Abstract
Background
Psychopathy is a mental health disorder characterized by callous and impulsive antisocial behavior, and is associated with a high incidence of violent crime, substance abuse, and recidivism. Recent studies suggest that the striatum may be a key component of the neurobiological basis for the disorder, though structural findings have been mixed and functional connectivity of the striatum in psychopathy has yet to be fully examined.
Methods
We performed a multimodal neuroimaging study of striatum volume and functional connectivity in psychopathy, using a large sample of adult male prison inmates (N=124). We conducted volumetric analyses in striatal subnuclei, and subsequently assessed resting-state functional connectivity in areas where volume was related to psychopathy severity.
Results
Total PCL-R and Factor 2 scores (which index the impulsive/antisocial traits of psychopathy) were associated with larger striatal subnuclei volumes and increased volume in focal areas throughout the striatum, particularly in the nucleus accumbens and putamen bilaterally. Furthermore, at many of the striatal areas where volume was positively associated with Factor 2 scores, psychopathy severity was also associated with abnormal functional connectivity with other brain regions, including dorsolateral prefrontal cortex, ventral midbrain and other areas of the striatum. The results were not attributable to age, race, IQ, substance use history, or intracranial volume.
Conclusion
These findings associate the impulsive/antisocial dimension of psychopathy with enlarged striatal subnuclei and aberrant functional connectivity between the striatum and other brain regions. Furthermore, the co-localization of volumetric and functional connectivity findings suggests that these neural abnormalities may be pathophysiologically linked.
Keywords: Striatum, Psychopathy, Nucleus Accumbens, Putamen, Functional Connectivity, Reward
INTRODUCTION
Psychopathy is a mental health disorder characterized by callous and impulsive antisocial behavior. Present in roughly a quarter of adult prison inmates, psychopathy is associated with a disproportionately high incidence of violent crime, substance abuse, and recidivism (1, 2). Identifying the psychological and neurobiological mechanisms underlying this disorder could thus have profound implications for the clinical and legal management of psychopathic criminals, as well as for the basic understanding of human social behavior. Based on the personality and behavioral characteristics of the disorder, such as impulsivity and deficits in passive avoidance (3), reversal learning (4), and perseverative responding to reward (5), it has long been postulated that psychopathy may be linked to abnormalities in processing reward and punishment (3, 6-9). Over several decades, a host of behavioral and psychophysiological studies have offered support for this theory (3, 4, 10, 11). More recently, brain imaging has been employed to address this hypothesis at the neural systems level. A number of these studies have focused on the ventral striatum, a subcortical target of mesolimbic dopamine neurons that responds to rewarding or pleasurable stimuli, as well as to abstract stimuli predicting their occurrence (3, 12, 13). While functional imaging studies in community samples have associated impulsive/antisocial psychopathic traits with heightened ventral striatum activity during the anticipation of monetary gain (14, 15), structural imaging studies have offered more mixed results. Some studies have associated psychopathy with increased ventral striatum volumes (16, 17), others have reported decreased ventral striatum volumes (18), and others have found volume increases (19) and decreases (20) in more dorsal and lateral regions of the striatum. The mixed findings among volumetric studies may be attributable to differences in subject populations (e.g., prison inmates vs. community samples), psychopathy severity, sample sizes, and substance use history.
In the present study, we used a unique mobile scanner to collect multimodal MRI from a large (N=124) sample of adult male prison inmates with a broad range of psychopathy severity in order to determine whether volumes of striatal subregions were linked to assessments of overall psychopathy severity as well as to assessments of distinct components of psychopathic traits (Factor 1: affective and interpersonal traits, Factor 2: antisocial and lifestyle traits). Furthermore, we analyzed resting-state fMRI data from the same participants to determine whether the observed striatal structural abnormalities were accompanied by alterations in striatal functional connectivity. This combination of analyses comprises the most comprehensive study of striatum structure and functional connectivity in psychopathy to date.
METHODS
Participants
124 adult male inmates, recruited from a medium-security Wisconsin correctional facility, participated in the present study. Informed consent was obtained both orally and in writing. Participants were selected based on the following inclusion criteria: (1) less than 45 years old; (2) IQ greater than 70; (3) no history of psychosis or bipolar disorder; (4) no history of significant head injury or post-concussion symptoms; (5) no current use of psychotropic medications, and (6) completed interview assessments for psychopathy and substance use disorder (see below). Of these 124 subjects, resting-state functional connectivity (RSFC) data were obtained for 115 subjects; 8 of these were excluded due to excessive motion in the scanner, leaving a total of 107 subjects for RSFC analysis.
