Abstract
Postpartum uterine bleeding is not uncommon and is caused by a variety of obstetrical and gynecological disorders, such as retained placenta, dysfunctional bleeding, and endometrial polyps. Placental polyps and uterine arteriovenous malformation are disorders often encountered in cases of abnormal uterine bleeding in the late puerperal period. These patients may experience life-threatening bleeding and require prompt intervention based on the correct differential diagnosis. The optimal treatments for both diseases differ as follows: intrauterine curettage or transcervical resection are chosen for placental polyps, while total abdominal hysterectomy or uterine artery embolization is preferred for uterine arteriovenous malformation since intrauterine curettage or transcervical resection has the risk of massive bleeding. However, since placental polyp and uterine arteriovenous malformation have similar clinical characteristics, it is important to accurately identify and differentiate between them to ensure optimal therapy. We report here cases that were suggestive of placental polyp or uterine arteriovenous malformation. We discuss the differential diagnoses and treatments for both diseases based on a literature review and propose a novel algorithm for managing such patients.
Keywords: arteriovenous malformation, diagnosis, placental polyp, transcervical resection, treatment
Introduction
Placental polyp is a retained piece of the placental tissues that persistently exist in the uterine cavity after abortion or parturition.1–3 Histologically, this fragment consists of organized villi and decidua, along with degenerated clots and regenerated endometrium. These components are firmly attached to the wall of the uterine cavity. The incidence of placental polypoid mass is less than 0.25% of all pregnancies. Furthermore, only 6% of placental polypoid masses are hypervascular and associated with severe hemorrhage.4 However, arteriovenous malformation (AVM) is an abnormal communication between arteries and veins, in which intervening capillaries are usually absent.5 AVMs are broadly classified as congenital or acquired, and the former type—believed to be caused by abnormal embryological development of vasculature—is generally more difficult to treat. Acquired uterine AVM, however, is thought to be formed in the uterus as a result of trauma associated with vaginal delivery, cesarean section, curettage procedures, retained products of conception, gestational trophoblastic disease, choriocarcinoma, or other gynecologic malignancies.6 Both placental polyp and uterine AVM can induce life-threatening uterine bleeding. The differential diagnosis for both diseases is based on various clinical findings, laboratory data, including serum β-human chorionic gonadotropin (hCG) levels, and the findings of transvaginal ultrasonography (TV-USG), power Doppler imaging, and computed tomography (CT) or magnetic resonance imaging (MRI) with contrast media. In some cases, a definitive diagnosis cannot be reached.
Total abdominal hysterectomy (TAH) is a radical surgical treatment for placental polyp or uterine AVM that causes serious uterine bleeding.5 Uterine artery embolization (UAE) is a useful choice to stop active bleeding in the postabortal or postpartum setting for placental polyp or uterine AVM.7,8 However, hysteroscopic transcervical resection (TCR) is used for treating placental polyps without hypervascularity but not uterine AVM.9 Conservative treatment is also used for both diseases in the absence of active bleeding.10 Methotrexate can be used to treat placental polyps11 but not uterine AVM, whereas hormonal compounds such as an estrogen/progestin mixture, danazol, and gonadotropin-releasing hormone agonists are administered for uterine AVM.12–14 Therefore, it is very important to diagnose these disorders promptly and accurately and provide the appropriate medical interventions to the patient. We report here two cases that were suggestive of placental polyp and uterine AVM and discuss the differential diagnoses and treatments for both diseases based on a literature review. We also propose a novel algorithm for the management of such patients.
Case reports
We obtained written informed consent from each patient to present their information and images in an international medical journal. Our institution does not require ethics approval for reporting individual cases.
Case 1
A 28-year-old woman (gravida 1, para 1) delivered by normal vaginal spontaneous delivery at 40 weeks and 5 day. Following the delivery of the placenta, subinvolution of the uterus was noted; therefore, bimanual uterine compression was performed and intravenous oxytocin was administered. The total blood loss was 875 g during the delivery. She had mild, intermittent uterine bleeding postpartum; however, no residual tissues or hematoma was observed in the uterus at the follow-up examination on postpartum day 5. On postpartum day 30, she visited our hospital for routine postpartum follow-up.
