Abstract
With advances in science and technology, there are more innovations in the approach to management of patients with breast cancer. Neoadjuvant chemotherapy that is designed to be used prior to surgical removal of a tumor has received significant attention. Currently, neoadjuvant chemotherapy is offered to patients with locally advanced breast cancer and also those breast cancer patients who may benefit from size reduction before conservation therapy. There is now sufficient evidence that if neoadjuvant chemotherapy leads to complete pathologic response, the patient will enjoy a better outcome. Therefore, assessment of the degree of response to neoadjuvant chemotherapy has a major impact on patient selection and the follow-up management of each patient and defines patient outcome.
Keywords: breast cancer, breast pathology report, neoadjuvant chemotherapy
Introduction
Defined as the administration of systemic therapy prior to surgical removal of a breast tumor, neoadjuvant chemotherapy was originally designed to be used in patients with locally advanced disease in order to convert inoperable tumors into operable tumors. Since the introduction of this concept, the significance of neoadjuvant chemotherapy in increasing the rate of conservation therapy and the associated reduced morbidity and better self-image has been fully acknowledged. Meanwhile, there have been concerns about local control after downstaging of the tumor and the delay to surgery in patients resistant to neoadjuvant chemotherapy.1–7 However, the report on randomized clinical trial comparing the effectiveness of neoadjuvant chemotherapy with adjuvant chemotherapy in operable breast cancer patients has shown equivalent results. This report was based on 5500 eligible women for this analysis. It was also reported that neoadjuvant chemotherapy avoids mastectomy in 25% of the patients. In contrast, <5% of the patients who were originally candidates for conservation therapy required a mastectomy because of disease progression while receiving neoadjuvant chemotherapy.8 Neoadjuvant chemotherapy is now commonly used for earlier, operable breast cancer patients. Currently, neoadjuvant chemotherapy is used for locally advanced breast cancer, inflammatory breast cancer, and downstaging of large tumors to allow for breast conservation therapy.9 The indication for neoadjuvant chemotherapy is now extended to clinically node negative breast cancer patients with unfavorable tumor profiles, in whom adjuvant systemic therapy is predicted.10
There are several benefits to the use of neoadjuvant chemotherapy. It offers a unique opportunity for the evaluation of treatment response with complete pathologic response acting as a surrogate marker of survival and for a more rapid assessment of the efficacy of new therapeutic agents and early cessation of ineffective treatment. In addition, in case of a resistance to therapy, adjusting the dose and/or change to another drug saves patients from the burden of toxicity and side effects. Furthermore, neoadjuvant chemotherapy provides an opportunity for individualized therapy and allows collection of tumor samples before, during, and after treatment for conducting translational research.11,12 This assessment of tumor behavior in situ during neoadjuvant chemotherapy and its correlation with clinical outcome is an excellent model to determine the predictive role of tumor characteristics. The ultimate goal of translational research associated with neoadjuvant chemotherapy is the introduction of individually tailored treatment strategies based on an individual risk profile.13 This review article is designed to highlight the predictors of response to neoadjuvant chemotherapy and provides suggestions for appropriate pathology reporting that has critical implications for patient care.
Predictors of response to neoadjuvant chemotherapy
Response to neoadjuvant chemotherapy is evaluated by the change in tumor size from pretreatment clinical and/or radiologic measurement to post-treatment status. The spectrum of response to neoadjuvant chemotherapy varies from complete response, partial response, to non-response. This concept is the same in breast tumors as well as axillary lymph nodes. Studies have shown that the rate of response to therapy varies from 15% to 30% depending on the type of tumor and the type of chemotherapy used.11,14–16 Patients who achieve complete response to neoadjuvant chemotherapy experience better outcomes, that is, long-term disease-free survival, and better overall survival when compared to those patients whose tumors do not respond to therapy.17
Definition of complete pathologic response as a surrogate endpoint predictor of long-term clinical benefit has remained variable. The three most commonly used definitions are absence of invasive cancer and in situ cancer in the breast, and axillary lymph nodes (ypTO ypNO), irrespective of ductal carcinoma in situ (ypTO/is ypNO), and absence of invasive cancer in the breast irrespective of ductal carcinoma in situ or lymph node involvement (ypTO/is). Obtaining data from 12 identified international trials with 11,955 patients, Cortazar et al.18 studied the relationship between pathological complete response and the patient outcome. The objective was to establish the definition of pathological complete response and identify breast cancer subtypes that are considered as best responders to neoadjuvant chemotherapy. The authors in this analysis reported that patients who showed pathologic complete response defined as ypTO ypNO or ypTO/is ypNO experienced better survival. This impact was more conspicuous in aggressive tumor subtypes such as triple-negative breast cancer and those with human epidermal growth factor receptor-2 (HER2) positive and hormone receptor negative tumors. However, they proposed that pathological complete response is a more stringent definition. As previously reported, the presence or absence of ductal carcinoma in situ in patients with complete absence of invasive carcinoma in the breast and lymph nodes did not adversely affect survival.19,20 This finding, however, is challenged by a German pooled analysis21 of seven neoadjuvant trials that had shown a patient without ductal carcinoma in situ has better survival compared to those patients with residual ductal carcinoma in situ with no residual invasive carcinoma in the breast.