Psychopathy was assessed with the Psychopathy Checklist Revised (PCL-R) by trained research assistants (21). The PCL-R is a 20-item scale completed based on a semi-structured interview and file review. Each item is scored as 0, 1, or 2 based on the severity of each trait. Total scores ≥30 (n=41) indicate psychopathy; scores >20 and <30 (n=48) are considered intermediate, and scores ≤20 (n=35) are non-psychopathic (2). Inter-rater reliability (intraclass correlation) for total PCL-R score was 0.98 based on 10 dual ratings. Total PCL-R, Factor 1 (interpersonal/affective traits), and Factor 2 (lifestyle/antisocial traits) scores were used for separate regression analyses (22).
Substance use disorder was assessed with the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-IV) (23). This measure classifies whether a subject meets criteria for lifetime history of substance abuse or substance dependence, for each of the following substances: alcohol, cannabis, cocaine, opioids, stimulants, sedatives, and hallucinogens. Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics
| All (n=124) | Non-Psychopathic (n=35) | Intermediate (n=48) | Psychopathic (n=41) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | pa | |
| Age | 31.6 | 7.3 | 31.3 | 7.9 | 31.8 | 6.7 | 31.5 | 7.7 | 0.93 |
| IQ | 98.1 | 11.5 | 97.3 | 12.0 | 95.3 | 11.6 | 101.5 | 10.3 | 0.19 |
| Total PCL-R score | 24.8 | 7.1 | 15.3 | 3.4 | 25.6 | 2.3 | 32.1 | 1.6 | <.001 |
| Factor 1 score | 9.2 | 3.3 | 5.5 | 2.1 | 9.3 | 2.3 | 12.3 | 1.8 | <.001 |
| Factor 2 score | 13.6 | 3.9 | 8.6 | 2.8 | 14.3 | 1.9 | 17 | 1.5 | <.001 |
| % | n | % | n | % | n | % | n | ||
| SUD: abuse | 24.2 | 30 | 22.9 | 8 | 25 | 12 | 24.4 | 10 | .88 |
| SUD: dependence | 55.6 | 69 | 40 | 14 | 56 | 27 | 68.3 | 28 | .01 |
| Race | |||||||||
| Caucasian | 56.6 | 70 | 60 | 21 | 45.8 | 22 | 65.6 | 27 | 0.52 |
| African-American | 41.1 | 51 | 34.3 | 12 | 52.1 | 25 | 34.1 | 14 | 0.52 |
| Hispanic | 1.6 | 2 | 2.9 | 1 | 2.1 | 1 | 0 | 0 | N/A |
| Native American | 0.8 | 1 | 2.9 | 1 | 0 | 0 | 0 | 0 | N/A |
Participant demographic and neuropsychological information is presented by group for non-psychopathic (PCL-R ≤20), intermediate (PCL-R >20 and <30) and psychopathic (PCL-R ≥30) inmates.
p-values are reported for two-sample t-tests (for age, IQ and psychopathy scores), Fisher's Exact Test (for Race) and Pearson Chi-Square test (for substance abuse and dependence) comparing psychopathic and non-psychopathic inmates.
MRI Acquisition
MRI data were acquired using the Mind Research Network's Siemens 1.5T Avanto Mobile MRI System equipped with a 12-element head coil. All participants underwent scanning on correctional facility grounds. A high-resolution T1-weighted structural image was acquired for each subject using a four-echo magnetization-prepared rapid gradient-echo sequence (TR=2530 ms; TE=1.64, 3.5, 5.36 and 7.22 ms; flip angle=7°; FOV=256×256 mm2 ; matrix=128×128; slice thickness=1.33 mm; no gap; voxel size=1×1×1.33 mm3;128 interleaved sagittal slices). All four echoes were averaged into a single high-resolution image (24). Resting-state functional images (T2*-weighted gradient-echo functional echo planar images (EPIs)) were collected while subjects lay still and awake, passively viewing a fixation cross for 5.5 min (158 volumes) (25), and were acquired with the following parameters: TR=2000 ms; TE=39 ms; flip angle=75°; FOV=24×24 cm; matrix=64×64; slice thickness=4 mm; gap=1 mm; voxel size=3.75×3.75×5 mm; 27 sequential axial oblique slices. Preprocessing and analyses of structural MRI data were conducted in both Freesurfer 5.3 (26) in Linux and Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). fMRI data analysis was performed using AFNI (27) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/).