Although a small amount of dark brown-colored vaginal discharge persisted, internal examination revealed no abnormal findings. However, a polypoid mass (18 mm × 14 mm) with high echoic regions was observed by TV-USG, arising from the posterior side of the uterine cavity (Figure 1(a)). TV-USG with color Doppler imaging showed vascular flow toward the polypoid mass from the uterine fundus (Figure 1(b)). Blood examination showed no abnormal findings, except for a positive serum hCG titer (3.68 mIU/mL). MRI of the pelvis was performed to further characterize this polypoid mass, which revealed that the polypoid mass projected into the uterine cavity with a non-uniform high intensity in T2-weighted images (Figure 2). CT angiography at postpartum day 39 showed a prominent vascular mass with its major vascular supply from the left uterine artery (Figure 3). At first, we considered performing TCR of the polypoid mass after selective embolization of the uterine artery. However, given the lack of significant uterine bleeding and progressive anemia and considering the patient’s wish for future pregnancy, we decided against this treatment. After 9 weeks of observation, the uterine polypoid mass naturally vanished, as confirmed by TV-USG.
Figure 1.
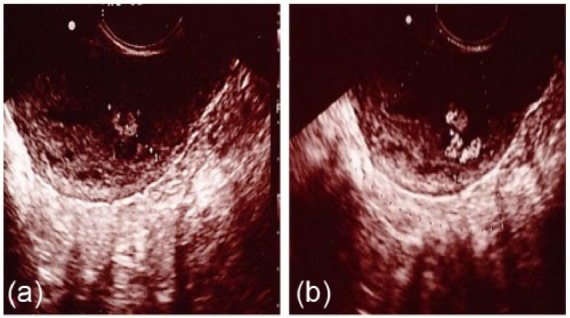
Imaging results of TV-USG (gray scale (a) and color Doppler (b)) in case 1. Note the (a) polypoid mass (18 mm × 14 mm) with high echoic regions and (b) vascular flow toward the polypoid mass from fundus of the uterus.
Figure 2.
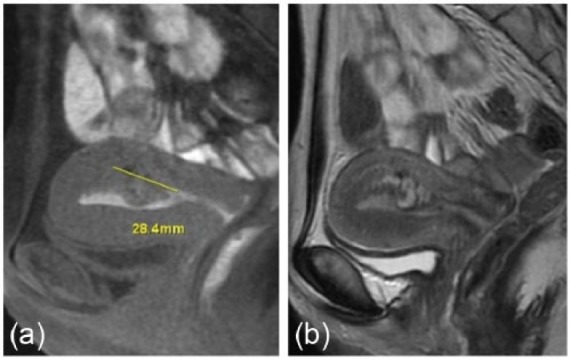
Imaging results of MRI in case 1. Note the (a) polypoid mass projecting into the uterine cavity in the T1-weighted image with (b) non-uniform high intensity in the T2-weighted image.
Figure 3.
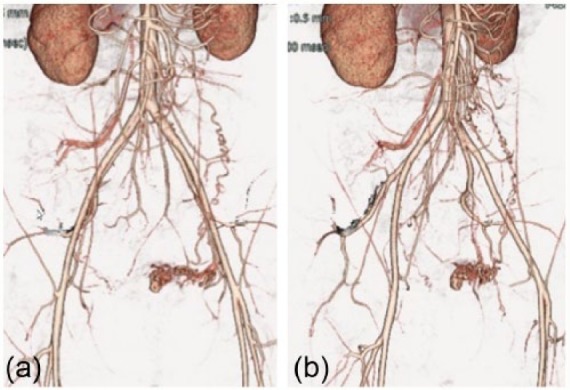
Imaging results of three-dimensional CT angiography in case 1. Note the prominent vascular mass with a major vascular supply from the left uterine artery.
Case 2
A 28-year-old woman (gravida 1, para 1) underwent cesarean section for breech presentation at another hospital. At routine follow-up examination on postpartum day 28, an abnormal mass measuring 26 mm × 16 mm was observed in the uterine cavity by TV-USG. Because the patient had no symptoms, further follow-up without intervention was recommended. On postpartum day 55, the patient experienced increased vaginal bleeding and was referred to our hospital for suspected placental polyp.
