Significance
During cell division, microtubules apply piconewton forces to segregate duplicated chromosomes into daughter cells. The kinetochore, located on the surface of the centromere chromatin, couples microtubules to the chromosomes. Little is known about the folding of the centromeric chromatin and how this templates the functional ultrastructure of the kinetochore. To better understand this fundamental problem, we used a microscopy technique that allowed the DNA associated with centromeric chromatin to be unfolded and accurately measured in the presence and absence of several key kinetochore components. By combining this microscopy method with statistical analysis of the unfolded chromatin fibers, we acquired data that allowed a subunit model of the kinetochore chromatin to be proposed.
Keywords: centromere, kinetochore, CENP-A, centrochromatin, boustrophedon
Abstract
During cell division, interactions between microtubules and chromosomes are mediated by the kinetochore, a proteinaceous structure located at the primary constriction of chromosomes. In addition to the centromere histone centromere protein A (CENP-A), 15 other members of the constitutive centromere associated network (CCAN) participate in the formation of a chromatin-associated scaffold that supports kinetochore structure. We performed a targeted screen analyzing unfolded centrochromatin from CENP-depleted chromosomes. Our results revealed that CENP-C and CENP-S are critical for the stable folding of mitotic kinetochore chromatin. Multipeak fitting algorithms revealed the presence of an organized pattern of centrochromatin packing consistent with arrangement of CENP-A–containing nucleosomes into up to five chromatin “subunits”—each containing roughly 20–30 nucleosomes. These subunits could be either layers of a boustrophedon or small loops of centromeric chromatin.
The centromere is the genetic locus located at the primary constriction of mitotic chromosomes that directs chromosome segregation. Biochemically, the centromere is defined by the presence of the histone H3 variant centromere histone centromere protein A (CENP-A) (1, 2) interspersed with canonical H3 nucleosomes carrying active chromatin marks (3, 4). This specialized chromatin class has been termed “centrochromatin” (5). During cell division, an elaborate multisubunit protein superstructure, the kinetochore, assembles on the surface of the centrochromatin to direct chromosome segregation.
Kinetochores contain ≥100 different proteins, 16 of which comprise the constitutive centromere associated network (CCAN). The CCAN remains associated with centromeric chromatin across the entire cell cycle (6–8). The CCAN includes CENP-A, CENP-C, and four multisubunit complexes: CENP-L/-N (9), CENP-H/-I/-K/-M (10), CENP-O/-P/Q-/R/-U (11), and CENP-T/-W/-S/-X (12–15).
Although numerous immuno-electron microscopy (16–18) and superresolution microscopy (19–21) studies have mapped the locations of CCAN components relative to one another, the packing of the chromatin fiber in centrochromatin remains unknown. Early studies of stretched chromosomes suggested a repeating “subunit” structure for the kinetochore (1, 22). One subsequent hypothesis was that centrochromatin is composed of “amphipathic” helices or loops, with CENP-A–containing nucleosomes facing the outer kinetochore and H3 chromatin oriented toward the interior (5). A recent study proposed that centrochromatin is folded back and forth into a sinusoidal patch or boustrophedon with a multilayered structure stabilized during mitosis by CENP-C (4).
Here, we have dissected centrochromatin organization by progressively unfolding the chromatin at low ionic strength in lysed interphase and mitotic cells. Measurement of the lengths of the resulting fibers revealed that centrochromatin unfolds in a series of discrete (∼0.5 µm) steps, consistent with a repeat substructure. CENP-C and CENP-S separately contribute to the stability of the centrochromatin structure during mitosis.
Results and Discussion
Step-Wise Unfolding of CENP-A Centrochromatin.
