Abstract
Obesity is currently recognized as a health epidemic worldwide. Its prevalence has doubled in the last three decades. Obesity is a complex clinical picture associated with physical, physiologic, hormonal, genetic, cultural, socioeconomic and environmental factors. The rate of obesity is also increasing in the pregnant women population. Maternal obesity is associated with less than optimal obstetrical, fetal and neonatal outcomes. It is also associated with significant adverse long-term effects on both obese parturients and the infants born from obese women. A number of guidelines have been published to educate health care workers and the general population in an attempt to develop effective interventions on a large scale to prevent obesity. These guidelines are multiple, confusing and inconsistent. There are no standard recommendations regarding gestational weight gaining goals, nutrients and additional elements necessary for certain obese women who have been treated with bariatric surgical procedures, screening for metabolic diseases such as diabetes, additional preventive health care services indicated for obese women in the pregnancy planning stages, during prenatal care, in the immediate post-partum period and as a long-term approach for health preservation. In 2013, the American Medical Association supported by several US national medical specialty organizations published Resolution 420 (A-13) recognizing obesity as a disease state with multiple pathophysiological aspects requiring a range of interventions to improve its prevention and treatment. The goal of this decision was to encourage a broader spectrum of health care benefits insurance coverage for the prevention and treatment of obesity. There are a number of myths and misconceptions associated with obesity. These perspectives present our views and clinical experience with a partial review of recent bibliography addressing the associations between obese reproductive age women and their risks during pregnancy.
Keywords: maternal obesity, pregnancy, severe maternal morbidity
Background
Obesity is defined by the World Health Organization (WHO) and the National Institutes of Health (NIH) in the United States as a person with a body mass index (BMI) above 30 kg/m2, calculated using the individual’s weight in kilograms divided by the square of the height (Table 1). For the benefit of the readers, we provide a perspective reflecting the basis for the definitions and the interest and concern that obesity has created in the scientific and clinical practice. We must also emphasize for readers, reviewers, commentators and editors that the extended date of the reference citations demonstrates the length of time that obesity has been a serious concern to basic and clinical investigators, epidemiologists and public health officials. Obesity is a worldwide public health issue of epidemic proportions. Obesity during pregnancy is associated with maternal and neonatal increased morbidity and mortality.9 The WHO10 recognizes obesity as a disease and has coined the term “globesity” to signify its major public health concern worldwide coexisting with malnutrition in developing countries. Of interest are opinions published by the International Federation of Gynecology and Obstetrics (FIGO). In 2013, it stated that the obstetric risks of maternal obesity may be lower than previously thought, as revealed by researchers from Oxford University. Their study seems to indicate that obese mothers exceeding a BMI of 30 K/m2 may not need to give birth in an obstetric unit.11 In 2015, FIGO12 reported “increasing health risks for mothers and babies” and that obese mothers should ideally lose weight before their pregnancy to decrease possible risks of preterm birth, fetal structural anomalies, large for gestational age babies and perinatal deaths. Reports from the National Health and Nutrition Examination Survey (NHANES) indicated that in 2012 58.5% of adult women in the United States were overweight or obese. The number of obese pregnant women continues to increase. Black and Mexican women showed a higher prevalence. Ideal control of maternal obesity should occur before conception. An increased risk of spontaneous abortion has been reported in obese women. Earlier studies showed that scientists investigated a possible link between metabolic status, body fat and fertility. Obesity as well as extreme malnutrition indicated similarities in the hormonal and metabolic patterns associated with physical or emotional stress. Obesity creates an oxydating physical environment leading to responses that cause damage to the mitochondria, decrease egg quality and interfere with normal embryo development.13 As we will review in the following pages, maternal obesity is associated with delayed conception, early spontaneous abortion, increased risk for fetal structural anomalies (except for gastroschisis), gestational diabetes, chronic and gestational hypertension, preeclampsia, obstructive sleep apnea (OSA), indicated or spontaneous preterm labor and preterm birth, prolonged pregnancy, increased use of labor induction and augmentation, dysfunctional labor, operative vaginal delivery, cesarean delivery, post-partum hemorrhage, post cesarean surgical site infection, thrombotic events and severe maternal morbidity and mortality.
Table 1.
| The Quételet Index (l’homme moyen) 1832 World Health Organization/National Institutes of Health (USA) Body mass index (BMI) = W/H2 or kg/m2 | |
|---|---|
| BMI (kg/m2) | |
| Recommended weight | 18.5–24.9 |
| Overweight | 25.0–29.9 |
| Obesity | ⩾30 |
| Class I | 30–34.9 |
| Class II | 35–39.9 |
| Class III (morbid obesity) | ⩾40 |
| with comorbidities | ⩾35 |
| Super (extreme) obesity (8.0%) | ⩾50 |
Preconception
Preconception counseling and patient education offer a clear opportunity for physicians caring for obese women planning a pregnancy. The use of appropriate contraception until the health status is improved must be discussed. Many published randomized trials involving contraceptive treatments do not include obese women. Elevated BMI is associated with a higher pregnancy rate in patients who use low-dose oral contraceptives. The Centers for Disease Control and Prevention (CDC) have published extensively on the criteria for contraceptive use.14 We refer the readers to this authoritative source since it is not within the scope of these perspectives to extensively discuss the different approaches to birth control alternatives on obese women.
Medical or surgical treatments to achieve near ideal body weight prior to conception have variably demonstrated to improve comorbidities and obstetrical outcome.15,16 Motivational interviewing through an individualized patient-centered approach aims at addressing prospective mothers’ unhealthy behavior and is an approach worth of further exploration.17 In our experience, even a moderate decrease of less than 10% in preconception weight improves maternal health and obstetrical outcomes. A variety of clinical interventions include special low glycemic or low calorie diets, exercise and diets, or just exercise.18–23 The readers should note the lack of uniformity on these approaches which are utilized in developed countries on different ethnic groups. Extremely obese patients have physical limitations to strenuous exercises and repeated counseling is essential. These interventions showed a 20% decrease in excessive gestational weight gaining. The frequency of fetal macrosomia showed a 15% decrease when the maternal BMI approaches the recommended level of less than 30 kg/m2. We favor this approach to counseling. We utilize it during preconception with emphasis on the maternal physical and emotional adverse effects of obesity, in addition to the concerns for future maternal and fetal well-being.
