Abstract
Delirium occurring in patients with dementia is referred to as delirium superimposed on dementia (DSD). People who are older with dementia and who are institutionalized are at increased risk of developing delirium when hospitalized. In addition, their prior cognitive impairment makes detecting their delirium a challenge. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision are considered the standard reference for the diagnosis of delirium and include criteria of impairments in cognitive processes such as attention, additional cognitive disturbances, or altered level of arousal. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision does not provide guidance regarding specific tests for assessment of the cognitive process impaired in delirium. Importantly, the assessment or inclusion of preexisting cognitive impairment is also not addressed by these standards. The challenge of DSD gets more complex as types of dementia, particularly dementia with Lewy bodies, which has features of both delirium and dementia, are considered. The objective of this article is to critically review key elements for the diagnosis of DSD, including the challenge of neuropsychological assessment in patients with dementia and the influence of particular tests used to diagnose DSD. To address the challenges of DSD diagnosis, we present a framework for guiding the focus of future research efforts to develop a reliable reference standard to diagnose DSD. A key feature of a reliable reference standard will improve the ability to clinically diagnose DSD in facility-based patients and research studies.
Keywords: Delirium, dementia, diagnosis, delirium superimposed on dementia, Alzheimer disease, Lewy Body dementia
Delirium is an acute neuropsychiatric disorder characterized by a disturbance in attention and awareness, which develops over a short period of time, with additional disturbance in cognition that are not explained by a preexisting cognitive impairment. Delirium that occurs in patients with dementia is referred to as delirium superimposed on dementia (DSD).1 People who are older with dementia and who are institutionalized are at increased risk of developing delirium when hospitalized. In addition, their prior cognitive impairment makes detecting their delirium a challenge. The prevalence of DSD in institutionalized patients ranges from 1.4% to 70% according to the diagnostic tools,2 whereas the prevalence in community and hospital populations ranges from 22% to 89%.1 It is associated with higher health care costs, worse functional outcomes, and higher mortality rates compared with patients with dementia alone.1,3–6 It was estimated that 35.6 million people lived with dementia worldwide in 2010, with the prevalence expected to nearly double every 20 years, to 65 million in 2030 and 115 million in 2050.7 Therefore, DSD by inference will affect millions of people worldwide especially in nursing home facilities.
The diagnosis of delirium currently relies on the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (Table 1).8,9 These 2 standard references are similar in the definition of delirium, but it should be noted that no specific criteria are provided to clinicians or researchers in either system to assist with the diagnosis of DSD. The DSM-5 specifies that the cognitive deficits should not be better explained by a preexisting, established, or evolving neurocognitive disorder. In the ICD-10, preexisting cognitive deficits are not considered.
Table 1.
DSM-5 and ICD-10 Diagnosis of Delirium
| DSM-5 | ICD-10 | |
|---|---|---|
| Attention | Disturbance in ability to direct, focus, sustain, or shift and | Reduced ability to focus, sustain, or shift attention. |
| Awareness | Disturbance in awareness environmental orientation | Clouding of consciousness, that is, reduced clarity of awareness of the environment |
| Timing/fluctuation | Develops quickly (hours to days) and represents a change from baseline and fluctuates over a day | Rapid onset and fluctuations of the symptoms over the course of the day. |
| Memory deficit | An additional disturbance in cognition (eg, memory deficit, disorientation, language, visuospatial ability, or perception). | Disturbance of cognition, manifest by both:
|
| Psychomotor deficit | None | At least 1 of the following psychomotor disturbances:
|
| Sleep disturbance | None | Disturbance of sleep or the sleep/wake cycle, manifest by at least 1 of the following:
|
| Corroborating Data | There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal, or exposure to a toxin, or is due to multiple etiologies. | Objective evidence from history, physical and neurologic examination, or laboratory tests of an underlying cerebral or systemic disease (other than psychoactive substance-related) that can be presumed to be responsible for the clinical manifestations. |
| Other cognitive disorders | Not better explained by a preexisting, established or evolving neurocognitive disorder and do not occur in the context of a severely reduced level of arousal, such as coma. | None |
Thirty years ago Lipowski10 provided a reference work for the definition of the concept of delirium. During the following years several terms have been used to define delirium. There has been an attempt to reduce the heterogeneity of delirium terminology to avoid “confusion” in clinical work and especially in research studies. The challenge to achieve clarity is even greater when delirium is considered in patients with preexisting dementia. In this context several terms have been used: sundowning, acute dementia, rapidly progressive dementia, acute confusion, delirium in cognitively impaired patients, and DSD.11
Although dementia may have more consistent terminology, the breadth of cognitive impairments in dementia nonetheless makes the assessment of baseline (and incident changes) in cognitive function challenging. Although some advocates prefer standardized testing, the progressive nature of dementia limits the battery of tests available. In addition, the growing diversity of the world population and the increasing challenge of dementia worldwide necessitate the availability of assessments in languages other than English.
