Significance
Hepatitis C virus (HCV) displays a narrow species tropism severely hampering development of small animal models that are required for vaccine and pathogenesis studies in vivo. The recent discoveries of HCV-related hepaciviruses in diverse hosts offer new opportunities with respect to the development of an immunocompetent animal model for HCV research. Among the hepaciviruses, the equine nonprimate hepacivirus (NPHV) represents the closest homolog of HCV discovered to date. We defined key aspects of natural immunity to NPHV challenge in the cognate host and provide evidence for natural protection from NPHV infection. Further characterization of the immune signatures that confer protection against NPHV could provide important information that may facilitate the development of new prophylactic strategies including protective vaccines against HCV.
Keywords: hepatitis C virus, nonprimate hepacivirus, infection, immune protection, rechallenge
Abstract
Hepatitis C virus (HCV) displays a restricted host species tropism and only humans and chimpanzees are susceptible to infection. A robust immunocompetent animal model is still lacking, hampering mechanistic analysis of virus pathogenesis, immune control, and prophylactic vaccine development. The closest homolog of HCV is the equine nonprimate hepacivirus (NPHV), which shares similar features with HCV and thus represents an animal model to study hepacivirus infections in their natural hosts. We aimed to dissect equine immune responses after experimental NPHV infection and conducted challenge experiments to investigate immune protection against secondary NPHV infections. Horses were i.v. injected with NPHV containing plasma. Flow cytometric analysis was used to monitor immune cell frequencies and activation status. All infected horses became viremic after 1 or 2 wk and viremia could be detected in two horses for several weeks followed by a delayed seroconversion and viral clearance. Histopathological examinations of liver biopsies revealed mild, periportally accentuated infiltrations of lymphocytes, macrophages, and plasma cells with some horses displaying subclinical signs of hepatitis. Following viral challenge, an activation of equine immune responses was observed. Importantly, after a primary NPHV infection, horses were protected against rechallenge with the homologous as well as a distinct isolate with only minute amounts of circulating virus being detectable.
Hepatitis C virus (HCV) infections constitute a major global health problem with ∼130 million people being chronically infected (1). Once infected, 70–90% of individuals progress to chronicity, rendering HCV the leading cause of liver diseases, including liver fibrosis, cirrhosis, and hepatocellular carcinoma (2). HCV is a blood-borne, positive-stranded RNA virus classified to the family of Flaviviridae within the genus Hepacivirus. Seven different HCV genotypes and numerous subtypes have been described, which differ on the nucleotide level by ∼30–35% between different genotypes and maximally 30% between subtypes (3). Furthermore, within infected individuals the virus circulates as a population of closely related but distinct genomes (4). Recently, significant progress has been made regarding the treatment of HCV with the implementation of direct-acting antiviral (DAA) drugs, which can be administered as all-oral IFN-free therapeutics and should help to reduce the global burden of HCV infections (5). However, there are still many caveats, including high treatment costs and the risk of reinfection after successful therapy, which is a problem especially in patient populations with frequent exposure to HCV. Furthermore, the inadequacy of current HCV screening programs often results in late diagnosis of chronic HCV infection and subsequent proceeding to end-stage liver diseases (6). All of these challenges highlight the necessity for a prophylactic vaccine against HCV, which despite extensive research remains elusive. This fact is partly due to the extensive heterogeneity of the virus, contributing to its ability to effectively escape immunity, facilitating viral persistence (7). Furthermore, the narrow host tropism of HCV for humans and the associated lack of an immunocompetent small animal model hamper vaccine development. Previous studies used chimpanzees (Pan troglodytes), which were the only fully immunocompetent animal model experimentally susceptible to HCV infection. These studies allowed analysis of viral pathogenesis and immune control and contributed to vaccine development (8). Recently, multiple novel animal hepaciviruses naturally infecting various species have been identified (9, 10). Viruses classified within the genus Hepacivirus have been discovered in dogs (11) and subsequently in horses (12), rodents (13–15), bats (16), Old World primates (17), cattle (18), and recently catsharks (19). These viruses display different homologies to HCV and offer new insights into the origin and evolution of Hepaciviruses. Furthermore, given the relationship of these animal viruses to HCV, they could facilitate the generation of immunocompetent surrogate animal models for HCV vaccine research. Among these newly discovered animal hepaciviruses, the equine nonprimate hepacivirus (NPHV) represents the closest homolog of HCV discovered to date. The virus was initially discovered in dogs (11); however, it was later shown to primarily infect horses with a high seroprevalence of ∼35% (12). NPHV shares a sequence homology of ∼50% to HCV (11). Several similarities to HCV could be observed upon equine hepacivirus infection. Similar to HCV, NPHV causes both acute and chronic infections in horses; although unlike HCV, the majority of animals are able to clear the infection (20–22). Experimental blood transmission has been shown, indicating that NPHV might be a blood-borne pathogen (23). Importantly, hepatotropism has been demonstrated for NPHV with viral replication occurring exclusively within the liver (20, 22, 23). Moreover, key viral evasion strategies are conserved between HCV and NPHV, as for instance, both viral proteases cleave their cognate innate immune adaptor protein MAVS (24, 25). Given the close similarities between HCV and NPHV, we aimed to investigate NPHV infection in the natural host to determine the course of infection and equine immune responses after experimental NPHV infection. Furthermore, we conducted challenge infections to investigate immune protection against secondary NPHV infections, elucidate hepaciviral biology, and the suitability of horses as surrogate models for HCV research.