In our practice, we note the frequent presence of comorbidities such as preexisting diabetes, gestational carbohydrate intolerance, hypertension, OSA, metabolic syndrome and gastro-esophageal reflux (GERD). It behooves us to rule them out or treat during the preconception planning to decrease or avoid obstetric complications.
Infertility
Obesity as well as undernutrition have been considered indicators of reproductive system dysfunction. However, in the case of assisted reproductive technology, this was disproven at both extremes of the patient’s BMI, while it was demonstrated that obese patients had lower peak estradiol concentrations than the non-obese group.24 Nutritional status, fat stores and fertility have been repeatedly investigated. It is reported that over and under nutrition may interfere with reproductive function, most likely when extreme.25 In our clinical experience, 6.5% of women between 18 and 45 years old with a BMI between 50 and 106 kg/m2 were delivered twice following unassisted conception in a period of less than 24 months (unpublished). Obesity is a frequent clinical finding in patients with polycystic ovarian syndrome (PCOS). It is a special concern in the adolescent-teen ager population. Obesity worsens the hormonal and metabolic profile in those patients and creates treatment concerns. Those women should be actively encouraged to lose weight, even in modest amounts (5%–10%), to improve their metabolic conditions.26 There is an associated increased risk of spontaneous and recurrent abortion in obese women as compared to non-obese controls (odds ratio (OR), 3.5; 95% confidence interval (CI), 1.03–12.01).27 Robker et al. have reported differences in ovarian metabolites in obese women and the association of the peroxidase proliferator-activated receptor gamma (PPARG) as a regulator of cellular functions such as adipogenesis and immune cell activator. This marker directs cyclic changes within ovarian tissue and has direct and indirect implications for ovarian function and female fertility.28 Treatments commonly used for diabetes (a frequent comorbid condition in obesity) could prevent any damage to the egg, restoring its quality, influencing embryo development and mitochondrial RNA levels equivalent to those of a healthy mother. Obese women who ovulate normally but are subfertile have a 4% lower pregnancy rate per kg/m2 per year compared with non-obese ovulating subfertile women. Increased BMI is associated with an increasing rate of recurrent spontaneous abortion than in non-obese women and is further increased in Asian women.29 In practice, it is important to provide obese women information about the association between their excessive weight on their fertility and the possible complications on the pregnancy. They should be encouraged to lose weight before embarking on fertility treatment and possible conception. In certain medical practices, fertility treatment is withheld from women above a certain BMI unless the woman is unable to lose weight in spite of serious efforts.30 The basis for these policies is related to the risks of the necessary treatments to the woman and the long-term effects of obesity on the future child and the mother. In considering all other maternal risks frequently diagnosed during pregnancy, we believe that there is no established evidence-based reason to deny treatment to these patients after discussing in a clear and complete counseling all potential complications of pregnancy in the obese woman. Recent information from a multicenter concurrent clinical trial treating women with PCOS in the United States utilizing clomiphene demonstrated the benefit of delayed infertility treatment preceded by lifestyle modification with weight loss compared to immediate treatment upon diagnosis.31
Prenatal course
This is the time to provide pertinent counseling, guidance and care. The objective is to reach fetal size and maturity adequate for safe delivery with the least burden of disease for mother and fetus and the best chance for optimal obstetric and perinatal outcome.
Obese pregnant women have been reported as having an increased risk for fetal structural anomalies such as open neural tube defects, fetal heart anomalies, hydrocephaly and limb malformations.32–34 Conversely, a significant decrease in fetal gastroschisis has been reported in obese mothers as compared with non-obese (OR, 0.17; 95% CI, 1.3–1.5). Maternal serum screening using various analytes is affected by elevated maternal BMI. Using weight adjustment improves detection of dorsal fetal wall defects and trisomy 18. It does not improve detection for trisomy 21 (Down syndrome).35 The diagnosis of fetal structural anomalies via ultrasound fetal anatomy survey is limited on the pregnant women with elevated BMI as reported by Dashed et al.36 in 2009. The detection of echogenic cardiac focus, echogenic fetal bowel or fetal nuchal fold appears not to be influenced by maternal BMI. There is evidence of increased congenital heart disease in fetuses of obese women. We obtain a fetal echocardiogram at week 18–20 to evaluate for the presence of fetal heart structural malformations. Recent fetal ultrasound guidelines published by the NIH stipulates that in the presence of suboptimal fetal segments after two consecutive ultrasound exams no further ultrasound exams are indicated and the mother should be counseled about the ultrasound limitations.37 The use of fetal magnetic resonance imaging (MRI) may reduce this problem; however, in our experience, MRI is not routinely used for fetal anatomy screening second to availability, need for expertise in its interpretation and cost.