The lack of standardization in the assessment of DSD may have potential significant clinical and research implications. The DSM-5 and the ICD-10 do not provide clinicians with indications of specific tests for assessment of attention or additional cognitive disturbances in cognition or altered level of arousal. Importantly, the assessment or inclusion of preexisting cognitive impairment is also not addressed by either the DSM-5 or ICD-10. Meagher et al12 have recently attempted to provide a standardized approach to the use of the DSM-5 criteria for delirium to avoid “confusion” even with the use of the standard reference.12
As the DSM-5 and the ICD-10 are considered the standard reference for the diagnosis of delirium, then several practical limitations should be considered.
First, attention has multiple domains,13 and there is not a clear DSM-5 and ICD-10 guidance regardingwhich domain orattention test should be used in patients with dementia, dementia stage, or dementia subtypes.
Second, the clinician is currently not provided with clear and predetermined methodology for how to ascertain the time of onset of delirium, the change from baseline, and fluctuation over the course of the day.
Third, there is no reference for how to determine the presence of a preexisting neurocognitive disorder or the level of arousal.
As a result, we formed a task force of European Delirium Association (www.europeandeliriumassociation.com), American Delirium Association (www.americandeliriumsociety.org), and Australasian Delirium Association (www.delirium.org.au) society members, collectively called iDelirium (www.idelirium.org), to (1) clarify the key elements for the diagnosis of DSD, (2) review evidence of DSD diagnosis, and (3) formulate a path for the future direction of research in DSD.
The Challenge of Neuropsychological Assessment of Delirium in Patient With Dementia
Attention Deficits
Inattention is considered a core feature of delirium (criterion A, DSM-5) and a valid assessment of attention is, therefore, central to the diagnosis.
Definition of attention
Attention is widely studied in neuropsychology, but there is not a single definition. A possible definition proposed by Cohen is the “ability to focus on a selected stimulus, sustaining that focus and shifting it at will.”14 Three major functions of attention are (1) orienting to sensory events, (2) detecting signals for focal processing, and (3) maintaining a vigilant or alert state.13,15 These are reflected to some extent in the DSM-5 criteria for delirium, which state that patients must demonstrate a reduced ability to direct, focus, sustain, and shift attention.8 However, elements of executive function fall under the umbrella name of inattention and include much higher level cognitive tasks that are likely to be impaired in dementia, such as shifting and divided attention (multitasking).
Attentional deficits in delirium and dementia
The extent to which different aspects of attention are affected in delirium is poorly understood. Marked impairments in focusing and sustaining attention (ie, the ability to maintain attention to stimuli over time) are considered the hallmark feature of delirium.16–18 One of the key challenges of delirium diagnosis is that the severity of attentional deficits in delirium varies greatly between patients (and also sometimes within the same patient at different time points), ranging from subtle impairments in complex working memory tasks, to more pronounced deficits in orienting and focusing attention, and finally to a state of lowered level of arousal whereby patients are unable to respond to simple commands. DSM-5 does offer some guidance on this matter, as it states that an inability to engage in standardized testing or interview should be classified as severe inattention.19
Complex attentional impairments may be found in the early stages of Alzheimer disease (AD) dementia (without delirium), but overall the ability to focus and sustain attention is relatively preserved in the earlier stages of AD.20 Conversely, it has been shown that sustained, divided, and selected attention are compromised in the moderate to severe stages of dementia.21 The type of dementia is also a factor. For example, patients with Lewy body dementia have considerably greater impairment of attention compared with patients with another type of dementia.22 Given the centrality of inattention to the definition of delirium, much more needs to be learned about the nature of attentional deficits in both dementia and delirium if criteria for the diagnosis of DSD are to be optimized.