Results
Horses Can Be Experimentally Infected with NPHV and Display Similar Hepatic Membrane Rearrangements to HCV.
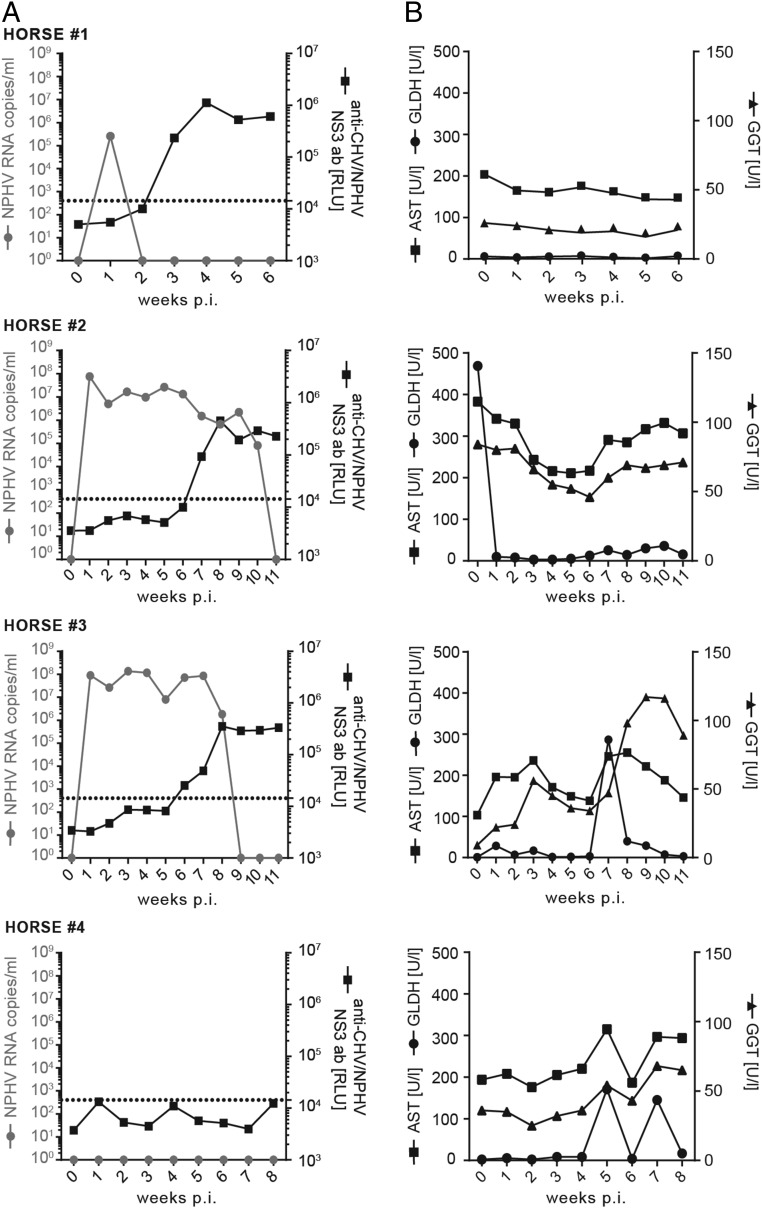
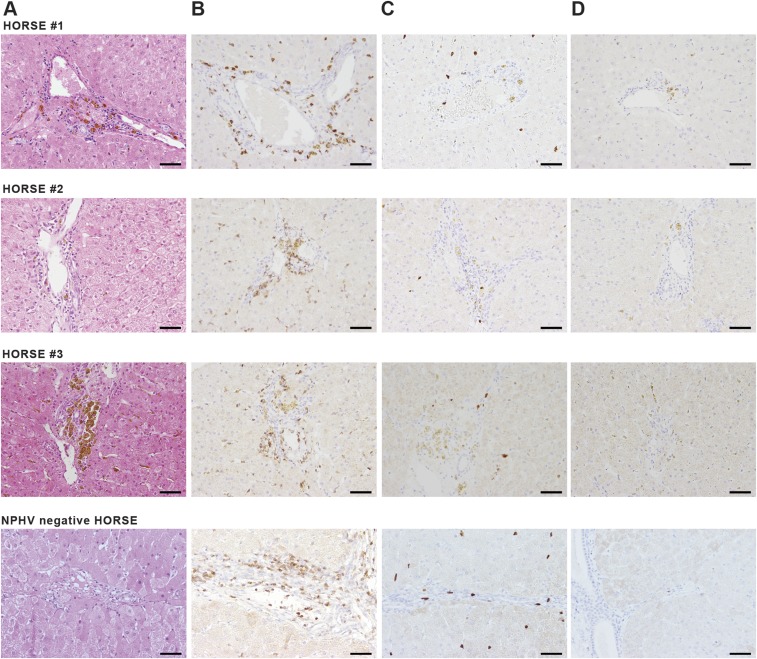
To study NPHV infections in vivo, we experimentally challenged three NPHV RNA− horses that were devoid of serum markers of a previous NPHV infection with different doses of NPHV-containing plasma (Fig. 1A). Horse 1 was i.v. challenged with 500 mL of donor plasma (GenBank accession no. KY124246, viral load 7.78 × 106 RNA copies per milliliter), whereas horses 2 and 3 received 100 mL of the same donor plasma each. Horse 4 received 100 mL of NPHV− plasma as control. Horses were monitored on a weekly basis for viral loads, seroconversion, and indications of liver injury. All NPHV-infected horses became viremic after 1 wk as determined by NPHV-specific quantitative RT-PCR (qRT-PCR) (Fig. 1A). Viral titers ranged between 105 copies per milliliter and 108 copies per milliliter. Horse 1 rapidly cleared the infection within 1 wk, whereas horses 2 and 3 stayed viremic for 8–10 wk before virus was cleared. A delayed seroconversion was observed in all horses with the appearance of NPHV-specific antibodies 3–7 wk postchallenge. The liver-specific enzymes γ-glutamyltransferase (GGT) and glutamate dehydrogenase (GLDH), which are specific markers of acute hepatocellular damage, and aspartate aminotransferase (AST), which complements the parameters in the screening enzymes, were monitored over the course of infection. Serum levels of liver enzymes stayed mainly within reference range with a mild elevation in horse 2 and horse 3 at seroconversion (Fig. 1B). Histopathological examinations revealed mild, multifocal, lymph–plasma–histiocytic infiltrates in periportal areas (Fig. S1A). Furthermore an intracytoplasmic, coarsely granular, yellow–brown pigment (hemosiderin) was multifocally demonstrated in hepatocytes, macrophages, and Kupffer cells at varying intensities. In addition, a mild, diffuse, hepatocellular vacuolation (most likely lipid storage) was found in one case (horse 3). Moreover, one biopsy exhibited single, multifocal, degenerating hepatocytes with an associated mild, lymphohistiocytic inflammation and a mild bile duct hyperplasia 1 (horse 2). In one biopsy, mild, multifocal, necrosuppurative hepatitis was present (horse 3). Similarly, a NPHV negative control horse displayed mild, multifocal, lymphohistiocytic infiltrates with single eosinophils in periportal areas and low amounts of intracytoplasmic deposits of a coarsely granular, yellow–brown pigment in hepatocytes, macrophages, and Kupffer cells. Furthermore, single, multifocal, degenerating hepatocytes with an associated mild neutrophilic inflammation were seen in the control animal. Immunohistochemical characterization of periportal infiltrates of NPHV-infected horses revealed 88.66–97.18% CD3+ T cells, 0.00–0.59% Pax-5+ B cells, and 2.23–11.34% MAC387+ macrophages (Fig. S1 B–D). Comparable results were obtained for periportal infiltrates of the control horse, where 92.1% CD3+ T cells, 0.85% Pax-5+ B cells, and 7.05% MAC387+ macrophages were found (Fig. S1 B–D).
Fig. 1.
Course of infection after experimental inoculation with NPHV+ plasma. Horse 1 was i.v. transfused with 500 mL NPHV containing plasma, whereas horses 2 and 3 were i.v. transfused with 100 mL NPHV containing plasma (GenBank accession no. KY124246, viral load 7.78 × 106 RNA copies per milliliter). Horse 4 was i.v. transfused with 100 mL NPHV− plasma. Serum samples were taken on a weekly basis postinoculation (weeks p.i.). (A) The viral load of NPHV RNA was determined by qRT-PCR and is depicted as gray dots (limit of quantification 50 RNA copies per serum sample). Anti-CHV (canine hepacivirus)/NPHV NS3 antibodies were measured by the luciferase immunoprecipitation (LIPS) assay and are depicted in black squares as relative light units (RLUs). A cutoff was calculated by the mean value of samples containing only buffer A, the renilla luciferase-NS3 (Ruc–NS3) fusion protein and A/G beads plus three SDs and is indicated by a dotted line. (B) Liver-specific enzymes in the serum were measured. Reference values are as follows: GLDH < 6 U/L, GGT < 20 U/L, and AST < 170 U/L.
Fig. S1.
Immunohistological analysis of liver biopsies. Liver biopsies were taken 6 wk (horse 1), 11 wk (horse 2), and 16 wk (horse 3) p.i. and embedded in paraffin. As control, a liver biopsy embedded in paraffin from a NPHV− horse was included. (A) Tissue sections were stained with hematoxylin and eosin. (B) T cells were detected using an anti-CD3 antibody. (C) Immunohistochemistry using an anti-Mac387 antibody was used for visualization of macrophages. (D) Low numbers of B lymphocytes were detected with anti–Pax-5 antibody. (Scale bars, 50 µm.)
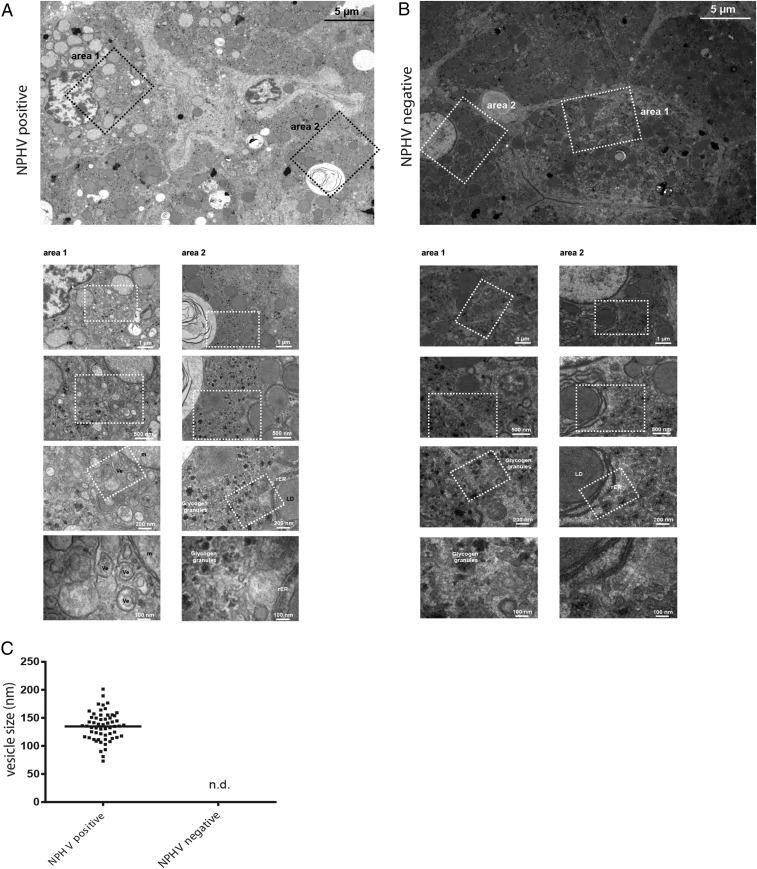
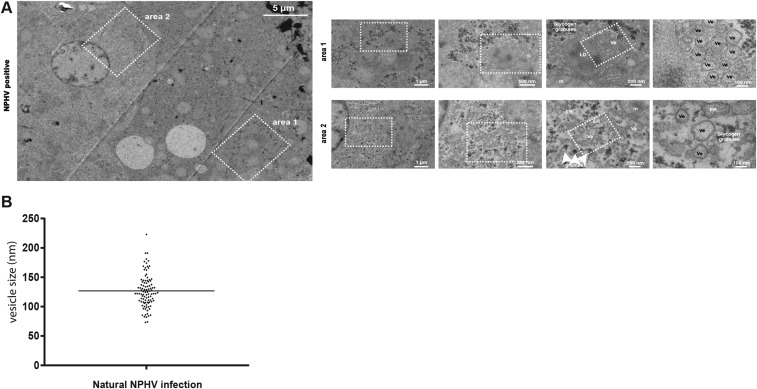
Hepatitis C virus is known to induce accumulations of vesicles forming a membranous web (MW) (26), consisting of mainly double membrane vesicles (DMVs) that are thought to be the site of viral RNA replication within the infected liver (27, 28). To examine whether MWs are formed in vivo within the liver of NPHV+ horses, we performed electron microscopy (EM)-based studies on liver biopsies of an infected horse, and in various cells, we observed numerous vesicles (Ve), which could not be detected in an uninfected horse (Fig. 2 A–C). These vesicles had an average diameter of about 120 nm and in some cases a double lipid bilayer could be observed, reminiscent of DMVs in HCV-infected cells (Fig. 2A). Furthermore, liver cells from the uninfected horse depicted a much more homogeneous appearance (Fig. 2B), in comparison with NPHV-infected liver cells (Fig. 2A). Importantly, these vesicles were also visible during a natural NPHV infection (Fig. S2 A and B), indicating that NPHV and HCV induce similar intracellular membrane rearrangements.
Fig. 2.
