The conversion of inert N2 gas to a metabolically tractable form, such as ammonia, is called nitrogen fixation. In biology, nitrogen fixation is a highly oxygen-sensitive process restricted to a select group of diverse microorganisms, often collectively referred to as diazotrophs, or “nitrogen eaters.” The sparse availability of fixed nitrogen, also known as fertilizer, has historically limited worldwide food production (1–4). Since about 1920, the situation has been significantly ameliorated by application of industrially produced fertilizer. Indeed, the Haber–Bosch process for industrial fertilizer production has been touted as the technological advance that has had the most impact on the modern world, driving the green revolution of the last century and fueling unprecedented population growth (1). However, the practice of applying industrially produced fertilizer to augment agricultural yield has also proven to incur severe economic, agronomic, and environmental penalties. Among these “penalites” are consumption of nonrenewable fossil fuels, prodigious production of greenhouse gases, spoiling of watersheds as a consequence of fertilizer run-off, costs associated with fertilizer distribution and application and, of course, socio-political issues associated with unbridled population growth (1–4).
Emergence of the recombinant DNA era in the mid-1970s led to the ambitious goal of endowing higher plants, in particular cereal plants, with the capacity to produce their own nitrogenous fertilizers by transfer of the microbial genetic determinants to plants (5). This effort was anticipated to result in sustainable agricultural practices that could reduce the demand for production of industrial fertilizers and avoid the costs and adverse effects associated with their transportation and application. Some 40 y later, this dream remains unrealized and can be reasonably considered as biotechnology’s most significant failure. Now, with a complete understanding of the fundamental genetic determinants necessary to sustain nitrogen fixation in microbes, and at least a rudimentary understanding of the mechanistic features of how nitrogen-fixation systems are assembled and operate, together with advances in organellar targeting and the advent of synthetic biology, there has been a rejuvenated interest in revisiting the nitrogen-fixation challenge (6). Yang et al. (7) now report in PNAS the remarkable observation that certain electron-transport chains of plant origin can be recruited to provide the reducing equivalents necessary to drive nitrogen fixation. This achievement represents an incremental, although profoundly important, milestone toward realizing the goal of endowing plants with the capacity for self-fertilization.
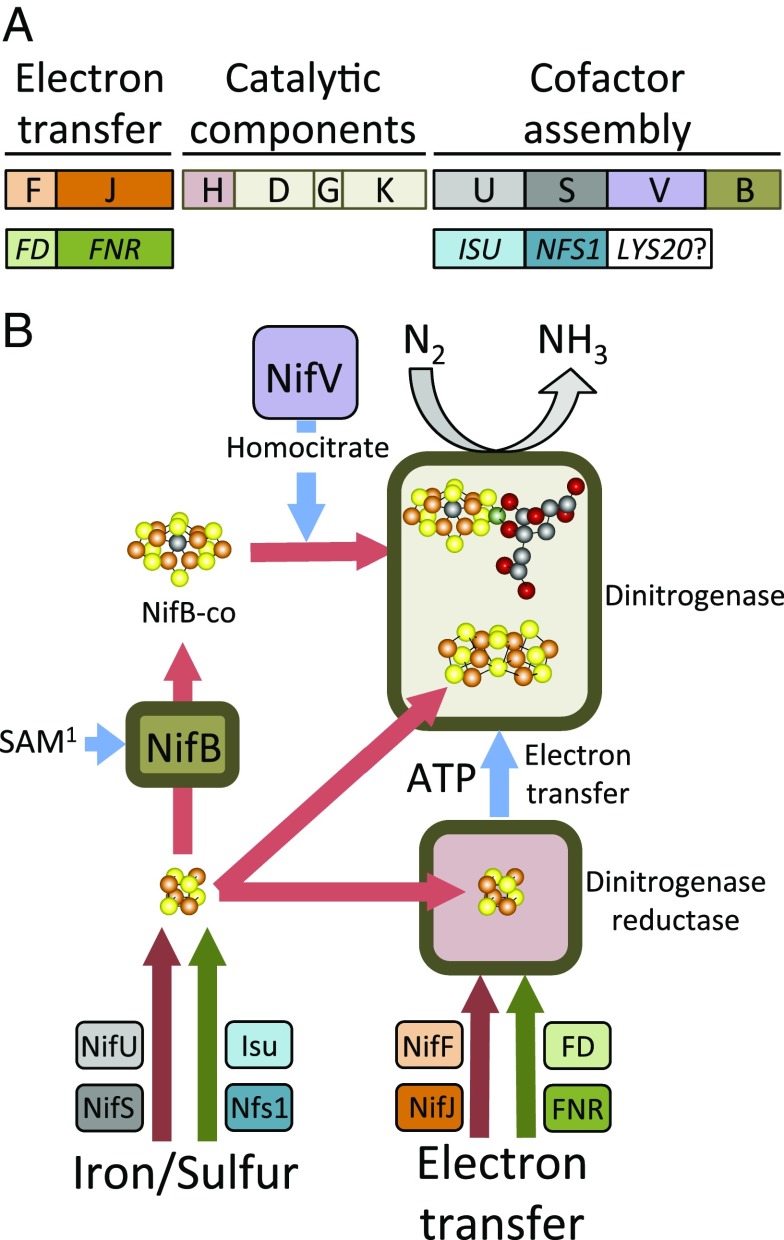