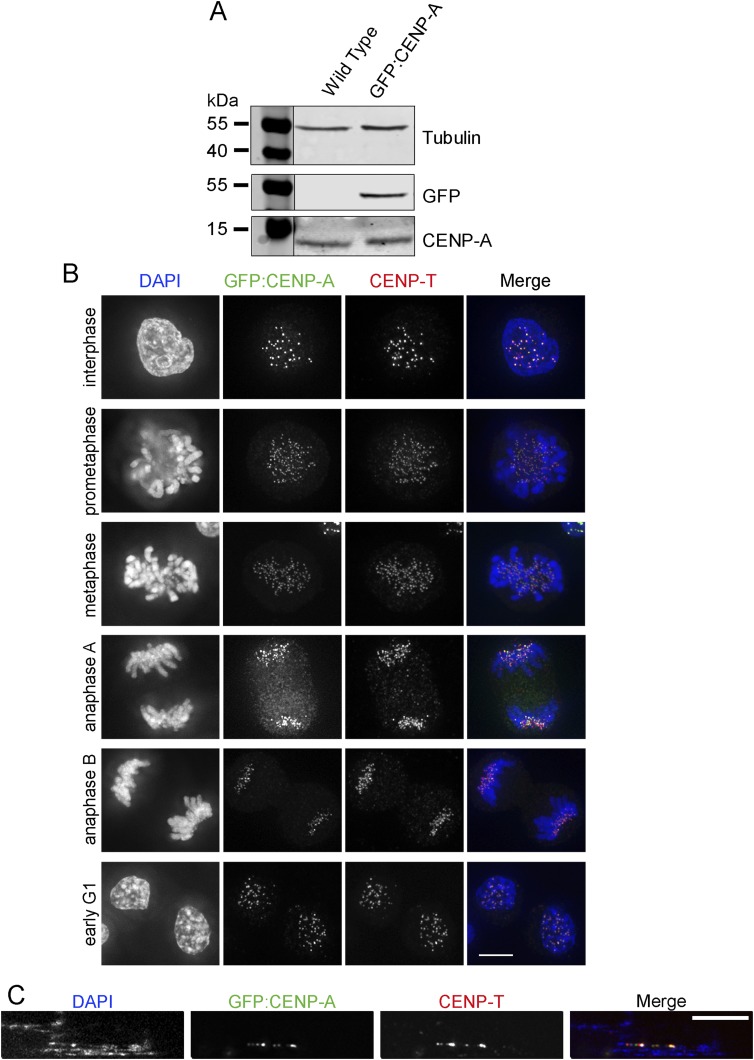
To characterize the folding of centromeric chromatin, we exploited the ability of low-salt TEEN buffer, which lacks divalent cations (Materials and Methods) to unravel highly compact kinetochore chromatin into extended fibers (23) (Fig. 1A). To identify unfolded centromeric regions, we generated cells expressing GFP:CENP-A from a DT40 wild-type cell line. Expression of exogenous GFP:CENP-A had no effect on endogenous CENP-A levels (Fig. S1A). Furthermore, GFP:CENP-A was not present on chromosome arms (Fig. S1B), a potential artifact associated with CENP-A overexpression. GFP:CENP-A was found exclusively at the kinetochore, colocalizing with CENP-T, at all cell-cycle stages (Fig. S1B)—even on unfolded centrochromatin (Fig. S1C). This cell line was used to analyze centromere unfolding in both interphase and mitotic cells (Fig. 1B).
Fig. 1.
Unfolding of centromere chromatin in interphase versus mitotic samples. (A) Schematic explaining the method used to unfold chromatin using TEEN buffer. TEEN has a low-salt concentration and contains EDTA as a divalent cation chelator. The excess of negative charges on the DNA and the hypotonic environment together cause cells to burst and the chromatin to unfold. (B) Representative fluorescence micrographs of unfolded centrochromatin fibers, detected using GFP:CENP-A and DAPI. (Scale bar, 1 μm.) (C) CLEM analysis of unfolded chromatin from asynchronous cells. DAPI and GFP:CENPA were used to identify typical unfolded fibers. The same regions were revisited using TEM. (Scale bar, 50 nm.) Fibers visualized by TEM were analyzed using multiple line scans (see representative line scan in black; scale bar, 50 nm) and pixel density measurements. The data were plotted in a line graph where the line profile represents an average of five line scans with SD. Vertical red lines mark the edges of electron dense regions (i.e., the width of the chromatin fiber). (D) Box and whisker plots showing the median fiber length for interphase and mitotic samples; the height of the box defines the interquartile range, and whiskers indicate the 10th and 90th percentile. Asterisks indicate statistical significance of differences in fiber length between interphase and mitosis (***P < 0.0001; Mann–Whitney U test) where n = 655fibers in total per each sample, over three independent experiments. (E) Bar chart showing GFP:CENP-A total fluorescence plotted as a function of centromere length (n = 50). A range of fiber lengths up to 2.5 μm were tested. Data are presented as mean ± SEM, with bins of 0.5-μm increments.
Fig. S1.
