein complex, which locks the gate of the receptors (Melcher et al., 2009). In contrast, monomeric receptors are in equilibrium between the gate-opened and gate-closed conformations in the absence of ABA. Thus, monomeric receptors can interact with and inhibit PP2C activity in an ABA-independent manner (Hao et al., 2011; Sun et al., 2012). The distinct roles of dimeric and monomeric receptors in ABA signaling remain elusive. However, recent reports demonstrated that the selective chemical activation of dimeric receptors could induce a full ABA response, which indicated that dimeric receptors are key factors in ABA signaling (Cao et al., 2013; Okamoto et al., 2013).
Small molecules, functioning as agonists or antagonists of ABA receptors, promote the discovery of ABA receptors and deepen our knowledge of the receptors’ mechanisms of action. Pyrabactin, the first identified ABA receptor-related small molecule, functions as an agonist of PYR1 and PYL1 and as a weak antagonist of PYL2 (Park et al., 2009; Melcher et al., 2010). Besides pyrabactin, quinabactin (also known as AM1), working as a selective agonist toward dimeric ABA receptors, is a promising agrochemical that elicits stomatal closure and enhances crop drought resistance (Cao et al., 2013; Okamoto et al., 2013). In addition, mandipropamid, a commercial agrochemical, functions as a selective agonist of engineered PYR1 (Park et al., 2015; Rodriguez and Lozano-Juste, 2015). ABA antagonists have been identified and designed before (Kim et al., 2011; Takeuchi et al., 2014; Ito et al., 2015). DFPM, a small molecule identified by chemical genetics screening, down-regulates ABA-dependent gene expression and also inhibits ABA-induced stomatal closure through the plant immune response pathway, and its target is still unclear (Kim et al., 2011). Small molecules AS6 and RK460 are two antagonists of ABA receptors (Takeuchi et al., 2014; Ito et al., 2015). AS6 was designed according to the ABA/PYR1 x-ray structure. Because of the structural similarity of ABA, AS6 is not an entire PRY/PYL antagonist, which is revealed by its intrinsic agonist activity toward ABA receptors. In addition, AS6 functions as an antagonist not to all the PYR/PYL-PP2C interactions. The complex structure of AS6 may result in a high production cost, and its chemical synthesis is complex, limiting its availability and utility as a chemical probe synthesized in large quantities (Takeuchi et al., 2014). RK460 is an antagonist toward PYR1 but not other PYR/PYLs, which cannot bypass the genetic redundancy of ABA receptors, and this may explain why RK460 has no antagonist activity to ABA-induced physiological processes (Ito et al., 2015).
The functional characterization of PYR/PYL members using conventional molecular genetic approaches would be daunting because of their genetic and functional redundancy. In this situation, a small molecule acting as a broad-spectrum antagonist toward all ABA receptors could be a powerful tool to sidestep this genetic redundancy. Such an ABA antagonist is particularly needed for ABA signaling studies in other plant systems with less available genetic resources. Moreover, it has been reported that some pathogens, such as Pseudomonas syringae, could hijack the ABA signaling pathway for their survival and cause plant disease (Fujita et al., 2006). ABA is thought to be one of the phytohormones that promote leaf senescence. ABA levels can be observed in many plants during leaf senescence (Tan et al., 2003). ABA induces expression of several senescence-associated genes (SAGs) and yellowing of the leaves, which are typical phenomena associated with leaf senescence (Liang et al., 2014). What's more, a new study showed that overexpression of PYL9 can promote Arabidopsis senescence via the core ABA signaling pathway (Zhao et al., 2016). Up-regulation of genes associated with ABA signaling and a dramatic increase in endogenous ABA promote fruit ripening and leaf senescence, indicating a promising agricultural application of ABA antagonist (Lim et al., 2007).
Here, we report a novel ABA antagonist, AA1, which was uncovered using our plant chemical genetics screening using commercial compound libraries. AA1 releases ABA-induced seed germination inhibition and stomatal closure. Biochemical assays demonstrated that AA1 inhibits the interactions between all 13 PYR/PYLs and PP2C by direct binding to PYR/PYL, which is a specific feature of all the PYR/PYL-related small molecules. Most importantly, AA1 down-regulates senescence-related gene expression and inhibits chlorophyll breakdown, and thus delays plant leaf senescence, which may provide some hints for how to apply ABA antagonists to agriculture.
RESULTS
Identification of an ABA Antagonist
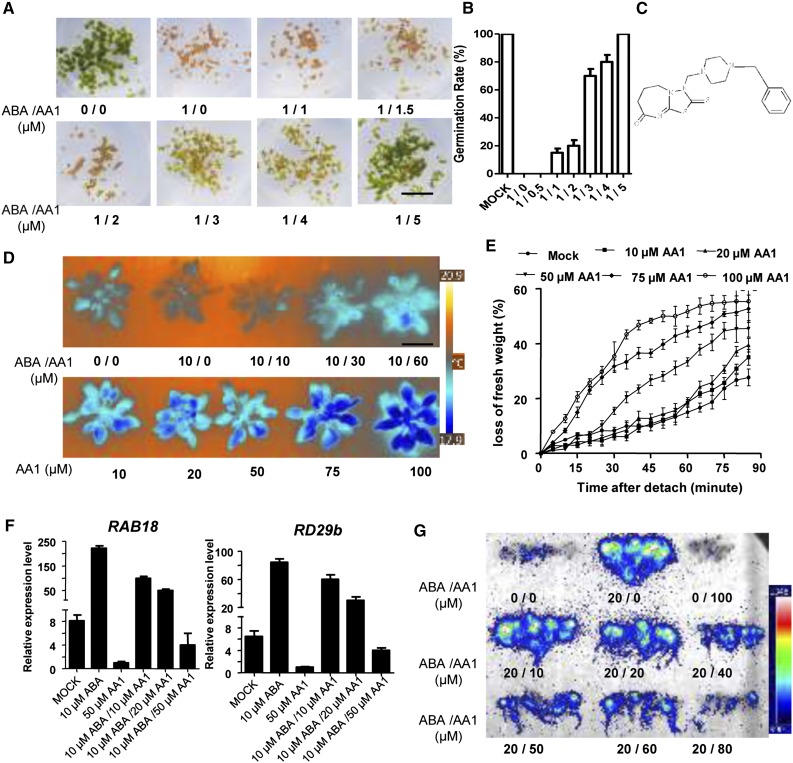
To identify small molecules that inhibit ABA signaling, we developed a chemical screen that relied on the seed-inhibitory effects of ABA (Zhao, 2012; Ye et al., 2016). Arabidopsis wild-type Columbia-0 (Col-0) seeds were grown in Murashige and Skoog (MS) medium with 0.7 μm ABA on 96-well microplates and then screened against 12,000 structurally novel and diverse synthetic small molecules. The resulting phenotypes after 5 d of growth allowed the identification of one small molecule that could relieve the inhibitory effects of ABA on seed germination, and the chemical was designated as AA1 (Fig. 1, A–C). We then assayed the effects of AA1 against the effect of ABA on seed germination by testing different ABA concentrations. The results showed that 100 μm AA1 could reverse the effect of 50 μm ABA on seed germination (Fig. 1; Supplemental Fig. S1). Next, we determined the 50% inhibitory concentration of AA1 by testing the activities of different concentrations of AA1 against 1 μm ABA, and the result showed that the 50% inhibitory concentration of AA1 is 2.546 μm (Fig. 1, A and B). Importantly, the suppression of the effect of ABA by AA1 was conserved in rice (Oryza sativa) and maize (Zea mays), which are monocotyledonous plants, and this indicates that the effect of AA1 is not restricted to dicotyledonous plants (Supplemental Fig. S1).
Figure 1.