Small tumor size, high tumor grade, high proliferation rate, tumor necrosis, and presence of tumor-associated lymphocytes are considered predictors of better response to neoadjuvant chemotherapy. In addition, patients with hormone receptor negative, HER2/neu oncogene positive tumors, and those with triple-negative breast cancer experience more favorable response to therapy.22,23
Predictors of non-response to neoadjuvant chemotherapy
Using gene expression profiling, it is also possible to predict partial or no response to therapy and avoid unnecessary chemotherapy. Recent studies using BluePrint and MammaPrint have shown that molecular subtyping may better identify patients who may not benefit from neoadjuvant chemotherapy. Gene profiling enables subdivision of luminal group into two types: luminal A and luminal B. Luminal A type tumors have an excellent prognosis and do not benefit from neoadjuvant chemotherapy24–26 (Figures 1 and 2). However, luminal B tumors often present a challenge in therapy. As they are more aggressive than luminal A types, they are generally treated by both endocrine therapy and chemotherapy, often with no effect. Recognizing that luminal B/HER2 tumors do not respond to chemotherapy provides an opportunity to plan a more effective endocrine therapy.27 To identify these patients who will not benefit from neoadjuvant chemotherapy, Balmativola et al. in 2014 reported their experience among a cohort of 490 cases showing pathological complete response and partial response compared with the group of non-responders. They confirmed the results of other studies that estrogen and progesterone positivity was associated with a lack of response to neoadjuvant chemotherapy.28 In addition, they demonstrated that lobular subtype of breast cancer and the absence of tumor-infiltrating lymphocytes were more significantly common in patients with no response to neoadjuvant chemotherapy. However, using multivariable analysis showed that the expression of estrogen report or progesterone receptors was independent variables for discriminating the no response category from those with complete and partial response. This observation may support the previously suggested idea by Delpich et al.29 that no response to neoadjuvant chemotherapy may be more related to intrinsic tumor characteristics than the expression of estrogen receptor. The authors also identified the cutoff values of 9 for mitosis and 18% for Ki-67 in differentiation between no responders versus partial and complete responders to the neoadjuvant chemotherapy.28
Figure 1.
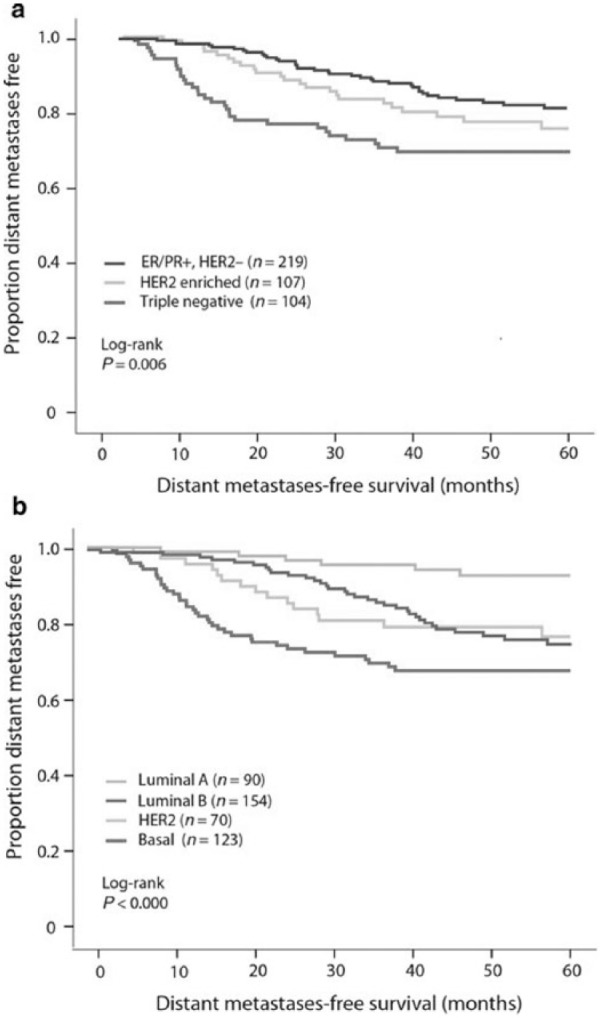
Survival rates according to stratification based on (a) IHC/FISH for ER, PR, and HER2 and (b) molecular subtyping using BluePrint and MammaPrint.
Source: Reprinted with permission from Gluck et al.24
IHC: immunohistochemistry; FISH: fluorescence in situ hybridization; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor-2.
Figure 2.