Structural MRI Preprocessing: Freesurfer
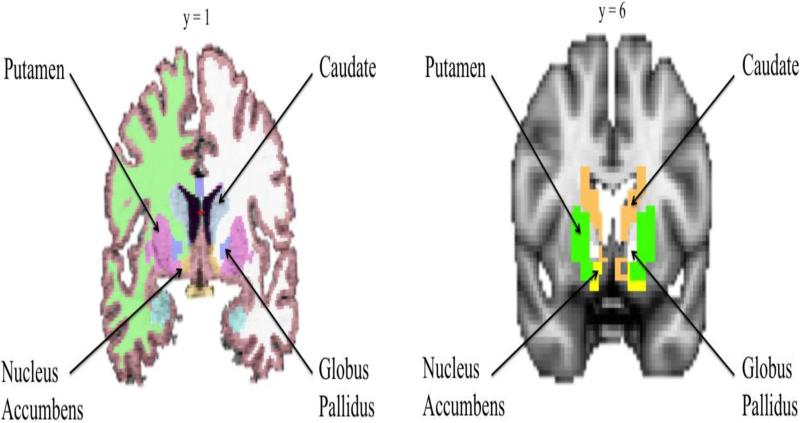
Freesurfer's automated preprocessing procedure includes skull-stripping, registration, intensity normalization, Talairach transformation, tissue segmentation, and surface tessellation (28). Freesurfer provides volume measurements for eight striatal subregions (left and right putamen, left and right caudate, left and right globus pallidus, and left and right nucleus accumbens) (Figure 1).
Figure 1.
Striatal subnuclei segmentation in Freesurfer 5.3 (left) and in SPM12 as defined by Individual Brain Atlases using Statistical Parametric Mapping 71 (IBASPM 71) (right).
Structural MRI Preprocessing: SPM
T1 images were manually realigned; segmented into gray matter, white matter, and cerebrospinal fluid; normalized to Montreal Neurological Institute (MNI)-152 space; modulated to preserve volume after normalization; and smoothed with an 8mm full-width at half-maximum (FWHM) Gaussian kernel (29). Individual Brain Atlases using Statistical Parametric Mapping 71 (IBASPM 71) (http://www.thomaskoenig.ch/Lester/ibaspm.htm) in the Wake Forest University (WFU) Pick Atlas Toolbox was used to create masks of the eight striatal subregion ROIs (Figure 1).
Functional MRI Preprocessing
The following preprocessing steps were performed: (1) EPI volumes were slice time corrected, (2) motion corrected by rigid body alignment, (3) deobliqued, (4) the first three volumes were omitted, (5) data were then motion corrected (3dvolreg in AFNI) and (6) despiked to remove extreme time series outliers and then (7) bandpass filtered (0.009<f <0.08) and spatially smoothed with a 6mm FWHM Gaussian kernel (30). The skull-stripped anatomical scan for each participant was rigidly coregistered with the EPI and diffeomorphically aligned to MNI-152 space (31). The transformation matrix from this registration was then used to align the EPI scans to MNI-152 space. Finally, the EPI scans were resampled to 3mm cubic voxels for subsequent functional connectivity analyses.
Because individual differences in subject motion can contribute to resting-state correlations (32-34), we excluded subjects with mean framewise motion displacement (i.e., volume to volume movement across the time series) >2mm and/or total scan time <4 min after censoring all time points with framewise motion displacement >0.2mm and extreme time series displacement (i.e., time points in which 10% of voxels were outliers (32-34). 8 participants were excluded for excessive motion, leaving a final sample of 107 participants.
Analytic Strategy
As results from studies of structural brain morphometry may vary as a function of analysis package (35), we used two separate software programs to measure the volumes of striatal subregions: Freesurfer and SPM. Both programs yield regional volume totals for the regions of interest (ROIs) of this study: putamen, caudate, globus pallidus and nucleus accumbens (see Table S1 for correlations between Freesurfer and SPM for the average volume of each subregion). Additionally, SPM was used to perform small volume-corrected voxel-wise analyses within ROIs – both because this type of analysis can detect focal relationships that may be missed in the regional volume analysis, and because this analysis allows for more specific localization of the areas where volume is most strongly linked to psychopathy severity.
We then examined whether the identified areas where volume correlated with psychopathy severity (in terms of total PCL-R, Factor 1 or Factor 2 scores) also had RSFC relationships to other brain regions that correlated with psychopathy severity. To do this, we created 3mm-radius spherical seeds around the peak coordinates of each focal cluster - identified via the within-ROI voxel-wise analysis - where volume was related to psychopathy severity, and subsequently assessed RSFC between these areas and other areas of the brain in relation to psychopathy ratings. Seeds were evaluated in RSFC regressions only in relation to the specific psychopathy score-type (Total PCL-R, Factor 1, and/or Factor 2) for which the seed had demonstrated a relationship within the volumetric analysis.
RSFC Analysis
RSFC was assessed for each seed ROI using the mean resting-state BOLD time series, extracted for each participant. The mean time series from each ROI was included in a GLM with 15 regressors of no interest: (1–12) six motion parameters (three translations and three rotations) obtained from the rigid-body alignment of EPI volumes and their six derivatives; (13) the white matter time series; (14) the CSF time series; and (15) a second-order polynomial to model baseline signal and slow drift. To further control for subject motion, volumes were censored for extreme time series displacement (i.e., time points in which 10% of voxels were outliers) and framewise motion displacement (i.e., volume to volume movement) >0.2 mm (32, 34). The output of R2 values from the GLM was converted to correlation coefficients (r), which were then converted to z scores via Fisher's r to z transform and corrected for degrees of freedom. The resulting z-score maps were entered into second-level statistical analyses (25).