Tests to evaluate inattention
There is no consensus on how attention should be assessed,23 and most existing tests differentiate the inattention found in delirium from that in dementia.24,25 This uncertainty impedes progress in clinical practice and research. Several neuropsychological tests are currently used to assess inattention in delirium including digit span, spatial span, months of the year and days of the week backwards, and serial sevens.18 A recent review of studies on attention assessment in delirium concluded that cancellation tests (ie, test to determine an individual’s ability to detect targets among a range of similar stimuli, usually digits or letters), span tests, and computerized tests of sustained attention appear to offer utility in discriminating delirium from dementia.18 Months of the year backwards, one of the most widely used attention test in clinical practice, appears to have high sensitivity to delirium in older hospitalized patients.26–28
In general, caution is warranted when generalizing these findings. Further, tests are often labeled as measuring inattention when they also depend on other cognitive components, which themselves are more likely to be affected in dementia. There is an overlap of attention, working memory, and executive control. For instance, digit span is used as a measure of inattention, but it is also used to measure executive function and working memory. Cognitively simple tests that rely on focusing and sustaining attention rather than manipulating information or testing memory may be especially useful in discriminating delirium from dementia. For instance, in preschool children, attention deficits have been evaluated moving slowly a simple picture in front of the patient’s face while verbally prompting them to look at the picture and evaluating their ability to look at the pictures when their eyes are open.29
Arousal, Awareness, and Motor Fluctuations
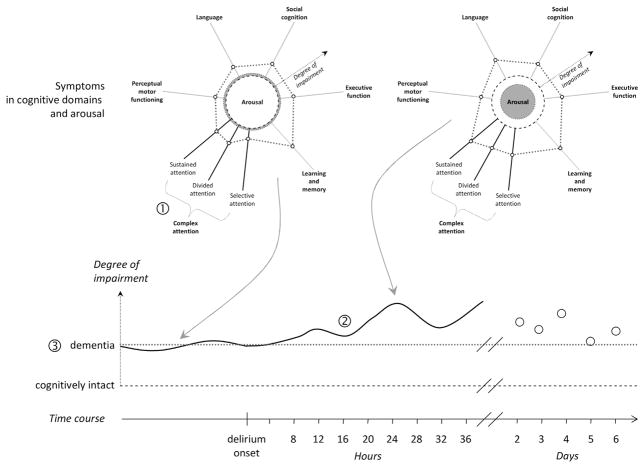
Different cognitive domains are affected in dementia. For example, although the ability to learn is reduced, an individual may still have little difficulty with perceptual motor functioning, and perhaps be slightly less impaired in selective attention in comparison with the ability to divide attention. Clearly, the ability to engage cognition depends on the degree of arousal (Figure 1). The term “arousal” can be defined as the level of sensory stimulation that is required to keep the patient attending to the examiner’s question or following a command.30
Fig. 1.
Cognitive domains and arousal. Different cognitive domains are affected in dementia, but the ability to engage cognition depends on the degree of arousal. In the radar plot to the left of the figure, this particular individual has almost normal arousal, perhaps being somewhat hyperalert (dashed circle signals “normal” arousal). With the onset of delirium, most cognitive domains deteriorate as arousal decreases (degree of arousal = dotted red circle) as could be the case given hypoactive delirium (radar plot to the right). In this constellation of an individual having shown cognitive impairment, including attention, prior to delirium, it is especially challenging to adequately test for specific change in any one of multiple attentional domains (marked 1). It is, moreover, as difficult to determine the time of onset given that change is not from a nonimpaired state (marked 2; the solid black line on the timeline signals the fluctuating degree of cognitive impairment, in close relationship to the severity of delirium symptoms), not knowing how severe the baseline impairment (dotted red line on the timeline) actually was (marked 3).