AA1 antagonizes the effects of ABA. A, AA1 relieves the ABA-induced inhibition of seed germination. Wild-type (Col-0) seeds were sown on one-half-strength MS medium containing the indicated chemicals. The concentrations of chemicals are shown as ABA/AA1 (μm/μm). Bar = 1.2 cm. B, Quantification of the seed germination rates in A (n = 3; error bars = se). C, Molecular structures of AA1. D, Leaf surface temperature as assessed by infrared thermal imaging. About 1-month-old Arabidopsis leaves were sprayed with the indicated chemicals. Leaf temperature was imaged by thermography after 12 h of chemical treatment. Bar = 4 cm. E, AA1 promotes water loss. Three-week-old Col-0 plants were sprayed with the indicated chemicals. The loss of fresh weight was measured after 12 h of chemical treatment (n = 3; error bars = se). F, Expression of ABA-responsive genes after chemical treatment (DMSO as mock). Col-0 seedlings were sprayed with the indicated chemicals for 6 h, after which gene expression levels were determined by quantitative real-time reverse transcription-PCR (n = 3; error bars = se). G, AA1 attenuates ABA-induced RD29a expression. Five-day-old pRD29-LUC transgenic seedlings were sprayed with the indicated chemicals, and the photographs were taken after 6-h chemical treatments. The concentrations of chemicals are shown as ABA/AA1 (μm/μm).
ABA controls stomatal aperture and transpiration rate, which is one of its critical physiological roles that can be measured indirectly through the leaf temperature (Kim et al., 2010; Gonzalez-Guzman et al., 2012). The leaf surface temperature increases when stomata close because of reduced evaporative cooling. The ABA-treated leaves displayed elevated leaf temperatures; this did not occur when the leaves were treated with AA1 at the same time (Fig. 1D). More importantly, AA1-treated leaves showed a decreased leaf temperature in an AA1 dose-dependent manner, indicating that AA1 can function in guard cells and can antagonize endogenous ABA (Fig. 1D). Consistent with the fact that AA1 increased transpiration (Fig. 1D), the AA1-treated leaves lost water more quickly (Fig. 1E). High salt concentration induces ABA biosynthesis and, hence, inhibits seed germination (Gonzalez-Guzman et al., 2012). To examine the effect of AA1 on high-salt responses, Arabidopsis seeds were sown on MS medium containing 200 mm NaCl, or AA1 plus NaCl. As shown in Supplemental Figure S1, the AA1-treated seeds effectively resisted high salt. Collectively, AA1, as an effective ABA antagonist, functions in ABA-mediated seed germination and growth regulation. What’s more, AA1 could be a useful tool for the study of ABA signaling in both monocots and dicots.
AA1 Inhibits ABA-Responsive Gene Expression
ABA signal transduction results in the up-regulation of stress-response genes (Cao et al., 2013). To characterize whether AA1 may affect ABA-induced gene expression, we detected the expression levels of RD29b and RAB18 in seedlings under different chemical treatments. These two genes were dramatically activated by ABA treatment, but their expression was reduced when the seedlings were cotreated with AA1 and ABA (Fig. 1F). ABA-induced stress-response gene expression also can be monitored in transgenic plants containing a firefly luciferase (LUC) reporter driven by the RD29a promoter, containing the DRE/C-repeat and ABRE elements (Ishitani et al., 1997; Cao et al., 2014). The resulting transgenic plants emit bioluminescence in response to cold, osmotic stress, or exogenous application of ABA (Ishitani et al., 1997). ABA treatment enhanced the expression of RD29A-LUC, whereas AA1 reversed the effect of ABA in a dose-dependent manner (Fig. 1G). Therefore, our physiology and gene expression results show that AA1 is a promising ABA antagonist for the dissection of the ABA signaling pathway.
AA1 Interferes with All the Interactions between PYR/PYL and PP2C to Release PP2C Activity
To test whether the antagonist activity of AA1 results from inhibited ABA biosynthesis, we measured the endogenous ABA concentration. The result showed that AA1 treatments had no effect on endogenous ABA content (Supplemental Fig. S2). In addition, we assessed the effects of AA1 on ABA transportation. We assessed ABA uptake in mesophyll protoplasts. Indeed, different concentrations of AA1 had no effect on the uptake of ABA into protoplasts (Supplemental Fig. S2). Taken together, the antagonist activity of AA1 is not due to the blocking of ABA biosynthesis or ABA transportation.
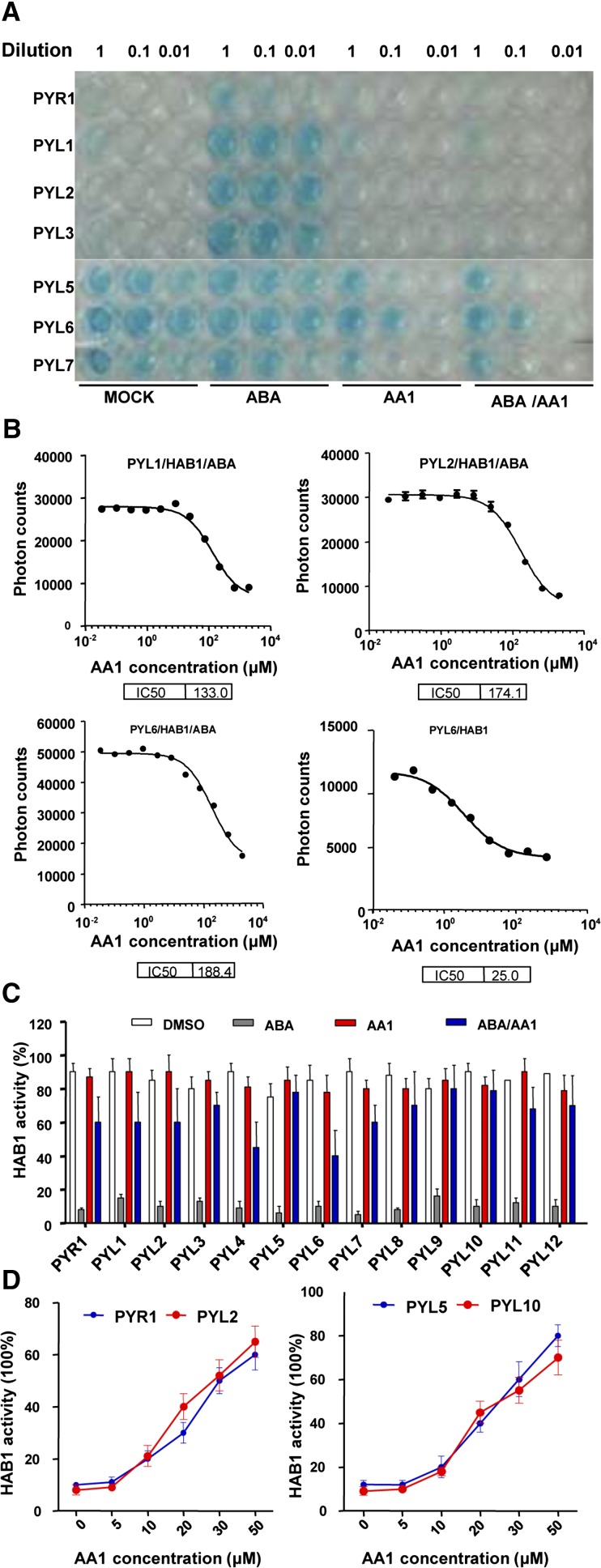
The selective agonist activities of pyrabactin and quinabactin result from their ability to act as ABA functional analogs to induce PYR/PYL-PP2C interactions, and AS6, a synthetic antagonist, is designed to be an antagonist of ABA-dependent PYR/PYL-PP2C interactions (Park et al., 2009, 2015; Cao et al., 2013; Okamoto et al., 2013). We tested the possibility that the ABA antagonist activity of AA1 results from a perturbation of PYR/PYL-PP2C interactions. Consistent with previous reports, ABA induced PYR1 and PYL1 to PYL4 interactions with HAB1, a member of the group A subfamily of plant PP2Cs (Fig. 2A), and the monomeric receptors interacted with HAB1 in the absence of ABA. As expected, AA1 blocked ABA-dependent interactions of PYR1 and PYL1 to PYL4 with HAB1 (Fig. 2A; Supplemental Fig. S2). In addition, AA1 attenuated ABA-independent PYR/PYL-HAB1 interactions, even in the absence of ABA (Fig. 2A; Supplemental Fig. S2), indicating that AA1 is possibly a potent broad-spectrum antagonist of PYR/PYL-PP2C interactions. Up to 100 μm AA1 could completely inhibit the interactions between PYR1 and PYL1 to PYL4 with HAB1 induced by 2 μm ABA. However, at this concentration, AA1 could not completely suppress the interactions between PYL5 to PYL12 and HAB1 in yeast cells (Fig. 2A; Supplemental Fig. S2). However, AS6, the designed antagonist of ABA, could induce the interactions of dimeric PYR1/PYLs with HAB1 in yeast two-hybrid assays, which is similar to the effects of ABA on PYR1/PYL interactions (Supplemental Fig. S3). And, up to 100 μm, AS6 could not block the interactions between monomeric PYLs and HAB1 (Supplemental Fig. S3). These results may be explained by the fact that AS6 also is a weak agonist of ABA receptors, and the weak agonist activity may be a side effect of AS6. Most importantly, AA1 is a complete antagonist of PYR/PYL-PP2C interactions without agonist activity. To further characterize the antagonist activity of AA1 for PYR/PYL-PP2C interactions, we carried out AlphaScreen assays (Melcher et al., 2009; Cao et al., 2013). These assays showed that AA1 inhibits the interaction intensity of dimeric PYL1 and PYL2 with HAB1 and that this inhibitory activity is dose dependent (Fig. 2B). ABA enhances the interaction of the monomeric receptor PYL6 with HAB1; this is revealed by a higher number of photon counts detected after ABA treatment (Fig. 2B), and such an increase in the interaction intensity required a higher dose of AA1 to reverse it (Fig. 2B). In summary, AA1 is a broad-spectrum antagonist of PYR/PYL-PP2C interactions, and AA1 acts on more PYR/PYL-PP2C interactions than AS6, which blocks ABA-dependent PYR/PYL-PP2C interactions. Like ABA, the effects of AA1 on dimeric and monomeric PYR/PYL-PP2C interactions display different modes, and the mode in each type of PYR/PYL is conserved.