There is an association reported between obese women and metabolic syndrome. As pregnancy advances insulin resistance increases, and this event may trigger subclinical metabolic dysfunction to progress to gestational diabetes, preeclampsia and fetal macrosomia. Obese pregnant women must be proactively risk-assessed from the time of the initial prenatal care visit.38 In our practice, we test early for carbohydrate intolerance via glucose challenge and retest in the third trimester (24–28 weeks) if the first result is negative. Our clinical experience shows that early screening for carbohydrate intolerance is important during the prenatal care of the obese. The objective is to maintain euglycemia for the duration of the pregnancy.39 Recently, Hong et al.40 reported in a limited retrospective study of singleton high-risk pregnancies, which included women with elevated BMI, no benefit to early screening for gestational diabetes mellitus and recommended a prospective trial. Overt or gestational diabetes is a frequent comorbidity that seriously complicates the clinical course of the obese pregnant women. The strong association between obesity and diabetes has created the term “diabesity” to signify the frequent association and to increase the awareness of the clinician.41 It also suggests a physiopathologic association between these two clinical entities.42 If treatment is required, our care plan includes daily blood glucose control to maintain glucose levels at or below 100 mg/dL fasting and 2 h up to 120 mg/dL as a desirable target with a Hb A1c at 5.4% as the ideal level.38,39 Nutritional and dietary counseling is provided early with repetitive instructions at each prenatal visit along with monitoring gestational weight gaining. Oral hypoglycemic agents may not suffice on the obese parturient; therefore, insulin may need to be initiated. Initiation and intensification of insulin may be necessary. Occasionally, the patient is in the hands of an endocrinologist with whom the obstetrician may co-manage through the length of the pregnancy. A number of patients may be utilizing an insulin pump which is usually adjusted by the endocrinologist as frequently as necessary to maintain the glucose levels suggested above. An alternative practice is to refer the obese diabetic pregnant woman to a perinatologist for management and delivery. As the pregnancy progresses, the diabetic patient will become increasingly dependent on exogenous medication to maintain normoglycemia. This is associated with increasing physiologic placental effects. Along with diabetes and gestational weight gaining control, we monitor fetal growth via ultrasound as the pregnancy approaches the last trimester. Delivery timing and route will follow the usual attempt at reaching full fetal potential and a metabolically stable mother. These perspectives do not intend to be a treatise on the management of diabetes in pregnancy. The readers are referred to the plethora of work published on the subject. Strict versus liberal blood glucose control show a wide variation of opinions based on retrospective small studies and meta-analysis.43
Chronic hypertension, gestational hypertension and preeclampsia are frequent comorbidities in the obese pregnant woman. Obesity is considered a state of chronic inflammation, and this factor has been reported as increasing the risk for preeclampsia.44 Additional maternal serum clinical chemical markers utilizing the determination of the ratio between angiogenic and antiangiogenic factors (sFlt-1/PlGF) have been recently reported to accurately forecast the absence of preeclampsia within 1 week of the determination. The patient’s blood pressure was not included in the original study and only 41.5% of the patients in the validation cohort had new onset or exacerbated hypertension. This test is not yet Food and Drug Administration (FDA)-approved for clinical use in the United States, nor uniformly available for clinicians all over the world; the test availability and utilization is therefore variable.45 We recommend blood pressure monitoring by daily self-determination with an appropriate size cuff. Morbidly and extremely obese patients (Class III) frequently maintain borderline blood pressure levels (140/90) and may require treatment. Careful instructions are given to the mother to observe for rapid weight gaining and the classic signs of emerging preeclampsia. Antihypertensive agents are added as indicated by the blood pressure levels. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers are contraindicated during pregnancy. The chronic use of diuretics restricts the physiologic intravascular volume expansion during pregnancy and is infrequently used on some patients who are affected by chronic hypertension and have achieved metabolic balance with these drugs. In such cases, the patient’s internist needs to be included in the treatment team for proper monitoring. A number of drugs such as clonidine, prazosin, methyldopa and labetalol are safe and effective during pregnancy. In cases of acute hypertensive crisis during pregnancy or in the post-partum period, established treatment protocols are available.46
In our practice, we screen obese pregnant women for OSA. OSA is characterized by episodes of nocturnal apnea, interrupted sleep and variable degrees of airway obstruction.9 The presence of OSA as a comorbidity is associated with increased risk of preeclampsia, eclampsia, maternal cardio vascular complications, abnormal thrombotic events and mortality. It is still undetermined whether there is an association with obstetric history.47,48 In addition, OSA in these patients may be associated with increased risks of preterm birth (frequently iatrogenic or indicated), fetal growth restriction (FGR) and stillbirth. OSA has been reported as a risk factor for liver injury independent of obesity.49 Once OSA is diagnosed, we recommend active treatment with C-pap with periodic determination of peak flow measurements. Liver and renal function tests are performed each trimester. Prenatal care visit frequency is tailored to the progress of the pregnancy and the woman response to instructions.
Maternal obesity has been reported as an independent risk factor for spontaneous extremely preterm delivery by Swedish investigators.50 A meta-analysis of 39 studies conducted in Brazil showed a reduced risk of spontaneous preterm delivery in overweight and Class I obese women and a modest increased risk for moderate preterm birth (32–36 weeks). Class II obese women have an increased risk in general, especially for very preterm birth (<32 weeks). Class III obese women have an even higher risk for very preterm birth (adjusted odds ratio (aOR) = 2.27; 95% CI, 1.76–2.94). High BMI does not change the risk for premature rupture of fetal membranes; however, it increases the risk for elective preterm birth.51 A recent meta-analysis utilizing US and Peruvian reports showed that the true science associated with the relationship between gestational weight gain in the obese women and preterm birth is limited. The investigators recommend further research to provide new information. The report showed that for obese women with gestational weight gain above the Institute of Medicine recommendations, the risk of indicated preterm birth increases (aOR, 1.54; 95% CI, 1.09–2.16) as compared with pregnant women with recommended BMI.52
Maternal obesity is associated with a 40% increased chance of suffering a stillbirth as the pregnancy approaches term. Black obese pregnant women have an adjusted hazard ratio of 1.9 (95% CI, 1.7–2.1), while White obese gravidas adjusted hazard ratio is 1.4 (95% CI, 1.3–1.5). Morbidly obese pregnant women as well as extremely obese pregnant women (BMI > 50 kg/m2) have an adjusted hazard of stillbirth of 1.4 and 1.69, respectively, at 30 to 33 weeks’ gestation with an increase to 3.20 and 2.95 at 37–39 weeks’ gestation and 3.30–8.95 at 40–42 weeks’ gestation compared with non-obese pregnant women. Those with a BMI of 50 kg/m2 (extremely obese) had a 5.7-fold and 13.6-fold greater risk of stillbirth at 39 and 41 weeks’ gestation, respectively, than the non-obese women.9 In this context, there is no evidence showing improvement in perinatal outcome with the implementation of fetal surveillance utilizing the visually interpreted non-stress test. There is no firm recommendation for or against routine fetal surveillance in obese pregnant women.9 There is no single specific fetal surveillance test that can accurately forecast fetal death. In summary, even modest increases in maternal BMI are associated with increased risk of fetal, neonatal and perinatal death.53 Furthermore, increased risks of stillbirth were observed at all gestational ages with some evidence of variable associations by race and parity.54 An increased risk of neonatal and infant death in obese women has also been reported.55 The corollary to the above information reaffirms the importance of reiterating the need for women planning a pregnancy or already pregnant to achieve recommended BMIs prior to conception and avoid or decrease excessive gestational weight gaining to decreased fetal, neonatal and infant morbidity and mortality. Pre-pregnancy elevated BMI has an OR from 1.32 for Class I obese to 1.73 for Class III obese for both neonatal and post neonatal mortality.56
During the second and third trimester, we utilize and recommend consultation with adult cardiology and anesthesia.