We performed linear regression analyses (3dRegAna in AFNI) to examine the relationship between psychopathy scores and RSFC for all seed ROIs. We performed separate regressions for Total PCL-R, Factor 1 (covarying for Factor 2), and Factor 2 (covarying for Factor 1). To correct for multiple comparisons, we used FWE correction at the cluster level using a whole brain mask (3dClustSim in AFNI) (36, 37) and applied cluster extent thresholding. The cluster extent threshold corresponded to the statistical probability (α=0.05, or 5% chance) of identifying a random noise cluster at a predefined voxel-wise (i.e., whole-brain) threshold of p=0.01 (uncorrected). Using this whole-brain FWE cluster correction, a cluster-corrected size of ≥106 voxels was significant at pFWE <0.05 in the regression analyses reported below for PCL-R scores.
Covariates
Total PCL-R scores and Factor 2 scores were significantly correlated with substance use disorder (r=0.338, p<0.001 and r=0.392, p>0.001, respectively). Because gray matter volume has been shown to relate to substance use (38-42), we included presence of substance use disorder (None, Abuse, or Dependence), using the diagnoses from the SCID-IV, as a covariate in all volumetric regression models. Furthermore, we observed a significant group effect (p<0.05) of race (between Caucasian and non-Caucasian subjects) on volume of many of the striatal ROI's, and thus race was included as a covariate in all volumetric regression models as well. In addition, we included age and intracranial volume as covariates in all volumetric regression models, as these factors have also been shown to have independent relationships with gray matter volume (43, 44). IQ was not related to psychopathy severity or striatal volumes, and so was not included as a covariate. In both volumetric and RSFC regressions where the main variable of interest was a factor score, the other factor score was included as a covariate. There was no significant relationship between PCL-R score and intracranial volume as measured by either SPM (p=0.499) or Freesurfer (p=0.343). In addition to regression analyses, we calculated zero-order (bivariate) correlations between PCL-R factor scores and structural volumes. The pattern of findings for these correlations was identical to that for the regressions unless otherwise noted.
RESULTS
Striatal Subnuclei Volumes
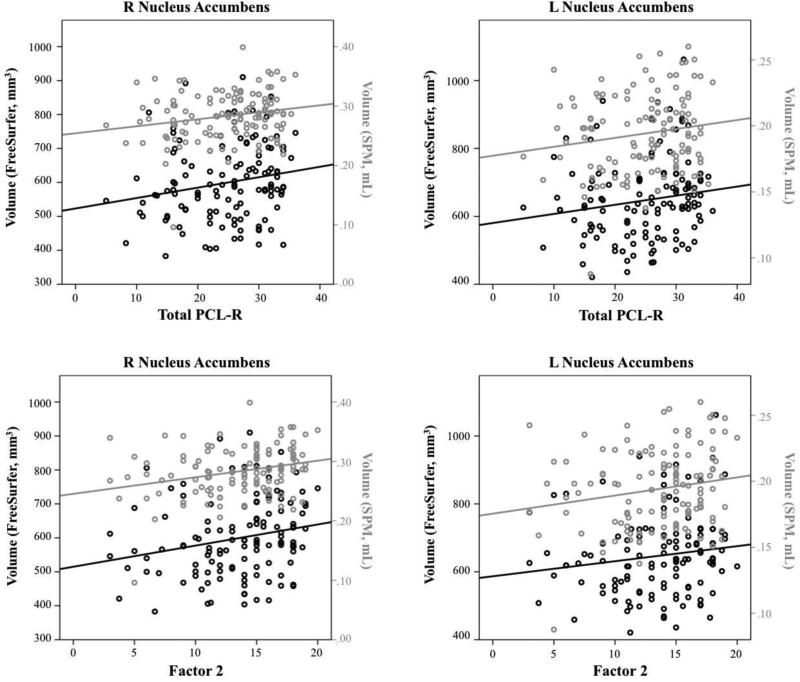
Total PCL-R scores were positively related to accumbens volumes in both Freesurfer and SPM (Figure 2). Factor 2 scores were positively related to volume in right putamen in both Freesurfer and SPM; in accumbens bilaterally, right globus pallidus, and right caudate in SPM; and in left putamen in Freesurfer. These findings were significant in the full regression model and zero-order correlations. In contrast, Factor 1 scores were negatively related to right putamen volume, though this relationship was not significant as a zero-order correlation and was only present in SPM. See Tables S2-S4 and Supplemental Results for complete results.
Figure 2.