The key role of arousal in delirium was first recognized over 3 decades ago and is now well established. In 1980, the Diagnostic and Statistical Manual of Mental Disorders (DSM) III version indicated that impaired consciousness was a core symptom of delirium, and this criterion was confirmed in subsequent years by almost all the classification systems. In what seems to be a reversal in this guidance, the current DSM-5 places greater emphasis on awareness, attention, and other cognitive disorders while de-emphasizing consciousness.8 Indeed, criterion D of DSM-5 states that inattention and changes in cognition must not occur in the context of a severely reduced level of arousal such as coma. Far from being a downgrading of the role of arousal in delirium, these criteria implicitly admit that arousal disorders are an integral aspect of this condition.19 In fact, both attention and arousal are hierarchically related; it is possible to have full arousal, but profound inattention (eg, hypervigilance), but not the other way around,31 (ie, when disorders of arousal exist inattention is always present).19 In other words, the DSM-5 criteria identify a threshold (ie, coma) under which a patient cannot be regarded as delirious, but does not reject the role of arousal in delirium phenomenology.19
Leonard et al32 have reported that delirium may be distinguished from dementia by virtue of the disproportionate impairment of vigilance and attention. Importantly, arousal is generally preserved in advanced chronic cognitive disorders,16 while attention and cognition (ie, orientation, memory, language, and executive functions) are usually already impaired in the moderate stages of dementia26,33 and especially when dementia is severe.34 A pathophysiological explanation relies on evidence that performance on attentional and general cognitive tasks, when administered, is contingent on brain activity in distributed cortical areas,35 which are generally damaged in the advanced stages of dementia, while arousal assessment tests structures in the brainstem.36 Because the hierarchical pattern of neuro-degeneration in AD begins in the entorhinal/perirhinal cortex, then progresses in the hippocampus and in association cortex, and finally in the primary neocortex (NIA-RI Consensus 1997), it could be hypothesized that attentional and other cognitive tasks cannot be assessed when the damage in the brain is advanced. On the contrary, arousal assessment may be feasible when dementia is severe. To indirectly support this observation, it is not surprising that studies to discriminate neuropsychological profiles of delirious and demented patients have generally recruited individuals with mild or, at most, moderate stages of dementia.
Tieges et al37 developed a new approach with the Observational Scale of Level of Arousal (OSLA)37 to detect delirium by observing eye opening, eye contact, as well as patient posture and movement. Delirium is not an isolated mental disorder but affects motor function as well.38,39 The evaluation of posture and movement might indeed be of interest in the context of patients with dementia. A study comparing 4 groups of 15 patients (with delirium alone, with dementia alone, with DSD, and with neither delirium nor dementia) found that when delirium develops, a worsening of motor performance also occurs.39 In patients with preexisting dementia, the resolution of delirium did not result in a significant improvement of specific cognitive functions, providing indirect evidence that when dementia is advanced, the cognitive testing does not provide good information to support the diagnosis of delirium. In this case, an assessment of motor abilities may assume a more specific diagnostic utility.39
In this regard, the Richmond Agitation and Sedation Scale (RASS),40,41 a scale originally evaluated to monitor the level of consciousness in critically ill patients, might provide important information on motor fluctuations given its potential to identify motor subtypes of delirium. Negative scores on the RASS may indicate hypoactive behavior in delirium and positive scores indicative of a hyperactive behavior. The scale has been recently modified and adapted for use in general wards (ie, the modified RASS or m-RASS).42 Both the RASS and the m-RASS are ultra-brief assessments of the level of consciousness, and recent studies have suggested that they have high specificity for delirium in geriatrics wards, emergency departments,37,42,43 and specifically in patients with dementia.44
It could, therefore, be hypothesized that when dementia is severe or very severe, and attention cannot be tested using tools that require a certain level of cognition, arousal, and basic motor performance may be routinely assessed to detect delirium. For example, the observation of an unexpected drowsiness or failure to sit up from the bed in patients who were previously alert and able to move could suggest delirium in patients with dementia. Mobility performances in patients with dementia may similarly be used to detect incipient delirium.
Dementia With Lewy Bodies and the Influence on Tests Used to Diagnose Delirium
The clinical phenotypes of dementias show some overlap with delirium. For example, fluctuating cognition is seen in AD and vascular dementia; however, the prevalence and severity of fluctuation is greatest in dementia with Lewy bodies (DLB) (Table 2).45 Similarly, complex visual hallucinations and delusions frequently occur in DLB, Parkinson disease dementia, and delirium but are seen in only 4%–8% of patients with AD.46 As a result, it is harder to differentiate DLB from delirium in comparison with other dementias according to the key features as identified by Meagher et al47 (attentional deficits, sleep-wake cycle disturbance, impaired higher level thinking disturbances). DLB is viewed by many as the most challenging dementia subtype in which to diagnose DSD.48
Table 2.
