Abstract
Burns are associated with activation of the innate immunity that can contribute to complications. Damage-associated molecular patterns (DAMPs) released after tissue injury play a critical role in the activation of the innate immunity, which appears to be mediated via toll-like receptors (TLRs). Previous findings have shown that TLRs and TLR-mediated responses are up-regulated after burn. Nonetheless, it is unclear what impact burn injury has on circulating levels of DAMPs. To study this, male C57BL/6 mice were subjected to a major burn injury or sham procedure. Three hours to 7 days thereafter, plasma was collected and assayed for the representative DAMPs (i.e., HMGB1, cytochrome C, DNA and S100A) and extracellular cleavage products (fibronectin and hyaluronan). HMGB1, cytochrome C, fibronectin and hyaluronan levels were elevated in a time-dependent manner after burn as compared to sham levels. A significant elevation in TNF-α, IL-6 and IL-10 cytokine plasma levels was also found after burn. All cytokine levels were increased as early as 3 hr and remained elevated up to 24 hr. Circulating CD11b+ monocytes were increased at 24 hr after burn and showed increased expression of TLR-2. In conclusion, these findings support the concept that burn-induced elevations in circulating DAMPs are in part responsible for monocyte activation and the development of inflammatory complications under such conditions and warrants further investigation.
Keywords: Danger Theory, Alarmins, Trauma, HMGB-1, Toll-like Receptors, CD11b
INTRODUCTION
It is well established that burn injury is associated with a marked inflammatory response and activation of the innate immune system which contributes to multiple complications. The burn induced inflammatory response is associated with the release of pro-inflammatory mediators which interact with the host and the subsequent development of a systemic inflammatory response syndrome (SIRS) and multiple organ failure [1;2].
Innate immunity activation, while an essential step in the early response to infection, is also important in the clearance of injured tissue and the initiation of tissue repair. With regard to burn, cellular injury can lead to the release of intracellular molecules known as damage-associated molecular patterns or DAMPs [3;4]. DAMPs are derived from a large array of cellular components including the plasma membrane, nucleus, endoplasmic reticulum, cytosol, and mitochondria [5]. Interestingly the existence of common recognition patterns for DAMPs and pathogen-associated molecular patterns (PAMPs) on toll-like receptors (TLRs) supports that concept of the mitochondria as an important source of DAMPs. A wide range of molecules of mitochondrial origin, including mitochondrial DNA, N-formyl peptides, cardiolipin, cytochrome C, carbamoyl-phosphate synthase 1 and ATP have been identified as DAMPs. These DAMPs are recognized by a number of different receptor types, including TLRs [6–8]. Activation of TLRs stimulates the innate immune system and can lead to inflammation and associated complications. Increased TLR reactivity has been implicated in a number of immunopathological aspects of trauma and burn [9–11].
The Danger Theory, proposed by Matzinger [12], hypothesizes that the mechanism of cell death governs whether an immune response is initiated. Controlled cell death (i.e., apoptosis), does not lead to DAMP generation; however, necrotic cell death (associated with tissue injury) generates DAMPs, which in turn activate the innate immune system. Potential DAMPs involved in the activation of the innate immune system via TLRs include mitochondrial DNA (mtDNA), HMGB-1 and S100A [13]. Zhang et al. recently demonstrated that mitochondrial DAMPs, released by cellular disruption after trauma, are present in the circulation and activate neutrophils [6]. The study herein was undertaken to investigate the relationships between burn injury, DAMPs and activation of innate immunity.
MATERIALS AND METHODS
Animals
C57BL/6 male mice (18–25 g, Charles River) were used for all experiments. Mice were allowed to acclimatize for at least one week prior to experimentation and maintained in ventilated cages under specific pathogen-free conditions. Animals were randomly assigned to either a sham or burn group. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio. This study was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
Burn procedure
Mice received a scald burn as described elsewhere [14]. Briefly, the mice were anesthetized by intraperitoneal (i.p.) injection of avertin (tribromoethanol prepared in amyl alcohol, Sigma Aldrich), at a dose of 275 mg/kg. The dorsal surface was shaved and the anesthetized mouse was placed in a custom-built, insulated mold exposing 12.5% of their total body surface area (TBSA) along the right dorsum. The mold was immersed in 70°C water for 10 sec to produce a 3rd degree burn. The burn procedure was repeated on the left dorsal side yielding a total burn size of 25% TBSA. Previous studies have verified this injury to be a full thickness with damage to the epidermal, dermal and sub-dermal layers [14]. The mice were then resuscitated with 1 mL of Ringer's lactate solution administered by i.p. injection and returned to their cages. The cages were placed on a heating pad until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia and resuscitation only. Three hours, 24 hrs, 3 days or 7 days later, blood and plasma were collected for analysis.