Ultrastructural analysis of horse liver biopsies. Biopsy samples taken from the liver of one NPHV− horse as well as from a positive control horse experimentally infected with NPHV (Fig. 6) were immediately fixed with glutaraldehyde. Samples were sectioned in small pieces and, subsequently, processed and analyzed by transmission electron microscopy (TEM) as described in SI Materials and Methods. EM micrographs of (A) a liver biopsy of an experimentally NPHV-infected horse, 7 wk postinfection, and (B) a NPHV− horse. An overview of several liver cells is depicted at Top. Higher magnification images of two different areas are shown at Bottom. The white boxed areas highlight the areas that are shown at Bottom as magnified views. (C) Quantification of vesicle sizes (in nanometers) from an experimentally NPHV-infected horse (n = 55) compared with a NPHV− horse. Ve, vesicle; LD, lipid droplet; m, mitochondria; rER, rough endoplasmic reticulum; n.d., not detected; nm, nanometer.
Fig. S2.
Ultrastructural analysis of horse liver biopsies after natural NPHV infection. (A) Liver biopsy sample of a naturally NPHV-infected horse (20) was taken and immediately fixed with glutaraldehyde. The sample was sectioned in small pieces and, subsequently, processed and analyzed by transmission electron microscopy (TEM) as described in SI Materials and Methods. An overview of several liver cells is depicted on the Left. Higher magnification images of two different areas are shown on the Right. The white boxed areas highlight the areas that are shown as magnified views to the Right. (B) Quantification of vesicle sizes from a naturally NPHV-infected horse (n = 100). Ve, vesicle; LD, lipid droplet; m, mitochondria; ER, endoplasmic reticulum; and nm, nanometer.
Experimentally Infected Horses Mount Immune Responses Against NPHV.
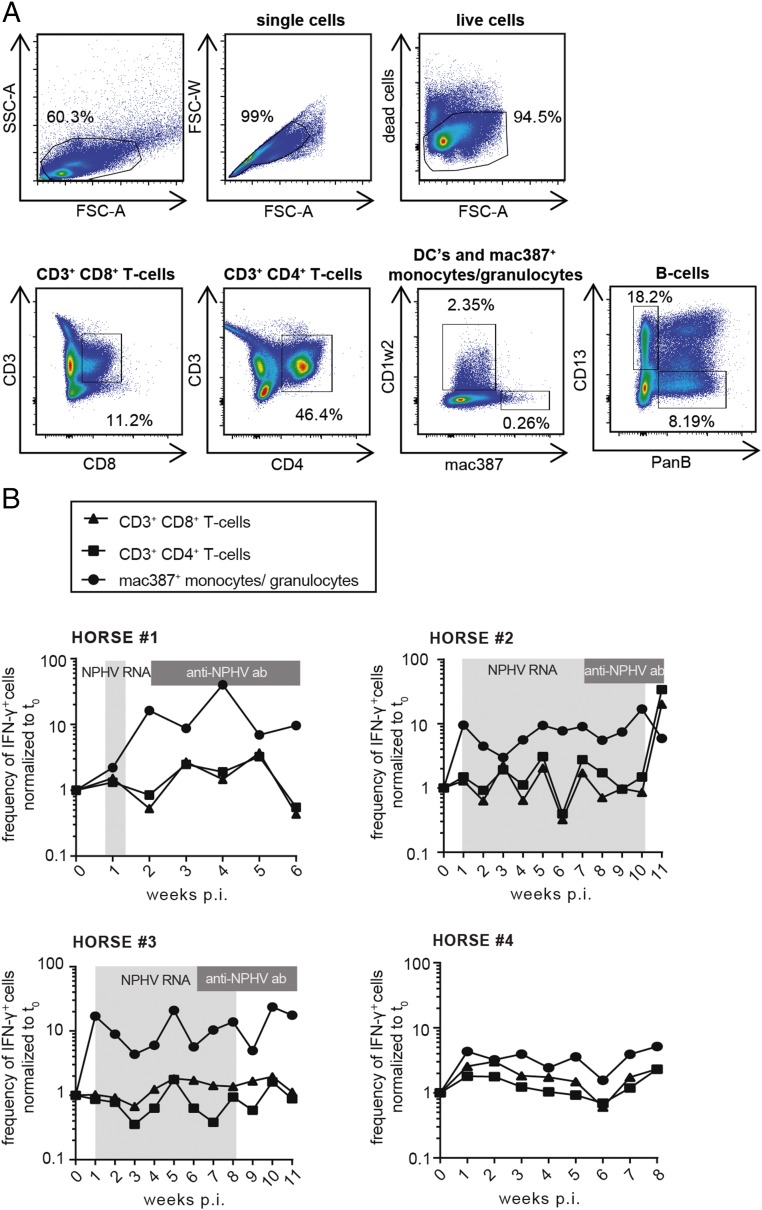
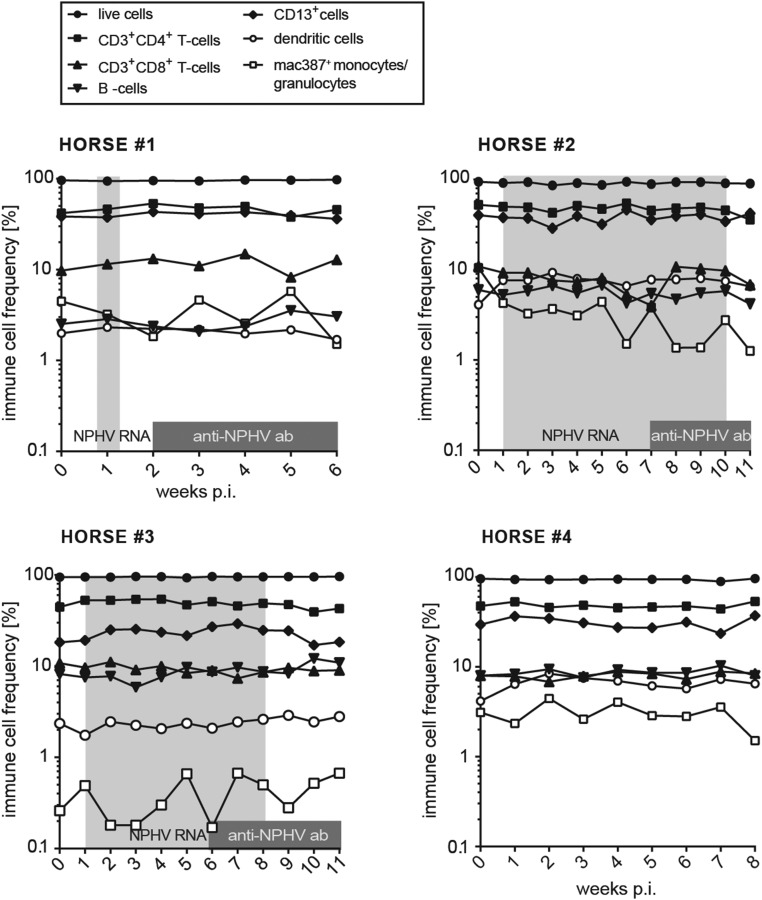
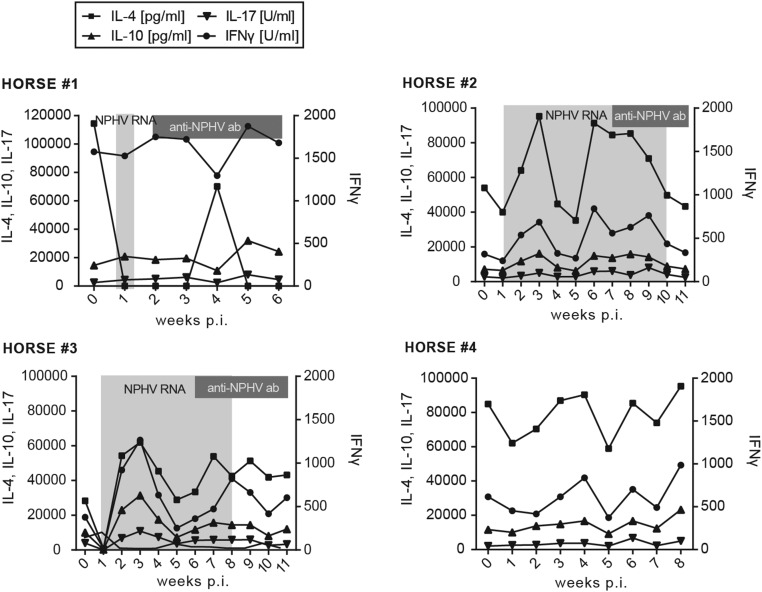
To better understand immune responses to NPHV infections, which could contribute to viral clearance, we established flow cytometric assays to monitor immune cell frequencies and activation status. We isolated peripheral blood mononuclear cells (PBMCs) from horses on a weekly basis and established a gating strategy to distinguish between CD3+CD4+ T-helper cells, CD3+CD8+ cytotoxic T cells, dendritic cells (DCs), B cells, and monocytes/granulocytes (Fig. 3A). Using this approach, we could analyze the peripheral immune cell frequency of individual subtypes; however, we did not observe any striking differences between individual horses (Fig. S3). Intracellular IFN-γ expression was monitored in individual immune cell subtypes to analyze the activation status. Interestingly, all NPHV-infected horses (horses 1–3) displayed an elevated frequency of IFN-γ–expressing monocytes/granulocytes following infection, indicating an activation of the innate immune response after experimental infection, which could not be observed to a similar extent in the negative control horse (Fig. 3B). To further analyze equine immune responses, we measured serum cytokine levels using a fluorescent bead-based multiplex assay. Serum cytokine levels varied between the individual horses. In two horses (horses 2 and 3) we observed a transient elevation of serum IL-4 and IFN-γ 3 wk p.i.; however, similar levels could also be observed in the negative control horse arguing for an NPHV-independent immune reaction (Fig. S4).