Figure 2.
AA1 interferes with PYR/PYL-PP2C interactions and PYR/PYL-dependent inhibition of PP2C activity. A, AA1 blocks ABA-dependent PYR/PYL-HAB1 interactions in yeast two-hybrid assays. Dimeric receptors (PYR1 and PYL1–PYL3) and monomeric receptors (PYL4–PYL6) were constructed as binding domain fusion proteins; HAB1 was fused with the activation domain. The yeast cells were grown on SD medium (-Leu/-Trp/-His plus 50 mg/L X-α-Gal and 5 mm 3-amino-triazole) with the indicated chemicals for 3 d. Working concentrations of the chemicals were 2 μm for ABA and 100 μm for AA1. The yeast cells were diluted into 1:10 (0.1) and 1:100 (0.01) using water. B, Binding affinity of PYR/PYL proteins to HAB1 measured by AlphaScreen assays. Dimeric receptors (PYL1 and PYL2) and a monomeric receptor (PYL6) were fused with His-SUMO; HAB1 was fused with biotin. The reactions were conducted with and without 100 μm ABA and the indicated concentrations of AA1 (n = 3; error bars = se). IC50, Fifty percent inhibitory concentration. C, AA1 attenuates the ABA-dependent inhibition of HAB1 activity via various ABA receptors. Various PYR/PYL-HAB1 combinations were incubated with the indicated chemicals (2 μm ABA, 100 μm AA1, or DMSO; n = 3; error bars = se). D, Dose-dependent effects of AA1 on PYR/PYL-dependent inhibition of PP2C activity. HAB1 activity was tested with dimeric receptors (PYR1 and PYL2) or monomeric receptors (PYL5 and PYL10) with 2 μm ABA and the indicated concentrations of AA1 (n = 3; error bars = se).
Next, the antagonist activity of AA1 was characterized by examining the way in which it reverses ABA-induced PP2C inhibition through PYR/PYL. All 13 Arabidopsis ABA receptors are activated after ABA binding, and ABA-bound receptors are responsible for PP2C inhibition. Hence, receptor activation can be monitored through PP2C activity inhibition. In the phosphatase assays, AA1 failed to activate any of the 13 receptors. This indicates that, unlike pyrabactin, which selectively activates (e.g. PYR1 and PYL1) or represses (e.g. PYL2) certain receptors, AA1 has no natural agonist activity (Fig. 2C). AA1 could repress all 13 receptors activated by ABA, revealed by its ability to reverse ABA-mediated PP2C inhibition, to certain levels (Fig. 2C). In addition, AA1 displayed unbiased antagonist activity to both dimeric receptors (PYR1 and PYL1–PYL3) and monomeric receptors (PYL4–PYL12; Fig. 2, C and D), which is consistent with the fact that AA1 interferes with all the interactions of 13 receptors with PP2C (Fig. 2A; Supplemental Fig. S2). More importantly, unlike AS6 or pyrabactin, AA1 is a broad-spectrum PYR/PYL antagonist without intrinsic agonist activity, and AA1 functions through both dimeric and monomeric receptor-PP2C interactions but not dimeric PYR/PYL-PP2C interactions, like AS6.
PYR/PYLs Are the Molecular Targets of AA1
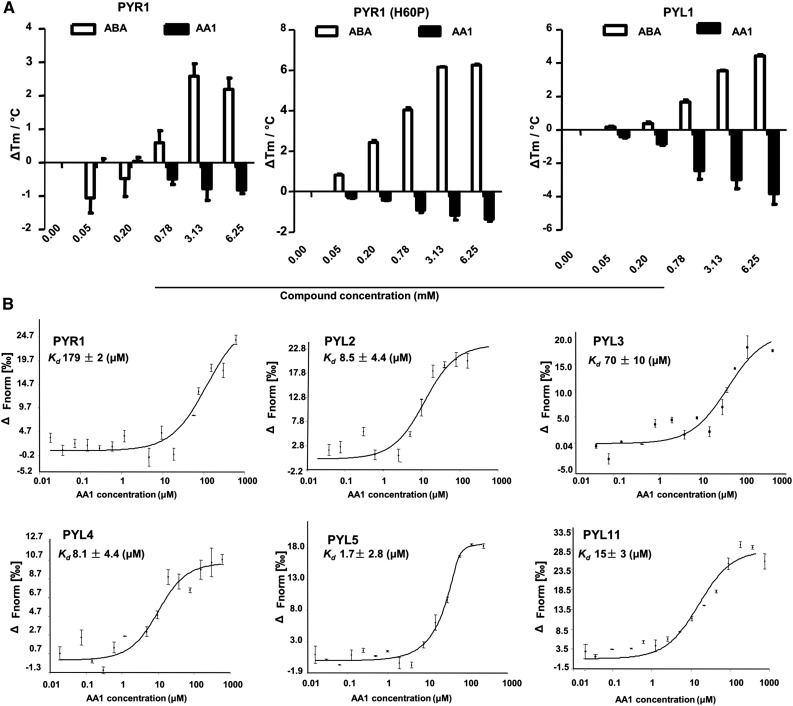
Given the results that AA1 antagonized the effects of ABA by interfering with PYR/PYL-PP2C interactions and relieved PP2C activity, we speculated that AA1 might act as an antagonist of PYR/PYL by binding to them directly. To test this hypothesis, we used a thermal shift assay (TSA) to quantify the changes in the thermal denaturation temperature of PYR/PYL under various conditions, including ligand-bound and ligand-free status (Soon et al., 2012). ABA-receptor complexes are more thermally stable than apo receptors (PYR1 and PYL1), and the melting temperatures (Tms) are up-regulated in an ABA dose-dependent manner (Fig. 3A). For AA1, the TSA dose-response plots showed an AA1 concentration-dependent decrease in ΔTm of AA1-bound receptors (Fig. 3A), indicating that AA1 might interact with PYR/PYL. More importantly, the receptors become more vulnerable upon AA1 binding compared with apo or ABA-bound receptors; this was revealed by the decreased ΔTm of AA1-bound receptors. Interestingly, ABA and AA1 have opposite effects on the thermostability of PYR/PYL.
Figure 3.
PYR/PYLs are direct targets of AA1. A, The TSA. Plots of ΔTm (°C) are shown for the effects of AA1 and ABA on PYR1, PYR1H60P, and PYL1 at the indicated chemical concentrations. The thermal stability of the chemical-PYR/PYL complex shows that the ABA-PYR/PYL complexes are more stable than the AA1-PYR/PYL complexes. B, MST data for the binding affinity of AA1 to ABA receptors. Plots show the fraction of dimeric receptors (PYR1, PYL2, and PYL3) and monomeric receptors (PYL4, PYL5, and PYL10) bound to AA1 at each tested AA1 concentration (n = 3; error bars = se).