Characterization of DT40 cells stably expressing GFP:CENP-A. (A) Western analysis of whole-cell lysate prepared from DT40 cells stably expressing GFP:CENP-A or the wild-type cell line (control). The membrane was probed with primary antibodies recognizing CENP-A, tubulin, and GFP. A LI-COR system was used for imaging. (B) Indirect immunofluorescence of cells expressing GFP:CENP-A. Cells were probed with anti–CENP-T antibody (red). Mitotic stages are indicated on the left of the panels. (Scale bar, 5 μm.) (C) Representative image of DT40 cells stably expressing GFP:CENP-A where CENP-A signal colocalizes with CENP-T on unfolded fibers. (Scale bar, 5 μm.)
We used correlative light and electron microscopy (CLEM) to determine the extent of chromatin unfolding induced by TEEN treatment. A line-scan analysis of correlative EM images confirmed the presence of fibers with a mean diameter of 12.6 ± 2.19 nm, consistent with the diameter of a single chromatin fiber (Fig. 1C). Thus, TEEN treatment can unfold chromatin to the level of single fibers.
Collective analysis of >1,300 individual centrochromatin fibers revealed that interphase prekinetochores unfolded to a significantly greater extent than mitotic kinetochores [unfolded length of CENP-A domain, 1.664 ± 0.049 µm versus 0.936 ± 0.025 µm (median ± SEM) respectively; Fig. 1D]. The increased stability presumably allows mitotic kinetochores to resist forces applied by spindle microtubules during chromosome movements.
To confirm that only single centromeres were analyzed, we measured GFP:CENP-A fluorescence levels as a function of chromatin fiber length (Fig. 1E). Total GFP:CENP-A amounts remained constant across a range of fiber lengths up to 2.5 µm (mean = 32,240.46 ± 3,872.53). This strongly suggests that each unfolded CENP-A subunit analyzed consists of a single unfolded centromere.
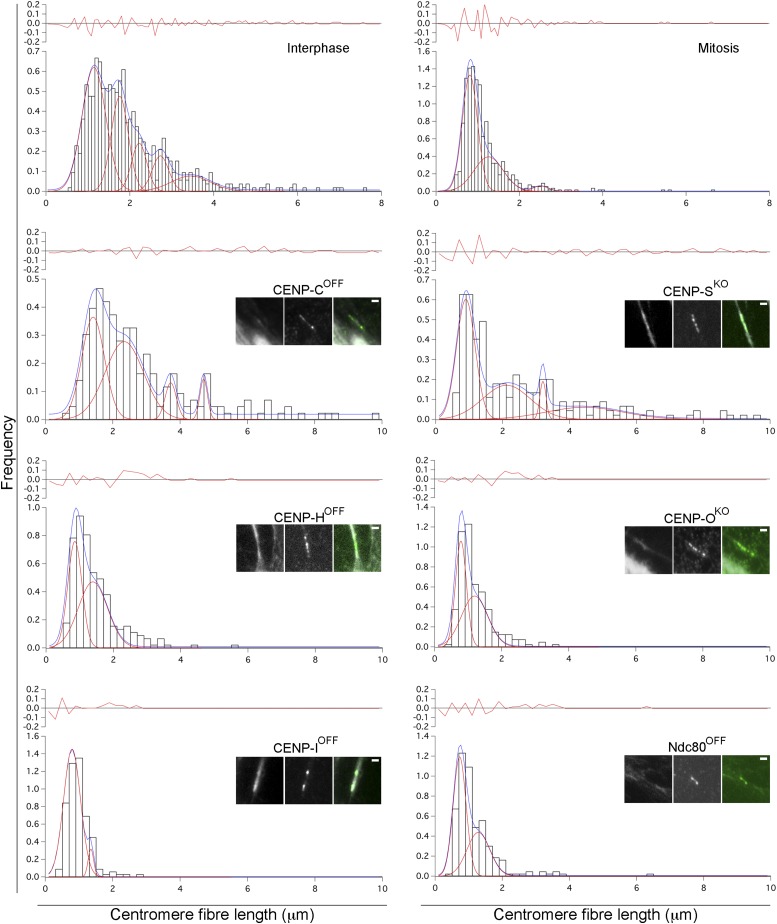
To determine whether centromeres unfold at random or in discrete steps, we analyzed the distribution of unfolded fiber lengths using frequency histograms and multipeak fitting algorithms to reveal periodicities in the data. Our comprehensive datasets (>650 measurements per sample) allowed us to generate high-resolution histograms (100 × 0.1 μm bins). This revealed the apparent presence of subpopulations of unfolded fibers, an observation masked with coarser bin widths (Fig. S2A). We then defined the periodicities observed (Fig. S2B), using the multipeak fitting software Igor Pro-6.2 (WaveMetrics, Inc.) (Materials and Methods). Five distinct peaks were recognized in interphase unfolded centrochromatin (Fig. 2A) and only three peaks in its mitotic counterpart (Fig. 2B). Each peak represented a node of accumulation of subpopulations of fibers, each corresponding to a potential subunit of centrochromatin released from the kinetochore.