Fig. 3.
Development of a flow cytometry-based method to measure immune cell frequencies and intracellular IFN-γ expression. PBMCs were isolated on a weekly basis postinoculation by centrifugation on a Ficoll-Hypaque density gradient and stored at −150 °C before flow cytometry staining. PBMCs were stained for the surface markers CD8, CD4, CD3, PanB, CD13, CD1w2, and Mac387 using equine-specific antibodies to detect T lymphocytes (CD3+CD4+ T-helper cells, CD3+CD8+ cytotoxic T cells), B lymphocytes (PanB+), CD13+ cells (expressed on blood neutrophils, basophils, monocytes, but not B or T cells), dendritic cells (CD1w2+), and monocytes/granulocytes (Mac387+), respectively. Additionally, intracellular IFN-γ staining was performed on all cells. Samples were analyzed by flow cytometry. (A) Exemplary depiction of the gating strategy. Dead cells as well as cell duplets were excluded before subsequent gating on the respective immune cell subset. (B) The frequency of IFN-γ–expressing immune cells (CD3+CD4+ T cells, CD3+CD8+ T cells, and mac387+ monocytes/granulocytes) of each time point postinoculation normalized to t0 is depicted for all four horses. Time points that tested positive for NPHV RNA and anti-CHV/NPHV NS3 antibodies are indicated in light- and dark-gray boxes, respectively.
Fig. S3.
Immune cell frequencies as determined by flow cytometry. PBMCs were stained with antibodies directed against CD8, CD4, CD3, PanB, CD13, CD1w2, and Mac387 to detect T lymphocytes (CD3+CD4+ and CD3+CD8+), B lymphocytes (PanB+), CD13+ cells (expressed on blood neutrophils, basophils, monocytes, but not B or T cells), dendritic cells (CD1w2+), and monocytes/granulocytes (Mac387+), respectively. All samples were analyzed by flow cytometry. Dead cells as well as cell duplets were excluded before subsequent gating on the respective immune cell subset. Depicted is the frequency of the respective immune cell subtype as percentage of live cells for each time point postinfection (p.i.). Time points that tested positive for NPHV RNA and anti-CHV/NPHV NS3 antibodies are indicated in light- and dark-gray boxes, respectively.
Fig. S4.
Sera cytokine levels. Collected serum samples were analyzed by a bead-based multiplex assay for the presence of IL-4, IL-10, IL-17, and IFN-γ. The lower limits of detection are 40 pg/mL for IL-4, 15 pg/mL for IL-10, and 10 units/mL for IL-10 and IFN-γ, respectively. Time points that tested positive for NPHV RNA and anti-CHV/NPHV NS3 antibodies are indicated in light- and dark-gray boxes, respectively.
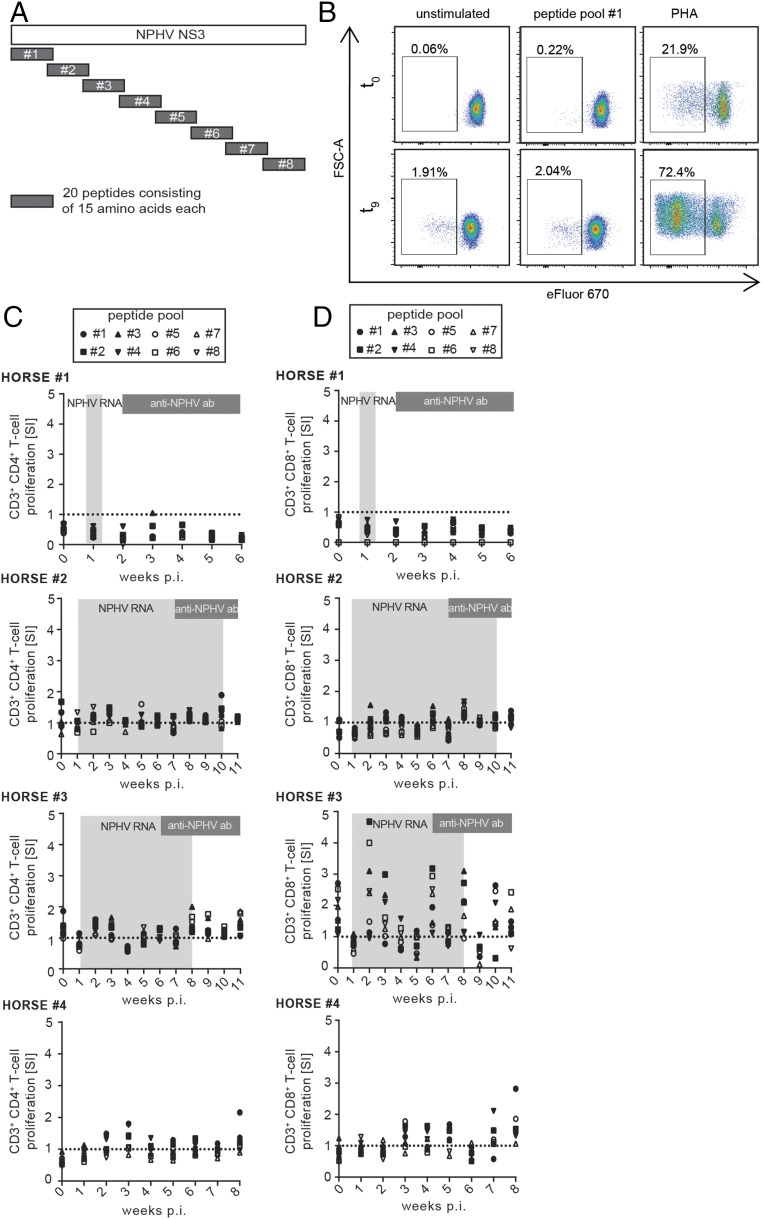
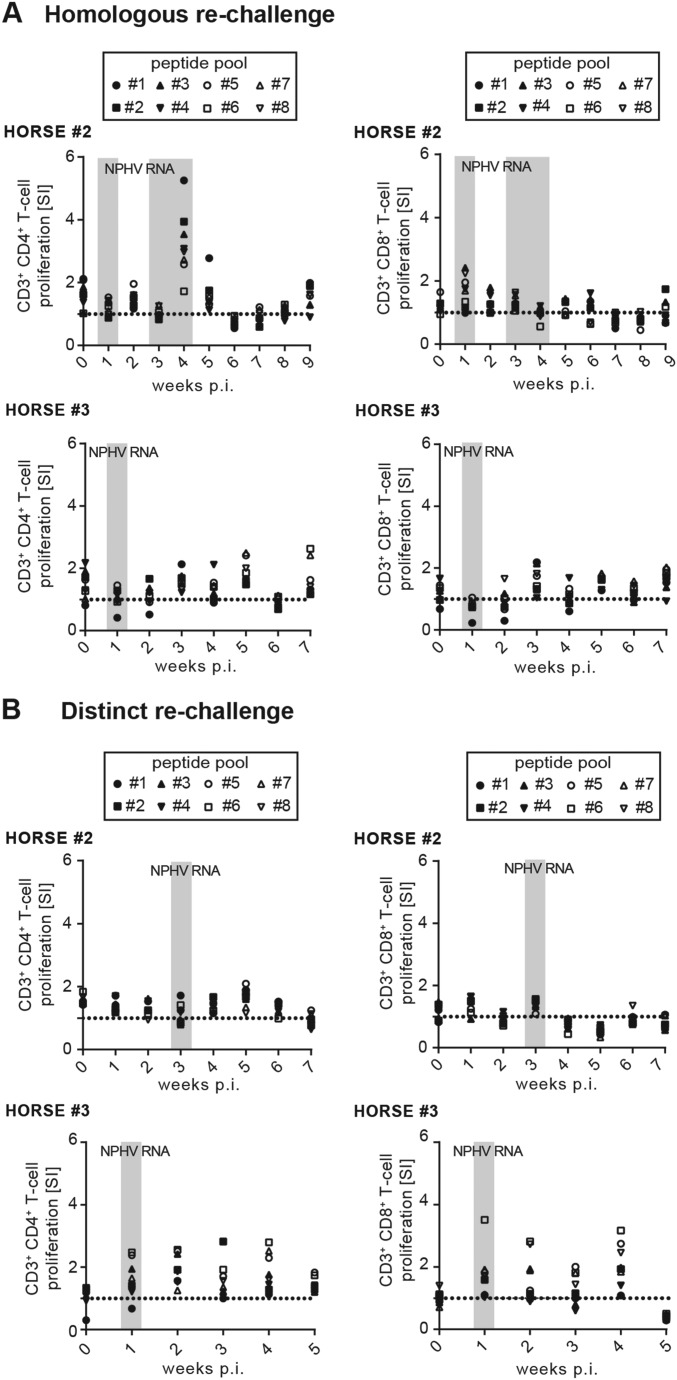
In humans and chimpanzees, a strong peripheral and intrahepatic T-cell immune response appears to be associated with HCV clearance (29). To analyze the impact of T cells on NPHV clearance, we established a NPHV-specific T-cell proliferation assay. We stimulated ex vivo isolated PBMCs with eight overlapping peptide pools spanning the whole nonstructural 3 (NS3) region of NPHV (Fig. 4A) and measured cell proliferation upon decrease of the fluorescence dye eFluor 670 (Fig. 4B). PBMCs were stimulated with PHA (phytohemagglutinin-M) as positive control (Fig. 4B). We gated on CD3+CD4+ T cells (Fig. 4C) and CD3+CD8+ T cells (Fig. 4D) and determined the respective proliferation as stimulation index (SI). No distinct NPHV-specific peripheral T-cell proliferation could be observed in horses 1 and 2 and the negative control horse 4; however, a weak CD3+CD8+ T-cell proliferation could be detected in horse 3 (Fig. 4D). Taken together, we established assays to analyze peripheral immune responses in horses and could show a moderate immune activation following experimental NPHV infection in horses, although differences between individuals were observed.