To explore whether AA1 also may interact with monomeric ABA receptors, we measured the ΔTm of PYR1H60P, the monomeric form of PYR1 with Pro introduced at position His-60 (Dupeux et al., 2011). The results showed that AA1 interacted with PYR1H60P, which shared a similar ΔTm trend with PYR1. Monomeric receptors have a higher binding affinity for ABA because they do not need a dimer dissociation step (Hao et al., 2011). Indeed, ABA affects the ΔTm of PYR1H60P about twice as strongly as that of wild-type PYR1 and PYL1, indicating that the introduction of this mutation leads to a significant increase in ABA binding affinity. However, this is not the case for AA1. Figure 3A shows that the ΔTm of PYR1H60P is higher than that of dimeric PYL1 (Fig. 3A), and this suggests that the binding of ABA and AA1 to PYR/PLY is through different action modes.
To further explore the binding of AA1 to ABA receptors, we developed a microscale thermophoresis (MST) assay and used it to determine the dissociation constant (Kd) for this binding. The MST assays were conducted using all 13 recombinant receptors, including dimeric and monomeric forms with a wide range of AA1 concentrations. The results revealed that AA1 bound to all the receptors tested, with different Kd values (Fig. 3B; Supplemental Fig. S4). The acquired Kd values ranged from 179 μm (PYR1) to 1.7 μm (PYL5) and indicated that PYL5 had the highest binding affinity of all 13 receptors tested (Fig. 3B; Supplemental Fig. S4). AA1 displayed low affinities to PYR1, PYL6, PYL7, PYL8, PYL9, and PYL10. To compare the binding affinity of AA1 and ABA, we used MST to measure the binding constant of ABA to two dimeric receptors (PYR1 and PYL2) and two monomeric receptors (PYL5 and PYL9; Supplemental Fig. S4). The Kd values we measured are close to those in previous reports (Miyazono et al., 2009; Yin et al., 2009; Hao et al., 2011; Sun et al., 2012). In terms of Kd, ABA prefers monomeric receptors over dimeric receptors, but this preference was not observed for AA1 (Fig. 3B; Supplemental Fig. S4). The binding constants of AA1 to PYL2 and PYL5 are smaller than that of ABA. However, with regard to PYR1 and PYL10, ABA has stronger binding affinity. Taken together, these results demonstrate that AA1 acts as an ABA antagonist by binding directly to all ABA receptors, and the binding affinities of ABA and AA1 are different toward given receptors.
Molecular Docking of AA1 to PYL2
To understand the molecular basis for the action of AA1 as an antagonist of PYR/PYL, we tried our assay on the crystal structure of AA1/PYR/PYLs, but we failed. This may be explained by our TSA, which demonstrated that AA1 will decrease the stability of PYR/PYLs (Fig. 3B), and the addition of AA1 to the PYR/PYL crystals could dissolve the crystal.
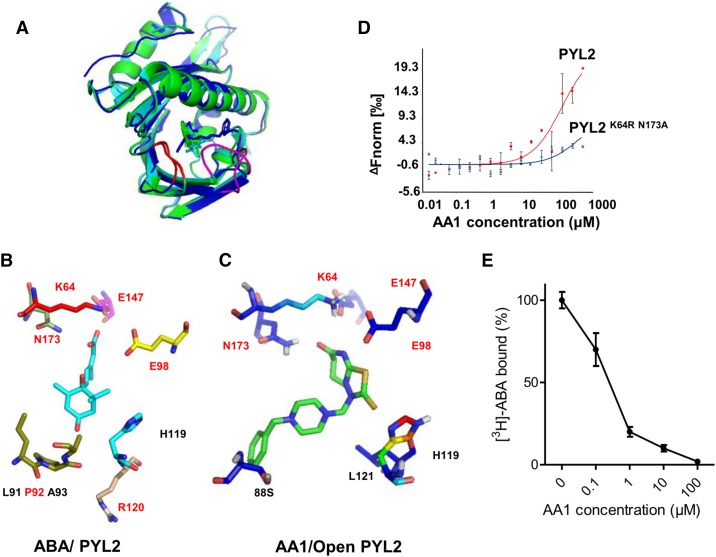
Finally, we predicated the structure of the AA1/PYL2 complex by molecular docking using the reported crystal structure of PYL2 (Melcher et al., 2009). AA1 was docked into the closed PYL2 (Protein Data Bank [PDB] no. 3KDI) and the open PYL2 (PDB no. 3KDH). The free binding energy values of AA1 to open and closed PYL2 were almost the same (Supplemental Table S1). Like ABA, AA1 is centered in the ligand-binding pocket of both closed and open PYL2 (Fig. 4A). Interestingly, AA1 undergoes an induced fit to accommodate the shape of the ligand-binding pockets of open and closed PYL2 (Fig. 4A; Supplemental Fig. S5).
Figure 4.
Molecular docking of AA1 to PYL2. A, Overall structure of the AA1/PYL2 complex. The docking of AA1 to open PYL2 (PDB no. 3KDH) and closed PYL2 (PDB no. 3KDI) is shown. The AA1/closed PYL2 complex is in green; the AA1/open PYL2 complex is in blue; ABA is in cyan; the latch loop of PYL2 is in red; and the gate loop is in magenta. B, Network of ABA (cyan-stick model, with red oxygen atoms) inside the PYL2-binding pocket. The amino acids that form hydrogen bonds with ABA are in red. C, Network of AA1 (green-stick model, with red oxygen atoms, blue nitrogen atoms, and yellow sulfur atoms) inside the open PYL2-binding pocket. The amino acids that form hydrogen bonds with AA1 are in red. D, Mutations in key ligand-binding pocket residues of PYL2 compromise AA1 binding as determined by MST (n = 3; error bars = se). E, Competitive binding of [3H]ABA to PYL2 in the presence of AA1. The reactions were performed in the presence of 0.1 μm [3H]ABA and the indicated amounts of AA1. Each assay was replicated three times, and the results were normalized relative to no AA1 (n = 3; error bars = se).
The carboxylate group of ABA interacts with the inner end of the pocket through hydrogen bonding, and the cyclohexene ring of ABA also interacts with the gate/latch loops via hydrogen bonding (Melcher et al., 2009). In quinabactin/PYL2 and pyrabactin/PYL1 complexes, the sulfonamide of quinabactin/pyrabactin functions like this carboxylate on ABA to dock into the inner side of the ligand-binding sites (Hao et al., 2010; Okamoto et al., 2013). ABA interacts with some of the same amino acids of PYL2 as AA1, especially those at the inner end of the ligand-binding pocket (Supplemental Tables S2 and S3). In the open PYL2/AA1 complex, the diazepin-8-one on AA1 docks into the inner end of the ligand-binding pocket by interacting with Lys-64, Glu-98, Glu-147, and Asn-173 through hydrogen bonding (Fig. 4B). Like ABA, AA1 also interacts with the latch and gate loops of PYL2, but via different amino acids (Fig. 4, B and C). Overall, AA1 enters into the ligand-binding pocket of PYL2 (Supplemental Fig. S5).
To confirm our molecular docking results, we detected the binding of AA1 to a PYL2 variant (K64R N173A). Lys-64 and Asn-173 are key amino acids of the ligand-binding pocket of PYL2, and these two amino acids locate in the inner face of PYL2.
AA1 enters into the ligand-binding pocket of PYL2 and forms hydrogen bonds with Lys-64 and Asn-173 (Fig. 4C; Supplemental Table S2). The mutations of Lys-64 and Asn-173 abolish the binding of ABA to PYL2 (Melcher et al., 2009; Nishimura et al., 2009). We tested the binding affinity of AA1 to PYL2K64R N173A by MST assays, and the results showed that AA1 could not bind to this PYL2 variant (Fig. 4D). Next, we performed competitive binding assays to further confirm that AA1 enters into the ABA-binding pocket of PYL2. AA1 competed efficiently with [3H]ABA for binding to PYL2 in a dose-dependent manner (Fig. 4E). These results confirmed our molecular docking results and illustrated that AA1 enters into the binding pocket of PYL2.