There is an increasing number of obese women who had undergone bariatric surgical procedures and conceived following the surgery. In our experience, most pregnancies following bariatric surgery are uneventful. The weight loss realized following the procedure provides improvement on the woman’s metabolic status, hypertension, abnormal high-density lipoprotein (HDL) cholesterol, metabolic syndrome, OSA and subfertility.57 There are two common types of bariatric procedures: restrictive (adjustable gastric band or AGB), where the size of the neo-stomach is reduced significantly and procedures that result in malabsorption (Roux-en-Y). There is an extensive literature dealing with the pros and cons of both procedures, side effects and complications.58 There is no firm opinion regarding the ideal interval between the bariatric surgical procedure and conception. A common approach is to ask the patients to wait for 12 months prior to conception. Our experience is that patients do not receive appropriate contraceptive counseling and medication post bariatric surgery and conceived sooner than advisable. Many patients who realize a significant reduction in their weight remain obese at the time of conception. There is no statistically significant difference in obstetric and neonatal outcomes on patients who conceived shortly after the bariatric procedure and those who wait for the prescribed length of time.59,60 Table 2 offers an example of the approach to the nutritional care of the pregnant woman following one of the bariatric surgical approaches. We offer these recommendations as the product of consolidated advice from a number of investigators and our own current practice. Currently, there is no standard approach to dietary supplements; we believe further studies and refinements of the current recommendations will be beneficial for this patient population as their ability to conceive increases after the surgical procedure, weight loss and health improvement. The care giver must be aware of the necessary adherence to dietary counseling and monitoring. A multidisciplinary prenatal care team is ideal to enhance all aspects of the care. Ideally, the patient should continue periodic contacts with the bariatric nutritionist. Diabetic screening is modified to accommodate the patient’s inability to tolerate a large acute glucose load and untoward side effects (dumping syndrome). We prefer to place the patient on a 1-week self-determined blood glucose level, fasting and random between weeks 24 and 26 and act according to results. Laboratory determinations for serum iron, ferritin, vitamin B12, folate, calcium and creatinine are repeated every trimester. If abnormal, treatment is carried out in tandem with the bariatric surgery team. After the post-partum visit and the couple’s future reproductive plans discussed, agreed upon contraceptive medication prescribed or initiated (vide supra). The patient is referred back to the bariatric surgery team. For completeness, we will briefly mention the current paucity of data associated with the long-term health of the offspring from obese women who underwent bariatric surgery prior to pregnancy.67
Table 2.
| Prenatal Care Post RYGB Combined procedure (restrictive and malabsorptive) |
|---|
| Protein deficiency: provide 60 g protein daily, balanced diet Iron deficiency (hypochlorhydria) anemia: in 10% of patients, 40–65 mg iron sucrose po daily Vitamin A deficiency: in ±10% of patients. 5000 IU daily Vitamin B12: cobalamin sublingual 10 µg daily or 1000 µg IM monthly Calcium: 1200–2000 mg citrate, 50–150 µg Magnesium: 200–1000 mg daily Vitamin D: 1000 IU daily Folic acid: 4 mg daily included in prenatal vitamins Iodine: no specific recommendations, depends on geographical location 250 µg daily Vitamin K: no specific recommendations Antioxidants: no specific recommendations |
RYGB: Roux-en Y gastric bypass.
As pregnancy progresses into the third trimester, maternal and fetal evaluation concentrates on the risk assessment tailored to the individual patient. Gestational weight gain, carbohydrate metabolism, blood pressure levels, fetal growth, cardiorespiratory function, adjustment of medications (insulin, oral hypoglycemic agents, antihypertensive, steroids, anticoagulants, antiasthmatics and more), patient working conditions when pertinent, cardiac function evaluation, cardiology clinical clearance for a major surgical procedure,68 anesthesia consultation and planning, prenatal education, immunizations and delivery planning, new born feeding, reproductive planning and post-delivery care are on the list of things to do and the concerns to anticipate. Parity becomes an important issue to discuss in association with management of labor. Review the history of previous deliveries, labor duration, delivery events, outcomes both maternal and neonatal if any of consequence for risk recurrence (i.e. failed induction, prolonged labor, macrosomia, shoulder dystocia, perineal lacerations, post-partum bleeding, surgical complications). Recently, an obstetric comorbidity index has been proposed to attempt to predict severe maternal morbidity.69 Further research may provide validation to incorporate this approach to improve the care of the obese pregnant woman.
Labor and delivery
The timing of labor is associated with the expression of the gene for corticotrophin-releasing hormone (CRH) by the placenta. This normal mechanism is distorted in the obese parturient. Post-term labor is common and induction may become necessary. There is no established specific oxytocin protocol for the obese parturient. These “perspectives” do not intend to provide a full discussion of the complexities of human parturition. The readers are referred to the specific literature on the subject.70,71 Monitoring the uterine contractions may be cumbersome and inaccurate, needing early amniorrhexis and use of intrauterine pressure catheters and fetal scalp lead for fetal heart monitoring. Cervical ripening becomes necessary in the presence of an unfavorable cervix. The association between maternal obesity and preterm (iatrogenic) birth or prolonged pregnancy and dysfunctional labor has been recognized.
In our experience, should regional anesthesia be part of the delivery plan, we request the placement of the epidural catheter early; initiate the test dose to evaluate results and then repeat according to need later in the labor process or utilize a continuous bedside infusion pump.72 The first stage of labor (until a 6 cm cervical dilation is reached) may be prolonged in the obese parturient (Figure 1, laborgram), while the second stage is relatively of the same duration as in the non-obese.73 Obese parturients are at increased risk of failed trial of labor after a previous cesarean as well increased in composite maternal morbidity and neonatal injury.74 We include these concerns during the counseling and discussion at the time of the prenatal visits.