On admission to our hospital, uterine bleeding was still observed but there was no active bleeding. Blood and laboratory examinations showed no anemia, and the serum hCG level was below 0.4 mIU/mL. The urinary pregnancy test was negative. TV-USG showed a polypoid mass measuring 23 mm × 19 mm in the uterine cavity (Figure 4). CT angiography showed a hypervascular mass measuring 30 mm × 30 mm within the uterine cavity (Figure 5), with the feeding artery being supplied by the left uterine artery. MRI also showed a mass measuring 30 mm in diameter, with non-uniform high intensity in the T1-weighted image (Figure 6(a)); the T2-weighted image revealed coil formation in the center of the tumor (Figure 6(b)). Thus, based on MRI findings, we diagnosed uterine AVM with clot. Considering the lack of active bleeding and the patient’s wish for future pregnancy, we decided against performing any medical intervention.
Figure 4.
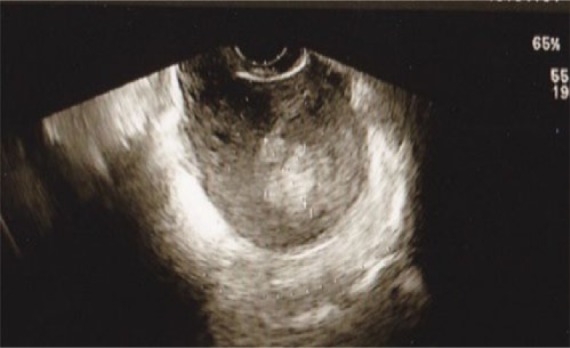
Imaging results of TV-USG in case 2. Note the hypervascular polypoid mass with a feeding artery originating from the fundus.
Figure 5.
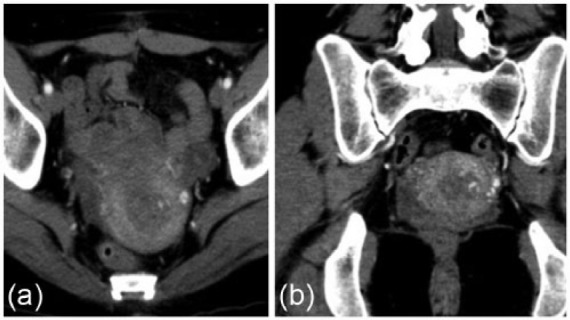
Imaging results of CT angiography in case 2. Note the hypervascular mass measuring 3 cm × 3 cm within the uterine cavity with the feeding artery being supplied by the left uterine artery. (a) Axial section (b) Coronal section.
Figure 6.
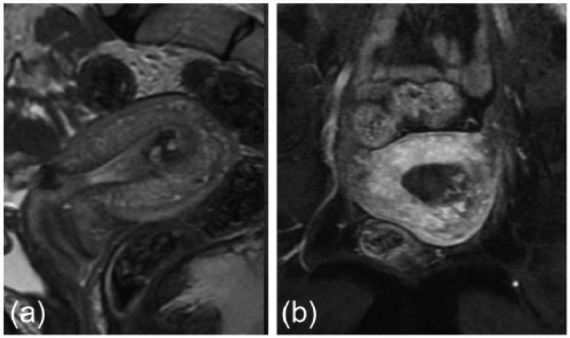
Imaging results of MRI in case 2. (a) A mass with non-uniform high intensity is observed on the T1-weighted image. (b) The T2-weighted image shows coil formation in the center of the tumor.
Discussion
We have often encountered patients bearing a polypoid mass in the uterine cavity with continuous vaginal bleeding at postpartum or after an artificial abortion. With severe vaginal bleeding, these disorders can be life threatening. Therefore, rapid diagnosis and appropriate management of such patients are essential. Since placental polyps and uterine AVM present with similar clinical features and laboratory findings, it is sometimes difficult to differentiate between these two entities and reach the correct diagnosis.