DAMP Analysis
Plasma levels of cytochrome C and HMGB1, S100A, fibronectin, and hyaluronan were assayed by ELISA according to the manufacturer’s recommendations. The ELISA kits for cytochrome C, HMGB1 and S100A were from www.antibodies-online.com. The kit for fibronectin was from Boster (Pleasanton, CA) and the kit for hyaluronan was from R&D Systems (Minneapolis, MN). Total double stranded DNA was isolated from plasma using a QIAamp DNA blood minikit (Qiagen, Valencia CA) following the manufacturer’s recommendations. DNA in the plasma was determined spectrophotometrically by measuring the absorbance of the sample at 260nm (A260) and adjusting the measurement for turbidity (measured by absorbance at 320nm). The concentration of DNA (µg/mL) = (A260 value – A320value) × the dilution factor × 50µg/mL (i.e., A260 of 1.0 = 50µg/mL double stranded DNA). The total yield of DNA was obtained by multiplying the DNA concentration by the sample volume.
Determination of cellular phenotype
Whole blood was collected from sham and burn mice at 24 hr after burn and stained with fluorescent-conjugated antibodies against CD11b (FITC), TLR-2 (PE), and TLR-4 (PE) to assess cellular phenotype. The manufacturer’s recommended methodology was employed (BD Pharmingen). Appropriate isotype controls were included. FITC and PE were analyzed with a Becton-Dickinson LSR II flow cytometer (BD Biosciences, Mountain View, CA). Monocyte populations were gated for analysis as determined for forward and side scatter). A total of 25,000 events were collected for each analysis and FlowJo software was used to analyze the results gating on the lymphocyte/monocytes gate as determined by forward and side scatter.
Cytokine Analysis