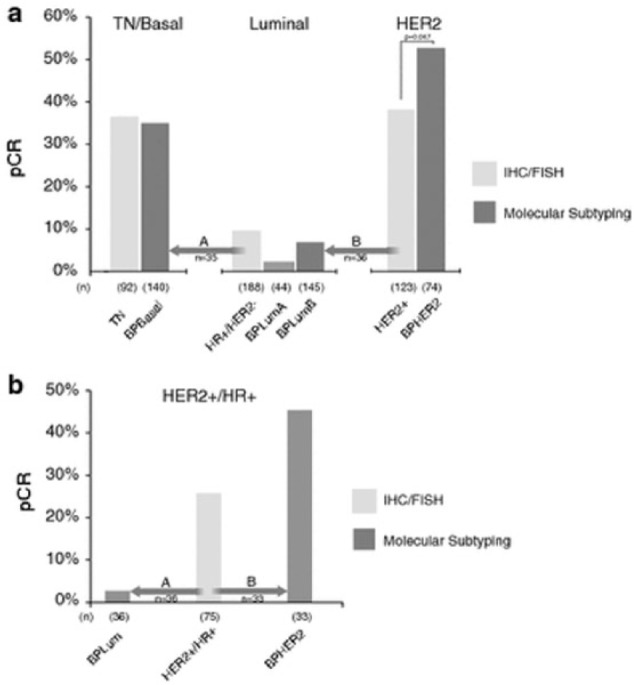
(a) pCR rates and major subtype reassignments for patients classified by BluePrint/MammaPrint molecular subtyping compared with IHC/FISH assessed subgroups. The analysis includes only patients treated with NCT (n = 403). The two major subtype reassignments were (A) conventional luminal (HR+/HER2−) patients, 35 of 188 (19%) patients reclassified by BluePrint as basal (arrow A) and (B) conventional HER2+ patients, 36 of 123 (29%) reclassified by BluePrint as luminal (arrow B). (b) pCR rates and major subtype reassignments for conventional HER2+/HR+ patients (“triple positive”) patients (95% treated with NCT/trastuzumab). A total of 36 of 75 (48%) of conventional HER2+/HR+ patients were reclassified by BluePrint as luminal—with only 1 pCR (3%) to NCT (arrow A). A total of 33 of 75 (44%) of conventional HER2+/HR+ patients were classified by BluePrint has HER2, with a pCR rate to NCT of 45% (arrow B). A total of six conventional HER2+/HR+ patients were reassigned to BPBasal (not shown).
Source: Reprinted with permission from Whitworth et al.25
Procedures required prior to neoadjuvant chemotherapy
In order to be able to accurately assess the status of the response to therapy, it is critically important to have a definitive diagnosis of breast cancer and obtain information about tumor type, tumor grade, presence or absence of necrosis, and/or lymphatic and vascular invasion.30 This information is helpful in making decisions about the initiation of neoadjuvant chemotherapy and selection of medication. A clip should be placed at the time of initial tissue sampling or during the first few cycles of neoadjuvant chemotherapy. This will make it possible to reliably identify the tumor bed after therapy. Access to sufficient tumor tissue is also required to be able to assess the status of biomarkers such as hormone receptors and HER2/neu oncogene. In addition, the status of axillary lymph nodes has to be known, clinically and by imaging, prior to neoadjuvant chemotherapy. Clinically positive axillary lymph nodes should be sampled by minimally invasive procedures such as fine needle aspiration biopsy and/or core needle biopsy. Clinically negative axillary lymph nodes should be sampled by sentinel lymph node biopsy.31–33
Evaluation of response to neoadjuvant chemotherapy
Response to neoadjuvant chemotherapy can be assessed by clinical examination, breast imaging studies, and, ultimately, pathologic examination of post-treatment specimen. Clinical examination is performed by assessment of the size of the tumor by palpation. This task is more difficult in tumors that have responded to therapy, as it is challenging to palpate the real tumor versus the treatment-induced changes.34 Breast imaging modalities such as mammography and ultrasound are not considered adequate for quantitative assessment of change in tumor size. However, more recent devices such as magnetic resonance imaging (MRI) and positron emission tomography (PET) have been shown to provide a better assessment of tumor response to neoadjuvant chemotherapy and better predict response to therapy.35–38 The frequency and nature of monitoring for the effects of neoadjuvant chemotherapy by imaging has remained controversial. There are individuals who believe that imaging should be repeated after completion of neoadjuvant chemotherapy before surgery to assess residual disease and plan surgical procedures. Imaging may be repeated in a timely fashion in order to document tumor response or disease progression. This assessment is critically important to minimize the toxicity to the patient and delay in surgical therapy in those patients who are not responding to neoadjuvant chemotherapy. When repeat imaging is used, the same modality for breast imaging should be used as the original baseline.39
Neoadjuvant chemotherapy induces changes in morphology of the tumor. Based on the degree of response, the distribution of residual tumor and the degree of treatment effect, morphologic features are different. These features need to be recognized and be included in the pathology report. The pathology report forms the foundation upon which the follow-up management of a breast cancer patient is planned.11,32,40
Pathology of response to neoadjuvant chemotherapy
Gross examination
Access to the information about the size and the location of the tumor prior to therapy is critical. Specimen radiography will assist in identification of clip or microcalcification. Associated with complete response to therapy, it is not possible to identify any grossly visible lesions. Identification of tumor bed and surrounding tissue is helpful in selecting the right area for tissue sectioning. Therefore, efforts should be made to evaluate the surgical margins since the tumor bed is usually seen as a non-descriptive area of irregular rubbery fibrous tissue that may also contain ill-defined areas of residual tumor. The size of the tumor bed should also be documented. Margin assessment is important in case residual tumor is later found by microscopic examination.32
Microscopic evaluation
Microscopically, it is important to find the previous biopsy changes characterized by stromal fibrosis, macrophages, lymphocytes, and, occasionally, multinucleated giant cell reaction. In the absence of this finding, the possibility of a sampling error in lumpectomy specimen should be highly considered. Similarly, recognition of the tumor bed is essential. Tumor bed is characterized by stromal fibrosis, necrosis, chronic inflammatory infiltrate, calcifications, and histiocytes11 (Figure 3).
Figure 3.

Photomicrograph of tumor bed with no residual tumor characterized by stromal fibrosis and chronic inflammatory infiltrate (H&E stain ×200).