Figure 1.
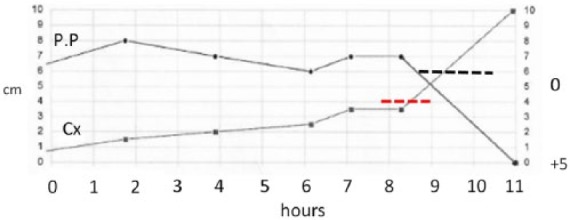
25-year-old gravida 2 para 0 Vertex (BMI, 68 kg/m2; induced labor; SVD; BW 3631 g).
Note protracted progress of labor. PP: presenting part; Cx: cervix; 0: station 0.
Operative vaginal delivery needs additional assistance at the bedside to support the patient’s legs. In our experience, the dorsal lithotomy position may be difficult to achieve comfortably by obese parturients; the team must be prepared for alternative positions and additional support. Increased risk for shoulder dystocia demands the team to be alert at all times to provide the necessary support and appropriate maneuvers conducive to alleviate the situation if it arises. The redundant pannus will create a concern to perform suprapubic pressure. Additional personnel must be available. Consider moving to deliver the posterior shoulder first or sling the anterior fetal axilla to slide it under the symphysis. We practice active management of the third stage of labor utilizing IV (10–40 U/500 or 1000 mL of crystalloid) or 10 U of oxytocin IM at the time of the first shoulder delivery will assist in decreasing the risk for post-partum hemorrhage. Readers should be aware of the inconsistencies of this approach across the world.75,76 We make certain that trained assistants, proper instruments and adequate lighting are ready to explore the uterus, vagina, cervix and perineum for possible lacerations.
Should the patient had a previous cesarean delivery (one or more), obtain accurate up-to-date delineation of placenta insertion. We evaluate the location and normal insertion should be mapped to allow for proper planning of incision placement and additional resources in case of an abnormally attached placenta (accreta, increta, percreta). It is important to review the previous cesarean(s) operative report along with the events of the post-operative care, the use of prophylactic antibiotics, presurgical preparations and local hygiene; discuss the increased risk for abnormal clotting events and its prevention with Sequential Compression Devices (SCDs), prophylactic anticoagulation starting 18–24 h post-surgery and continue until discharge from the hospital or further. Discuss the importance of early ambulation and the eventual need for intensive care unit admission and blood products transfusion. Transporting the patient to the operating room table may need additional equipment (inflatable mattress). The operating room table should be tailored to the patient’s weight. Should the regional anesthesia fail, endotracheal intubation may be necessary and anesthesia should be ready for this approach on a patient with significantly reduced neck motion.72 Obese parturients are at increased risk of primary cesarean during the intrapartum period.77 Abdominal incision planning assists in deciding the location, type, size and the proper pannus management according to its size. If concerns are warranted regarding placenta insertion or location, the indication for a hysterectomy should be discussed with the patient and her family and shared with Obstetrics (OB) nursing personnel, anesthesia, transfusion services and neonatology who will be involved in the care. If during prenatal care the diagnosis of abnormal placentation is firm, consider referring the patient for her delivery to a higher level institution with experience in these cases and additional surgical support. When an elective surgical delivery is planned, attempt to schedule it early in the day when the obstetrical unit is fully staffed. Make certain that the appropriate (bariatric) surgical instruments are available. In our experience, we frequently utilize a self-retaining protector-retractor (Alexis® O)78 or similar to reduce the number of instruments in the field and extra assistants at the table side. Abdominal incision location preference is left at the surgeon’s election as well as the cesarean technique. Consider having a portable vacuum extractor or obstetrical forceps for the delivery, since occasionally the distance between the patient’s skin and the uterus is significant and the usual manual maneuvers are cumbersome. The usual and customary “fundal pressure” is for the most part of little use. If there has been a prolonged labor or any period of bearing down efforts prior to the cesarean, and the fetal presenting part is deeply engaged, cephalad displacement by a trained vaginal hand (push up approach) may become necessary. Be prepared for such contingency and have a knowledgeable assistant in charge of displacing the fetal presenting part upward. In our experience, we use this approach and do not perform the hysterotomy until the presenting part is above the symphysis to avoid prolapse of the fetal shoulder or upper extremity. Conversely, delivering the fetal pole on the upper uterine segment (breech extraction) may be an alternative maneuver in certain cases. In case of a multiple pregnancy, we recommend presurgical planning according to the fetal position.
Treat the third stage of labor with equal attention as it is on a vaginal delivery. Closing the hysterotomy and the abdominal wall are left to the decision of the individual surgeon, its training and experience in this very special population. Hemostasis is essential and it should be accomplished with limited use of cautery that create areas of local necrosis leading to increased chance for post-operative complications (seroma, hematoma), wound disruption and fasciitis. Fascial closure may be performed as routinely done or via Smythe Jones technique. The pannus must be closed avoiding pockets with poor opposition that may be an additional source of wound disruption. Two or three layers of interrupted absorbable sutures may be needed. We also recommend closing the skin with sutures and not staples, since this last approach is associated with a higher number of post-operative wound complications. Consider early and daily close surveillance of wound care; if staples were used, delayed removal appears to be beneficial. Upon discharge, the patient and her immediate family members should be instructed to participate in wound care. These patients are frequently unable to access the incision on their own for proper hygiene.
Postpartum
Long-term care of the newly delivered obese women must insure monitoring of weight loss, cardiac and metabolic follow-up, blood pressure monitoring and necessary live style adjustment for health preservation. Obstetricians may select to refer these women to a primary care physician for follow-up.79 Reproductive planning must be discussed during prenatal care to determine the most appropriate approach as was discussed under contraception. Appropriate referrals to a primary care physician who understands the intricacies of obesity should ideally be provided at the time of hospital discharge. That will include access to a comprehensive weight management program including dietary adjustments, physical activity and behavioral modifications. Multidisciplinary care programs suitable for individual patients are available in most health care systems or community programs and are recommended. Expanded counseling may involve the evaluation for bariatric surgery procedures and a proper referral provided. In cases where the pregnancy has been completed on a patient who had pre-pregnancy bariatric surgery, a return to the bariatric surgeon should be secured to allow for any necessary follow-up care, especially of those women with an adjustable device (lap band). We utilize the term “bariatric obstetrics” to identify all efforts to care for this population and provide all indicated interventions to decrease or reverse obesity and its short- and long-term adverse effects on parental and infant health.