Zero-order correlation plots for significant relationships between PCL-scores (Total, Factor 2) and striatal subnuclei.
Voxel-Wise Volume Analysis
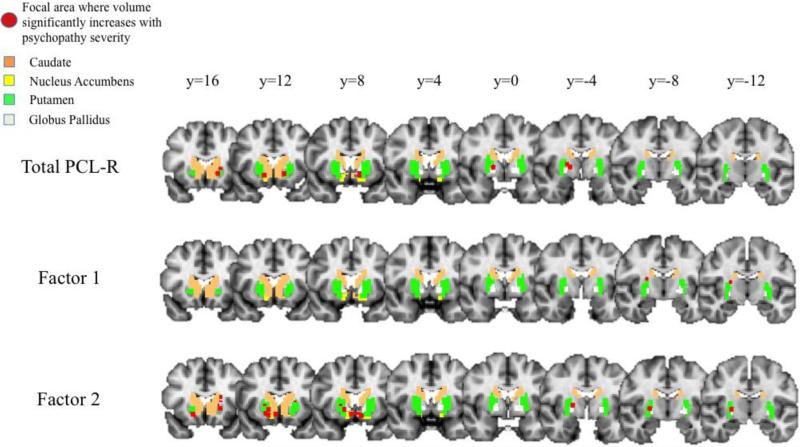
Voxel-wise regressions revealed a number of focal regions in the striatum in which volume increases with increasing psychopathy severity (Figure 3). Total PCL-R scores were positively related to focal volume clusters in the accumbens bilaterally, in the globus pallidus bilaterally, and in the left putamen; Factor 1 scores were positively related to a focal volume cluster in the right putamen; and Factor 2 scores were positively related to focal volume clusters in the left caudate, in the accumbens bilaterally, in the right globus pallidus, and in the putamen bilaterally. Lastly, despite our finding of negative relationships between Factor 1 score and regional volume of the right putamen, there were no focal volume clusters negatively related to psychopathy scores. See Tables S5-S7 for full results.
Figure 3.
Focal areas within striatum (shown in red) where volume has a positive relationship with PCL-R scores (Total, Factor 1, Factor 2).
Resting-State Functional Connectivity
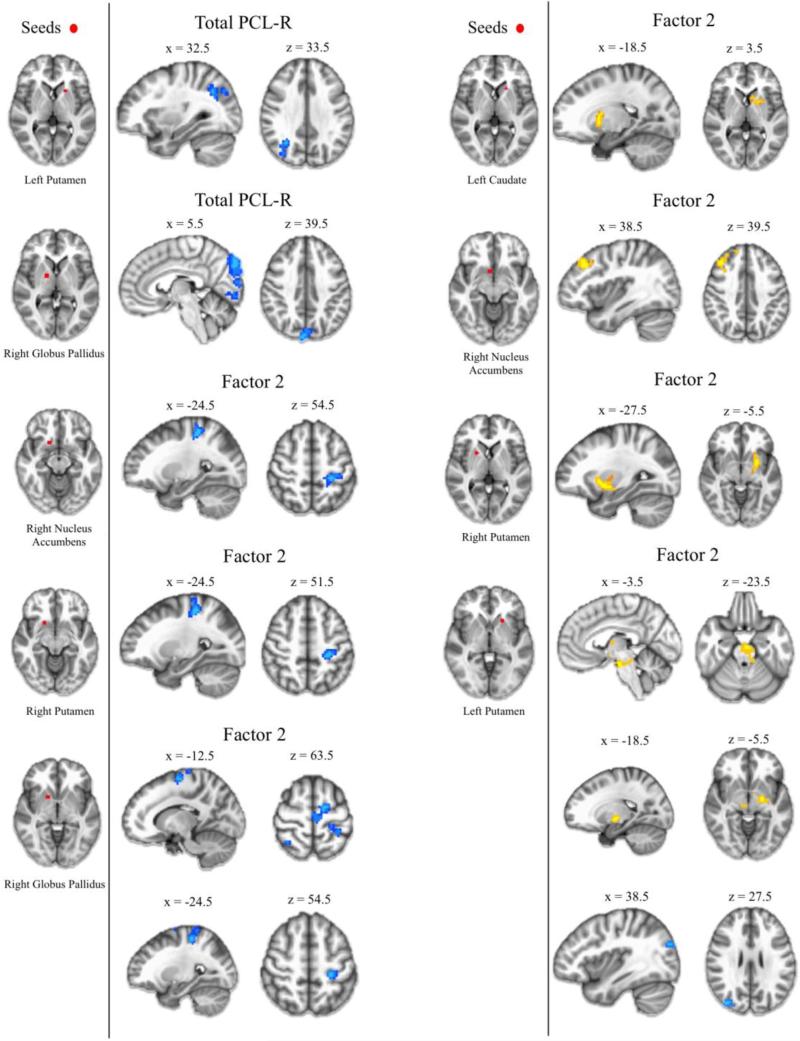
Total PCL-R scores were inversely related to RSFC between left putamen and right superior lateral occipital cortex, and also between right globus pallidus and right occipital cortex (Figure 4). Factor 1 scores were not related to RSFC for any seeds. Factor 2 scores were positively related to RSFC between striatal seeds and the ventral midbrain, dorsolateral prefrontal cortex, and other areas of the striatum. Factor 2 scores were inversely related to RSFC between striatal seeds and precentral gyrus, postcentral gyrus, and lateral occipital cortex (Figure 4). See Tables S8-S9 for full results.