The spectrum of changes after therapy is defined as complete response (no residual tumor identified; Figure 4), partial response (residual tumor seen singly or in clusters; Figure 5), and non-response (no change identified; Figure 6). Occasionally, it is difficult to distinguish between reactive/inflammatory atypia and residual tumor cells. Changes in non-neoplastic breast tissue include cytoplasmic and nuclear enlargement and sclerosis of basement membranes that may mimic residual tumor. In these circumstances, immunostaining for epithelial markers such as cytokeratin AE-1/AE-3 or CK7 and CD68 for histiocytes and myoepthelial markers are appropriate diagnostic adjuncts.11
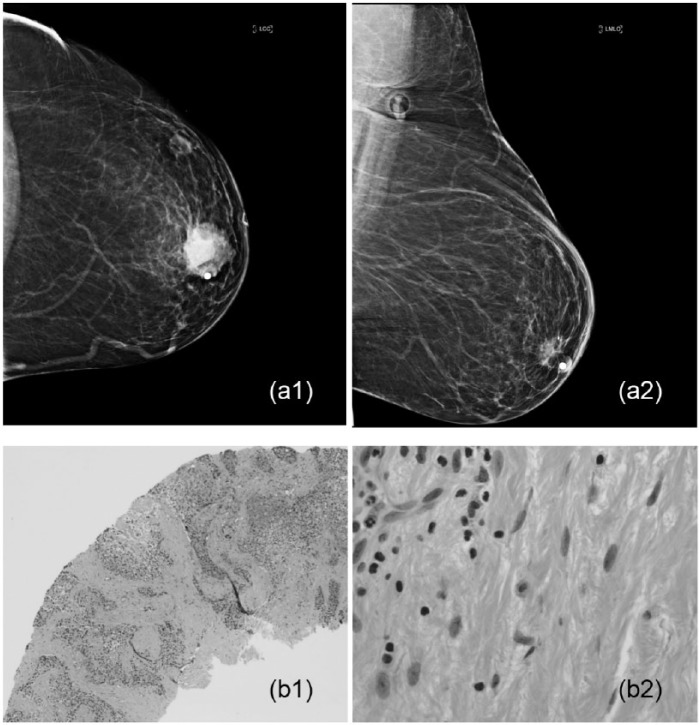
Figure 4.
An example of complete pathologic response in a 52-year-old woman with palpable mass who underwent neoadjuvant chemotherapy: (a1) the tumor measured 4.1 × 3.6 × 3.2 cm3 by breast imaging, (a2) the tumor disappeared after therapy, (b1) poorly differentiated infiltrating ductal carcinoma on core needle biopsy, and (b2) there is no residual tumor seen on post-neoadjuvant chemotherapy lumpectomy sample (H&E stain ×200 and ×400).
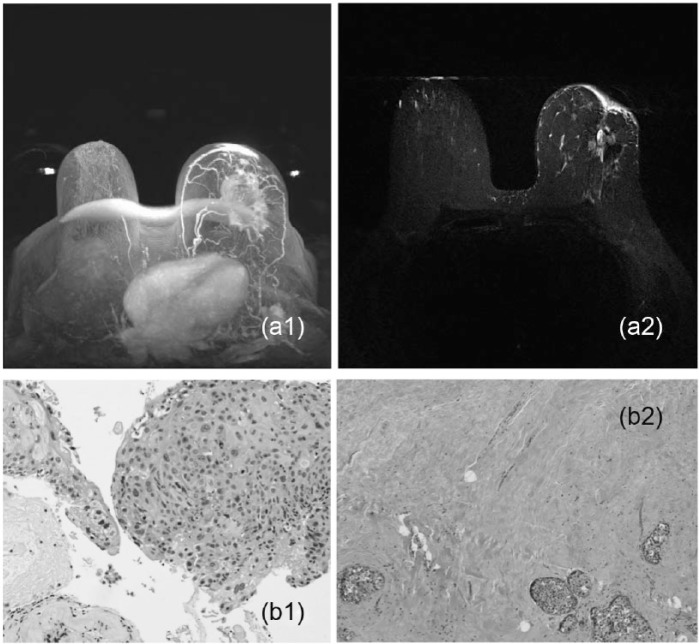
Figure 5.
(a1) An example of partial response in a 58-year-old woman with a palpable breast lesion presenting with an enhancing mass in the left breast by imaging measuring 7.9 × 5.6 × 4.7 cm3 who underwent neoadjuvant chemotherapy. (a2) Partial response is seen by a difference in the size of the lesion. (b1) This case was diagnosed as a poorly differentiated infiltrating ductal cell carcinoma (H&E stain ×400). (b2) Residual tumor cells are seen characterized by a few clusters of tumor cells in the background of fibrosis in this case (H&E stain ×200).
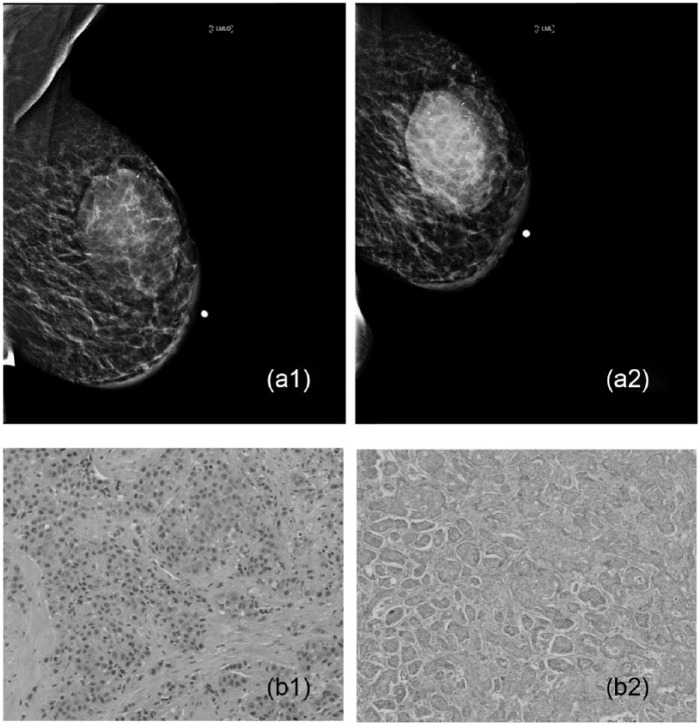
Figure 6.