Plasma samples collected from sham and burn mice at 24 hr after burn were assayed for IL-6, IL-10 and TNF-α cytokines that were determined by commercial sandwich ELISA according to the manufacturer’s recommendations (OptiEIA; BD Pharmingen, La Jolla CA).
Statistics
Data are expressed as mean ± SEM for 4–7 mice/group. Comparisons were analyzed using ANOVA and SigmaPlot 11.0 software (Systat Software Inc, San Jose CA). A p-value < 0.05 was considered to be statistically significant for all analyses.
RESULTS
Plasma DAMP levels
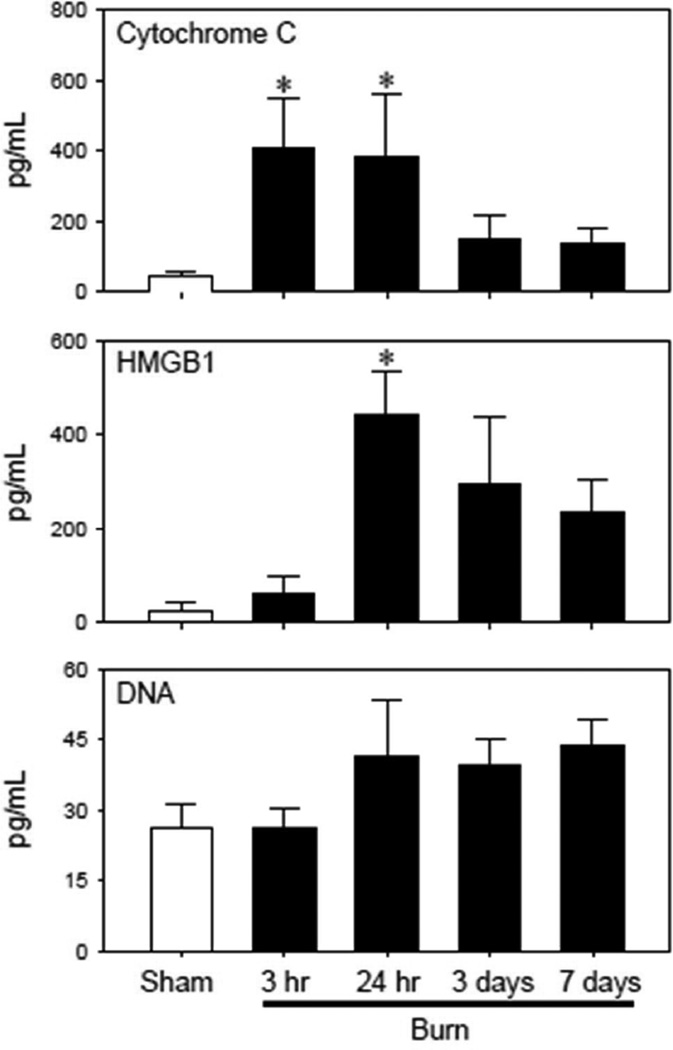
Circulating DAMPs levels were measured from 3 hr to 7 days after sham and burn procedure (Fig 1). Cytochrome C levels, a marker of mitochondrial damage, were elevated (~8-fold) as early as 3 hr post-injury and remained elevated at 24 hr. At 3 days post-injury they returned to sham levels. HMGB1 levels were elevated ~10-fold at 24 hr after injury, but at no other times after injury. Plasma DNA levels trended upwards at 1–7 days after burn, but they were not significantly different from sham mice. S100A was not measurable at any time point (data not shown)
Figure 1. Plasma DAMP levels.
Plasma samples were collected from sham and burn mice and assessed for HMGB1, cytochrome C, DNA, and S100A8 (which was not measurable) as described in the Materials & Methods. Data are expressed as the mean ± SEM for 5–7 mice/group. *p<0.05 vs. respective sham group.
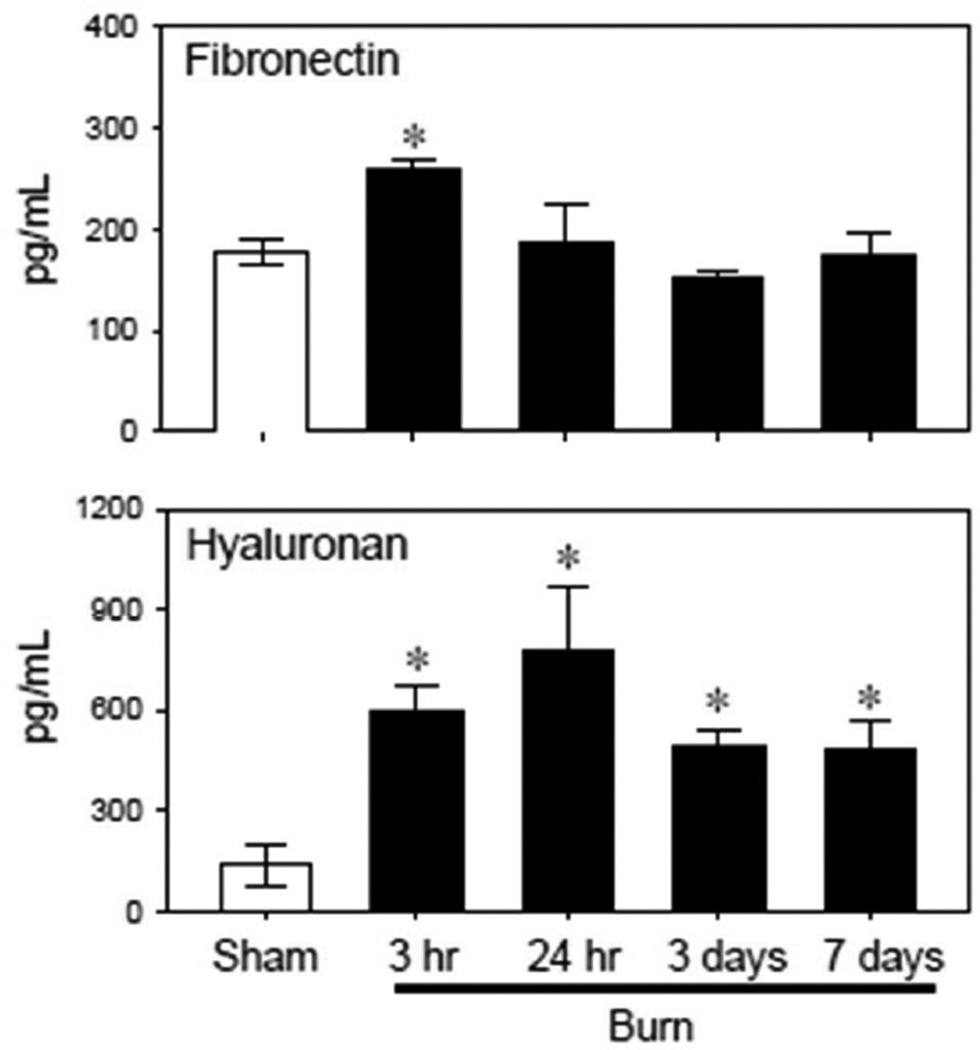
Circulating levels of the extracellular matrix proteins fibronectin and hyaluronan were also elevated after burn injury (Fig 2). Fibronectin plasma levels increased by ~50% 3 hrs after burn and returned to normal (i.e., sham) levels by 24 hrs. In contrast, hyaluronan levels were elevated 4 to 5-fold at all times assessed after injury (3 hr – 7 days).