Structure-Activity Relationship Study of AA1
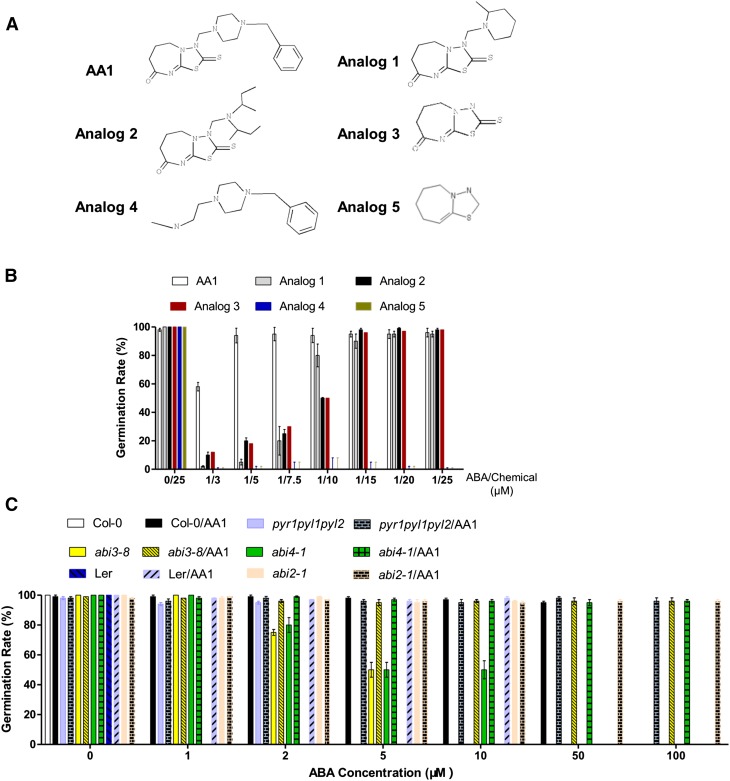
The molecular docking results display that the diazepin-8-one group of AA1 enters into the binding pocket of PYL2, indicating that this is an important group of AA1s. We performed a structure-activity relationship study to find the core substructure of AA1. We tested five AA1 analogs with various structures (Fig. 5, A and B; Supplemental Fig. S6). Analogs 1 and 2 have different side chains compared with AA1, and such differences did not change the activity of AA1 (Supplemental Fig. S6). Most importantly, the core group without any side chains shows similar antagonist activity to AA1 (Fig. 5, A and B; Supplemental Fig. S6). According to the docking modeling, the oxygen atom, the nitrogen atom of the heptatomic ring, and the sulfur atom form hydrogen bonds with PYL2. Consistent with the results of docking, the antagonist activity disappeared when the core group lost these atoms (Fig. 5, A and B; Supplemental Fig. S5). These results indicate that the diazepin-8-one group, which docks into the ligand-binding pocket of PYL2, is necessary for the activity of AA1, and we found a novel group that functions as the carboxylate group of ABA and sulfonamide of quinabactin/pyrabactin, which can dock into the ligand-binding pocket of ABA receptors (Supplemental Fig. S5).
Figure 5.
Structure-activity relationship and genetic study of AA1. A, Structures of AA1 analogs. B, Germination rates of Col-0 seeds under different combinations of ABA/chemical treatments (n = 3; error bars = se). The seeds were sown on the indicated chemical-containing one-half-strength MS plates, and the results were collected after 4 d of growth. C, Germination rates of distinct mutants under the indicated chemical treatments. Seeds of different backgrounds were sown on the indicated chemical-containing one-half-strength MS plates, and the results were collected after 5 d of growth. pyr1pyl1pyl2, abi3-8, and abi4-1 are in the Col-0 background, and abi2-1 is in the Landsberg erecta (Ler) background (n = 3; error bars = se).
Genetic Relationship Study of AA1 with Core Components of ABA Signaling
Multilocus loss-of-function mutants are required for strong ABA signaling phenotypes (Park et al., 2009; Gonzalez-Guzman et al., 2012), which is due to the extensive redundancy between the receptors, and such genetic redundancy impedes the genetic study of ABA receptors with other components of ABA signaling. The ABA core signaling pathway includes receptors, PP2Cs, SnRKs, and downstream transcription factors (Fujii et al., 2009). However, due to the difficulty of obtaining the multilocus loss-of-function mutants of ABA receptors with other core components, the genetic relationship study of ABA receptors with other components has not been realized yet.
AA1, the broad-spectrum PYR/PYL/RCAR antagonist, could block all 13 members, and this feature makes AA1 an effective genetic tool with which to study the genetic relationships between the core components of ABA signaling Thus, we tested the genetic relationship of ABA receptors with other components by treating the related mutants with AA1. Our results showed that AA1 could enhance the insensitivities of all the ABA-insensitive mutants we tested, including pyr1pyl1pyl2, abi2-1, abi3-8, and abi4-1 (Fig. 5C; Supplemental Fig. S6). Such results are similar to the fact that abi1 and abi2 could significantly enhance the resistance of transcription factor mutants (abi3 and abi4) to ABA (Finkelstein and Somerville, 1990; Finkelstein, 1994). Our results and previous studies showed that the upstream genetic factors (receptors and PP2Cs) could enhance the phenotypes of downstream genetic factors (transcription factors), indicating that the ABA signaling pathway is not a simple straightforward genetic pathway, and the members at each level (13 receptors, nine PP2Cs, three SnRKs, and several transcription factors) may form complex genetic networks.
AA1 Delays Leaf Senescence and Fruit Ripening
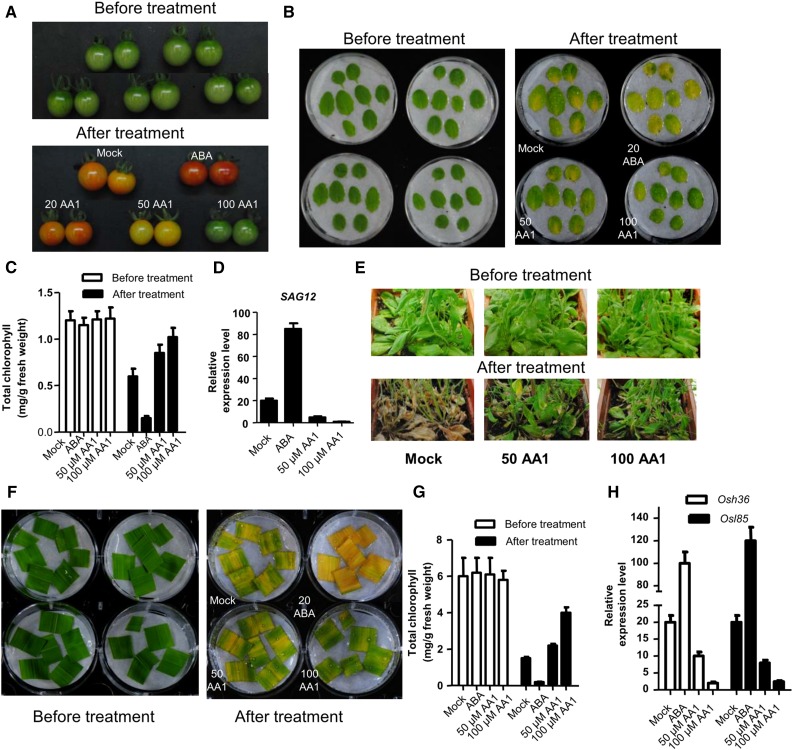
Besides its role in seed germination and stress regulation, ABA is an important regulator in fruit ripening (Zhang et al., 2009; Leng et al., 2014; Weng et al., 2015). ABA production is gradually elevated to its maximal level when ripening occurs (Zhang et al., 2009). ABA content-decreased mutants display shortened fruit ripening, and the delayed ripening may prolong the storage time for fresh fruits. We tested whether AA1 may delay fruit ripening by treating tomato (Solanum lycopersicum) fruits of the green mature stage. The application of ABA accelerated the changes in skin color compared with the control (Fig. 6A). In contrast to mock and ABA treatment, AA1 delayed the ripening time of tomato in a dose-dependent manner, indicating its potential application in fruit storage.