Fig. 4.
Analysis of NPHV-specific T-cell proliferation. (A) Eight distinct but overlapping peptide pools originating from the NS3 region of NPHV were synthesized based on the NS3 reference sequence of NPHV isolate H10 (GenBank accession no. KP640276). Individual peptides were pooled with each pool containing 20 peptides consisting of 10 overlapping amino acids each. Isolated PBMCs were stimulated in duplicates with the peptide pools and incubated for 7 d at 37 °C before flow cytometry analyses. As positive control, PBMCs were stimulated with PHA. (B) Gating strategy after flow cytometry-based analysis of T-cell proliferation. Seven days postinfection, PBMCs were stained for CD3, CD4, and CD8 surface markers to gate on the respective cell population as described above. A decrease in the proliferation dye eFluor 670 indicates proliferation of the respective population. Representative plots of horse 2 at t0 and t9 are shown. The amount of proliferating CD3+CD4+ (C) and CD3+CD8+ (D) T cells was calculated by dividing the stimulated samples by the unstimulated control of each time point depicted as stimulation index (SI). The respective values of each peptide pool are indicated with a single symbol for all of the respective time points measured in duplicates. The dotted line indicates the unstimulated control. Time points that tested positive for NPHV RNA and anti-CHV/NPHV NS3 antibodies are indicated in light- and dark-gray boxes, respectively.
Horses Are Protected Against Rechallenge with Homologous or Distinct NPHV Isolates.
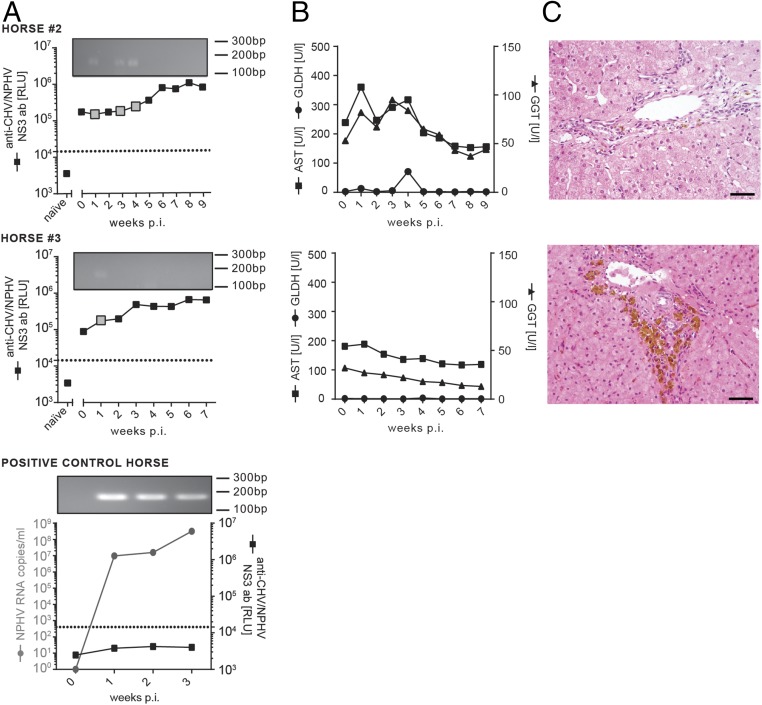
Previous studies in chimpanzees and also reports from infected patients have shown that HCV reinfection is possible after resolving a primary HCV infection (30–32). To analyze whether horses are protected against a secondary NPHV reinfection, we experimentally rechallenged two of the horses, which had previously resolved a NPHV infection (horses 2 and 3) with 100 mL of the homologous virus isolate (GenBank accession no. KY124246, viral load 7.78 × 106 RNA copies per milliliter). Inclusion of a previously naïve horse (positive control horse) confirmed that the used plasma was still infectious (Fig. 5A, Lower graph). Both rechallenged horses retained a high NPHV-specific antibody titer before secondary rechallenge (naïve vs. t = 0; Fig. 5A). Interestingly, no productive infection could be established in the rechallenged animals with only trace amounts of viral RNA being detectable (gray boxes and agarose gel) (Fig. 5A). Detected viral genomes were sequenced to confirm homologous genome identity. NPHV-specific antibody titers further increased in the rechallenged horses 3–6 wk postinfection (Fig. 5A). Liver-specific enzymes mainly stayed within the reference range (Fig. 5B) and histopathological examination revealed mild, multifocal, lymph–plasma–histiocytic infiltrates in periportal areas. Furthermore, an intracytoplasmic, coarsely granular, yellow–brown pigment (hemosiderin) was multifocally demonstrated in hepatocytes, macrophages, and Kupffer cells in varying intensities (Fig. 5C).
Fig. 5.
Homologous NPHV reinfection of horses. Horses 2 and 3 were rechallenged ∼5 mo post initial viral clearance with 100 mL homologous inoculum (GenBank accession no. KY124246, viral load 7.78 × 106 RNA copies per milliliter). Serum and PBMC samples were taken on a weekly basis and stored at −20 °C/−150 °C before analysis. (A) Course of the experimental, homologous reinfection. All serum samples were analyzed for the presence of NPHV RNA and anti-CHV/NPHV NS3 antibodies, respectively. Anti-CHV/NPHV NS3 antibodies were measured by the LIPS assay in duplicates and the mean values are depicted as black squares as relative light units (RLUs). A cutoff was determined as described above and is depicted as dotted line. The value of the naïve sample represents the first serum sample taken before the initial inoculation in the first part. NPHV RNA values of horses 2 and 3 were below the detection limit at all time points (limit of quantification 50 RNA copies per serum sample). To detect trace amounts of NPHV RNA, all qRT-PCR samples were loaded on an agarose gel as shown at the Top of each graph. Positive signals in the agarose gel are highlighted in each graph by a big gray square. As control, one naïve horse was inoculated with 100 mL homologous plasma. (B) Monitoring of liver enzyme values within sera. AST, GLDH, and GGT were determined in all serum samples of horses 2 and 3. Reference values are as follows: GLDH < 6 U/l, GGT < 20 U/l, and AST < 170 U/l. (C) Three weeks postinoculation, liver biopsies were taken and stained with hematoxylin and eosin (H&E). Periportally, biopsies revealed a low number of lymphocytes and macrophages. In addition, a variable degree of coarsely granular, brown pigment within the cytoplasm of hepatocytes and macrophages was present. (Scale bars, 50 µm.)
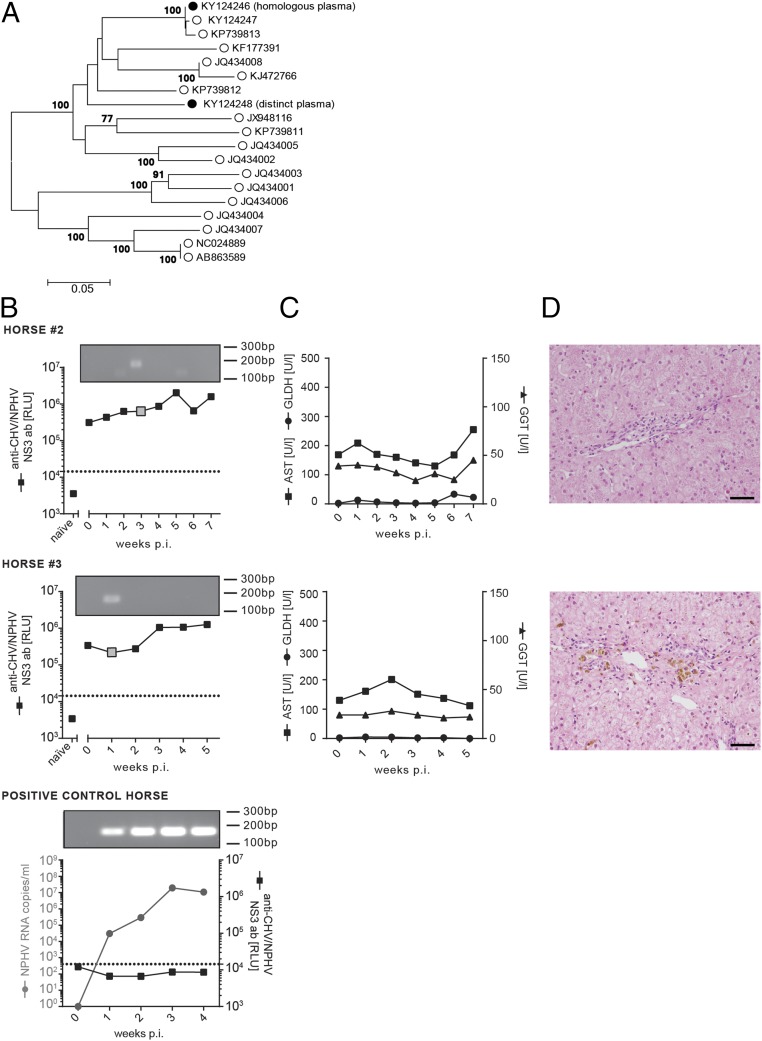
To examine the breadth of immune protection, we rechallenged the same horses (horses 2 and 3) with 100 mL of a distinct NPHV isolate (GenBank accession no. KY124248, viral load 6.0 × 105 RNA copies per milliliter) that differs from the primary inoculum at 11.54% of nucleotide sites of the near-completely sequenced E1E2 glycoproteins, thus mimicking a rechallenge with a NPHV subtype (Fig. 6A). Similar to the first rechallenge, previously infected horses were protected against reinfection compared with a naïve horse (positive control horse) (Fig. 6A). Only trace amounts of viral RNA could be detected 3 wk and 1 wk postinfection, respectively. The naïve positive control horse rapidly developed robust viremia 1 wk postinfection with high viral titers (Fig. 6B). Sequencing of viral trace amounts confirmed the correct viral sequence of the inoculum. Rechallenged horses (horses 2 and 3) displayed a slight increase in NPHV-specific antibody titers (Fig. 6B); however, no infection-associated clinical signs for hepatitis could be observed upon biochemical and histopathological examination (Fig. 6 C and D). In chimpanzees, after reinfection a rapid virological control has been connected to HCV-specific T-cell responses (31, 33, 34). We therefore analyzed peripheral T-cell responses against NPHV after rechallenge; however, we could detect only weak T-cell proliferation in both horses after homologous or distinct rechallenge (Fig. S5 A and B). In conclusion, we could show that horses are protected against homologous and distinct viral rechallenge, although no sterilizing immunity is induced as trace amounts of viral RNA can be detected.