How to distinguish between placental polyp and uterine AVM
To distinguish placental polyp from uterine AVM, the measurement of serum level of hCG is crucial. In the presence of placental polyp, serum hCG levels are likely to be above the assay’s detectability threshold, whereas the hCG titer is usually negative in patients with uterine AVM.6 Generally, serum hCG levels are elevated during gestation, ranging from 27,300 to 233,000 mIU/mL at 8–11 weeks of gestation15,16 and following delivery, they gradually decrease to the levels seen in non-pregnant women (0–5 mIU/mL) within 4 weeks postpartum.17–22 Thus, within 4 weeks postpartum, a relatively high level of serum hCG is not a critical determinant of a placental polyp. In our first case diagnosed with placental polyp, the serum level of hCG was 3.68 mIU/mL at 30 days after delivery; therefore, the value was not very informative with respect to the diagnosis.
Color Doppler ultrasonography (USG), CT, and MRI are sometimes helpful for the diagnosis as well as the selection of the treatment. On gray-scale USG, a placental polyp is usually visualized as a hypo- or anechoic tubular structure in the uterine cavity. However, color Doppler USG can provide significant information about the presence of blood flow in placental polyp tissue as well as the size of the vascularized mass and arteries that supplies blood to the placental polyp.23 The findings of blood flow and the vascularized structures are very useful when selecting TCR as the treatment. TCR should primarily be selected for treating hypovascular polyps; it can be combined with UAE if the placental polyps exhibit vascularity. On the other hand, gray-scale USG commonly shows a heterogeneous myometrium with multiple hypo- or anechoic tubular structures in cases of uterine AVM. However, such myometrial structures are sometimes difficult to distinguish from a polypoid mass because of the enlargement of the myometrial structures. In uterine AVM, color Doppler USG also reveals various abnormalities that cannot be differentiated from the findings of placental polyps.24
CT angiography can reduce the study time and radiation dose and is a useful technique for preoperative anatomical conceptualization for the gynecologist. Three-dimensional CT angiography can identify feeding arteries in placental polyps or visualize the afferent artery–nidus–efferent artery component in uterine AVM.25 On MRI, most placental polyps are visualized as high-intensity lesions in T2-weighted images and low-intensity lesions in T1-weighted images; in contrast-enhanced MRI, a flow void can be observed in the fundus of the polyp, implying the existence of rapid blood flow.26 On the other hand, uterine AVM is basically detected as a vascular tumor expanding and meandering within the myometrium. However, MRI and MR angiography are expensive, with longer acquisition times compared with CT angiography.25 Therefore, the combined use of TV-USG (alone or with color Doppler) and CT angiography might be suitable for reaching the appropriate differential diagnosis in patients with suspected placental polyp or uterine AVM who have active vaginal bleeding. Enhanced MRI would be useful for identifying the feeding artery, especially in patients without active bleeding.
Thus, no simple, distinguishing finding or characteristic is present for aiding the diagnosis of both these diseases. The combined use of USG, CT angiography, and MRI along with the results of serum hCG levels is critical for reaching the final diagnosis.
How to manage placental polyp and uterine AVM
In the case of placental polyp or uterine AVM, the first medical management should be chosen according to the severity of the vaginal bleeding. In cases with continuous and/or massive vaginal hemorrhage, rapid medical intervention is required. TAH is a curative treatment;5 however, uterine arterial embolization (UAE) should also be considered as the intervention of choice, especially for patients desirous of having future pregnancies. It should be noted that after UAE, there is a possibility of infertility due to decreased ovarian function.27 Furthermore, it is important to inform patients of the pregnancy-associated complications after UAE. Indeed, previous reports have demonstrated increased rates of abortion and premature birth following UAE28 along with higher rates of fetal malpresentation and bleeding during delivery.29 Moreover, the occurrence of post-embolization syndrome, with symptoms such as pelvic pain and fever, has also been reported.30,31 If continuous vaginal bleeding remains uncontrollable even after UAE, TAH should be performed promptly to avoid fatal blood loss.