Figure 6.
AA1 delays fruit ripening and leaf senescence. A, Morphological differences between tomato fruits treated with exogenous ABA or AA1 and DMSO-treated fruits (Mock). In the green mature stage, tomato fruits were harvested and treated with 0.5 mL per fruit of 20 μm ABA or 50 or 100 μm AA1. Tomato fruits were treated with the indicated chemicals every 4 d for 2 weeks. B, AA1 delays the dark-induced leaf senescence of Arabidopsis. Detached leaves from Col-0 were incubated with the indicated chemicals (DMSO as mock, 20 μm ABA, or 50 or 100 μm AA1) for 5 d in darkness. C, Chlorophyll content in leaves of Col-0 treated with the indicated chemicals (n = 3; error bars = se). D, Expression levels of SAG12 in Col-0 leaves treated with the indicated chemicals (n = 3; error bars = se). E, One-month-old plants were treated with the indicated chemicals every week for 1 month. Photographs were taken after 1 month of chemical treatment. F, AA1 delays dark-induced leaf senescence in Zhonghua 11 rice. Detached flag leaves from wild-type plants at the heading stage were incubated with the indicated chemicals (DMSO as mock, 20 μm ABA, or 50 or 100 μm AA1) for 5 d in darkness. G, Chlorophyll content in leaves of rice treated with the indicated chemicals (n = 3; error bars = se). H, Expression levels of Osh36 and Osl85 in rice leaves treated with the indicated chemicals (n = 3; error bars = se).
Due to the role of ABA in leaf senescence, AA1, as a general antagonist of PYR/PYLs, may delay leaf senescence. To test this hypothesis, we used dark-induced leaf senescence as a system in which to study the effect of AA1. ABA accelerated the yellowing of detached Arabidopsis leaves. While AA1 had the opposite effects compared with ABA, AA1 can delay the yellowing of the detached leaves (Fig. 6B). The most striking feature of leaf senescence is the breakdown of chlorophyll during chloroplast degeneration. The content of chlorophyll in ABA-treated leaves was only one-fifth of that of non-chemical-treated leaves (Fig. 6B). However, AA1 inhibited the breakdown of chlorophyll, which was consistent with the delayed leaf yellowing, demonstrating that chlorophyll degradation proceeded at a significantly slower pace in the AA1-treated leaves (Fig. 6, B and C). SAGs are molecular markers of ABA-induced senescence (Zhao et al., 2016). Consistent with the delayed yellowing of AA1-treated leaves, SAG12, the chlorophyll degradation-related gene, was down-regulated by AA1 (Fig. 6D). What’s more AA1 also delayed the age-dependent leaf senescence in Arabidopsis (Fig. 6E). AA1 also delayed the senescence of detached flag leaves of rice in a dose-dependent manner (Fig. 6F). Similarly, the content of chlorophyll in 100 μm AA1-treated leaves was 3 times greater than that in control leaves (Fig. 6G). Osh36 and Osl85, the senescence marker genes (Liang et al., 2014), were down-regulated by AA1 treatment (Fig. 6H). It is known that cytokinin antagonizes ABA, and cytokinin potently suppresses senescence (Ha et al., 2012). To test whether AA1 delays leaf senescence through cytokinin signaling, we detected the effects of AA1 on cytokinin-responsive gene expression (Bhargava et al., 2013). Cytokinin significantly induced cytokinin-responsive gene expression, but AA1 had no such effects (Supplemental Fig. S7). These results showed that AA1 delays leaf senescence not through cytokinin signaling.
DISCUSSION
Several small molecules that act as agonists or antagonists of phytohormone receptors have been identified using a chemical genetics approach and have advanced the study of plant hormones, the indispensable regulators in plant biology (Hayashi et al., 2008; Park et al., 2009; Hicks and Raikhel, 2012; Meesters et al., 2014). Active molecules uncovered through chemical genetics studies have provided unique molecular genetic tools with which to study specific life processes in tissues and their development, and in general, their use is not limited to a particular species (Hicks and Raikhel, 2012). In this research, using a high-throughput chemical genetics screen, we identified a novel ABA antagonist, AA1, that acts on both monocots and dicots (Fig. 1A; Supplemental Fig. S1), with a potential application in agriculture to help solve problems related to stresses such as drought and salinity (Supplemental Fig. S1).
AA1 was demonstrated to act as an ABA antagonist that targets all 13 PYR/PYLs and affects their interactions with PP2Cs (Fig. 2, A and B; Supplemental Fig. S2). Compared with AS6 and other antagonists, we think AA1 has some advantages. (1) AA1 is the first small molecule acting on all PYR/PYL-PP2C interactions, while AS6 acts only on ABA-dependent PYR/PYL-PP2C interactions (Figs. 2 and 3; Supplemental Figs. S2–S4). (2) AA1 is the first broad-spectrum ABA receptor antagonist. (3) AA1 has no intrinsic agonist activity, while, acting as a weak antagonist (this may be a side effect of AS6), it could induce the interactions of PYR/PYLs with PP2C. The structure of AA1 is novel, and we can design more agonists or antagonists of PYR/PYL according to AA1. In addition, this novelty makes AA1 a more powerful tool with which to study ABA receptors. (4) To our knowledge for the first time, we have studied the genetic relationships between ABA receptors with other components of ABA signaling. (5) Also, to our knowledge for the first time, we have demonstrated that plant senescence can be chemically controlled through ABA receptors, indicating the potential application of the antagonist of ABA receptors. We believe that this is a major advance in agriculture. Most importantly, for agricultural application, the structure of AA1 is simple and easy to synthesize at lower cost, allowing its availability and utility as a chemical probe synthesized in large quantities, making it a very promising agrochemical, which is a big advantage over AS6. Previous reports showed the cocrystal structure of AS6-PYL2, and the observations provided direct evidence that, like the agonist, AS6 induced the gate-closed conformation of PYL2, leading to a more stable complex. AS6 has intrinsic antagonist activity, allowing the conformation change of PYL2 to make it more stable, while AA1 is a complete antagonist, and this difference may be the reason why we failed to determine the cocrystal structure of AA1 with PYR/PYLs. However, we believe that, in the future, the crystal structure will promote our understanding of ABA receptors, especially for analog 3, which has the simplest structure.
Considering the difficulty, particularly in nonmodel plants, of simultaneously obtaining multigene knockout mutants of ABA receptors and the high functional redundancy among these receptors, it would be very difficult to analyze their possible epistasis or hypostasis roles in certain genetic pathways (Hicks and Raikhel, 2012). As AA1 targets all ABA receptors, the functional redundancy among PYR/PYL members can be overcome, thus making such research more expedient and effective. The commercial AA1 can be used as a powerful tool with which to study nonmodel plants and the effects of ABA signaling at specific developmental stages. In this study, to our knowledge for the first time, we used AA1 to study the genetic relationship of ABA receptors with other ABA signaling components.
The MST and TSA demonstrated that PYR/PYLs are direct molecular targets of AA1. More importantly, AA1 binds to all 13 ABA receptors of Arabidopsis, which is consistent with its role in suppressing all PYR/PYL-PP2C interactions and its broad-spectrum activity (Fig. 3; Supplemental Fig. S4). Several structural studies have demonstrated a gate-latch-lock mechanism for ABA receptors to recognize ABA, in which the dimeric ABA receptors have an inner ligand-binding pocket that is guarded by two functionally important β-loops, termed the gate and latch loops. The ligand-binding pocket is blocked in the receptor homodimers; therefore, binding of ABA to the receptor requires receptor dimer dissociation, which results in the ABA-binding affinity being approximately 2 orders of magnitude lower than that of the monomeric ABA receptors (Melcher et al., 2009; Miyazono et al., 2009; Nishimura et al., 2009; Santiago et al., 2009; Yin et al., 2009). However, we did not observe a similar result for AA1, which indicates that AA1 has an unbiased binding affinity toward dimeric and monomeric ABA receptors (Figs. 2B and 3). The molecular docking assays demonstrated that AA1 enters into the ligand-binding pocket of PYL2 through its diazepin-8-one group (Fig. 4), indicating that such a group may be promising for the design of agonists toward PYR/PYL.