An example of no response with progressive disease in a 59-year-old woman with a 5-cm palpable mass seen on breast imaging who underwent neoadjuvant chemotherapy. (a1 and a2) Please note the progression of this tumor and (b1 and b2) in comparison of core needle biopsy findings versus lumpectomy sample diagnosed as poorly differentiated infiltrating ductal cell carcinoma (H&E stain ×200 and ×400).
Complete pathologic response involves the disappearance of all invasive carcinoma in the breast and axillary lymph nodes after completion of therapy. Residual ductal carcinoma in situ may also be present; however, this finding does not alter survival. It appears that ductal carcinoma in situ and vascular tumor emboli are relatively resistant to neoadjuvant chemotherapy compared to the associated invasive carcinoma.40 Assessment of post-treatment lymph nodes involves recognition of pronounced lymphoid depletion, atrophy, and fibrosis where there is evidence of complete response to therapy. Residual tumors may be better identified by immunostaining for cytokeratin11 (Figures 7 and 8).
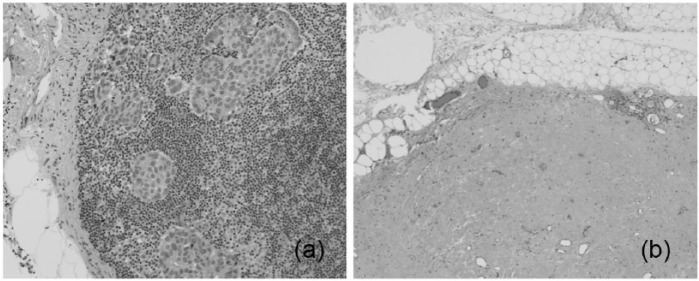
Figure 7.
(a) An example of complete response to neoadjuvant chemotherapy in a lymph node positive breast cancer patient with metastatic tumor seen in H&E staining of sentinel lymph node and (b) please note the disappearance of tumor cells in the same lymph node after neoadjuvant chemotherapy (H&E stain ×400).
Figure 8.
(a) An example of a HER2/neu oncogene positive breast cancer patient who underwent neoadjuvant chemotherapy and (b) please note the change to HER2/neu oncogene negative status after therapy (HER2/neu gene amplification by FISH technology).
Treatment effect
Tumor size and tumor cellularity
The pretreatment clinical stage and post-treatment pathologic stage are the determinant of the progression of breast cancer patients undergoing neoadjuvant chemotherapy. Tumor size is easy to measure if there is no or minimal response to therapy. This assessment becomes challenging as tissue response to therapy makes the measurement of the actual isolated and clusters of residual tumor difficult. The literature indicates that these patients who experience complete disappearance of their tumor following neoadjuvant chemotherapy enjoy a better outcome.41,42 Overall, it appears that smaller tumor size represents a good prognostic factor, and residual tumors of >2 cm are associated with higher rates of locoregional tumor recurrence after neoadjuvant chemotherapy.23 Tumor cellularity can also be used as a measure of response to therapy. However, this assessment may be complicated by the presence of associated chemotherapy-induced tissue reaction resulting in overestimation of cellularity. Assessment of tumor cellularity requires access to tumor tissue prior to chemotherapy. It is critical to describe tumor cellularity and type of stroma (sclerotic vs edematous) in biopsy samples prior to neoadjuvant chemotherapy in order to compare these findings in post-chemotherapy samples. It may be a good idea to secure microscopic images of pre-neoadjuvant chemotherapy biopsy for better assessment of tumor cellularity.30,43 Loss of tumor cellularity correlates with better clinical outcome.34,44
Tumor grade and tumor type
The grade of tumor may change to be higher or lower grade as the result of neoadjuvant chemotherapy. However, ultimately, the pretreatment tumor grade remains to be an independent prognostic factor.45 The selection of neoadjuvant chemotherapy and its impact on breast cancer outcome mainly depend on molecular subtypes, the degree of proliferation rate, and various biomarkers.