Figure 2. Plasma levels of extracellular matrix cleavage products.
Plasma samples were collected from sham and burn mice and assessed for fibronectin and hyaluronan levels as described in the Materials & Methods. Data are expressed as the mean ± SEM for 4–5 mice/group. *p<0.05 vs. respective sham group.
Plasma cytokine levels
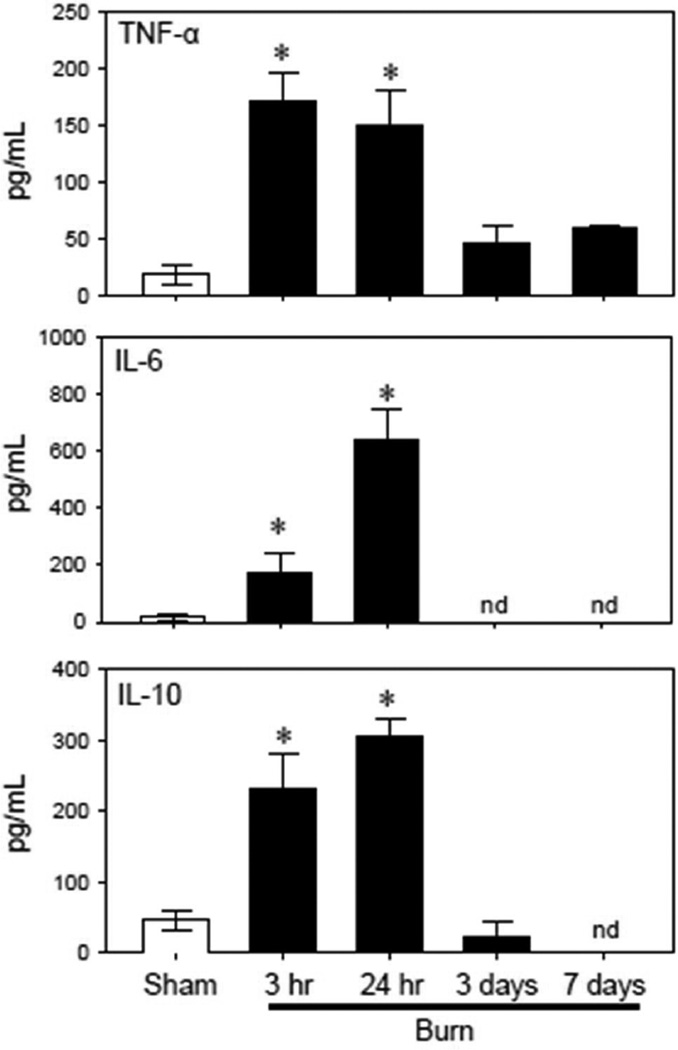
The inflammatory response was assessed by measuring the cytokine content in plasma after burn or sham treatment. In parallel with DAMPs, plasma cytokine levels were also elevated after burn injury (Fig. 3). TNF-α levels increased as early as 3 hr post-burn and remained elevated up to 24 hr. IL-6 and IL-10 levels were also significantly increased at early time point of 3 hr post-injury and were further elevated by 24 hr after burn as compared with that of sham mice. At 3 and 7 days post-injury all cytokine levels had returned to normal or were not detectable.