Conclusion
Caring for obese pregnant women offers a number of challenges for the care giver, the system, the patient herself and the family. The preceding perspectives offer a capsulized scenario to have in mind while caring for this population. In spite of ongoing efforts to prevent obesity, the number of obese pregnant women with significant comorbidities continues to increase. All aspects of their obstetrical care deserve special attention. These perspectives convey our education, training and clinical experience in the evaluation and management of obesity associated with pregnancy. Readers must be informed that currently there are approximately 26 national and international guidelines associated with different aspects of the management of obesity in pregnancy. These guidelines include patients studied through decades.80–85 These guidelines are based on retrospective cohort studies and guidelines reviews and show significant discrepancies among them while showing limited guidance to clinical practitioners. They all recommend further randomized, double blind, population control trials and the development of standard guidelines.
Future
Maternal obesity has been defined as a worldwide epidemic. Its effects are long lasting on both the mother and the offspring. A concerted social and medical effort includes collaboration to ameliorate the effects of this disease on the future generation. As it has been demonstrated, delaying conception until the maternal BMI reaches the recommended levels seems to improve the obstetrical and neonatal outcomes. Public health educational programs and community outreach efforts should increase. Adequate funding is necessary. Legislators must be informed of this condition that threatens public health. Elected officials are mandated to preserve the health of the public. One example is a collaborative community multiinstitutional approach to achieve the healthy people 2020 goals.86 Further research in improved medical and surgical treatments of obesity may bring alternatives for patients not responding to current methods. The AspireAssist™ device (Aspire Bariatrics, King of Prussia, PA, USA) has been recently approved by the FDA in the United States. A small clinical trial of 111 patients has been completed (unpublished), demonstrating a 12% loss in total body weight versus 3.6% loss in the control group after 1 year of use. The opportunity to predict future obesity in children may start at 4 months of age as recently reported by Smego at the 2016 National Endocrine Society meeting (unpublished). This will allow to identify those children at risk for adult obesity and counsel the families toward healthier lifestyles.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Eknoyan G. Adolphe Quetelet (1796–1874): the average man and indices of obesity. Nephrol Dial Transpl 2008; 23(1): 47–51. [DOI] [PubMed] [Google Scholar]
- 2. Keys A, Fidanza F, Karvonen MJ, et al. Indices of relative weight and adiposity. J Chron Dis 1972; 25: 329–343. [DOI] [PubMed] [Google Scholar]
- 3. Apell SP, Wahlsten O, Gawlitza H. Body mass index-a physics perspective. SE-412.97, September 2011. Göteborg: Department of Applied Physics, Chalmers University of Technology. [Google Scholar]
- 4. WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO technical report series 854, 1–3 November 1995. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 5. WHO. Obesity: preventing and managing the Global epidemic. WHO technical report series 894, 3–5 June 2000. Geneva: Switzerland, World Health Organization. [PubMed] [Google Scholar]
- 6. National Heart Lung and Blood Institute. The NHLBI practical guide; identification, evaluation and treatment of overweight and obesity in adults. NIH publication No. 00–4084 or 02–4084. Bethesda, MD: National Institutes of Health, 2001. [Google Scholar]
- 7. Sand AS, Emaus N, Lian O. Overweight and obesity in young adult women: a matter of health or appearance? The Tromsø study: fit futures. Int J Qual Stud Health Well-being. Epub ahead of print September 22 2015. DOI: 10.3402/qhwv10.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komaroff M. For researchers on obesity: historical review of extra body weight definitions. J Obes 2016; 2016: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American College of Obstetricians & Gynecologists. Practice bulletin # 156: obesity in pregnancy. Obstet Gynecol 2015; 126(6): e112–e126. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Global database on body mass index (Last update 26 July 2013). Geneva: World Health Organization, 2006. [Google Scholar]
- 11. International Federation of Gynecology and Obstetrics (FIGO). Gynaecology and women’s health (BJOG). London: FIGO, 2013. [Google Scholar]
- 12. International Federation of Gynecology and Obstetric (FIGO). New born health. London: FIGO, 2015. [Google Scholar]
- 13. Robker R. and International Federation of Gynecology and Obstetrics (FIGO). Fertility. London: FIGO, 2015. [Google Scholar]
- 14. Centers for Disease Control and prevention. US medical eligibility criteria for contraceptive use. MMWR 59, 28 May 2010. (pp. 1–86). Atlanta, GA: Centers for Disease Control and prevention. [Google Scholar]
- 15. Beard JH, Bell RL, Dyffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg 2008; 18: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 16. Stang J, Huffman LG. Position of the Academy of Nutrition and dietetics: obesity, reproduction and pregnancy outcomes. J Acad Nutr Diet 2016; 116(4): 677–691. [DOI] [PubMed] [Google Scholar]
- 17. Krukowski RA, DiLillo V, Ingle K, et al. Design and methods of a synchronous online motivational interviewing intervention for weight management. JMIR Res Protoc 2016; 5(2): e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang JW, Foster KE, Pumarino J, et al. Perspectives on prevention of type 2 diabetes after gestational diabetes: a qualitative study of Hispanic, African-American and white women. Matern Child Health J 2015; 19(7): 1526–1534. [DOI] [PubMed] [Google Scholar]