If the vaginal bleeding is not life threatening, reaching the diagnosis of placental polyp or uterine AVM is important for ensuring optimal treatment. As mentioned above, a placental polyp is usually accompanied by persistent elevation of serum hCG levels above the non-pregnant level (5 mIU/mL) even after 4 weeks postpartum.17–22 However, we should note that the rate of decrease in postpartum serum hCG levels varies widely, and these levels are not always key to the diagnosis, especially among patients in the early postpartum period. In addition, in patients with extremely high serum hCG levels, gestational trophoblastic disease (GTD) should be suspected. It is known that the presence of total hydatidiform mole accounts for 20% of GTD.32 Serum levels of hCG are also known to be high in patients with placental site trophoblastic tumor (PSTT).
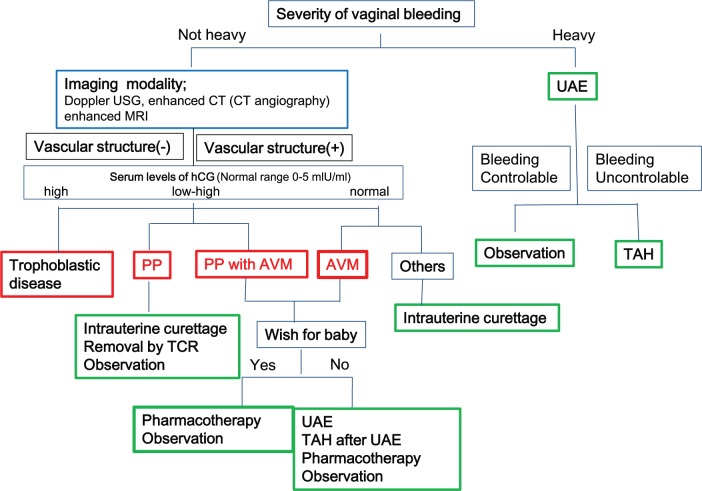
Based on the information from the literature as well as our experience, we propose a novel algorithm to differentiate and treat these two similar diseases that require specific treatments (Figure 7). The presence of active or life-threatening bleeding is the key determinant of prompt intervention for hemostasis. UAE can be the first choice of treatment, followed by observation in cases where bleeding is controlled or hysterectomy in cases with uncontrollable bleeding. The lack of active or life-threatening bleeding provides an opportunity to measure serum hCG levels and to attempt imaging studies, such as color Doppler USG, enhanced CT including CT angiography, and enhanced MRI. Since the test results for serum hCG values typically require several days, imaging studies are more valuable for establishing the initial diagnosis. If there is no vascular formation in the suspected placental polyp, the removal of the uterine mass by TCR or curettage is recommended. In contrast, if there is any vascular structure in the lesion, UAE should precede TCR to avoid unexpected bleeding. In a patient with a suspected AVM or a placental polyp associated with AVM, TCR cannot be recommended due to bleeding from vessels following this treatment. Furthermore, if the patient does not desire further pregnancy, UAE followed by hysterectomy can be considered to avoid bleeding. In contrast to these medical interventions for placental polyp or AVM, follow-up by simple observation is another potential choice of management in patients with few or no symptoms. Previous studies have reported that both placental polyp and uterine AVM can disappear without any medical intervention.24,33,34 In fact, in both the cases presented here, the patients recovered without any intervention. Although obstetricians and gynecologists often prefer to select intense or active interventions in cases with uterine bleeding, it is important to note that the “wait and see” option is a potent treatment choice. A number of drugs, such as methylergonovine maleate, danazol, gonadotropin-releasing hormone agonist, and estrogen/progesterone, have been reported to successfully contribute to the disappearance of AVM;12–14,35 thus, pharmacotherapy followed by observation represents a potential option. Furthermore, serum hCG levels are helpful for distinguishing between GTD and placental polyp, with the former generally showing extremely high titers while the latter exhibits various levels, depending on the postpartum period.
Figure 7.
Proposed algorithm for the diagnosis and management of placental polyp and AVM.
UAE: uterine artery embolization; TAH: total abdominal hysterectomy; PP: placental polyps; AVM: arteriovenous malformation; TCR: transcervical resection.