Fig. S2.
Increasing the number of bins increases the resolution of the histograms. (A) Two columns of frequency histograms containing fiber length data for interphase and mitotic samples. Columns show progressive improvements in the histogram resolution by adjusting the number of bins and bin width (bin width indicated in brackets); 50 bins (200 nm), 60 bins (166 nm), 80 bins (125 nm), and 100 bins (100 nm). The scale on the y axis has been kept different for interphase and mitosis to allow a better visualization of the peaks in the two datasets. (B) Frequency histograms containing both datasets allotted into 100 bins (100 nm) are merged together in one plot with the same scale on the y axis.
Fig. 2.
The centromere is composed of multiple dynamic chromatin layers. Multipeak analysis using datasets of unfolded centromere chromatin fibers from interphase and mitotic samples. A and B show probability density histograms (white bars). The x and y axes show centromere fiber length (μm) and frequency, respectively. Data are allotted into 100 bins, each with a resolution of 100 nm per bin. Multipeak fitting algorithm identified putative populations of fiber lengths within the datasets, depicted by discrete peaks (red lines). Best fitting curve is also shown (blue line) for both samples.
To estimate the amount of DNA present in each unfolding subunit, we determined the density of nucleosome packing in mitotic chromatin fibers unfolded under these conditions by analyzing TEM of chromosome spreads prepared in TEEN buffer. The average center-to-center distance between adjacent nucleosomes was 20.43 ± 0.68 nm (Fig. S3A). The comparable distance from chicken erythrocyte interphase chromatin in low-salt buffer was 38.71 ± 1.6 nm (Fig. S3B). These numbers could not be measured specifically at centromeres and thus provide only baseline values for estimating the chromatin packing.
Fig. S3.
Quantification of the internucleosome distance. (A) TEM of mitotic chromosomes centrifuged onto a carbon film after TEEN buffer treatment. The center-to-center distance between adjacent nucleosomes (n = 203) was determined to be 20.4 ± 0.68 nm (mean ± SEM). The Inset represents a 2× zoom. (Scale bar, 50 nm.) (B) Micrograph of interphase chromatin from chicken erythrocytes spread onto grids and imaged by TEM following rotary shadowing. The center-to-center distance between adjacent nucleosomes (n = 65) was determined to be 38.71 ± 1.6 nm (mean ± SEM). The Inset represents a 2× zoom. (Scale bar, 50 nm.) (C) Schematic of the calculations used to predict the DNA content at the chicken centromere.
Having measured peak locations and an approximate internucleosome distance, we could estimate the amount of DNA present within the kinetochore. The distances between the fiber origin, the first peak, and pairs of adjacent peaks (steps) were interpreted as measures of chromatin length per unfolding subunit. Interphase fibers unfolded in five steps with a mean step size of 0.69 ± 0.24 µm (Fig. 3A). Assuming 200 bp per average nucleosome, this corresponds to 17.8 Kbp of DNA (Fig. S3C). In mitosis, we identified three more variable steps (0.83 ± 0.33 µm) corresponding to roughly 24.4 Kbp of chromatin (Fig. 3B and Fig. S3C). These estimates of DNA content assume that the centrochromatin unfolds completely and are therefore almost certainly underestimates.
Fig. 3.
Quantification of the steps of unfolding. Schematic displaying the subpopulations of the peaks (gray triangle, μm) and the distance of the interval between two consecutive peaks (μm). (A and B) Chromatin unfolding periodicities observed for wild-type chromosomes in interphase and mitosis, respectively. (C–H) Chromatin unfolding periodicities from mitotic cells corresponding to the various mutants listed to the left of each panel. Data obtained from multipeak fitting analysis are in Fig. S7.
If we exclude the first step, subsequent steps of unfolding of interphase chromatin are remarkably reproducible, with an average step size of 0.58 ± 0.1 µm, corresponding to roughly 15 nucleosomes (Fig. 3A). Interestingly, the first step is almost exactly twice this. A similar consideration of the mitotic unfolding is more speculative, given the apparent variable spacing and small number of steps. However, the minimum observed step (0.45 µm, ∼22 nucleosomes) is close to the average step size observed for interphase chromatin and, again, almost exactly half the length of the first step.