It is clear that low-grade invasive breast carcinomas such as tubular carcinoma, lobular carcinomas, invasive cribriform carcinoma, papillary carcinoma, and mucinous carcinoma with low proliferation rates will not benefit from neoadjuvant chemotherapy and are not commonly a candidate for this option. In contrast, poorly differentiated high-grade carcinomas, basal cell type, and triple-negative breast cancer patients with high proliferation rate often are responsive to neoadjuvant chemotherapy. Naturally, neoadjuvant chemotherapy has no effect to change the breast subtypes.46,47
Lymph nodes
The status of lymph nodes after therapy is the most important prognostic factor. This can be achieved clinically and by imaging. Performing sentinel lymph node biopsy after neoadjuvant chemotherapy for patients with clinically negative axilla at the time of diagnosis is an acceptable approach. As it has already been mentioned, clinically or radiologically suspicious lymph nodes should undergo minimally invasive sampling procedures such as ultrasound-guided fine needle aspiration biopsy prior to neoadjuvant chemotherapy.30 Patients with negative results are then candidates for sentinel lymph node biopsy prior to chemotherapy by histopathology and can be spared from axillary dissection after neoadjuvant chemotherapy.48 Patients with residual tumor in lymph nodes have worse prognosis compared to patients who have no residual tumor in lymph nodes. This underscores the necessity of finding residual tumor cells in lymph nodes of patients who undergo neoadjuvant chemotherapy that may require immunostaining for epithelial matter. The evidence of treatment effects in lymph nodes with residual metastasis is associated with a better prognosis.49,50
Prognostic factors
Patients who achieve pathologic complete response experience better outcome regardless of tumor subtypes. As previously stated, patients with negative hormone receptors and positive HER2/neu oncogene and those with triple-negative tumors often respond to neoadjuvant chemotherapy. However, patients with HER2/neu oncogene positive and triple-negative breast cancer who fail to respond to therapy have a worse outcome compared to patients with hormone receptor positive tumors.22,51 Studies have shown that there is 8%–33% discrepancy between pre- and post-treatment status of hormone receptors. The discrepancy about HER2/neu oncogene is up to 32% (Figure 9). The stated reasons for these discrepancies are related to tissue processing and fixation, laboratory errors, tumor heterogeneity, and change in biology of the tumor.52,53
Figure 9.
(a) An example of a breast cancer patient with a tumor presenting with a high proliferation rate, as evidenced by brown nuclear staining of a majority of tumor cells, who underwent neoadjuvant chemotherapy and (b) please note the decrease in the number of proliferating cells after therapy (Ki-67, immunostain 200 × 400).
The predictive significance of change in tumor biomarkers following neoadjuvant chemotherapy is not yet known. There is, however, a suggestion that when there is a change from hormone receptor negativity to a positive reaction, this may indicate a better outcome.54 Similarly, among HER2/neu oncogene positive patients who were treated with neoadjuvant trastuzumab, a reduction in the expression of HER2/neu oncogene was associated with poor recurrence-free survival.22 Currently, the change in the rate of Ki-67 as a proliferation marker is regarded as a marker for response to neoadjuvant chemotherapy, particularly in patients with hormone receptor positive tumors who receive endocrine therapy55–57 (Figure 10).
Figure 10.
(a) An example of a lymph node of a 64-year-old woman with an infiltrating ductal cell carcinoma who underwent neoadjuvant chemotherapy. The routine staining of the lymph shows a small area of suspicion for residual tumor. Please see circled portion (H&E stain ×200). (b) The presence of isolated and clusters of residual tumor cells are seen in the same lymph node upon immunostaining for cytokeratin. Please note the brown membrane staining of residual tumor cells (immunostained slide ×200).
Pathologic reporting
Pathologic evaluation of tumor response to neoadjuvant chemotherapy is of critical importance in the treatment planning and prognosis of patients who undergo this therapy. Pathology report must include assessment of response to tumor, size of the tumor bed, size and extent of residual tumor, tumor cellularity compared to primary tumor and tumor grading. In addition, the information about the viability of the tumor cells measured by Ki-67 immunostaining, presence of mitosis, presence of necrosis, lymphovascular invasion, presence of ductal carcinoma in situ, and the status of margin with respect to tumor bed are important factors to include in pathology reporting. Inclusion of prefix pT for pathologic staging is also required. There is no doubt that the absence of tumor bed and previous biopsy changes must urge for further evaluation and possible re-excision of the missed tumor in these patients. Pathology reports must also include the number of lymph nodes, number of lymph nodes with metastasis and the size of the largest deposit, the status of tumor response, and the presence or absence of extra-nodal involvement. Despite the emphasis on the significance of pathology reporting, there are still reports of variability among pathologists. The areas of discrepancies are laterality, grading of tumor, the status of lymph node metastasis, and response to therapy. Measures should be taken to standardize pathology reporting and monitor the adherence to already established guidelines in breast pathology.58–63
Future perspective
Breast cancer has remained a heterogeneous disease that presents with several different clinical presentations, morphologic features, and molecular characteristics. Breast cancer is not a single disease, and every tumor is characterized by different sizes and types, lymph node status, hormone receptor status, and HER2/neu oncogene. It occurs and behaves differently among different ethnic groups. This heterogeneity most likely is the reflection of several genetic pathways that eventually determine the type and biology of breast cancer. With advances in science and technology, it is anticipated that more progress will be made in understanding of the biology of this devastating disease.
There is no doubt that the global interest in betterment of the lives of breast cancer patients continues to lead into the discovery of more molecular-targeted therapies that influence the survival of patients with this disease. There is now sufficient evidence to recognize the merits of neoadjuvant chemotherapy for selected group of breast cancer patients. As there are still breast cancers that do not respond to the currently available chemotherapeutic agents, more efforts will be placed to better characterize the molecular makeup of each individual breast cancer. This information is the appropriate pathway for drug discoveries, so that there will be more possibility of offering patients personalized breast cancer care. It is hoped that these efforts make an impact on the mortality from breast cancer across the globe.