In summary, the presence of placental polyp or uterine AVM is common in postpartum patients presenting with unexpected vaginal bleeding. Since these disorders can be lethal, especially if there is massive bleeding, it is crucial to reach a prompt and accurate diagnosis and provide the appropriate medical interventions to the patient, for which our proposed algorithm is expected to be helpful.
Conclusion
We report here two cases that were suggestive of placental polyp and uterine AVM and discuss the differential diagnoses and treatments for both diseases based on a literature review. We also propose a novel algorithm for the management of such patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Cunningham FG. Williams obstetrics. 23rd ed. New York: McGraw-Hill, 2010, p. 648. [Google Scholar]
- 2. Swan RW, Woodruff JD. Retained products of conception. Histologic viability of placental polyps. Obstet Gynecol 1969; 34(4): 506–514. [PubMed] [Google Scholar]
- 3. Takeda A, Koyama K, Imoto S, et al. Placental polyp with prominent neovascularization. Fertil Steril 2010; 93(4): 1324–1326. [DOI] [PubMed] [Google Scholar]
- 4. Marques K, Looney C, Hayslip C, et al. Modern management of hypervascular placental polypoid mass following spontaneous abortion: a case report and literature review. Am J Obstet Gynecol 2011; 205(2): e9–e11. [DOI] [PubMed] [Google Scholar]
- 5. Fleming H, Ostor AG, Pickel H, et al. Arteriovenous malformations of the uterus. Obstet Gynecol 1989; 73(2): 209–214. [PubMed] [Google Scholar]
- 6. O’Brien P, Neyastani A, Buckley AR, et al. Uterine arteriovenous malformations: from diagnosis to treatment. J Ultrasound Med 2006; 25(11): 1387–1392; quiz 1394–1395. [DOI] [PubMed] [Google Scholar]
- 7. Markoff G, Quagliarello J, Rosen RJ, et al. Uterine arteriovenous malformation successfully embolized with a liquid polymer, isobutyl 2-cyanoacrylate. Am J Obstet Gynecol 1986; 155(3): 659–660. [DOI] [PubMed] [Google Scholar]
- 8. Palmaz JC, Newton TH, Reuter SR, et al. Particulate intraarterial embolization in pelvic arteriovenous malformations. Am J Roentgenol 1981; 137(1): 117–122. [DOI] [PubMed] [Google Scholar]
- 9. Mu YL, Liu M, Li Q, et al. Clinical value of transcervical resection under hysteroscope for placental remnants. Zhonghua Fu Chan Ke Za Zhi 2007; 42(8): 523–525. [PubMed] [Google Scholar]
- 10. Pelage JP, Fohlen A, Le Pennec V. Role of arterial embolization in the management of postpartum hemorrhage. J Gynecol Obstet Biol Reprod 2014; 43(10): 1063–1082. [DOI] [PubMed] [Google Scholar]
- 11. Yamamasu S, Nakai Y, Nishio J, et al. Conservative management of placental polyp with oral administration of methotrexate. Oncol Rep 2001; 8(5): 1031–1033. [DOI] [PubMed] [Google Scholar]
- 12. Oride A, Kanasaki H, Miyazaki K. Disappearance of a uterine arteriovenous malformation following long-term administration of oral norgestrel/ethinyl estradiol. J Obstet Gynaecol Res 2014; 40(6): 1807–1810. [DOI] [PubMed] [Google Scholar]
- 13. Takeuchi K, Yamada T, Iwasa M, et al. Successful medical treatment with danazol after failed embolization of uterine arteriovenous malformation. Obstet Gynecol 2003; 102(4): 843–844. [DOI] [PubMed] [Google Scholar]
- 14. Nonaka T, Yahata T, Kashima K, et al. Resolution of uterine arteriovenous malformation and successful pregnancy after treatment with a gonadotropin-releasing hormone agonist. Obstet Gynecol 2011; 117(2 Pt 2): 452–455. [DOI] [PubMed] [Google Scholar]
- 15. Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem 1997; 43(12): 2233–2243. [PubMed] [Google Scholar]