Delirium and DLB: Overlap and Differences
| Delirium | DLB |
|---|---|
| Onset: Acute onset | Onset: Gradual and progressive onset |
| Cognitive impairment: fluctuation in attention, executive functioning, memory, visuospatial deficits | Cognitive impairment: fluctuation in attention, executive functioning, vigilance, memory, visuospatial deficits |
| Perceptual disturbances: visual and auditory hallucinations, delusions | Perceptual disturbances: recurrent structured visual hallucinations, delusions |
| Sleep wake-cycle: disturbance in sleep wake-cycle | Sleep wake-cycle: REM sleep behavior disorder |
| Motor signs: hyperactive or hypoactive motor signs | Motor signs: parkinsonism motor signs |
| Neuroleptic sensitivity: non present | Neuroleptic sensitivity: severe neuroleptic sensitivity |
DLB, dementia with lewy bodies; REM, rapid eye movement.
There is a pressing need to develop methods to distinguish delirium and DLB. Lewy body disease is the second most common form of dementia in older people with a prevalence of 15%–20% of all dementias at autopsy.49 The prevalence of older adults with 2 or more DLB symptoms in nursing homes ranges from 16% to 20%.50 Furthermore, patients with DLB also appear to be more acutely sensitive to minor insults (even subclinical events) and, thus, more delirium “prone.” Consequently, and unsurprisingly, delirium is more commonly diagnosed in DLB than AD51 with 32% of outpatients with DLB experiencing delirium as opposed to 15% of outpatients with AD.52
Differentiating DSD from DLB or delirium alone is vital to guide initial management and safe prescribing. Incorrectly diagnosing an acute change as a deterioration in patients with existing DLB rather than delirium can result in the under-investigation of common causes of delirium, such as infections, medications, and pain and inadequate management of this potentially life threatening medical emergency. Conversely, the lack of recognition of undiagnosed DLB in someone with delirium may result in patients being treated with antipsychotic medications. Such agents can significantly worsen features of their DLB, such as parkinsonism and cognitive impairment/arousal, and significantly increase the risk of mortality.53 Furthermore, a failure to diagnosis DLB may mean that patients miss out on appropriate treatments, such as cholinesterase inhibitors, which have been shown to improve hallucinations, delusions, and cognition in patients with DLB.54 Given findings that suggest cholinesterase inhibitors might worsen delirium outcomes,55 it is imperative to make an accurate diagnosis of DLB in these settings.
Because of these overlapping clinical phenotypes, collateral history is essential to the identification of delirium in patients with DLB. Clinicians need to be vigilant to an acute, distinct change compared with baseline, including increased fluctuations in someone who previously had predominantly parkinsonism and hallucinations. In those with delirium, a previous history of symptoms including autonomic dysfunction (ie, recurrent dizziness, blackouts, constipation, urinary urgency, etc), rapid eye movement (REM) sleep behavior disorder, anosmia, visuoperceptual disturbances, as well as psychosis and episodes of delirium, along with clinical examination findings of parkinsonism (eg, bradykinesia, hypomimia, tremor, rigidity, change in posture, recurrent falls, or altered gait), may all suggest an undiagnosed DLB. The best methods to elicit these collateral histories may provide a focus for future diagnostic work to assist with the diagnosis of delirium in patients with DLB.
Research into how to recognize delirium in people with DLB may also focus on developing methods to test features that are most likely to differ, including cognitive domains such as orientation, vigilance, and psychomotor disturbances.49 Currently, when there is a suspicion of DLB, diagnostic biomarkers, such as a DaT SCAN may be helpful,56 although from a practical point of view, these usually need to be performed after the delirium episode ends. The role of objective biomarkers measurements, such as electroencephalography (EEG) to distinguish delirium and DLB, is appealing, however, EEG patterns in delirium overlap with those seen in DLB, making the differentiation more difficult. More advanced methods of EEG processing/quantification may illuminate other specific nuances between delirium and DLB and could provide a focus for future research.