Fig. 6.
Nonhomologous NPHV reinfection of horses. Horses 2 and 3 were rechallenged ∼5 mo after viral clearance of the homologous reinfection (1 y post initial infection) with 100 mL of a distinct inoculum (GenBank accession no. KY124248, viral load 6.0 × 105 RNA copies per milliliter). Serum and PBMC samples were taken on a weekly basis and stored at −20 °C/−150 °C before analysis. (A) Phylogenetic analysis of NPHV isolates. The consensus sequence of inoculum used for the homologous infection (GenBank accession no. KY124246) and the distinct rechallenge (GenBank accession no. KY124248) were sequenced in the near complete E1E2 region and are depicted in relation to reference NPHV isolates. The evolutionary history was inferred by using the maximum likelihood method based on the general time reversible model. Depicted is the tree with the highest log likelihood; bootstrap values are indicated at tree nodes; and scale bar refers to branch lengths measured in the number of substitutions per site. (B) Course of the experimental, nonhomologous reinfection. Anti-CHV/NPHV NS3 antibodies were determined by the LIPS assay and the mean values of the duplicate samples are presented as black squares as relative light units (RLUs). The naïve sample corresponds to the t0 value before the first infection. NPHV RNA values were below the detection limit (limit of quantification 50 RNA copies per serum sample) and qRT-PCR products are visualized by separation on an agarose gel. Positive signals are indicated with a gray big square. A naïve (NPHV seronegative and RNA negative) horse was inoculated with the distinct plasma as positive control. (C) Determination of the AST, GGT, and GLDH level in the serum. Reference values are as follows: GLDH < 6 U/l, GGT < 20 U/l, and AST < 170 U/l. (D) Three weeks postinoculation, liver biopsies were taken and stained with hematoxylin and eosin. Periportally, biopsies revealed a low number of lymphocytes and macrophages. In addition, a variable degree of coarsely granular, brown pigment within the cytoplasm of hepatocytes and macrophages was present. (Scale bars, 50 µm.)
Fig. S5.
T-cell proliferation following rechallenge. Eight distinct but overlapping peptide pools originating from the NS3 region of NPHV were synthesized based on the NS3 reference sequence NS3 reference sequence of NPHV isolate H10 (GenBank accession no. KP640276). Individual peptides were pooled, with each pool containing 20 peptides consisting of 10 overlapping amino acids each. Isolated PBMCs were stimulated in duplicates with the peptide pools and incubated for 7 d at 37 °C before flow cytometry analyses. As positive control PBMCs were stimulated with PHA. PBMC were collected after (A) homologous NPHV rechallenge or (B) distinct NPHV rechallenge and T-cell proliferation was analyzed as described before. The amount of proliferating CD3+CD4+ and CD3+CD8+ T cells was calculated by dividing the stimulated samples by the unstimulated control of each time point depicted as stimulation index (SI). The respective values of each peptide pool are indicated with a single symbol for all of the respective time points measured in duplicates. The dotted line indicates the unstimulated control. Time points that tested positive for NPHV RNA are indicated in light-gray boxes.
Discussion
Persistent HCV infections account for ∼500,000 deaths annually and are the leading indication for liver transplantation (35). With the development of robust in vitro cell culture systems the viral replication cycle could be studied in detail and ultimately contributed to the development and approval of efficient treatment options in the form of DAAs (36). Nevertheless, a protective vaccine is still lacking, which prevents effective global control and eradication of this virus. The lack of a traceable immunocompetent animal model for HCV hampers vaccine development, as cell culture systems do not permit detailed studies of virus–host interactions and cannot completely elucidate mechanisms that contribute to viral clearance versus persistence. So far, chimpanzees are the only fully immunocompetent host supporting a HCV infection, but due to ethical concerns, use of chimpanzees for research has been banned in the United States and the European Union. Therefore, the development of a new tractable immunocompetent animal model is of utmost importance. The recent discovery of animal hepaciviruses provides new opportunities to broaden our knowledge about hepaciviral virus–host interactions and could lead to the development of new surrogate experimental models for HCV. To estimate the significance of these novel viruses as surrogate models for HCV, a detailed understanding and biological characterization is crucial. This study provides insights into the closest homolog of HCV discovered to date, the equine NPHV. Previous studies already demonstrated similarities between HCV and NPHV, including molecular features as well as the mode of transmission and hepatotropism (20, 22, 23, 37, 38). However, only a few studies have addressed detailed analysis of NPHV infection in vivo. We could confirm previously published data that horses can be experimentally infected by i.v. inoculation (23). All inoculated horses became viremic 1 wk postinfection and with the exception of one horse (horse 1) stayed viremic for several weeks. Reasons for the different clearance rates between the animals need to be further investigated, but one could imagine that undetected previous natural infections with NPHV might contribute to the observed differences. Ultrastructural studies of liver biopsies from infected versus naïve horses revealed that NPHV infection induces the formation of vesicles within the liver, similar to the vesicles that have been previously observed in liver biopsies of an HCV-infected chimpanzee (26) and comparable to the structures that are induced by HCV upon infection of culture cells (27). These findings indicate that HCV and NPHV might share common strategies for viral replication, resulting in the formation of similar replication structures, also known as replication factories. It is believed that formation of such vesicles not only facilitates the coordination of the different steps of the viral replication cycle, but could also contribute to immune evasion by shielding viral RNA from recognition by innate sensors such as retinoic acid-inducible gene I (RIG-I) or melanoma differentiation-associated gene 5 (MDA5) (27). However, it is not completely understood how host immune responses contribute to viral clearance compared with the establishment of chronic infection (39). Studies of early immune events during a HCV infection in humans are difficult due to various reasons, including the late diagnosis in the majority of cases rendering animal models valuable surrogate models. Furthermore, several studies indicated that HCV-specific CD4 and CD8 T-cell responses in both humans as well as chimpanzees are weak after HCV infection and that spontaneous clearance is associated with a stronger cellular response. The latter is typically broad, polyfunctional, and sustained, indicating that cellular immunity is an important factor (40). To analyze the impact of the equine immune system on viral clearance, we established a variety of immunological assays to evaluate equine immune responses. However, given the fact that the horse is not a commonly used animal model, immunological tools are limited compared with murine model systems. Nevertheless, we explored innate and adaptive immune responses and despite viral clearance in our horses, only weak immune responses following viral challenge were detected. An earlier study using immune-deficient foals indicated that disease outcome varies based on the development of the immune system. Normal immunocompetent foals and immune-deficient foals were not able to clear NPHV infection, whereas young adult horses did clear the virus (23). Future studies directly addressing intrahepatic immune responses could further clarify the roles of specific immune cells in viral clearance. A previously resolved HCV infection does not necessarily confer sterilizing immunity against reinfection, which impedes global control of HCV infections (32). Reinfection following sustained virological response (SVR) has been reported in several studies among high-risk populations, including people who inject drugs (PWIDs) and prisoners. However, these studies are rare and sometimes show inconclusive results (32, 41). Important insights regarding reinfection and protection have been gained from earlier experiments in chimpanzees. Challenge experiments have demonstrated that despite apparently efficient immune responses in primary infection resulting in viral clearance, reinfection was possible in chimpanzees with both homologous and heterologous viruses. However, reinfection seems to be associated with improved control of viral replication, a short course of infection, and an increased viral clearance rate compared with primary infections (30, 31, 33, 42). To analyze immune protection upon previous NPHV infection, we rechallenged previously infected horses with homologous as well as with nonhomologous NPHV isolates. As NPHV exhibits low sequence diversity between isolates, classical heterologous rechallenge could not be conducted. However, homologous or distinct rechallenge experiments revealed that horses were partially protected with only trace amounts of viral RNA being detectable. This finding indicates the existence of immune mechanisms that contribute to protection. In chimpanzees, rapid virological control after reinfection has been connected to HCV-specific T-cell responses (31, 33, 34). However, first analyses of NPHV specific T-cell responses did not indicate a strong peripheral T-cell response. One limitation of the used assay is that T-cell responses were only determined with NS3-specific peptides and other immunodominant epitopes outside NS3 were not tested. Further analyses of equine hepatic T-cell responses might provide additional insights into protective immune-mediated mechanisms. In addition, the role of virus-specific antibodies needs to be further addressed. In humans, HCV strain-specific neutralizing antibodies have been found to be associated with spontaneous recovery (43, 44). Our reinfected horses displayed a higher titer of NPHV-specific antibodies before rechallenge, which even further increased after a new exposure. Although it will be interesting to analyze the presence and importance of neutralizing antibodies, these studies are currently not feasible as no cell culture system is available for NPHV.