Figure 4.
Resting-state functional connectivity results for focal volume clusters within the striatum. Positive relationships between focal clusters and PCL-R scores (Total, Factor 1, Factor 2) are shown in yellow. Negative relationships are shown in blue.
DISCUSSION
This study used a multimodal neuroimaging approach to investigate the neural underpinnings of psychopathy in the striatum. First, we investigated how volumes of striatal subnuclei relate to psychopathy severity as measured by total PCL-R, Factor 1, and Factor 2 scores. In general, we found that psychopathy severity was linked to larger striatal subnuclei volumes – most robustly in accumbens and putamen – and that this enlargement was more strongly linked to Factor 2 scores than Factor 1 scores.
Next, we performed voxel-wise analyses to identify the focal areas within the striatum where volume was most strongly related to psychopathy severity. These results aligned with those of the regional volume analyses, as volume in focal areas throughout the striatum, including the nucleus accumbens and putamen, were positively associated with psychopathy severity – driven predominantly by Factor 2 scores.
We then performed RSFC analyses to examine whether areas of the striatum for which structural analyses had revealed abnormal volumes associated with psychopathy also displayed functional connectivity abnormalities. Indeed, we found that at many of these striatal areas, psychopathy severity was also associated with abnormal RSFC to other areas of the brain. Psychopathy severity was positively associated with RSFC between striatal areas and other areas of the striatum, dorsolateral prefrontal cortex, and ventral midbrain; conversely, psychopathy severity was inversely related to RSFC between striatal areas and areas within the parietal and occipital lobes. As in the structural analyses, Factor 2 scores predominantly drove the RSFC findings.
Overall, these findings help to clarify the structural and functional features of the striatum in psychopathy. Our results are consistent with studies finding volume increases of the striatum in psychopathy (16, 17), and also provide the first detailed analysis of how these structural abnormalities may correspond to abnormalities in functional connectivity.
Of particular note is the strong relationship observed here between Factor 2 scores and striatal neurobiology. As the Factor 2 dimension of psychopathy is characterized in part by impulsive behavior (2, 22) and excessive need for stimulation, our finding that striatal neurobiology related most strongly to Factor 2 is consistent with a large literature implicating abnormality of the striatum in deficits in reward-processing and impulse control (45-51). For instance, we found that Factor 2 severity was positively associated with functional connectivity between the nucleus accumbens and dorsolateral prefrontal cortex. Evidence suggests that individual differences in reward-processing and impulse control are related to the integrity of fronto-striatal circuitry (52, 53). A diffusion-tensor imaging study found that increased structural connectivity between the striatum and prefrontal cortex was associated with greater reward dependence (54). This is consistent with our finding of a positive relationship between Factor 2 scores and functional connectivity between the striatum and prefrontal cortex. . Relatedly, we observed that Factor 2 severity was positively associated with functional connectivity between the striatum and ventral midbrain. The ventral midbrain is known to communicate with the striatum via dopaminergic transmission as part of the reward processing circuit (13). Furthermore, we observed three distinct instances of elevated striato-striatal functional connectivity in relation to Factor 2 severity. Collectively, cortico-striato-midbrain circuitry is thought to be central to the brain's reward system (55), and our finding of abnormal fronto-striatal, striatomidbrain, and striato-striatal functional connectivity in relation to Factor 2 severity provides evidence for a neural substrate for the deficits in reward processing observed in psychopathy (52), and may be related to the heightened mesolimbic dopamine response to reward associated with impulsive-antisocial psychopathic traits, as previously demonstrated in a community (non-offender) sample (14).
Several striatal subregions also showed inverse relationships between psychopathy severity and functional connectivity with areas of the pre- and postcentral gyri. Imaging studies in humans have shown cortical thinning in the precentral gyrus bilaterally in psychopathy (24), as well as thinning in the sensorimotor cortex more generally in a community sample of violent individuals with antisocial personality disorder (56). Our results suggest that the volumetric abnormalities observed in these areas in relation to psychopathic traits may be related to abnormalities in functional connectivity with the striatum.