Framework for Improving the Diagnosis of DSD
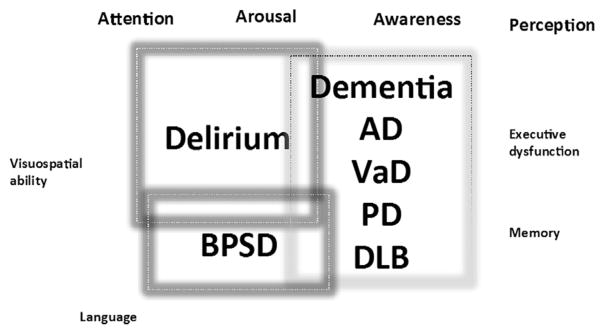
There are many gaps in our knowledge about how to diagnose DSD with a high degree of accuracy. The current state of the science does not provide guidance on which clinical tests and biomarkers should be specifically tested in patient with DSD. Additional challenges are related to the possible difference in DSD presentation in patients with different types of dementia (Figure 2).
Fig. 2.
The challenge of delirium across different types of dementia and possible overlap with behavioral and psychological symptoms of dementia (BPSD). AD, Alzheimer dementia; DLB, dementia with lewy bodies; VaD, vascular dementia; PD, Parkinson dementia.
Here we provide a consensus from the iDelirium group on research that is needed to clarify important key points in the diagnosis of DSD (Table 3).
Table 3.
Framework for Improving the Diagnosis of DSD
| Improving attention testing |
|
| Impact of arousal/awareness/motor fluctuations |
|
| Clinical evaluation of DSD | Define how clinicians should test DSD using clinical information collected from family members, medical record review, and evaluation of basic and instrumental activities of daily living |
| Clinical exam | Define which component of the neurologic examination might be useful or indicative in the diagnosis of DSD |
| Laboratory testing |
|
| Neuroimaging |
|
Improving Attention Testing
(1) Define which types of attention should be tested; (2) Define what type of attention should be tested according to the type of dementia and severity of dementia; (3) Define what attention tests should be used according to the type of dementia and severity of dementia.
Impact of Arousal/Awareness/Motor Fluctuations
(1) Further define the implications for using the RASS, m-RASS, or OSLA to diagnosis DSD and the possible implications on outcomes; (2) Define the role of the use of objective evaluation of the level of motor fluctuation (eg, actigraphy monitoring) for the diagnosis of DSD; (3) Define the importance and the possible use of clinical evaluation of motor fluctuation using bed-side tests (eg, Tinetti scale, hierarchical assessment of balance and mobility).
Clinical Evaluation of DSD
Define a specific protocol to collect information from family members, review of medical records, to determine basic and instrumental activities of daily living, and to determine prior cognitive function using a standardized collateral informant questionnaire, such as the SF-IQCODE (Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly) or the AD8.
Clinical Examination
Define which component of the neurologic examination might be useful or indicative in the diagnosis of DSD (eg, tendon reflexes, plantar reflexes, UPM/extrapyramidal, archaic reflexes, myoclonia; eye examination including pupil size, light reflex, oculocephalic response; cough reflex, grimace).
Laboratory Testing
(1) Define a core set of laboratory evaluation for the clinical diagnosis of DSD; (2) Define a core set of laboratory evaluation for the research diagnosis of DSD.
Neuroimaging
(1) Clarify the role of neuroimaging in the clinical diagnosis of DSD; (2) Provide indications for the use of neuroimaging techniques for a research standard of diagnosis of DSD (eg, brain magnetic resonance imaging, DaT SCAN).
Summary
DSD is a clinical syndrome that can result in very poor outcomes. Accurate identification has so far been elusive. In this report, we have presented descriptions of its presentation and associated clinical findings, and the insufficiency of current diagnostic assessment tools. We hope that our framework will be helpful for the improved delineation of this highly problematic geriatric clinical syndrome.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 2.de Lange E, Verhaak PF, van der Meer K. Prevalence, presentation and prognosis of delirium in older people in the population, at home and in long term care: A review. Int J Geriatr psychiatry. 2013;28:127–134. doi: 10.1002/gps.3814. [DOI] [PubMed] [Google Scholar]
- 3.Sampson EL, Blanchard MR, Jones L, et al. Dementia in the acute hospital: Prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195:61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 4.Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8:500–505. doi: 10.1002/jhm.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellelli G, Frisoni GB, Turco R, et al. Delirium superimposed on dementia predicts 12-month survival in elderly patients discharged from a postacute rehabilitation facility. J Gerontol Ser A Biol Sci Med Sci. 2007;62:1306–1309. doi: 10.1093/gerona/62.11.1306. [DOI] [PubMed] [Google Scholar]
- 6.Morandi A, Davis D, Fick DM, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc. 2014;15:349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer Dementia. 2013;9:63–75. e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association A. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 2013. [Google Scholar]
- 9.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010. 2013 Available at: http://apps.who.int/classifications/icd10/browse/2010/en-/F00-F09.2013.