- 19. Muktabhant B, Lawrie TA, Lumbiganon P, et al. Diet or exercise or both, for preventing excessive weight gain in pregnancy. Cochrane DB Syst Rev 2015; 15(6): CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bain E, Crane M, Tieu J, et al. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane DB Syst Rev 2015; 12(4): CD010443. [DOI] [PubMed] [Google Scholar]
- 21. Kalter-Leibovici O, Younis-Zeidan N, Atamna A, et al. Lifestyle intervention in obese Arab women: a randomized control trial. Arch Intern Med 2010; 170(11): 970–976. [DOI] [PubMed] [Google Scholar]
- 22. Chasan-Taber L. Lifestyle interventions to reduce risk of diabetes among women with prior gestational diabetes mellitus. Best Pract Res Clin Obstet Gynecol 2015; 29(1): 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chasan-Taber L, Marcus BH, Rosal MC, et al. Proyecto mama: a lifestyle intervention in overweight and obese Hispanic women: a randomized controlled trial-study protocol. BMC Pregnancy Childbirth 2015; 15: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lashen H, Ledger W, Bernal AL, et al. Extremes of body mass do not adversely affect the outcome of superovulation and in-vitro fertilization. Hum Reprod 1999; 14(3): 712–715. [DOI] [PubMed] [Google Scholar]
- 25. Mutsaerts MAQ, Groen H, ter Bogt NCW, et al. The lifestyle study: costs and effects of a structured lifestyle program in overweight and obese sub fertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomized controlled trial. BMC Women Health 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoeger KM. Role of lifestyle modification in the management of polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab 2006; 20(2): 293–310. [DOI] [PubMed] [Google Scholar]
- 27. Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case control study. Hum Reprod 2004; 19: 1644–1646. [DOI] [PubMed] [Google Scholar]
- 28. Robker RL, Akison LK, Bennett BD, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Endocrinol Metab 2009; 94(5): 1533–1540. [DOI] [PubMed] [Google Scholar]
- 29. Lo W, Rai R, Hameed A, et al. The effect of body mass index on the outcome of pregnancy in women with recurrent miscarriage. J Fam Commun Med 2012; 19(3): 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koning AM, Mol BW, Dondorp WJ. Obesity: argument for withdrawing fertility treatment. Ned Tijdschr Genees 2014; 158: A7258. [PubMed] [Google Scholar]
- 31. Legro RS, Dodson WC, Kunselman AR, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocr Metab 2016; 101(7): 2658–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marengo L, Farag NH, Canfield M. Body mass index and birth defects: Texas, 2005–2008. Matern Child Health J 2013; 17(10): 1898–1907. [DOI] [PubMed] [Google Scholar]
- 33. Block SR, Watkins SM, Salemi JL, et al. Maternal prepregnancy body mass index and risk of selected birth defects: evidence of a dose response relationship. Pediatr Perinat Epidemiol 2013; 27: 521–531. [DOI] [PubMed] [Google Scholar]
- 34. Hildebrand E, Gottvall T, Blomberg M. Maternal obesity and detection rate of fetal structural anomalies. Fetal Diagn Ther 2013; 33(4): 246–251. [DOI] [PubMed] [Google Scholar]
- 35. Stothard KJ, Tennant PW, Bell R, et al. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009; 301(6): 636–650. [DOI] [PubMed] [Google Scholar]
- 36. Dashed JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstet Gynecol 2009; 113(5): 1001–1007. [DOI] [PubMed] [Google Scholar]
- 37. Reddy UM, Abuhamad AZ, Levine D, et al. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists. American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. J Ultrasound Med 2014; 33(5): 745–757. [DOI] [PubMed] [Google Scholar]
- 38. Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2): 221–230. [DOI] [PubMed] [Google Scholar]
- 39. American Diabetes Association. Management of diabetes in pregnancy. Sec 12: In standards of medical care in diabetes 2015. Diabetes Care 2015; 38(suppl. 1): 577–579. [Google Scholar]
- 40. Hong WY, Biggio JR, Tita A, et al. Impact of early screening for gestational diabetes on perinatal outcomes in high risk women. Am J Perinat 2016; 33(8): 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chadt A, Schermeck S, Joost HG, et al. Molecular links between obesity and diabetes “diabesity.” In: Endotext (eds De Groot LJ, Beck-Peccoz P, Chrousos G, et al.), South Dartmouth, MA, 1 May 2014. [Google Scholar]
- 42. Farag YMK, Gaballa M. Diabesity: an overview of a rising epidemic. Nephrol Dial Transpl 2011; 26: 28–35. [DOI] [PubMed] [Google Scholar]
- 43. Martis R, Brown J, Alsweiler J, et al. Different intensities of glycemic control for women with gestational diabetes mellitus. Cochrane Database Syst Rev, 7 April 2016; 4: CD011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spradley FT, Palei AC, Granger JP. Immune mechanisms linking obesity and preeclampsia. Biomolecules 2015; 5: 3142–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016; 374(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 46. American College of Obstetricians and Gynecologists. Task force on hypertension in pregnancy. Obstet Gynecol 2013; 122: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 47. Louis JM, Mogos MF, Salemi JL, et al. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014; 37(5): 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bin YS, Cistulli PA, Ford JB. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med 2016; 12(6): 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Byrne TJ, Parish JM, Somers V, et al. Evidence for liver injury in the setting of obstructive sleep apnea. Ann Hepatol 2012; 11(2): 228–231. [PubMed] [Google Scholar]
- 50. Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013; 309(22): 2362–2370. [DOI] [PubMed] [Google Scholar]
- 51. Torloni MR, Betrán AP, Daher S, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J Matern Fetal Neonat Med 2009; 22(11): 957–970. [DOI] [PubMed] [Google Scholar]
- 52. Faucher MA, Hastings-Tolsma M, Song JJ, et al. Gestational weight gain and preterm birth in obese women: a systematic review and meta-analysis. BJOG 2016; 123(2): 199–206. [DOI] [PubMed] [Google Scholar]
- 53. Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death. A systematic review and meta-analysis. JAMA 2014; 311(15): 1536–1546. [DOI] [PubMed] [Google Scholar]