Another intriguing observation in this study is the stark difference in the neural correlates of Factor 1 and Factor 2 scores. Whereas Factor 2 scores were uniformly positively associated with both regional and focal volumes in the ventral striatum, Factor 1 scores did not have robust or consistent relationships with striatal subregion volumes. Consideration of Factor 1 findings is important as Factor 1 traits are unique to psychopathy, whereas Factor 2 traits may be shared with other externalizing disorders such as antisocial personality disorder. In the present study, the only findings related to Factor 1 were somewhat inconsistent (negative association with putamen in the overall striatal subnuclei volume analysis, but positive association within putamen in the voxel-wise volume analysis) and were not significant in zero-order correlations. By contrast, a previous study found a positive relationship between overall lenticular nucleus (putamen plus globus pallidus nuclei) volumes and Factor 1 scores (19). Furthermore, whereas Factor 2 scores were associated with multiple patterns of abnormal striatum functional connectivity, there were no such correlations with Factor 1 scores. These distinct relationships suggest that Factor 1 and Factor 2 traits, despite being highly correlated in terms of PCL-R subscores, are clearly dissociable at the neural level. This conclusion is consistent with recent neuroimaging studies examining white matter microstructure as well as cortical functional connectivity (25, 57, 58).
One issue that warrants consideration is the substantial rate of substance use disorder in this sample, which correlated significantly with both Total PCL-R and Factor 2 scores. Multiple studies have linked substance use disorder to structural and functional abnormalities in the striatum (40, 59, 60). We included a substance use variable in our regression models to account for this feature of the study population. Hence, the findings we report here do not appear to be due to individual differences in substance abuse histories. In a future study, we will more fully examine the relationships between substance use characteristics and striatum structure and function in this sample. Another issue worth addressing in future studies is the relationship between volumetric and RSFC findings. While our approach to choosing seeds for the RSFC analysis allowed us to directly assess whether structural and functional abnormalities co-occurred at the same sites, this method may be considered liable to statistical non-independence (61), in that the volume and RSFC of striatal subnuclei may be inherently linked. Future studies, in both clinical and non-clinical samples, could establish whether this is indeed the case. Another consideration to address is the relationship between the findings of this study and those of previous imaging studies of psychopathy from our group. Two volumetric studies from Ermer et al. (62, 63) – both in samples entirely distinct from the sample of the present study – did not report a relationship between psychopathy and striatal volume in a whole-brain analysis in SPM. However, the striatum was not investigated via an ROI approach, as the focus of these investigations was paralimbic regions. Another volumetric study by Pujara et al. (16) – from which there is an overlap of 12 of the 124 subjects in the present study – used an extreme group design (psychopathic vs. non-psychopathic inmates) with a relatively small overall sample size (n=41) and did not assess the relationship between psychopathy and striatal volume across the full, continuous range of severity, nor did it examine individual factor scores. Furthermore, a prior RSFC study by Philippi et al. (25), which used the same subjects as the present study, only examined cortico-cortical relationships. Finally, it is important to note the differences in regional volume results yielded by Freesurfer and SPM. While Table S1 demonstrates significant correlations between volumes for most striatal subnuclei, the correlation values themselves are moderate. Disparities in volume calculations between the two programs – likely due to differences in image processing methods and ROI definitions – demonstrates the importance of evaluating volumetric relationships in multiple software packages in future studies.
In sum, we have analyzed a unique set of multimodal neuroimaging data from a large sample of incarcerated criminal offenders to characterize the relationships between specific striatal subnuclei and distinct clusters of psychopathic traits. Our findings provide evidence that enlarged striatal subnuclei and aberrant functional connectivity between the striatum and other regions of the brain may contribute to the impulsive/antisocial dimension of psychopathy. Furthermore, our finding that abnormalities in volume and functional connectivity often co-occurred at the same sites suggests that these abnormalities may be pathophysiologically linked.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the many individuals at the Wisconsin Department of Corrections who made this research possible, and are especially indebted to Warden Judy Smith, Warden Randy Hepp, and Dr. Kevin Kallas. This research was supported by grants from the National Institutes of Health (MH070539, DA026505, MH087525, MH090169).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCOLUSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Smith SS, Newman JP. Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. J Abnorm Psychol. 1990;99:430–439. doi: 10.1037//0021-843x.99.4.430. [DOI] [PubMed] [Google Scholar]
- 2.Hare RD. The Hare psychopathy checklist-revised. 2nd ed. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- 3.Lykken DT. A study of anxiety in the sociopathic personality. J Abnorm Psychol. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- 4.Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J Abnorm Psychol. 2006;115:552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- 5.Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. J Abnorm Psychol. 1987;96:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- 6.Cleckley H. The Mask of Sanity. St. Louis: Mosby. 1941 [Google Scholar]