Figure 3. Plasma cytokine levels.
Plasma samples from sham and burn mice were assessed for cytokine levels as described in the Materials & Methods. Data are expressed as the mean ± SEM pg/ml for 4 mice/group. nd = not detectable. *p<0.05 vs. respective sham group.
Analysis monocyte phenotype and activation
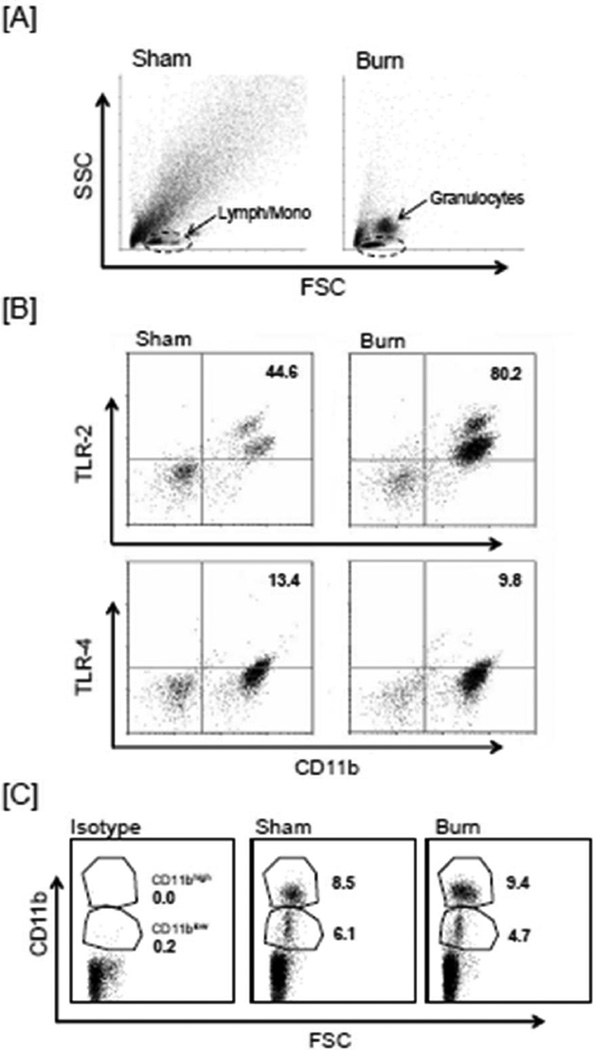
To investigate whether monocytes were altered after burn, blood samples at 24 hr after sham procedure or burn injury were stained for CD11b expression. A qualitative change in the circulating cell population was observed after burn with an apparent increase in the granulocyte numbers as determined by forward and side scatter (Fig.4A). Lymphocyte and monocytes numbers appeared to be comparable in the sham and burn mice. A marked increase in the levels of circulating CD11b+ monocytes was observed after burn in the lymphocyte monocytes gate, as compared with shams (87% ± 2% and 54% ± 6% for burn and sham respectively; mean = SEM, n=4/group). The CD11b+ monocytes showed increased expression of TLR-2, but not TLR-4 after the injury as compared to sham mice (Fig 4B). A significantly greater proportion of the CD11b+ monocytes population was TLR-2 positive as compared with sham mice. In contrast to TLR-2 expression, TLR-4 expression on monocytes remained comparable in both sham and burn groups.
Figure 4. Analysis of CD11b and TLR expression on monocytes.
Blood samples were collected after 24 hr of sham or burn procedure and the blood cells were stained with CD11b alone or in combination with TLR-2 and TLR-4 antibodies as described in the Materials & Methods. The monocyte population was gated as determined by forward and side scatter. Panel A shows the entire cell population with the lymphocyte/monocytes gate (Lymph/mono, as determined by forward, FSC and side scatter, SSC) used for monocytes analysis. In panel B the upper right quadrant of the graphs represents the monocytes positive for CD11b and TLR-2 or TLR-4. The numbers are the mean percentage of the gated population. Panel C shows the CD11b+ monocytes based on the forward scatter (FSC) and CD11b expression. The numbers are the percentages of the CD11blow and CD11bhigh populations. Data shown are representative of 4 independent experiments.