ABA responses are blocked in plants without stress; however, the concentration of ABA in vivo is sufficient for PYR/PYL activation to initiate ABA signaling. This observation suggested that, if not all, at least some of the ABA receptors need to be inhibited in plants without stress; however, how plants manage this is unknown at present. We believe that AA1 could shed light on this question, and we imagine that there might be some natural molecules, other than ABA, that target PYR/PYL to inhibit ABA responses in unstressed plants. These molecules could function similarly to AA1 and even share some structural similarity with AA1. The study of the mechanism of the effects of AA1 provides a new vision for ABA signaling research and indicates that exciting results may yet emerge in this field in the future.
A previous report demonstrated that down-regulated ABA content leads to delayed leaf senescence and an extended grain-filling period, resulting in increased grain yield in rice (Liang et al., 2014). Our results showed that AA1 can delay leaf senescence in both Arabidopsis and rice. Thus, the application of AA1 should be a useful strategy for improving crop yield in the future. In summary, our chemical genetics screen identified AA1, which acts as an ABA antagonist on all ABA receptors by binding to them directly. AA1 may provide an essential key to address important unanswered questions in ABA signaling research.
Significance
We describe the discovery and mechanistic characterization of a small molecule targeting to all ABA receptors, AA1, which acts in plants by repressing ABA-induced responses. We have demonstrated that plant senescence can be chemically controlled through ABA receptors, indicating the potential application of antagonists of ABA receptors. We believe that this is a major advance in agriculture. Most importantly, for agricultural application, the structure of AA1 is simple and easy to synthesize at lower cost, allowing its availability and utility as a chemical probe synthesized in large quantities, which is a big advantage over other antagonists of ABA receptors.
MATERIALS AND METHODS
Plant Chemical Genetics Screening
The chemical genetics screens were conducted as described previously (Zhao, 2012; Ye et al., 2016). A small synthetic molecular chemical library of 12,000 structurally novel and diverse chemicals was used (Life Chemical). To identify the small molecules, reversal of the seed germination inhibitory activity of ABA was assessed, in which Col-0 seeds were grown on 96-well microplates containing one-half-strength MS agar medium supplemented with 0.7 μm ABA and 100 μm of the chemical. The seed plates were incubated at 4°C for 3 d for stratification and grown at 22°C for an additional 6 d. The chemical hits uncovering seed germination under ABA treatment were retested, and the strongest hit and its analogs were used for further study. The chemical names from Life Chemical are as follows: AA1 (F0544-0152), Analog1 (F0544-0123), Analog2 (F0544-0096), Analog3 (F0544-0123), and Analog4 (F2163-0147).
Seed Germination Assay
The seeds were sterilized with a NaClO/HCl mixture for 2 h. Then, the seeds were planted on one-half-strength MS agar medium in the presence or absence of the indicated amounts of chemicals. After 3 d of synchronization in a 4°C freezer, the plates were grown in a phytotron (23°C, 16 h of light, 8 h of dark). Seed germination rates were determined by calculating the average germination rates of three replicates, and about 100 seeds in each replicate were tested.
Thermal Imaging
Three-month-old Arabidopsis (Arabidopsis thaliana) seedlings were treated with the indicated chemicals for 12 h. Thermal images were obtained using Testo 881-2 thermography (Testo). Images were saved on a computer memory card and analyzed using IRSoft software (Testo).
Gene Expression Analysis
Total RNA was isolated using the TRIzol reagent (Invitrogen). Up to 500 ng of RNA was used for the synthesis of cDNA according to the manufacturer’s instructions (Prime Script RT-PCR kit with gDNA Eraser; Takara). Quantitative real-time RT-PCR was carried out using the Bio-Rad CFX96 with Realtime PCR Master Mix (SYBR Green; Toyobo). To quantify the expression level of ABA-responsive genes, total RNAs were extracted from 5-d-old seedlings, which were treated with the indicated chemicals for 6 h; qRT-PCR was carried out using the primers described before (Cao et al., 2013).
pRD29A-LUC Expression
One-week-old seedlings with pRD29a-LUC (C24 ecotype) grown on one-half-strength MS solid medium were transferred to filter paper soaked with DMSO control or the indicated chemicals for 6 h. LUC imaging was carried out as described before (Ye et al., 2016).
[3H]ABA Uptake into Arabidopsis Protoplasts
Arabidopsis protoplasts were incubated in 1 mL of a bathing solution containing 4.5 nm [3H]ABA (Perkin-Elmer) and the indicated amounts of AA1 (with DMSO as a control). After washing with 3× washing buffer, the mixture was resuspended in 100 µL of water and scintillation fluid. The radioactivity of the bound [3H]ABA was measured using a scintillation counter. The chlorophyll content of the protoplasts was used as an internal control to normalize the protoplast input.
Yeast Two-Hybrid Assay
The yeast two-hybrid assay was carried out as described previously (Cao et al., 2013; Ye et al., 2016). The coding sequences of 13 PYR/PYL genes and HAB1 were amplified from cDNA of Col-0 plants by PCR and cloned into pBD-GAL4 (Stratagene) and pGADT7 (Clontech), respectively. The vectors were cotransformed into AH109 (Clontech) with corresponding PYR/PYL and HAB1 vector combinations. After 2 d of incubation at 30°C on SD(-Leu/-Trp) agar plates, well-grown clones were diluted further to 1:10 and 1:100 dilution gradients. Up to 1 μL of the dilution was added onto SD(-Leu/-Trp/-His) agar plates containing 1 mm 3-amino-triazole, 50 mg L−1 X-α-Gal, 2 μm ABA, and 100 μm quinabactin. Photographs were taken after 3 d of growth at 30°C incubation.
AlphaScreen Assay
The AlphaScreen assay was conducted according to previous reports (Melcher et al., 2009; Cao et al., 2013). Briefly, 100 nm recombinant H6-SUMO-PYR/PYL bound to a nickel acceptor and 100 nm biotin-PP2Cs bound to streptavidin acceptor beads were mixed with the indicated chemicals. The interactions were assessed by AlphaScreen technology (Perkin-Elmer).
HAB1 Phosphatase Activity Assay
Phosphatase assays were performed according to a previous report (Park et al., 2015). All the receptors and HAB1 were expressed in Escherichia coli BL21 as H6-SUMO fusion proteins, as described before (Cao et al., 2013), except that PYL11 and PYL12 were expressed as fusion proteins in vector pET51b. Briefly, 60 pmol of receptors and 60 pmol of His-SUMO-HAB1 were mixed in 80 μL of reaction buffer (100 mm Tris [pH 7.9] and 100 mm NaCl), and then the probe molecules were added for 30 min. Reactions were started by adding 20 μL of 5 mm 4-methylumbelliferyl phosphate (Sigma) in 156 mm Tris-OAc (pH 7.9) and 330 mm KOAc substrate buffer. The phosphatase activity was detected immediately using a microplate reader (Thermo Scientific Varioskan flash; 355 nm excitation and 460 nm emission).
TSAs
All the reactions were conducted as described previously (Soon et al., 2012). The reactions were in final volumes of 10 μL on 96-well plates with SYPRO Orange (Invitrogen) and incubated with compounds on ice for 30 min. Thermal melting experiments were carried out using the StepOnePlus Real-Time PCR System (Applied Biosystems) melt curve program, with a ramp rate of 1°C and a temperature range of 25°C to 90°C for all other experiments. All experiments were conducted in triplicate.
Microscale Thermophoresis
The MST assays were conducted according to the user manual (NanoTemper Technologies). Approximately 10 μm recombinant receptors was labeled with red fluorescent dye (NT-647-NHS; NanoTemper Technologies). A range of concentrations of the required ligand (ranging from 0.02 to 500 μm) was incubated with 1 µm purified protein for 1 h in assay buffer (20 mm Bis-Tris [pH 7.9] and 150 mm NaCl). The sample was loaded into NanoTemper glass capillaries, and microthermophoresis was carried out using 20% LED power and 80% MST. The Kd was calculated using the mass action equation via NanoTemper software from duplicate reads of triplicate experiments. The instrument used was a NanoTemper monolith NT.115.