Given the close genetic relatedness between HCV and NPHV, our study contributes to the biological characterization and to the understanding of hepaciviral pathogenesis and associated immunity. Detailed characterization of hepaciviral infections in their natural hosts will aid our understanding of viral and host determinants, which in the end, contributes to elucidate the mechanisms of HCV persistence and immunity.
Materials and Methods
Animals.
Animal experiments were first examined by the animal welfare representatives of the University of Hannover Foundation, and then approved by the Lower Saxony's official authorities (LAVES 13/1262).
Determination of Liver-Specific Enzymes.
To monitor occurrence of hepatitis, heparinized blood samples were taken at weekly time points and analyzed for the liver-specific enzymes GLDH, GGT, and AST in the laboratory of the small animal clinic at the University of Veterinary Medicine Hannover Foundation.
Extraction of Viral RNA and qRT-PCR.
Viral RNA was purified from serum samples by the High Pure Viral RNA Kit (Roche) as described in the manufacturer’s instructions. Purified RNA was subsequently transcribed into cDNA by using the Prime Script RT Master Mix Kit (Takara) with random hexamer primers. cDNA was stored at −20 °C until further analysis. A SYBR Green-based qRT-PCR was performed with SYBR Premix Ex Taq II (Takara) and previously described NPHV-specific primers targeting the 5′-untranslated region (5′-UTR) (12). Absolute quantification of NPHV RNA copies was conducted. Serial dilution of a plasmid containing the NPHV 5′-UTR sequence was used to generate a standard curve (limit of quantification 50 RNA copies per serum sample) and to calculate viremia in individual samples.
SI Materials and Methods
Animals.
The following horse breeds and genders were included in the study: Horse 1, warmblood mare; horse 2, warmblood gelding; horse 3, warmblood mare; horse 4, warmblood gelding; positive control horse (homologous infection), standardbred mare; and positive control horse (distinct infection), warmblood gelding. The ages ranged from 5 to 22 y. All horses tested negative for the presence of equine pegiviruses as described previously (45). Briefly, RNAs from serum samples were prepared with the QIAamp Viral RNA Kit (Qiagen) according to the manufacturer’s recommendations and subsequently analyzed by a Taqman-based triplex real-time qRT-PCR with probes specific for equine pegivirus 1 (EPgV 1), Theiler’s disease associated virus (TDAV), and an internal control (in vitro transcribed EGFP RNA).
Luciferase Immunoprecipitation System.
Anti-CHV/NPHV NS3 antibodies were determined as described previously (12, 20). Relative light units (RLUs) were measured in a plate luminometer (LB 960 XS3, Berthold). A cutoff was calculated by the mean value of wells containing only buffer A, the Ruc–NS3 fusion protein, and buffer A plus three SDs.
Examination of Liver Biopsies by Transmission Electron Microscopy.
Liver biopsies from healthy or NPHV-infected horses were taken and fixed with 5% (vol/vol) glutaraldehyde. The biopsies were sectioned into small pieces of ∼1 mm3 with a razor blade, washed with 0.1 M sodium cacodylate buffer, and postfixed with 1% OsO4 (Electron Microscopy Sciences) and 1.5% (wt/vol) potassium hexacyanoferrate (III) [K3Fe(CN)6; Merck] in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h on ice. Subsequently, sections were washed with H2O and fixed with 1% uranylacetate (UA) during 1 h at room temperature before they were rinsed with H2O, dehydrated with increasing concentrations of acetone [50, 70, 90, and 100% (vol/vol)], and embedded with graded series of resin (Epon, Serva). The resin-infiltrated pieces of liver biopsies were then incubated at 60 °C for 2 d. After polymerization of the resin, embedded cells were sectioned using a Leica Ultracut UCT ultramicrotome and a 35° diamond knife (Diatome). Sections with a thickness of 60 nm were collected onto 100-mesh copper grids coated with Formvar (Plano) and carbon. Sections were counterstained with 2% (wt/vol) lead citrate in H2O for 2 min and analyzed with a Biotwin CM120 Philips electron microscope (100 kV) equipped with a bottom-mounted 1 K charge-coupled device (CCD) camera (Keen View; SIS).
Histology and Immunohistochemistry.
Equine liver biopsies were fixed overnight in 10% (vol/vol) neutral-buffered formalin and embedded in paraffin wax. Tissue sections (2–3 µm) were stained with hematoxylin and eosin (H&E) or used for immunohistochemistry. Immunohistochemistry for characterization of periportal infiltrates was performed at 6, 11, or 16 wk postinoculation. For immunohistochemistry, the ABC method was applied by using antibodies directed against CD3 (rabbit polyclonal antibody, DakoCytomation), PAX-5 (mouse monoclonal antibody 24/Pax-5, BD Transduction) and myeloid/histiocyte antigen (mouse monoclonal antibody MAC387, DakoCytomation) as described (46, 47). In all cases a pretreatment with boiling in citrate buffer for 20 min was performed. For analysis, the total number of immunopositive cells in periportal areas per liver biopsy was counted for each staining. Furthermore, the obtained number of immunopositive cells was divided by the total number of inflammatory, periportally located cells in all three labelings to yield the relative amount of each inflammatory cell type.
Isolation of Peripheral Blood Mononuclear Cells.
Whole blood in sodium-heparinized blood tubes of horses was collected at different time points and PBMCs were isolated using Ficoll-Hypaque purification (Biochrom) and cryopreserved in the presence of 10% (vol/vol) dimethyl sulfoxide in FBS. PBMCs were thawed, washed, taken up in X-Vivo Medium (Lonza), and counted before use in assay.
Flow Cytometry.
For flow cytometry analysis, thawed PBMCs were seeded at a density of 1 × 106 cells and either stimulated with Cell Stimulation Mixture (eBioscience) 1:500 or left untreated. For intracellular IFN-γ staining, PBMCs were treated with a protein transport inhibitor mixture containing brefeldin A and monensin (eBioscience) and incubated for 6 h at 37 °C and 5% CO2 before flow cytometry staining. A live death staining was performed using zombie aqua (eBioscience) according to the manufacturer’s instructions. To differentiate between immune cell subsets, cells were stained for 15 min at 4 °C with combinations of antibodies specifically binding to CD8 (mouse anti-horse, clone CVS2; Bio-Rad), CD4 (mouse anti-horse, clone CVS4, Bio-Rad), PanB (mouse anti-horse, clone CVS36, Bio-Rad), CD13 (mouse anti-horse, clone CVS19, Bio-Rad), CD1w2 (mouse anti-bovine, clone CC20, Bio-Rad), and Mac387 (mouse anti-human, clone MAC387, Bio-Rad). The cells were subsequently washed with 1 mL flow cytometry buffer (PBS, 1% FCS) and fixed for 20 min with fixation/permeabilization solution (BD Biosciences). Intracellular staining was performed by addition of fluorescently labeled markers for IFN-γ (mouse anti-bovine, clone CC302, Bio-Rad) and CD3 (rat anti-human, clone CD3-12, Bio-Rad) diluted in BD Perm/Wash Buffer (BD Biosciences). Samples were measured using a FACS LSR II, and data were analyzed with FlowJo 7.6.5 software (TreeStar). Dead cells as well as cell duplets were excluded before subsequent analysis.
T-Cell Proliferation.
T-cell proliferation assays were performed as previously described with some modifications (48, 49). Briefly, 7 × 106 PBMCs were stained with 5 µM of Cell Proliferation Dye eFluor 670 (eBioscience) and incubated at 37 °C in a waterbath for 8 min. Staining was stopped by addition of 5 mL of cold FCS and incubation for 5 min on ice. After several washing steps, 2 × 105 cells were seeded into each well of a 96-well plate. PBMCs were stimulated in duplicate wells with eight distinct, overlapping NPHV NS3 peptide pools. In detail, NPHV NS3 overlapping peptides (15-mers in eight pools, 20 peptides per pool, overlapping by 10 aa) spanning the whole NS3 region of the genome corresponding to the amino acid sequences of the NPHV isolate H10 (sequence partially available under GenBank accession no. KP640276) were synthesized from PEPscreen custom peptide library (Proimmune). Individual synthetic peptides were dissolved in DMSO to a concentration of 50 mg/mL. The final concentration of each peptide within the pool upon stimulation was 5 µg/mL. As control, two wells were left untreated and supplemented with the same concentration of DMSO and two wells were stimulated with 5 µg/mL phytohemagglutinin-M (PHA-M; Sigma) as positive control. PBMCs were incubated at 37 °C and 5% CO2. After 3–4 d a medium change was performed. After 7 d PBMCs were stained with T-cell markers CD8 (mouse anti-horse, clone CVS2; Bio-Rad), CD4 (mouse anti-horse, clone CVS4, Bio-Rad), CD3 (rat anti-human, clone CD3-12, Bio-Rad), and analyzed by flow cytometry as described above.
Cytokine Detection.
Cytokines (IL-4, IL-10, IL-17, and IFN-γ) were determined in equine sera with a fluorescent bead-based, 5-plex cytokine multiplex assay using equine-monoclonal antibodies and a Luminex 200 System as described previously (50). Detection limits were as follows: IFN-γ (10–5,000 units/mL), IL-4 (40–80,000 pg/mL), IL-10 (15–35,000 pg/mL), and IL-17 (10–10,000 units/mL). The assay was performed at the Animal Health Diagnostic Center at Cornell University.
Phylogenetic Analysis.