CD11b+ monocytes were further investigated in terms of their CD11b low (CD11blow) and high surface (CD11bhigh) expression (Fig 4C). Two populations of CD11b+ cells were clearly evident in the monocytes population. While the overall percentage of CD11b+ cells increased after burn, the percentages of CD11blow and CD11bhigh cells from the blood of burn mice were comparable to that of blood from sham mice.
DISCUSSION
Burn injury causes a number of inflammatory complications that lead to significant morbidity and mortality [9]. Activation of the innate immune system plays a vital role in clearing injured tissue and beginning the process of tissue repair. Cellular injury resulting from burn can lead to the release of DAMPs [3], which bind a variety of receptors including TLRs and stimulate the innate immune system. Once activated, the innate immune response can result in inflammation, SIRS and inflammatory complications such as acute respiratory distress syndrome (ARDS) or multiple organ dysfunction syndrome (MODS) [15]. Mitochondrial DAMPs are present in the circulation after trauma and have been shown to activate neutrophils [6]. Moreover, elevated levels of DAMPs following trauma were also shown to be related to SIRS, MODS and mortality [16]. The present study shows a strong association between DAMPs, inflammation and the activation of monocytes after burn.
Given the massive amount of tissue necrosis associated with the site of injury, it is not surprising that DAMP levels are elevated early after burn. The injured skin is the most likely source of the elevation of plasma DAMPs including extracellular cleavage products (fibronectin and hyaluronan), but other sources cannot be excluded, as distal organ (i.e., liver, lung, etc) injury is associated with major burns [17]. We observed that circulating DAMP levels (HMGB, cytochrome C, and fibronectin) were significantly increased in the early post-burn period (3–24 hrs) and subsequently decreased over time. In contrast, hyaluronan levels remained elevated post-injury. These findings suggest that the burn injury is creating cellular destruction and breakdown that is serving as a source of DAMPs. The decrease in some of the DAMP levels over time may be accounted for by the acute inflammatory response subsiding after the initial burn insult. In contrast, one study in injured humans has shown that the elevation in DAMPs following trauma is sustained over 1 week [16]. Further studies are needed to better characterize DAMP levels in humans and mice after burn or trauma.
TLRs are a connection between tissue injury following burn and the inflammatory response. Our group has shown that circulating leukocytes were responsive to TLR-induced activation, and that TLR-mediated inflammatory responses were enhanced 3–7 days post-injury [18]. Experimental models of burn have also shown enhanced TLR-mediated reactivity in the spleen, microvasculature, heart, lungs and intestines [19–22]. Inhibition of TLR-2 or TLR-4 signaling after burn has also been shown to suppress HMGB1-induced Kupffer cell cytokine production [23].
Circulating monocytes were activated at 24 hrs following burn as evidenced by increased expression of CD11b and TLR-2, but not TLR-4. The reason for the burn-induced changes in TLR expression in our model is unclear, but may be related in part to TNF-α, which was elevated early after injury. Previous in vitro studies have shown that stimulation of hepatocytes or macrophages with TNF-α will lead to preferential increases in TLR-2 mRNA expression without affecting TLR-4 expression [24;25]. Alternatively, tissue injury (i.e., burn) and the exposure of extracellular matrix proteins may be responsible for the elevation in TLR-2 expression, as TLR-2 is activated by exposure to extracellular matrix proteins, whereas TLR-4 is inhibited by intact matrix [26]. While the current study did not specifically address the role of TNF-α it would of particular interest to evaluate this idea in future studies.
Monocytes and macrophages are instrumental cells in the early inflammatory response to injury. We have previous shown that CD11b+ population increases in the burn wound with a shift towards the CD11bhigh population [27]. A shift towards the CD11bhigh population was not observed in the study herein, which is likely related to the source of the cells (injury site vs. circulation) and the time post-injury (3 days vs. 24 hrs).