Biological nitrogen fixation is exclusively catalyzed by a complex and extremely oxygen-sensitive metalloenzyme, called nitrogenase. Although a diverse set of microorganisms can perform nitrogen fixation, they all produce nitrogenases, which share common features with respect to catalytic mechanism and assembly of the metal-containing cofactors critical to their functions (8). Nitrogenases from all organisms described so far comprise two catalytic partners. One of those partners, designated dinitrogenase reductase, serves as a nucleotide-dependent agent of electron delivery to the other partner, dinitrogenase (9) (Fig. 1). After dinitrogenase accumulates a sufficient number of electrons from the reductase, it binds and reduces N2 to form ammonia (10) (Fig. 1). The metabolic burden of nitrogen fixation can be appreciated from the perspective that eight electrons and 16 ATP are consumed for the reduction of each N2. In addition to the nitrogenase catalytic components, there are proteins required for assembly and insertion of the metal clusters necessary to activate the catalytic unit (11). Furthermore, there are proteins required to couple cellular metabolism to nitrogen-fixation–specific electron transfer (7). Thus, there are at least two fundamental aspects that must be achieved before the production of nitrogen-fixing cereals using a direct gene-transfer approach can be considered a tractable goal. It must be demonstrated that a minimum set of genetic determinants for nitrogen fixation can be transferred to a host eukaryote and produce active nitrogenase components. Also, it must be shown that the eukaryotic host can provide the reducing power and energy necessary to sustain nitrogenase catalysis. Although neither of these objectives has been fully realized, there is now compelling proof-of-principle that both objectives can and will be achieved.
Fig. 1.
Simplified schematic of nitrogen fixation. (A, Upper) Minimal set of genes that can sustain nitrogen fixation in E. coli (9). (Lower) Eukaryotic genes that can or might functionally replace bacterial counterparts. Color schemes correspond to functional properties of the gene products shown in B. (B) Functional features of gene products shown in A and described in the text. Genes and corresponding functions are indicated by matched colors. Green arrows indicate functional replacement by eukaryotic proteins. For the bacterial system, the iron and sulfur needed to assemble all nitrogenase cofactors are supplied by the product of NifU and NifS. Cofactors are shown as ball-and-stick models: yellow (sulfur), orange (iron), black (carbon), red (oxygen). SAM1 indicates S-adenosyl methionine. Participants of microbial origin expected to be irreplaceable are indicated by thick borders.