To determine the genetically more distant inoculum for the second challenge with the more distinct NPHV isolate, NPHV glycoproteins E1E2 were amplified and sequenced according to previously published protocols (51). In brief, cDNA was produced from extracted RNA using the High-Fidelity cDNA Synthesis Kit (Roche) according to the manufacturer’s recommendations using 4 μL (100 μM) of the E1E2-specific primer A-O-hepaci-NS2 (5′-CAATATTCAAGCCACCATTAAC-3′) and subsequently amplified via nested PCR methods using the Platinum Taq DNA Polymerase High-Fidelity (Invitrogen) with the following primer pairs: S-O-EQ5UTRIAS (5′-CTGATAGGATGCTTGCGAGGGC-3′) and A-O-hepaciNS2 for the outer PCR; S-dCW02 (5′-GCCGATCTCGCTGGGTACG-3′) and A-E2W02 (5′-GCCTCATAGTATCTGAAAG-3′) for the inner run. Information about the cycling parameters is available upon request. After amplification, samples were purified from an agarose gel using the NucleoSpin Gel and PCR Clean-Up Kit (Macherey-Nagel). Sequencing was performed on an Illumina MiSeq (details on library preparation available upon request). Raw reads were mapped to previously obtained sequences (Sanger) in our laboratory and new consensus sequences were extracted using CLC Genomics Workbench 7.5.1 (Qiagen). Multiple sequence alignment was generated for the near-complete E1E2 region with MEGA6 software using MUSCLE alignment (52). Identified sequences are available in GenBank (GenBank accession nos. KY124246–KY124248). Phylogenetic tree was constructed using the maximum liklihood method based on the general time reversible model. Robustness of the analysis was assured by 1,000 iterations depicted as bootstrap value. A discrete gamma distribution was used to model evolutionary rate differences among sites [4 categories (+G, parameter = 0.4457)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 0.0000% sites). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 19 nucleotide sequences including all three codon positions. There were a total of 1,578 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (52).
Acknowledgments
We thank Susanne Möller, Anja Seemann-Jensen, Suneetha Pothakamuri, Michael Engelmann, Bettina Buck, and Petra Grünig for technical support; Peter D. Burbelo (NIH) for providing the Renilla luciferase–NS3 fusion plasmid; Uta Haselmann for her excellent technical support; all members of the Institute of Experimental Virology, Twincore, for helpful suggestions and discussions; and the Electron Microscopy Core Facilities (University of Heidelberg) and the European Molecular Biology Laboratory (Heidelberg, Germany) for providing access to their equipment. The monoclonal antibodies against equine cytokines were developed with funding from Agriculture and Food Research Initiative Competitive Grants 2005-01812 and 2015-67015-23072, supported by the USDA National Institute of Food and Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KY124246 and KY124248).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619380114/-/DCSupplemental.
References
- 1.WHO 2016 Hepatitis C: Fact Sheet no. 164. Available at www.who.int/mediacentre/factsheets/fs164/en/. Accessed February 28, 2017.
- 2.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 3.Simmonds P. Genetic diversity and evolution of hepatitis C virus: 15 years on. J Gen Virol. 2004;85(Pt 11):3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 4.Martell M, et al. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: Quasispecies nature of HCV genome distribution. J Virol. 1992;66(5):3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy KR, et al. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: Observations through HCV-TARGET database. Alimentary Pharmacology and Therapeutics. 2017;45(1):115–126. doi: 10.1111/apt.13823. [DOI] [PubMed] [Google Scholar]
- 6.Baumert TF, Fauvelle C, Chen DY, Lauer GM. A prophylactic hepatitis C virus vaccine: A distant peak still worth climbing. J Hepatol. 2014;61(1) Suppl:S34–S44. doi: 10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436(7053):961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 8.Verstrepen BE, Boonstra A, Koopman G. Immune mechanisms of vaccine induced protection against chronic hepatitis C virus infection in chimpanzees. World J Hepatol. 2015;7(1):53–69. doi: 10.4254/wjh.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaender S, Brown RJP, Pietschmann T, Steinmann E. Natural reservoirs for homologs of hepatitis C virus. Emerg Microbes Infect. 2014;3(3):e21. doi: 10.1038/emi.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartlage AS, Cullen JM, Kapoor A. The strange, expanding world of animal hepaciviruses. Annu Rev Virol. 2016;3(1):53–75. doi: 10.1146/annurev-virology-100114-055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor A, et al. Characterization of a canine homolog of hepatitis C virus. Proc Natl Acad Sci USA. 2011;108(28):11608–11613. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo PD, et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J Virol. 2012;86(11):6171–6178. doi: 10.1128/JVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drexler JF, et al. Evidence for novel hepaciviruses in rodents. PLoS Pathog. 2013;9(6):e1003438. doi: 10.1371/journal.ppat.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor A, et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. MBio. 2013;4(2):e00216-13. doi: 10.1128/mBio.00216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firth C, et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio. 2014;5(5):e01933–e14. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan PL, et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc Natl Acad Sci USA. 2013;110(20):8194–8199. doi: 10.1073/pnas.1303037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauck M, et al. A novel hepacivirus with an unusually long and intrinsically disordered NS5A protein in a wild Old World primate. J Virol. 2013;87(16):8971–8981. doi: 10.1128/JVI.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baechlein C, et al. Identification of a novel hepacivirus in domestic cattle from Germany. J Virol. 2015;89(14):7007–7015. doi: 10.1128/JVI.00534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M, et al. Divergent viruses discovered in arthropods and vertebrates revise the evolutionary history of the Flaviviridae and related viruses. J Virol. 2015;90(2):659–669. doi: 10.1128/JVI.02036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaender S, et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology. 2015;61(2):447–459. doi: 10.1002/hep.27440. [DOI] [PubMed] [Google Scholar]
- 21.Reuter G, Maza N, Pankovics P, Boros A. Non-primate hepacivirus infection with apparent hepatitis in a horse: Short communication. Acta Vet Hung. 2014;62(3):422–427. doi: 10.1556/AVet.2014.011. [DOI] [PubMed] [Google Scholar]
- 22.Scheel TK, et al. Characterization of nonprimate hepacivirus and construction of a functional molecular clone. Proc Natl Acad Sci USA. 2015;112(7):2192–2197. doi: 10.1073/pnas.1500265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsay JD, et al. Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus. Hepatology. 2015;61(5):1533–1546. doi: 10.1002/hep.27689. [DOI] [PubMed] [Google Scholar]
- 24.Parera M, Martrus G, Franco S, Clotet B, Martinez MA. Canine hepacivirus NS3 serine protease can cleave the human adaptor proteins MAVS and TRIF. PLoS One. 2012;7(8):e42481. doi: 10.1371/journal.pone.0042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anggakusuma, et al. Hepacivirus NS3/4A proteases interfere with MAVS signaling in both their cognate animal hosts and humans: Implications for zoonotic transmission. J Virol. 2016;90(23):10670–10681. doi: 10.1128/JVI.01634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger D, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76(12):5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Brey I, et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8(12):e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero-Brey I, Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6(7):2826–2857. doi: 10.3390/v6072826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thimme R, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99(24):15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett SE, et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33(6):1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 31.Prince AM, et al. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192(10):1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 32.Grebely J, et al. International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: Towards a vaccine. Lancet Infect Dis. 2012;12(5):408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nascimbeni M, et al. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77(8):4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoukry NH, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197(12):1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaender S, von Hahn T, Steinmann J, Ciesek S, Steinmann E. Prevention strategies for blood-borne viruses in the era of vaccines, direct acting antivirals and antiretroviral therapy. Rev Med Virol. 2016;26(5):330–339. doi: 10.1002/rmv.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bukh J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65(1) Suppl:S2–S21. doi: 10.1016/j.jhep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, et al. Hallmarks of hepatitis C virus in equine hepacivirus. J Virol. 2014;88(22):13352–13366. doi: 10.1128/JVI.02280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter S, et al. Ion channel function and cross-species Determinants in viral assembly of nonprimate hepacivirus p7. J Virol. 2016;90(10):5075–5089. doi: 10.1128/JVI.00132-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014;146(5):1193–1207. doi: 10.1053/j.gastro.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 40.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436(7053):946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 41.Midgard H, et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65(1) Suppl:S33–S45. doi: 10.1016/j.jhep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Lanford RE, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78(3):1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavillette D, et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79(10):6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logvinoff C, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101(27):10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Postel A, et al. Frequent presence of hepaci and pegiviruses in commercial equine serum pools. Vet Microbiol. 2016;182:8–14. doi: 10.1016/j.vetmic.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 46.Lehmbecker A, et al. Neurolymphomatosis in three horses with multicentric T-cell-rich B-cell lymphoma. J Comp Pathol. 2014;151(2–3):181–185. doi: 10.1016/j.jcpa.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich F, et al. Immunophenotyping of immune cell populations in the raccoon (Procyon lotor) Vet Immunol Immunopathol. 2015;168(3–4):140–146. doi: 10.1016/j.vetimm.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Suneetha PV, et al. Effect of peptide pools on effector functions of antigen-specific CD8+ T cells. J Immunol Methods. 2009;342(1–2):33–48. doi: 10.1016/j.jim.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Schlaphoff V, et al. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathogens. 2011;7(5):e1002045. doi: 10.1371/journal.ppat.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner B, Freer H. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol. 2009;127(3–4):242–248. doi: 10.1016/j.vetimm.2008.10.313. [DOI] [PubMed] [Google Scholar]
- 51.Gather T, et al. Vertical transmission of hepatitis C virus-like nonprimate hepacivirus in horses. J Gen Virol. 2016;97(10):2540–2551. doi: 10.1099/jgv.0.000561. [DOI] [PubMed] [Google Scholar]
- 52.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]