TLR-2 is expressed by both monocytes and macrophages and recognizes antigen or PAMPs through bacterial triacyl lipoproteins and has common ligands with TLR-4, such as HMGB1 [23]. In this study, TLR-2 expression was increased early after injury as would be expected in conjunction with the elevation in DAMP levels seen. Conversely, TLR-4 was expressed at much lower levels on circulating monocytes and was not affected by burn. A number of DAMPs have been shown to interact with TLRs to induce an inflammatory response. Yang et al. found that TLR-4 binds the nuclear protein HMGB1 and activates TNF release from macrophages [28]. Others have shown that DAMPs can function to potentiate an immunoinflammatory response in addition to serving as endogenous ligands for TLRs. HMGB1 has been shown to bind inflammatory mediators, such as LPS, DNA or IL-1β. For instance, LPS can induce TLR-4 activation and activate HMGB1 release which then, in turn, binds more LPS and transports it to TLR-4 to initiate NF-κβ driven inflammation [29]. In this regard, elevations in HMGB1, in the absence of infection or these additional components, may not initiate TLR-mediated activation of the innate immune response following burn. Furthermore, DAMPs may assist in activating the immune response by forming complexes that bind TLRs to initiate a response rather than by individually binding the receptor to elicit a response.
It is also worth considering that DAMPs may activate TLR-4, but may also down-regulate TLR expression rather than increasing receptor expression. This may be why TLR-4 expression did not significantly increase at 24 hrs after burn injury. Inflammatory products such as cytokines have been shown to modulate TLR expression. IL-4 can inhibit TLR-4 mRNA and decrease the surface expression of TLR-4 on peripheral blood monocytes [30;31]. TLR-4 expression may be low in circulating monocytes but may potentially be increased in monocytes or other cell types at the local site of burn injury, such as the skin. In a study evaluating the role of DAMPs in traumatic brain injury and cerebral edema, TLR-4 expression was restricted to microglial/macrophage cells in the cortex surrounding the brain contusion.
Mitochondrial DNA, released after injury, has been shown to activate human polymorphonuclear neutrophils (PMNs) through direct interaction with TLR-9 to elicit an innate immune response by causing PMN migration and degranulation [6]. Others have demonstrated that DNA that is released into the circulation following injury can form complexes with HMGB1 that then bind TLR-9 [32;33]. TLR-9, in this case, may serve as an important mediator in the inflammatory response following burn. While the present study did not assess TLR-9 activity, we found that circulating DNA levels trended upward but did not reach statistical significance. Other DAMPs (i.e., cytochrome C and HMGB-1) were significantly elevated following burn. One explanation for the observed difference is that our methodology of spectrophotometric analysis of the circulating DNA is less sensitive and measures total DNA, whereas PCR used by others is specific of mitochondrial DNA. PCR may have allowed greater detection of circulating DNA and could be utilized in the future to address this difference. Future work might also include analysis of TLR-9 expression.
The pro-inflammatory cytokines TNF-α, IL-6 and IL-10 were elevated in the early post-burn period (3–24 hrs) and in part mirrored the increase in DAMPs. These findings, while associative, support the concept that burn-induced elevation in circulating DAMPs are causative in innate immune activation, via activation of TLRs, and contribute to the development of post-burn inflammatory complications. Nonetheless, this relationship has yet to be fully characterized and this study herein has a number of limitations. These limitations include the following; the relationship between DAMPs and changes in TLR expression and monocytes activation are only associative and may be occurring independently, assessment of monocytes activation was limited to a single time point, monocytes phenotyping was limited to CD11b expression and forward and side scatter and changes in circulating monocytes numbers after burn were not determined. In conclusion, while the findings herein are only descriptive and associative the diagnostic and therapeutic potential of understanding the relationships between inflammatory complications, DAMPs, and activation of TLRs on immune cells in burn patients remains promising and warrants further investigation.
Highlights.
Burn induced an elevation in circulating DAMPs 3–24 hr after injury
Elevated DAMPs levels were associated with increased IL-6, IL-10 and TNFα
Elevated DAMPs were associated with increased expression of TLR-2 on monocytes
Acknowledgments
Support was provided by NIH grant GM079122. These finding were presented in part at the Shock Society meeting. MR was responsible for the animal experiments, cell isolation, flow cytometry, data analysis, and drafting of the manuscript. SN was responsible for interpretation and assisted in the drafting of the manuscript. QZ was responsible for the animal experiments, cell isolation and flow cytometry. MGS was responsible for scientific conception, design and interpretation and assisted in the final drafting of the manuscript. All authors read and approved the final version of the manuscript. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest.
The authors declare that they have no competing interests.