In recent studies it has been shown that an active dinitrogenase reducatase can be produced in eukaryotes, provided it is targeted to either mitochondria in yeast or plastids in tobacco (12, 13). These achievements are noteworthy for two reasons. First, although dinitrogenase reductase is structurally much simpler than dinitrogenase, it is much more sensitive to inactivation by oxygen. It is therefore anticipated that an active dinitrogenase can also be produced in such experimental systems if the appropriate assembly requirements are satisfied. It also appears that the much anticipated “oxygen problem” might be circumvented by mitochondrial targeting or, perhaps, plastid-targeting strategies combined with controlled conditions for expression, as suggested by Yang et al. (7). Second, dinitrogenase reductase contains an iron-sulfur cofactor that is essential for its activity (10). In nitrogen-fixing bacteria this cofactor is assembled by the products of two nitrogen-fixation–specific genes, designated nifU, encoding a cofactor assembly scaffold, and nifS, encoding a cysteine desulfurase (14). However, neither NifU nor NifS is necessary for the production of an active nitrogenase reductase in either yeast or tobacco (12, 13), indicating that counterparts involved in assembly of iron-sulfur cofactors in eukaryotic organisms—for example Isu and Nfs1 (15)—can substitute for the nitrogen-fixation–specific assembly components. It should be noted that NifU and NifS also supply the iron-sulfur building blocks necessary for formation of the complex cofactors associated with dinitrogenase (Fig. 1).
Yang et al. (7) have now addressed a similar functional replacement question but used a reverse approach: namely, they asked if plant-specific electron-transfer chains could replace the normal bacterial components. To execute these experiments, minimal gene modules were constructed that comprise the essential features necessary for nitrogen fixation to occur in the experimental microbe Escherichia coli. These include: (i) a module encoding the nitrogenase catalytic proteins, (ii) a module encoding proteins necessary for metal-containing cofactor assembly, and (iii) a module encoding proteins required to provide the reducing equivalents necessary for catalysis. Although there are a variety of different electron-transfer components that can support nitrogen fixation in microbes, the minimal electron-transport module selected for these experiments consists of two protein partners, NifJ and NifF. NifJ is a pyruvate flavodoxin oxidoreductase and NifF is a flavodoxin. NifJ couples the oxidation of pyruvate to the reduction of NifF, which, in turn, serves as a direct electron donor to dinitrogenase reductase. Yang et al. substituted NifF by various electron carriers of plastid origin, known as ferredoxins, and obtained functional hybrid electron-transfer modules (7). In this case the functional unit required that NifJ remain intact; thus, a hybrid module consisting of a bacterial protein and a plastid protein is effective in coupling cellular metabolism to nitrogen fixation. It was next shown that both NifJ and NifF could be functionally replaced by intact plant plastid electron-transport chains comprised of various ferredoxin–NADPH oxidoreductases and their cognate ferredoxins. These results demonstrate that plant plastids have the capacity to couple cellular metabolism to nitrogen fixation without the need for participation of any microbial electron-transfer partner. In contrast to the situation with substitution by plastid electron-transfer modules, mitochondrial ferredoxins cannot functionally replace NifF, nor can a complete mitochondrial electron-transfer unit replace the intact NifJ–NifF module. However, a hybrid electron-transfer module consisting of a mitochondrial adrenodoxin oxidoreductase and plant-like ferredoxins could be used to replace the NifJ–NifF module, indicating that plant mitochondria might be engineered to have the capacity to support nitrogen fixation in the absence of bacterial electron-transfer components.