- 16. Batzer FR. Hormonal evaluation of early pregnancy. Fertil Steril 1980; 34(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 17. Aral K, Gurkan Zorlu C, Gokmen O. Plasma human chorionic gonadotropin levels after induced abortion. Adv Contracept 1996; 12(1): 11–14. [DOI] [PubMed] [Google Scholar]
- 18. Steier JA, Bergsjo P, Myking OL. Human chorionic gonadotropin in maternal plasma after induced abortion, spontaneous abortion, and removed ectopic pregnancy. Obstet Gynecol 1984; 64(3): 391–394. [PubMed] [Google Scholar]
- 19. Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol 2004; 104(1): 50–55. [DOI] [PubMed] [Google Scholar]
- 20. Korhonen J, Alfthan H, Ylostalo P, et al. Disappearance of human chorionic gonadotropin and its alpha- and beta-subunits after term pregnancy. Clin Chem 1997; 43(11): 2155–2163. [PubMed] [Google Scholar]
- 21. Marrs RP, Kletzky OA, Howard WF, et al. Disappearance of human chorionic gonadotropin and resumption of ovulation following abortion. Am J Obstet Gynecol 1979; 135(6): 731–736. [DOI] [PubMed] [Google Scholar]
- 22. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol 2004; 104(5 Pt 1): 975–981. [DOI] [PubMed] [Google Scholar]
- 23. Takeda A, Koyama K, Imoto S, et al. Computed tomographic angiography in diagnosis and management of placental polyp with neovascularization. Arch Gynecol Obstet 2010; 281(5): 823–828. [DOI] [PubMed] [Google Scholar]
- 24. Timmerman D, Wauters J, Van Calenbergh S, et al. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet Gynecol 2003; 21(6): 570–577. [DOI] [PubMed] [Google Scholar]
- 25. Aiyappan SK, Ranga U, Veeraiyan S. Doppler sonography and 3D CT angiography of acquired uterine arteriovenous malformations (AVMs): report of two cases. J Clin Diagn Res 2014; 8(2): 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki N, Suzuki M, Terada S, et al. Placental polyp: MR imaging findings. Am J Roentgenol 1995; 165(6): 1554. [DOI] [PubMed] [Google Scholar]
- 27. Sentilhes L, Gromez A, Clavier E, et al. Fertility and pregnancy following pelvic arterial embolisation for postpartum haemorrhage. BJOG 2010; 117(1): 84–93. [DOI] [PubMed] [Google Scholar]
- 28. Berkane N, Moutafoff-Borie C. Impact of previous uterine artery embolization on fertility. Curr Opin Obstet Gynecol 2010; 22(3): 242–247. [DOI] [PubMed] [Google Scholar]
- 29. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol 2002; 100(5 Pt 1): 869–872. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Chen J, Shi H, et al. Efficacy and safety of embolization in iatrogenic traumatic uterine vascular malformations. Clin Radiol 2012; 67(6): 541–545. [DOI] [PubMed] [Google Scholar]
- 31. Ghai S, Rajan DK, Asch MR, et al. Efficacy of embolization in traumatic uterine vascular malformations. J Vasc Interv Radiol 2003; 14(11): 1401–1408. [DOI] [PubMed] [Google Scholar]
- 32. Soper JT. Gestational trophoblastic disease. Obstet Gynecol 2006; 108(1): 176–187. [DOI] [PubMed] [Google Scholar]
- 33. Kayem G, Davy C, Goffinet F, et al. Conservative versus extirpative management in cases of placenta accreta. Obstet Gynecol 2004; 104(3): 531–536. [DOI] [PubMed] [Google Scholar]
- 34. Kitahara T, Sato Y, Kakui K, et al. Management of retained products of conception with marked vascularity. J Obstet Gynaecol Res 2011; 37(5): 458–464. [DOI] [PubMed] [Google Scholar]
- 35. Onoyama I, Fukuhara M, Okuma A, et al. Successful pregnancy after the noninvasive management of uterine arteriovenous malformation. Acta Obstet Gynecol Scand 2001; 80(12): 1148–1149. [DOI] [PubMed] [Google Scholar]