Summary
Currently, neoadjuvant chemotherapy has become a major trend in breast cancer care. Assessment of the response to neoadjuvant chemotherapy requires an established integrated multidisciplinary care among pathologists, radiologists, surgeons, and oncologists. In order to provide an appropriate reporting of pathologic response to therapy, access to diagnostic and prognostic/predictive information of breast tumors and sentinel lymph nodes prior to neoadjuvant chemotherapy is essential. Responsibilities of a pathologist include proper handling and processing of post-treatment surgical specimens, such as identification of tumor bed for assessment of response to therapy; comprehensive sampling of tumor bed; measurement of the size of the residual tumor; and determination of the extent of the residual tumor, cellularity, and treatment effects in both breast and lymph nodes. Immunostains for epithelial markers and markers for macrophages may be used for assessment of the presence or absence of residual tumor. Ki-67 may be used to grade the degree of response to neoadjuvant chemotherapy. The status of hormone receptors HER2/neu oncogene may change as the result of neoadjuvant chemotherapy. It is critically important to adhere to the established guidelines for accurate reporting of the impact of neoadjuvant chemotherapy for proper management of breast cancer patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999; 17(2): 460–469. [DOI] [PubMed] [Google Scholar]
- 2. Smith IE, Lipton L. Preoperative/neoadjuvant medical therapy for early breast cancer. Lancet Oncol 2001; 2: 561–570. [DOI] [PubMed] [Google Scholar]
- 3. Kaufmann M, Von Minckwitz G, Smith R, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol 2003; 21(13): 2600–2608. [DOI] [PubMed] [Google Scholar]
- 4. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005; 97: 188–194. [DOI] [PubMed] [Google Scholar]
- 5. Mieog J, Van der Hage JA, Van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007; 2: CD005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu SV, Melstrom L, Yao K, et al. Neoadjuvant therapy for breast cancer. J Surg Oncol 2010; 101: 283–291. [DOI] [PubMed] [Google Scholar]
- 7. Shin HC, Han W, Moon HG, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol 2013; 20: 2582–2589. [DOI] [PubMed] [Google Scholar]
- 8. Mieog JS, Van der Hage JA, Van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007; 94(10): 1189–1200. [DOI] [PubMed] [Google Scholar]
- 9. Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005; 11: 5678–5685. [DOI] [PubMed] [Google Scholar]
- 10. Vugts G, Maaskant-Braat AJG, Nieuwenhuijzen GAP, et al. Patterns of care in the administration of neo-adjuvant chemotherapy for breast cancer. A population based study. Breast J 2016; 22(3): 316–321. [DOI] [PubMed] [Google Scholar]
- 11. Sahoo S, Dabbs DJ, Bhargava R. Pathology of neoadjuvant response of breast carcinoma. In: Dabbs DJ. (ed.) Breast pathology (vol. 1). Philadelphia, PA: Elsevier Saunders, 2012, pp. 519–535. [Google Scholar]
- 12. Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002; 95: 681–695. [DOI] [PubMed] [Google Scholar]
- 13. Precht IM, Lowe KA, Atwood M, et al. Neoadjuvant chemotherapy of breast cancer: tumor markers as predictors of pathologic response, recurrence, and survival. Breast J 2010; 16: 362–368. [DOI] [PubMed] [Google Scholar]
- 14. Dawood S, Ueno NT, Valero V, et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer 2011; 117(9): 1819–1826. [DOI] [PubMed] [Google Scholar]
- 15. Van Deurzen CH, Vriens BE, Tjan-Heijnen VC, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer 2009; 45: 3124–3130. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz GF, Tannenbaum JE, Jernigan AM, et al. Axillary sentinel lymph node biopsy after neoadjuvant chemotherapy for carcinoma of the breast. Cancer 2010; 116: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 17. Kaufmann M, Von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 2007; 18(12): 1927–1934. [DOI] [PubMed] [Google Scholar]
- 18. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 19. Feldman LD, Hortobagyi GN, Buzdar AU, et al. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res 1986; 46: 2578–2581. [PubMed] [Google Scholar]
- 20. Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 2007; 25: 2650–2655. [DOI] [PubMed] [Google Scholar]
- 21. Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 22. Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 Trial (CALGB 150007/150012). Breast Cancer Res Treat 2012; 132: 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Von Minckwitz G, Untch M, Muesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 2011; 125(1): 145–156. [DOI] [PubMed] [Google Scholar]
- 24. Gluck S, de Snoo F, Stork-Sloots L, et al. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat 139(3): 759–767. 2013. [DOI] [PubMed] [Google Scholar]
- 25. Whitworth P, Stork-Sloots L, De Snoo FA, et al. Chemo sensitivity predicted by bluePrint 80-gene functional subtype and MammaPrint in the prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol 2014; 21(10): 3261–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romero A, Prat A, Garcia-Saenz JA, et al. Assignment of tumor subtype by genomic testing and pathologic-based approximations: implications on patient’s management and therapy selection. Clin Transl Oncol 2012; 16(4): 386–394. [DOI] [PubMed] [Google Scholar]
- 27. Turner N, Biganzoli L, Malorni L, et al. Adjuvant chemotherapy: which patient? What regimen? Am Soc Clin Oncol Educ Book 2013; 4: 3–8. [DOI] [PubMed] [Google Scholar]
- 28. Balmativola D, Marchio C, Maule M, et al. Pathological non-response to chemotherapy in a neoadjuvant setting of breast cancer: an inter-institutional study. Breast Cancer Res Treat 2014; 148: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delpech Y, Coutant C, Hsu L, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer 2013; 108(2): 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchio C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr 2011; 43: 86–90. [DOI] [PubMed] [Google Scholar]