- 10.Lipowski ZJ. Delirium: Acute confusional states. New York: Oxford University Press; 1990. [Google Scholar]
- 11.Marcantonio ER. In the clinic. Delirium. Ann Intern Med. 2011:154. doi: 10.7326/0003-4819-154-11-201106070-01006. ITC6–1, ITC6–2, ITC6–3, ITC6–4, ITC6–5, ITC6–6, ITC6–7, ITC6–8, ITC6–9, ITC6–10, ITC6–11, ITC6–12, ITC6–13, ITC6–14, ITC6–15; quiz ITC6–16. [DOI] [PubMed] [Google Scholar]
- 12.Meagher DJ, Morandi A, Inouye SK, et al. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12:164. doi: 10.1186/s12916-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen RA. The Neuropsychology of Attention. New York: Plenum Press; 1993. [Google Scholar]
- 15.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 16.Brown LJ, Fordyce C, Zaghdani H, et al. Detecting deficits of sustained visual attention in delirium. J Neurol Neurosurg Psychiatry. 2011;82:1334–1340. doi: 10.1136/jnnp.2010.208827. [DOI] [PubMed] [Google Scholar]
- 17.O’Keeffe ST, Gosney MA. Assessing attentiveness in older hospital patients: Global assessment versus tests of attention. J Am Geriatr Soc. 1997;45:470–473. doi: 10.1111/j.1532-5415.1997.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 18.Tieges Z, Brown LJ, MacLullich AM. Objective assessment of attention in delirium: A narrative review. Int J Geriatr psychiatry. 2014;29:1185–1197. doi: 10.1002/gps.4131. [DOI] [PubMed] [Google Scholar]
- 19.Boustani MRJ, Shaughnessy M, Gruber-Baldini A, et al. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014;12:141. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- 21.Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: Relationship to episodic and semantic memory impairment. Neuropsychologia. 2000;38:252–271. doi: 10.1016/s0028-3932(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 22.Calderon J, Perry RJ, Erzinclioglu SW, et al. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meagher DJ, Maclullich AM, Laurila JV. Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214. doi: 10.1016/j.jpsychores.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2012;60:2005–2013. doi: 10.1111/j.1532-5415.2012.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronnick K, Emre M, Lane R, et al. Profile of cognitive impairment in dementia associated with Parkinson’s disease compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1064–1068. doi: 10.1136/jnnp.2006.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Regan NA, Ryan DJ, Boland E, et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85:1122–1131. doi: 10.1136/jnnp-2013-307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamis D, Meagher D, Murray O, et al. Evaluating attention in delirium: A comparison of bedside tests of attention. Geriatr Gerontol Int. 2016;16:1028–1035. doi: 10.1111/ggi.12592. [DOI] [PubMed] [Google Scholar]
- 28.Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10:645–650. doi: 10.1002/jhm.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HA, Gangopadhyay M, Goben CM, et al. The preschool confusion assessment method for the ICU: Valid and reliable delirium monitoring for critically ill infants and children. Crit Care Med. 2016;44:592–600. doi: 10.1097/CCM.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner JB, Saper CB, Schiff ND, Plum F. Multifocal, diffuse, and metabolic brain diseases causing delirium, stupor, or coma. New York, NY: Oxford University Press; 2007. pp. 179–296. [Google Scholar]
- 31.Bhat R, Rockwood K. Delirium as a disorder of consciousness. J Neurol Neurosurg Psychiatry. 2007;78:1167–1170. doi: 10.1136/jnnp.2007.115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard M, McInerney S, McFarland J, et al. Comparison of cognitive and neuropsychiatric profiles in hospitalised elderly medical patients with delirium, dementia and comorbid delirium-dementia. BMJ Open. 2016;6:e009212. doi: 10.1136/bmjopen-2015-009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolanowski AM, Fick DM, Yevchak AM, et al. Pay attention! The critical importance of assessing attention in older adults with dementia. J Gerontol Nurs. 2012;38:23–27. doi: 10.3928/00989134-20121003-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: A caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–39. [PubMed] [Google Scholar]