In the simplest model system developed so far, there are 10 proteins required to sustain nitrogen fixation in E. coli (9) (Fig. 1). With respect to the goal of transferring that capacity to plant cells, it is now established that proteins necessary for coupling cell metabolism to nitrogen fixation (NifJ and NifF) and proteins required for mobilizing iron and sulfur for metal cofactor assembly (NifU and NifS) can be supplied by the plant host. Among the 10 proteins required in the minimal microbial system is NifV, which catalyzes formation of homocitrate, an organic constituent of the metal-containing cofactor that provides the nitrogenase active site (16). Because homocitrate is a metabolite already produced by some eukaryotes (17), it might be possible to separately engineer production of homocitrate in development of the first-generation nitrogen-fixing eukaryote. Thus, in theory expression of only five microbial proteins could be required to develop a first-generation nitrogen-fixing plant (Fig. 1). In the minimal set of genes required for microbial nitrogen fixation are encoded four proteins associated with formation of the nitrogenase catalyic partners. Also included is nifB, which is an S-adenosylmethionine–dependent enzyme (11) that provides the metal-sulfur core, called NifB-co (18) (Fig. 1) of the nitrogenase active site. It seems highly unlikely that replacement of either of the catalytic components, or NifB, by proteins of strictly plant origin can ever be accomplished. Nevertheless, the current simplification of the nitrogen-fixation challenge is critically important because it lowers the number of players that must be mobilized into plant organelles and it will be easier to assess the particular function that is missing or limiting in efforts to produce plant cells that have the capacity for nitrogen fixation. In aggregate, recent advances have provided optimism that it will be possible to develop a first-generation nitrogen-fixing plant using a model system. However, the development of a truly robust system that provides significant economic and agronomic benefit will probably require a reverse of the reductionist approach used so far, and this could take yet another several decades.
Footnotes
The authors declare no conflict of interest.
See companion article on page E2460.
References
- 1.Smil V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- 2.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA. 2011;108(50):20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton MA, et al. Too much of a good thing. Nature. 2011;472(7342):159–161. doi: 10.1038/472159a. [DOI] [PubMed] [Google Scholar]
- 5.Hardy RWF, Havelka UD. Nitrogen fixation research: A key to world food? Science. 1975;188(4188):633–643. doi: 10.1126/science.188.4188.633. [DOI] [PubMed] [Google Scholar]
- 6.Beatty PH, Good AG. Plant science. Future prospects for cereals that fix nitrogen. Science. 2011;333(6041):416–417. doi: 10.1126/science.1209467. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Xie X, Yang M, Dixon R, Wang Y-P. Modular electron-transport chains from eukaryotic organelles function to support nitrogenase activity. Proc Natl Acad Sci USA. 2017;114:E2460–E2465. doi: 10.1073/pnas.1620058114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dos Santos PC, Fang Z, Mason SW, Setubal JC, Dixon R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics. 2012;13:162. doi: 10.1186/1471-2164-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Xie X, Wang X, Dixon R, Wang YP. Reconstruction and minimal gene requirements for the alternative iron-only nitrogenase in Escherichia coli. Proc Natl Acad Sci USA. 2014;111(35):E3718–E3725. doi: 10.1073/pnas.1411185111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seefeldt LC, Hoffman BM, Dean DR. Mechanism of Mo-dependent nitrogenase. Annu Rev Biochem. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Ribbe MW. Nitrogenase assembly. Biochim Biophys Acta. 2013;1827(8-9):1112–1122. doi: 10.1016/j.bbabio.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Torrejón G, et al. Expression of a functional oxygen-labile nitrogenase component in the mitochondrial matrix of aerobically grown yeast. Nat Commun. 2016;7:11426. doi: 10.1038/ncomms11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivleva NB, Groat J, Staub JM, Stephens M. Expression of an active subunit of nitrogenase via integration into plant organelle genome. PLoS One. 2016;11(8):e0160951. doi: 10.1371/journal.pone.0160951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos PC, et al. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J Biol Chem. 2004;279(19):19705–19711. doi: 10.1074/jbc.M400278200. [DOI] [PubMed] [Google Scholar]
- 15.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460(7257):831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 16.Hoover TR, Imperial J, Ludden PW, Shah VK. Homocitrate is a component of the iron-molybdenum cofactor of nitrogenase. Biochemistry. 1989;28(7):2768–2771. doi: 10.1021/bi00433a004. [DOI] [PubMed] [Google Scholar]
- 17.Andi B, West AH, Cook PF. Stabilization and characterization of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae. Arch Biochem Biophys. 2004;421(2):243–254. doi: 10.1016/j.abb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Shah VK, Allen JR, Spangler NJ, Ludden PW. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Purification and characterization of NifB cofactor, the product of NIFB protein. J Biol Chem. 1994;269(2):1154–1158. [PubMed] [Google Scholar]