- 31. Pu RT, Schott AF, Sturtz DE, et al. Pathologic features of breast cancer associated with complete response to neoadjuvant chemotherapy: importance of tumor necrosis. Am J Surg Pathol 2005; 29(3): 354–358. [DOI] [PubMed] [Google Scholar]
- 32. Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med 2009; 133(4): 633–642. [DOI] [PubMed] [Google Scholar]
- 33. Cox CE, Cox JM, White LB, et al. Sentinel node biopsy before neoadjuvant chemotherapy for determining axillary status and treatment prognosis in locally advanced breast cancer. Ann Surg Oncol 2006; 13(4): 483–490. [DOI] [PubMed] [Google Scholar]
- 34. Pierga JY, Mouret E, Laurence V, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. The role of clinical response. Eur J Cancer 2003; 39(8): 1089–1096. [DOI] [PubMed] [Google Scholar]
- 35. Keune JD, Jeffe DB, Schootman M, et al. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg 2010; 199: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184(6): 1774–1781. [DOI] [PubMed] [Google Scholar]
- 37. Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol 2004; 14: 1371–1379. [DOI] [PubMed] [Google Scholar]
- 38. Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer 2008; 112(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 39. Partridge SC, Vanantwerp RK, Doot RK, et al. Association between serial dynamic contrast-enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. J Magn Reson Imaging 2010; 32(5): 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharkey FE, Addington SL, Fowler LJ, et al. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol 1996; 9(9): 893–900. [PubMed] [Google Scholar]
- 41. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008; 26: 778–785. [DOI] [PubMed] [Google Scholar]
- 42. Cleator SJ, Makris A, Ashley SE, et al. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol 2005; 16(2): 267–272. [DOI] [PubMed] [Google Scholar]
- 43. Marchio C, Maletta F, Annaratone L, et al. The perfect pathology report after neoadjuvant therapy. J Natl Cancer Inst Monogr 2015; 2015(51): 47–50. [DOI] [PubMed] [Google Scholar]
- 44. Rajan R, Poniecka A, Smith TL, et al. Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer 2004; 100(7): 1365–1373. [DOI] [PubMed] [Google Scholar]
- 45. Schneeweiss A, Katretchko J, Sinn HP, et al. Only grading has independent impact on breast cancer survival after adjustment for pathological response to preoperative chemotherapy. Anticancer Drugs 2004; 15(2): 127–135. [DOI] [PubMed] [Google Scholar]
- 46. Haffty BG, Perrotta PL, Ward BE, et al. Conservatively treated breast cancer: outcome by histologic type. Breast J 1997; 3: 7 [Google Scholar]
- 47. Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13(8): 2329–2334. [DOI] [PubMed] [Google Scholar]
- 48. Mamounas E, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012; 30(32): 3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carey LA, Metzger R, Dees FC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst 2005; 97: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 50. Hennessy BT, Hortobagyi GN, Rousier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary lymph node metastases following primary chemotherapy. J Clin Oncol 2005; 23(36): 9304–9311. [DOI] [PubMed] [Google Scholar]
- 51. Bhargava R, Beriwal S, Dabbs DJ, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predict survival after neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer 2010; 116: 1431–1439. [DOI] [PubMed] [Google Scholar]
- 52. Van de, Ven S, Smit VT, Dekker TJ, et al. Discordances in ER, PR, and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 2011; 37: 422–430. [DOI] [PubMed] [Google Scholar]
- 53. Vincent-Salomon A, Jouve M, Genin P, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer 2002; 94: 2169–2173. [DOI] [PubMed] [Google Scholar]
- 54. Tacca O, Penault-Llorca F, Abrial C, et al. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist 2007; 12: 636–643. [DOI] [PubMed] [Google Scholar]
- 55. Lee J, Im YH, Lee SH, et al. Evaluation of ER and Ki-67 proliferation index as prognostic factors for survival following neoadjuvant chemotherapy with doxorubicin/docetaxel for locally advanced breast cancer. Cancer Chemother Pharmacol 2008; 61: 569–577. [DOI] [PubMed] [Google Scholar]
- 56. Jones RL, Salter J, A’Hern R, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009; 116: 53–68. [DOI] [PubMed] [Google Scholar]
- 57. Decensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol 2011; 22: 582–587. [DOI] [PubMed] [Google Scholar]
- 58. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25(28): 4414–4422. [DOI] [PubMed] [Google Scholar]
- 59. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003; 12(5): 320–327. [DOI] [PubMed] [Google Scholar]
- 60. Von Minckwitz G, Kaufmann M, Kuemmel S, et al. Correlation of various pathologic complete response (pCR) definitions with long-term outcome and the prognostic value of pCR in various breast cancer subtypes: results from the German neoadjuvant meta-analysis. J Clin Oncol 2011; 29: 1028. [Google Scholar]
- 61. Provenzano E, Vallier A-L, Champ R, et al. A central review of histopathology reports after breast cancer neoadjuvant chemotherapy in the neo-tango trial. Br J Cancer 2013; 108: 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peintinger F, Sinn B, Hatzis C, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol 2015; 28: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. College of American Pathologists. Protocol for the examination of specimens from patients with invasive carcinoma of the breast, http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/BreastInvasive_12protocol_3100.pdf (2012, accessed 30 October 2014).