Remarkably, a recent paper looking at human centromeres calculated that 1 in 25 centromeric nucleosomes contains CENP-A (24). Although the corresponding measurements have not been made for DT40 cells, the correlation with the average subunit size measured here is striking. It is therefore possible that each centrochromatin subunit is organized around a single CENP-A nucleosome in DT40 cells.
It is tempting to speculate that unfolding of centrochromatin in low ionic strength buffer begins for interphase chromatin with two “subunits,” followed by four individual steps, and in mitotic chromatin with two steps of one single subunit. The differences in total length suggest either that the CENP-A chromatin domain is smaller in mitosis compared with interphase or (more likely) that the mitotic chromatin is more constrained and unfolds only partly.
What are the subunits likely to be? Given that they correspond to roughly 20–30 nucleosomes, we suggest that it is unlikely that they would correspond to gyres of a chromatin helix (Fig. 4A). The solenoid as described by Finch and Klug was proposed to have from 4 to 10 subunits per turn (25), and increasing this two- or threefold would give rise to chromatin fibers much wider than typically seen. They could, however, correspond to folded loops (Fig. 4B) or to successive layers of a boustrophedon (a stack of planar sinusoidal patches) (Fig. 4C) (4). Interestingly, the typical width of a kinetochore plate measured by electron microscopy in DT40 cells is 227 nm (26). This would easily accommodate layers containing 25 nucleosomes interlinked by other components of the CCAN.
Fig. 4.
Comparison of models for centrochromatin structure. (A) Solenoid in which CENP-A (red) and H3 (gray) nucleosomes are organized at centromeres into helical gyres (1 gyre per subunit) or (B) loops clustered next to each other (1 loop per subunit). These two models were first proposed by ref. 3. Gyres and loops diagrammed here could both generate unfolded fibers in the presence of TEEN with an unfolded length of roughly 0.5 μm. (C) Boustrophedon model consisting of a stack of planar sinusoidal patches (layers) of centrochromatin (1 layer is a subunit). As a response to TEEN buffer, single layers of the boustrophedon might unfold into chromatin fibers 0.5 μm long.
The Role of CCAN Proteins in the Maintenance of Kinetochore Chromatin Folding.
To further dissect the role of individual CCAN components in stabilizing subunit interactions in centrochromatin, we analyzed fiber unfolding following the depletion of specific CENPs. This analysis used conditional knockouts (designated GENENAMEON/OFF) for CENP-C; CENP-H and CENP-I (from the CENP-H/-I/-K/-M complex); CENP-N (from the CENP-L/-N complex), CENP-T/-W (from the CENP-T/-W/-S/-X complex), Ndc80 (from the Ndc80 complex), and absolute knockouts (designated GENENAMEKO) for the nonessential proteins CENP-S and CENP-O (from the CENP-O/-P/-Q/-R/-U complex). Generation of these knockout cell lines was previously described (4, 7, 14, 26–32). We confirmed that the growth properties of each cell line remained as previously described for the original knockouts (Figs. S4B and S5B).
Fig. S4.
Characterization of DT40 conditional knockouts or deletion cell lines stably expressing GFP:CENP-A. (A) Indirect immunofluorescence of cells expressing GFP:CENP-A probed with anti–CENP-T antibody (red). The localization of GFP:CENP-A is at the kinetochore in all of the conditions analyzed. (Scale bar, 5 μm.) (B) Growth curves of mutant cell lines plus or minus doxycycline and DT40 control cell line. (C) Western blot of whole-cell lysate of samples before the addition of doxycycline.
Fig. S5.
Characterization of CENP-N and CENP-W conditional knockouts or inducible auxin-degron for CENP T-cell lines stably expressing GFP:CENP-A. (A) Indirect immunofluorescence of cells expressing GFP:CENP-A and probed with anti–CENP-T antibody (red). The localization of GFP:CENP-A is at the kinetochore in all of the conditions analyzed. (Scale bar, 5 μm.) (B) Growth curves of mutant cell lines in the presence of doxycycline. The growth curves for AID-CENP-T:CENP-TON/OFF cells plus and minus auxin are shown in ref. 32, figure 2C. These cells begin to die within 24 h of the addition of auxin. (C) Western blot of whole-cell lysate of samples with no addition of doxycycline or auxin.