- 35.Wildgruber D, Kischka U, Ackermann H, et al. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Brain Res Cogn Brain Res. 1999;7:285–294. doi: 10.1016/s0926-6410(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 36.Tindall SC. Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Chapter 57. Boston, MA: Butterworths; 1990. [PubMed] [Google Scholar]
- 37.Tieges Z, McGrath A, Hall RJ, Maclullich AM. Abnormal level of arousal as a predictor of delirium and inattention: An exploratory study. Am J Geriatr Psychiatry. 2013;21:1244–1253. doi: 10.1016/j.jagp.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Adamis D, Treloar A, Gregson N, et al. Delirium and the functional recovery of older medical inpatients after acute illness: The significance of biological factors. Arch Gerontol Geriatr. 2011;52:276–280. doi: 10.1016/j.archger.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Bellelli G, Speciale S, Morghen S, et al. Are fluctuations in motor performance a diagnostic sign of delirium? J Am Med Dir Assoc. 2011;12:578–583. doi: 10.1016/j.jamda.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 41.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 42.Chester JG, Harrington BM, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han JH, Vasilevskis EE, Schnelle JF, et al. The diagnostic performance of the Richmond agitation sedation scale for detecting delirium in older emergency department patients. Acad Emerg Med. 2015;22:878–882. doi: 10.1111/acem.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morandi A, Han JH, Meagher D, et al. Detecting delirium superimposed on dementia: Evaluation of the diagnostic performance of the Richmond agitation and sedation scale. J Am Med Dir Assoc. 2016;17:828–833. doi: 10.1016/j.jamda.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker MP, Ayre GA, Cummings JL, et al. Quantifying fluctuation in dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. Neurology. 2000;54:1616–1625. doi: 10.1212/wnl.54.8.1616. [DOI] [PubMed] [Google Scholar]
- 46.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 47.Meagher DJ, Leonard M, Donnelly S, et al. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry. 2010;81:876–881. doi: 10.1136/jnnp.2009.200956. [DOI] [PubMed] [Google Scholar]
- 48.Richardson S, Teodorczuk A, Bellelli G, et al. Delirium superimposed on dementia: A survey of delirium specialists shows a lack of consensus in clinical practice and research studies. Int Psychogeriatr. 2016;28:853–861. doi: 10.1017/S1041610215002288. [DOI] [PubMed] [Google Scholar]
- 49.Gore RL, Vardy ER, O’Brien JT. Delirium and dementia with Lewy bodies: Distinct diagnoses or part of the same spectrum? J Neurol Neurosurg Psychiatry. 2015;86:50–59. doi: 10.1136/jnnp-2013-306389. [DOI] [PubMed] [Google Scholar]
- 50.Zahirovic I, Wattmo C, Torisson G, et al. Prevalence of dementia with Lewy body symptoms: A cross-sectional study in 40 Swedish nursing homes. J Am Med Dir Assoc. 2016;17:706–711. doi: 10.1016/j.jamda.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Vardy E, Holt R, Gerhard A, et al. History of a suspected delirium is more common in dementia with Lewy bodies than Alzheimer’s disease: A retrospective study. Int J Geriatr Psychiatry. 2014;29:178–181. doi: 10.1002/gps.3986. [DOI] [PubMed] [Google Scholar]
- 52.Hasegawa N, Hashimoto M, Yuuki S, et al. Prevalence of delirium among out-patients with dementia. Int Psychogeriatr IPA. 2013;25:1877–1883. doi: 10.1017/S1041610213001191. [DOI] [PubMed] [Google Scholar]
- 53.McKeith IG, Perry RH, Fairbairn AF, et al. Operational criteria for senile dementia of Lewy body type (SDLT) Psychol Med. 1992;22:911–922. doi: 10.1017/s0033291700038484. [DOI] [PubMed] [Google Scholar]
- 54.Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: A systematic review and meta-analysis. Am J Psychiatry. 2015;172:731–742. doi: 10.1176/appi.ajp.2015.14121582. [DOI] [PubMed] [Google Scholar]
- 55.van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: A multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 56.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]