- 7.Fowles DC. The three arousal model: implications of gray's two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301–315. [PubMed] [Google Scholar]
- 9.Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmauk FJ. Punishment, arousal, and avoidance learning in sociopaths. J Abnorm Psychol. 1970;76:325–335. doi: 10.1037/h0030398. [DOI] [PubMed] [Google Scholar]
- 11.Arnett PA, Smith SS, Newman JP. Approach and avoidance motivation in psychopathic criminal offenders during passive avoidance. J Pers Soc Psychol. 1997;72:1413–1428. doi: 10.1037//0022-3514.72.6.1413. [DOI] [PubMed] [Google Scholar]
- 12.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 13.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjork JM, Chen G, Hommer DW. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biol Psychol. 2012;89:408–415. doi: 10.1016/j.biopsycho.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujara M, Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Neural correlates of reward and loss sensitivity in psychopathy. Soc Cogn Affect Neurosci. 2014;9:794–801. doi: 10.1093/scan/nst054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffer B, Muller BW, Scherbaum N, Hodgins S, Forsting M, Wiltfang J, et al. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry. 2011;68:1039–1049. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- 18.Boccardi M, Bocchetta M, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, et al. Atypical nucleus accumbens morphology in psychopathy: another limbic piece in the puzzle. International journal of law and psychiatry. 2013;36:157–167. doi: 10.1016/j.ijlp.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira JB, Ferreira-Santos F, Almeida PR, Barbosa F, Marques-Teixeira J, Marsh AA. Psychopathic traits are associated with cortical and subcortical volume alterations in healthy individuals. Soc Cogn Affect Neurosci. 2015;10:1693–1704. doi: 10.1093/scan/nsv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare RD. The Hare psychopathy checklist-revised. 2nd ed. Multi-Health Systems; Toronto: 2003. [Google Scholar]
- 22.Harpur T, Hare R, Hakstian A. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment: A Journal of consulting and clinical Psychology. 1989:1. [Google Scholar]
- 23.First M. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) Biometrics Research: New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 24.Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, et al. Cortical thinning in psychopathy. Am J Psychiatry. 2012;169:743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M. Altered resting-state functional connectivity in cortical networks in psychopathy. J Neurosci. 2015;35:6068–6078. doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 30.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23(Suppl 1):S139–150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagopalan V, Pioro EP. Disparate voxel based morphometry (VBM) results between SPM and FSL softwares in ALS patients with frontotemporal dementia: which VBM results to consider? BMC Neurol. 2015;15:32. doi: 10.1186/s12883-015-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 37.Carp J. The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage. 2012;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, et al. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 40.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- 44.Miller AK, Alston RL, Corsellis JA. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 45.Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, et al. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nat Med. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- 46.Amen DG, Stubblefield M, Carmicheal B, Thisted R. Brain SPECT findings and aggressiveness. Ann Clin Psychiatry. 1996;8:129–137. doi: 10.3109/10401239609147750. [DOI] [PubMed] [Google Scholar]
- 47.Soderstrom H, Hultin L, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced frontotemporal perfusion in psychopathic personality. Psychiatry Res. 2002;114:81–94. doi: 10.1016/s0925-4927(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 48.Vollm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 2007;156:151–167. doi: 10.1016/j.pscychresns.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, et al. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J Abnorm Psychol. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avila C, Garbin G, Sanjuan A, Forn C, Barros-Loscertales A, Bustamante JC, et al. Frontostriatal response to set switching is moderated by reward sensitivity. Soc Cogn Affect Neurosci. 2012;7:423–430. doi: 10.1093/scan/nsr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glenn AL, Yang Y. The potential role of the striatum in antisocial behavior and psychopathy. Biol Psychiatry. 2012;72:817–822. doi: 10.1016/j.biopsych.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 52.Blair KS, Newman C, Mitchell DG, Richell RA, Leonard A, Morton J, et al. Differentiating among prefrontal substrates in psychopathy: neuropsychological test findings. Neuropsychology. 2006;20:153–165. doi: 10.1037/0894-4105.20.2.153. [DOI] [PubMed] [Google Scholar]
- 53.Lapierre D, Braun CM, Hodgins S. Ventral frontal deficits in psychopathy: neuropsychological test findings. Neuropsychologia. 1995;33:139–151. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- 54.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 55.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayan VM, Narr KL, Kumari V, Woods RP, Thompson PM, Toga AW, et al. Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry. 2007;164:1418–1427. doi: 10.1176/appi.ajp.2007.06101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf RC, Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Decety J, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36:4202–4209. doi: 10.1002/hbm.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Contreras-Rodriguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, et al. Functional Connectivity Bias in the Prefrontal Cortex of Psychopaths. Biol Psychiatry. 2015;78:647–655. doi: 10.1016/j.biopsych.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 60.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. J Am Acad Child Adolesc Psychiatry. 2013;52:94–103. e103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.