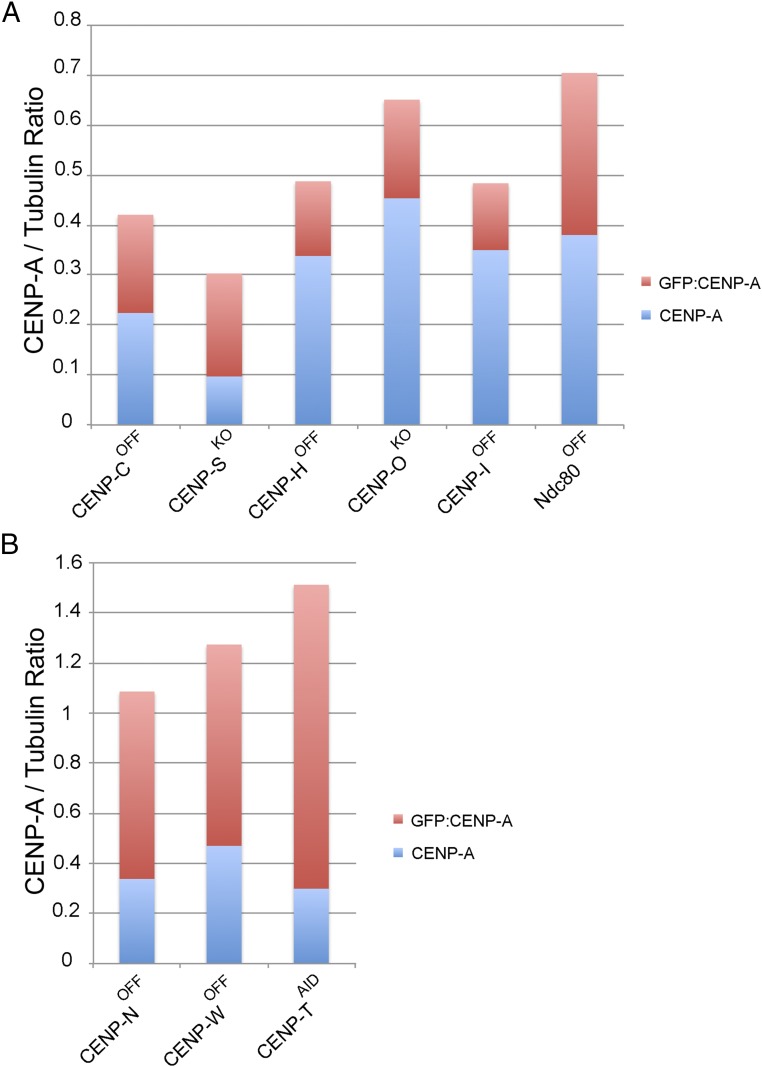
Each mutant cell line was engineered to stably express GFP:CENP-A. GFP:CENP-A colocalized with CENP-T at centromeres both in the presence or absence of doxycycline (Fig. S4A). CENP-T localization was decreased following CENP-H and CENP-I depletion and abolished in CENP-N, CENP-T, and CENP-W mutants (Fig. S5A). Immunoblotting analysis showed some variability in the expression levels of the total CENP-A across the cell lines (Figs. S4C, S5C, and S6).
Fig. S6.
Quantification of CENP-A levels. The quantification of the bands for endogenous CENP-A, GFP:CENP-A, and tubulin from the blots shown in Fig. S4C (A) or Fig. S5C (B) was performed using ImageJ. The graphs show the levels of endogenous CENP-A (blue) and GFP:CENP-A (red) after normalization with tubulin.
To examine the role of individual proteins in the stability of mitotic kinetochore chromatin, cells were depleted of target proteins (+dox or +aux) and synchronized in mitosis, before processing for fiber analysis. A striking difference in the median unfolded length was seen between mitotic centrochromatin fibers from wild type (0.936 ± 0.025 µm) and both CENP-COFF (2.207 ± 0.135 µm) and CENP-SKO (1.66 ± 0.143 µm) cells (Fig. 5A). Strikingly, CENP-C depletion resulted in an even greater extension of the mitotic centromere than was seen with wild-type interphase fibers (1.664 µm ± 0.049; P < 0.0001) (Fig. 5A).
Fig. 5.
Unfolding of centromere chromatin in CENP mutants. (A and B) Box and whisker plots displaying the spread of the datasets of unfolded fiber length measured in (A) wild type and the indicated mutants, after being blocked in nocodazole, and (B) wild-type and indicated mutant asynchronous cells. The height of the box defines the interquartile range; whiskers indicate the 10th and 90th percentile. The n number is specified in each box. (A) Each conditional knockout cell line has been tested for significant difference against unfolded fibers from control interphase or control mitosis. (B) Mann–Whitney U test was performed confirming statistically significant differences between the mutants and wild-type fibers. (A and B) Mann–Whitney U test: ***P < 0.001; ns, not significant; P > 0.05.