- 54. Carmichael SL, Blumenfeld YJ, Mayo J, et al. Prepregnancy obesity and risks of stillbirth. PLoS ONE 2015; 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen A, Feresu SA, Fernandez C, et al. Maternal obesity and the risk of infant death in the United States. Epidemiology 2009; 20(1): 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Declerq E, MacDorman M, Cabral H, et al. Prepregnancy body mass index and infant mortality in 38 US states 2012–2013. Obstet Gynecol 2016; 127(2): 279–287. [DOI] [PubMed] [Google Scholar]
- 57. ACOG. ACOG practice bulletin # 105: bariatric surgery and pregnancy. Obstet Gynecol 2009; 113(6): 1405–1413. [DOI] [PubMed] [Google Scholar]
- 58. Johansson K, Cnattingius S, Näslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med 2015; 372: 814–824. [DOI] [PubMed] [Google Scholar]
- 59. Kominiarek MA. Preparing for and managing a pregnancy after bariatric surgery. Semin Perinatol 2011; 35(6): 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norgaard LN, Gjerris ACR, Kirkegaard I, et al. Fetal growth in pregnancies conceived after gastric bypass surgery in relation to surgery-to-conception interval: a Danish National cohort study. PLoS ONE 2014; 9(3): e90317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ziegler O, Sirveaux MA, Bruneaud L, et al. Medical follow up after bariatric surgery; nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 2009; 35(6 Pt 2): 544–557. [DOI] [PubMed] [Google Scholar]
- 62. Gesquiere I, Foulon V, Augustiins P, et al. Micronutrient intake, from diet and supplements, and association with status markers in pre and post-RYGB patients. Clin Nut. Epub ahead of print 23 August 2016. DOI: 10.1016/j.clnu.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 63. Peterson LA, Zeng X, Caufield-Noll CP, et al. Vitamin D status and supplementation before and after bariatric surgery: a comprehensive literature review. Surg Obes Relat Dis 2016; 12(3): 693–702. [DOI] [PubMed] [Google Scholar]
- 64. Mischler RA, Armah SM, Wrigh BN, et al. Influence of diet and supplements on iron status after gastric bypass surgery. Surg Obes Relat Dis 2016; 12(3): 651–658. [DOI] [PubMed] [Google Scholar]
- 65. Boyce SG, Goriparthi R, Clark J, et al. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg 2016; 26(5): 966–971. [DOI] [PubMed] [Google Scholar]
- 66. Miller GD, Norris A, Fernandez A. Changes in nutrients and food groups intakes following laparoscopic Roux-en-Y gastric bypass (RYGB). Obes Surg 2014; 24(11): 1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Catalano PM. Management of obesity in pregnancy. Obstet Gynecol 2007; 109(2 Pt1): 419–433. [DOI] [PubMed] [Google Scholar]
- 68. Gaesser GA, Tucker WJ, Jarrett CL, et al. Fitness versus fatness: which influences health and mortality risk the most? Curr Sports Med Rep 2015; 14(4): 327–332. [DOI] [PubMed] [Google Scholar]
- 69. Baterman BT, Gagne JJ. The obstetric comorbidity index predicts severe maternal morbidity. BJOG 2015; 122(13): 1756. [DOI] [PubMed] [Google Scholar]
- 70. Carlson NS, Lowe NK. Intrapartum management associated with obesity in nulliparous women. J Midwifery Wom Heal 2014; 59: 43–53. [DOI] [PubMed] [Google Scholar]
- 71. Carlson NS, Hernandez TL, Hurt J. Parturition dysfunction in obesity: time to target the pathobiology. Reprod Biol Endocrin 2015; 13: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soens MA, Birnbach DJ, Ranasinghe JS, et al. Obstetric anesthesia for the obese and morbidly obese patient: an ounce of prevention is worth more than a pound of treatment. Acta Anaesth Scand 2008; 52(1): 6–19. [DOI] [PubMed] [Google Scholar]
- 73. Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol 2011; 205(3): 244.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hibbard JU, Gilbert S, Landon MB, et al. For the NIH Maternal Fetal Medicine Units network. Obstet Gynecol 2006; 108(1): 125–133.16816066 [Google Scholar]
- 75. Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol 2015; 213(1): 76.e1–10. [DOI] [PubMed] [Google Scholar]
- 76. Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2015; 3: CD007412. [DOI] [PubMed] [Google Scholar]
- 77. Kawaita T, Reddy UM, Landy HJ, et al. Indications for primary cesarean delivery relative to body mass index. Am J Obstet Gynecol 2016; 215(4): 515.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Applied Medical. Alexis® O C-section retractor. Rancho Santa Margarita, CA: Applied Medical. [Google Scholar]
- 79. McPherson ME, Vanderkruik R, Reims K, et al. The healthy weight collaborative: quality improvement methods promoting healthy weight. J Health Care Poor U 2012; 23(suppl. 3): 21–33. [DOI] [PubMed] [Google Scholar]
- 80. Hamad R, Cohen AK, Rehkopf DG. Changing national guidelines is not enough: the impact of 1990 IOM recommendations on gestational weight gain among US women. Int J Obes (Lond) 2016; 40: 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schumann NL, Brinsden H, Lobstein T. A review of national health policies and professional guidelines on maternal obesity and weight gain in pregnancy. Clin Obes 2014; 4(4): 197–208. [DOI] [PubMed] [Google Scholar]
- 82. Grivell RM, O’Brien CM, Dodd JM. Managing obesity in pregnancy: a change in focus from harm minimization to prevention. Semin Reprod Med 2016; 34(2): e38–e46. [DOI] [PubMed] [Google Scholar]
- 83. Denison FC, Norwood P, Bhattacharya S, et al. Association between maternal body mass index during pregnancy, short-term morbidity and increased health service costs: a population based study. BJOG 2014; 121(1): 72–81. [DOI] [PubMed] [Google Scholar]
- 84. Barakat R, Pelaez M, Cordero Y, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol 2016; 214(5): e1–e8. [DOI] [PubMed] [Google Scholar]
- 85. Kominiarek MA, Chauhan SP. Obesity before, during, and after pregnancy: a review and comparison of five national guidelines. Am J Perinat 2016; 33(5): 433–441. [DOI] [PubMed] [Google Scholar]
- 86. Basu S, Seligman H, Winkleby M. A metabolic-epidemiological microsimulation model to estimate the changes in energy intake and physical activity necessary to meet the healthy people 2020 obesity objective. Am J Public Health 2014; 104(7): 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]