REFERENCES
- 1.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10(2):79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Ann Surg. 1996;223(1):14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flohe SB, Flohe S, Schade FU. Invited review: deterioration of the immune system after trauma: signals and cellular mechanisms. Innate Immun. 2008;14(6):333–344. doi: 10.1177/1753425908100016. [DOI] [PubMed] [Google Scholar]
- 4.Beyer C, Stearns NA, Giessl A, Distler JH, Schett G, Pisetsky D. The extracellular release of DNA and HMGB1 from Jurkat T cells during in vitro necrotic cell death. Innate Immun. 2012;18(5):727–737. doi: 10.1177/1753425912437981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75(6):995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 9.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 10.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol. 2004;75(3):400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 11.Maung AA, Fujimi S, Miller L, Macconmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:568–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 13.Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P. Regulation of dendritic-and T-cell fate by injury-associated endogenous signals. Crit Rev Immunol. 2009;29(1):69–86. doi: 10.1615/critrevimmunol.v29.i1.30. [DOI] [PubMed] [Google Scholar]
- 14.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14(6):623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 15.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68(6):1328–1332. doi: 10.1097/TA.0b013e3181dcd28d. [DOI] [PubMed] [Google Scholar]
- 16.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–596. doi: 10.1097/SLA.0b013e3182a4ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan P, Frew Q, Green A, Martin R, Dziewulski P. Cause of death and correlation with autopsy findings in burns patients. Burns. 2013;39(4):583–588. doi: 10.1016/j.burns.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Schwacha MG, Zhang Q, Rani M, Craig T, Oppeltz RF. Burn enhances Toll-like receptor induced responses by circulating leukocytes. Int J Clin Exp Med. 2012;5(2):136–144. [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin JW, Wu MH, Guo M, Reynoso R, Yuan SY. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock. 2008;29(3):349–355. doi: 10.1097/shk.0b013e3181454975. [DOI] [PubMed] [Google Scholar]
- 20.Bruns B, Maass D, Barber R, Horton J, Carlson D. Alterations in the cardiac inflammatory response to burn trauma in mice lacking a functional Toll-like receptor 4 gene. Shock. 2008;30(6):740–746. doi: 10.1097/SHK.0b013e318173f329. [DOI] [PubMed] [Google Scholar]
- 21.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61(2):293–298. doi: 10.1097/01.ta.0000228969.46633.bb. [DOI] [PubMed] [Google Scholar]
- 22.Oppeltz RF, Rani M, Zhang Q, Schwacha MG. Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells. Cytokine. 2011;55(3):396–401. doi: 10.1016/j.cyto.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen XL, Sun L, Guo F, Wang F, Liu S, Liang X, et al. High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs-dependent signaling pathway after burn injury. PLoS One. 2012;7(11):e50668. doi: 10.1371/journal.pone.0050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumura T, Ito A, Takii T, Hayashi H, Onozaki K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J Interferon Cytokine Res. 2000;20(10):915–921. doi: 10.1089/10799900050163299. [DOI] [PubMed] [Google Scholar]
- 25.Oshikawa K, Sugiyama Y. Regulation of toll-like receptor 2 and 4 gene expression in murine alveolar macrophages. Exp Lung Res. 2003;29(6):401–412. doi: 10.1080/01902140303756. [DOI] [PubMed] [Google Scholar]
- 26.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19(7):872–874. doi: 10.1096/fj.04-3211fje. [DOI] [PubMed] [Google Scholar]
- 27.Rani M, Zhang Q, Schwacha MG. Gamma delta T cells regulate wound myeloid cell activity after burn. Shock. 2014;42(2):133–141. doi: 10.1097/SHK.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107(26):11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mita Y, Dobashi K, Endou K, Kawata T, Shimizu Y, Nakazawa T, et al. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol Lett. 2002;81(1):71–75. doi: 10.1016/s0165-2478(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 31.Fiset PO, Tulic MK, Skrablin PS, Grover SM, Letuve S, Mazer BD, et al. Signal transducer and activator of transcription 6 down-regulates toll-like receptor-4 expression of a monocytic cell line. Clin Exp Allergy. 2006;36(2):158–165. doi: 10.1111/j.1365-2222.2006.02370.x. [DOI] [PubMed] [Google Scholar]
- 32.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 33.Krieg AM. TLR9 and DNA 'feel' RAGE. Nat Immunol. 2007;8(5):475–477. doi: 10.1038/ni0507-475. [DOI] [PubMed] [Google Scholar]