Molecular Docking
The AutoDock4.0/Vina package was used to model the docking of AA1 with open PYL2 (PDB no. 3KDH) and closed PYL2 (PDB no. 3KDI). The principle of AutoDock4.0/Vina has been described previously (Morris et al., 2009; Trott and Olson, 2010). During the docking process, the exhaustiveness of the global search was set to 20, and the maximum number of conformers was set to 15. The docking results were analyzed by the Pymol software (http://pymol.org/).
Competitive Binding Assays
His-PYL2 bound to Ni-NTA beads was incubated with 0.1 μm [3H]ABA (Perkin-Elmer) alone or with the indicated amounts of AA1 for 1 h. After washing three times, the beads were resuspended in 100 µL of water and mixed with scintillation fluid. The radioactivity of the bound [3H]ABA was measured using a scintillation counter.
Dark-Induced Leaf Senescence
About 3-week-old Col-0 leaves were detached and incubated with the indicated chemicals. For rice (Oryza sativa), fully expanded flag leaves were excised carefully. Detached leaves were cut into ∼3-cm pieces and treated with the indicated chemicals in petri dishes. The samples were incubated at 28°C in darkness for 5 d.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Col-0 seeds sown in the presence of the indicated chemicals.
Supplemental Figure S2. Ten-day-old seedlings incubated with the indicated chemicals.
Supplemental Figure S3. Effects of AS6 on the interactions of HAB1-PYR1/PYLs by yeast two-hybrid assay.
Supplemental Figure S4. AA1 binding to ABA receptors in MST assays.
Supplemental Figure S5. Overall structures of the PYL2/AA1/ABA complexes.
Supplemental Figure S6. Structure-activity relationship study of AA1.
Supplemental Figure S7. Effects of AA1 on cytokinin-responsive gene expression.
Supplemental Table S1. Binding free energy of AA1 to PYI2.
Supplemental Table S2. The interacting amino acids of open PYL2 with AA1.
Supplemental Table S3. The interacting amino acids of closed PYL2 with AA1.
Supplementary Material
Acknowledgments
We thank Dr. Yasushi Todoroki (Shizuoka University) for the gift of AS6 and the National Center for Protein Science Shanghai (Protein Expression and Purification System) for support with the instruments and technical assistance.
Glossary
- ABA
abscisic acid
- Col-0
Columbia-0
- MS
Murashige and Skoog
- TSA
thermal shift assay
- MST
microscale thermophoresis
- PDB
Protein Data Bank
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 31171293 and 31371361 to Y.Z.) and the Chinese Academy of Sciences (to Y.Z and J.-K.Z.).
References
- Bhargava A, Clabaugh I, To JP, Maxwell BB, Chiang Y, Schaller EG, Carolina N, Loriaine A, Kiber J (2013) Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-seq in Arabidopsis. Plant Physiol 162: 272–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Liu X, Zhang Y, Xue X, Zhou XE, Melcher K, Gao P, Wang F, Zeng L, Zhao Y, et al. (2013) An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res 23: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao MJ, Wang Z, Zhao Q, Mao JL, Speiser A, Wirtz M, Hell R, Zhu JK, Xiang CB (2014) Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J 77: 604–615 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Dupeux F, Santiago J, Betz K, Twycross J, Park SY, Rodriguez L, Gonzalez-Guzman M, Jensen MR, Krasnogor N, Blackledge M, et al. (2011) A thermodynamic switch modulates abscisic acid receptor sensitivity. EMBO J 30: 4171–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR. (1994) Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5: 765–771 [Google Scholar]
- Finkelstein RR, Somerville CR (1990) Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol 94: 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9: 436–442 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al. (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17: 172–179 [DOI] [PubMed] [Google Scholar]
- Hao Q, Yin P, Li W, Wang L, Yan C, Lin Z, Wu JZ, Wang J, Yan SF, Yan N (2011) The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol Cell 42: 662–672 [DOI] [PubMed] [Google Scholar]
- Hao Q, Yin P, Yan C, Yuan X, Li W, Zhang Z, Liu L, Wang J, Yan N (2010) Functional mechanism of the abscisic acid agonist pyrabactin. J Biol Chem 285: 28946–28952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H (2008) Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc Natl Acad Sci USA 105: 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV (2012) Small molecules present large opportunities in plant biology. Annu Rev Plant Biol 63: 261–282 [DOI] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9: 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kondoh Y, Yoshida K, Umezawa T, Shimizu T, Shinozaki K, Osada H (2015) Novel abscisic acid antagonists identified with chemical array screening. ChemBioChem 16: 2471–2478 [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Hauser F, Ha T, Xue S, Böhmer M, Nishimura N, Munemasa S, Hubbard K, Peine N, Lee BH, et al. (2011) Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr Biol 21: 990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng P, Yuan B, Guo Y (2014) The role of abscisic acid in fruit ripening and responses to abiotic stress. J Exp Bot 65: 4577–4588 [DOI] [PubMed] [Google Scholar]
- Liang C, Wang Y, Zhu Y, Tang J, Hu B, Liu L, Ou S, Wu H, Sun X, Chu J, et al. (2014) OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci USA 111: 10013–10018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Meesters C, Mönig T, Oeljeklaus J, Krahn D, Westfall CS, Hause B, Jez JM, Kaiser M, Kombrink E (2014) A chemical inhibitor of jasmonate signaling targets JAR1 in Arabidopsis thaliana. Nat Chem Biol 10: 830–836 [DOI] [PubMed] [Google Scholar]
- Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Park SY, Weiner JJ, Fujii H, Chinnusamy V, et al. (2009) A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher K, Xu Y, Ng LM, Zhou XE, Soon FF, Chinnusamy V, Suino-Powell KM, Kovach A, Tham FS, Cutler SR, et al. (2010) Identification and mechanism of ABA receptor antagonism. Nat Struct Mol Biol 17: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Miyakawa T, Sawano Y, Kubota K, Kang HJ, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y, et al. (2009) Structural basis of abscisic acid signalling. Nature 462: 609–614 [DOI] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30: 2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED (2009) Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 326: 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Peterson FC, Defries A, Park SY, Endo A, Nambara E, Volkman BF, Cutler SR (2013) Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc Natl Acad Sci USA 110: 12132–12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR (2015) Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520: 545–548 [DOI] [PubMed] [Google Scholar]
- Rodriguez PL, Lozano-Juste J (2015) Unnatural agrochemical ligands for engineered abscisic acid receptors. Trends Plant Sci 20: 330–332 [DOI] [PubMed] [Google Scholar]
- Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Márquez JA (2009) The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 462: 665–668 [DOI] [PubMed] [Google Scholar]
- Soon FF, Suino-Powell KM, Li J, Yong EL, Xu HE, Melcher K (2012) Abscisic acid signaling: thermal stability shift assays as tool to analyze hormone perception and signal transduction. PLoS ONE 7: e47857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Wang H, Wu M, Zang J, Wu F, Tian C (2012) Crystal structures of the Arabidopsis thaliana abscisic acid receptor PYL10 and its complex with abscisic acid. Biochem Biophys Res Commun 418: 122–127 [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Okamoto M, Akiyama T, Muto T, Yajima S, Sue M, Seo M, Kanno Y, Kamo T, Endo A, et al. (2014) Designed abscisic acid analogs as antagonists of PYL-PP2C receptor interactions. Nat Chem Biol 10: 477–482 [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35: 44–56 [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L, Zhao F, Li R, Xu C, Chen K, Xiao H (2015) The zinc finger transcription factor SlZFP2 negatively regulates abscisic acid biosynthesis and fruit ripening in tomato. Plant Physiol 167: 931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Gong Z, Lu X, Miao D, Shi J, Lu J, Zhao Y (2016) Germostatin resistance locus 1 encodes a PHD finger protein involved in auxin-mediated seed dormancy and germination. Plant J 85: 3–15 [DOI] [PubMed] [Google Scholar]
- Yin P, Fan H, Hao Q, Yuan X, Wu D, Pang Y, Yan C, Li W, Wang J, Yan N (2009) Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat Struct Mol Biol 16: 1230–1236 [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60: 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. (2012) A chemical genetics method to uncover small molecules for dissecting the mechanism of ABA responses in Arabidopsis seed germination. Methods Mol Biol 876: 107–116 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chan Z, Gao J, Xing L, Cao M, Yu C, Hu Y, You J, Shi H, Zhu Y, et al. (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Natl Acad Sci USA 113: 1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.