Small albeit statistically significant changes were also observed following the depletion of CENP-H, CENP-T, CENP-I, and CENP-N (Fig. 5A). These effects are small, and in an earlier, less extensive, study, depletion of CENP-H appeared not to affect the stability of the mitotic kinetochore (4). No destabilization was observed for mitotic centrochromatin fibers from CENP-OKO, Ndc80OFF, or CENP-WOFF cells.
Analysis of the unfolding step sizes for mitotic centrochromatin revealed the existence of two classes of mutants (Fig. 3 and Figs. S7 and S8). The first, composed of CENP-HOFF, CENP-IOFF, Ndc80OFF, CENP-OKO, CENP-NOFF, CENP-WOFF, and CENP-TAID cells (Fig. 3 and Figs. S7 and S8), showed a mean unfolding step of 0.49 ± 0.05 µm (compared with 0.45 µm for wild-type mitotic centrochromatin), which was preceded by a mean step of 0.76 ± 0.04 µm (0.81 µm in wild type). No third step was seen in these samples, possibly due to the decreased sample size, as that step corresponded to only 3% of unfolded kinetochore fibers from mitotic wild-type cells. These data reveal that mitotic centrochromatin from CENP-HOFF, CENP-IOFF, Ndc80OFF, CENP-OKO, CENP-NOFF, CENP-WOFF, and CENP-TAID cells apparently unfolds like wild-type centrochromatin. Therefore, these kinetochore components play at most a minor role in stabilizing the mitotic kinetochore chromatin packing as detected by this assay.
Fig. S7.
Multipeak analysis of centromere chromatin fibers unfolding datasets in CENP mutant cell lines. For all of the panels, the bottom part of each graph shows probability density histograms, where x and y axes show frequency and centromere fiber length (μm), respectively; the datasets are divided into 100 bins (interphase and mitosis wild type; Fig. 2 and Fig. S2) with a resolution of 100 nm or 50 bins (mutants) with a resolution of 200 nm per bin. Histograms of centromere fiber length showing putative populations of fiber lengths within the datasets underlined by the diverse peaks (red lines). The scale on the y axis has been kept different for the different mutants to allow a better visualization of the peaks in the datasets. The best fitting curve is shown (blue line) for all of the samples. (Inset) Fluorescent images of centromere fibers are shown for each mutant; DAPI, GFP, and merge, respectively. (Scale bar, 1 μm.) The upper part of each graph highlights the amount of residuals.
Fig. S8.
Centrochromatin unfolding analysis of mitotic CENP-N–, CENP-W–, and CENP-T–depleted cells. (A) Schematic displaying the subpopulations of the peaks (μm) and the distance of the interval between two consecutive peaks (μm). Data were obtained from multipeak fitting analysis in B. (B) For all panels, the bottom part of each graph shows probability density histograms, where x and y axes show frequency and centromere fiber length (μm), respectively; the datasets are divided in 50 bins with a resolution of 200 nm per bin. Histograms of centromere fiber length showing putative populations of fiber lengths within the datasets underlined by the diverse peaks (red lines). The scale on the y axis has been kept different for the different mutants to allow a better visualization of the peaks in the datasets. The best fitting curve is shown (blue line) for all of the samples. (Inset) Fluorescent images of centromere fibers are shown for each mutant; DAPI, GFP, and merge respectively. (Scale bar, 1 μm.) The upper part of each graph highlights the amount of residuals.
In contrast, the unfolding pattern exhibited by centrochromatin from CENP-COFF and CENP-SKO cells was very different from that seen with either wild type or the other mutants (Fig. 3 and Fig. S7). Unfolding involved four steps instead of the three seen in wild type, and in contrast to the other examples, the unfolding proceeded in relatively equal steps (i.e., not two subunits followed by one). Furthermore, each step was roughly twice the length of the minimum step seen for wild type. Thus, mitotic centrochromatin from CENP-COFF and CENP-SKO cells unfolded with a mean step size of 1.172 ± 0.12 µm and 1.110 ± 0.08 µm, respectively (Fig. 3 C and D). This is consistent with a model where CENP-C and CENP-S are required to link adjacent centrochromatin subunits (e.g., loops or layers of a boustrophedon) together.
In addition to the different step size, unfolded centrochromatin fibers from CENP-SKO and CENP-COFF cells average 1.8–2.4 times longer than the corresponding fibers from wild type (Fig. 5A). Thus, in addition to causing a different pattern of unfolding, the loss of CENP-C and CENP-S also results in a greater overall extent of kinetochore unfolding. This reinforces the conclusion that the organization of the kinetochore chromatin in these mutant cells is significantly different from that in wild type.
Importantly, interphase centrochromatin from CENP-COFF and CENP-SKO cells behaves very differently (Fig. 5B). CENP-COFF interphase centrochromatin unfolds to the same extent as wild type, but CENP-SKO interphase centrochromatin unfolds to a significantly greater extent. This strongly suggests that even though both proteins are required for the stabilization of centrochromatin, they may do so via distinct mechanisms.
Our data support previous conclusions that the inner kinetochore protein CENP-C (17, 20) forms a nexus for multiple interactions that stabilize the mitotic kinetochore, among other things, determining the diameter of the outer kinetochore plate (33). Indeed, CENP-C is required to efficiently recruit both inner and outer kinetochore components during kinetochore assembly (34–37).
The destabilization of mitotic kinetochores in CENP-SKO cells was surprising. CENP-S is part of the heterotetrameric CENP-T/-W/-S/-X complex (12). However, loss of CENP-W had no effect and CENP-T only a minor effect on centrochromatin stability in our assay. Detectable (though reduced) levels of CENP-T are retained at kinetochores of CENP-SKO cells, and chromosomes appear to contain both CENP-T/-W and CENP-T/-W/-S/-X complexes (37) (Fig. S4A). Similarly to CENP-C, an electron microscopy study reported that CENP-S depletion could affect kinetochore plate size (26).
Consistent with these effects on kinetochore plate size, quantitation of immunoblots revealed that the total CENP-A levels were lowest in CENP-COFF and CENP-SKO cell lines (Fig. S6). However, human cells normally contain excess CENP-A molecules (24), and chicken cells survive up to 4 days following CENP-A depletion, by which time CENP-A molecules have been diluted 12-fold (38). The lower level of CENP-A is unlikely to explain the centrochromatin destabilization in those mutants, as there is very little difference in total CENP-A levels between CENP-COFF and CENP-IOFF cells even though the centrochromatin is destabilized in one and normal in the other.
Possible roles of CENP-S at the kinetochore are complicated by the fact that this protein also functions in DNA repair. CENP-S/MHF1 has a role in the resolution of DNA interstrand crosslinks and sister chromatid exchanges (SCEs) by the Fanconi Anemia complex. CENP-S is required for chromatin targeting and stability of the FANCM subcomplex, of which it is a member together with CENP-X/MHF2 (39, 40). In DT40 cells, SCEs increased 3–4-fold when CENP-S/MHF1 was depleted (39, 40). Thus, CENP-S involvement in centrochromatin stability may reflect a more general role in chromatin higher order structure across the cell cycle.
Our results suggest that CENP-H/-I/-K/-M and Ndc80 complexes act within individual centrochromatin subunits or between subunits and nonchromatin components of the kinetochore. In contrast, CENP-C has a web of interactions with other CCAN members, including CENP-A and CENP-H/-I/-K/-M (35, 36, 41) as well as outer kinetochore components (10, 42). CENP-T/W/S/X also interacts both with CENP-H/-I/-K/-M (10) and the Ndc80 complex (13, 43). We considered whether interactions between the inner and outer kinetochore might stabilize mitotic centrochromatin, but this is unlikely, as kinetochores lacking Ndc80 have a subunit organization and mitotic stability similar to wild type (Figs. 3H and 5A).
The dependencies on centrochromatin stability observed here do not appear to correspond to the recent description of “core” and “expandable” kinetochore modules described recently in Xenopus extracts (44). There, CENP-A, CENP-H/-I/-K/-M, CENP-T/W/S/X, and Ndc80 were all found to be part of the core kinetochore that was unaffected by the loss of microtubules, whereas CENP-C was involved in the expansion that occurred when microtubules were absent. The authors suggested that this expansion did not involve the centrochromatin but rather corresponded to a polymerization of protein complexes, in which the multifunctional CENP-C played a key role. This is consistent with our observation that both a core component (CENP-S) and an expandable component (CENP-C) are involved in mitotic centrochromatin stability.
The role of CENP-O/-P/-Q/-R/-U in kinetochore organization remains enigmatic. Studies in Saccharomyces cerevisiae report a role for the COMA complex (CENP-O/-P/-Q/-R/-U complex homolog) in the looping of centromere chromatin (45). However, our results plus other recent studies have failed to identify a function for this complex in vertebrate kinetochores (37, 41).
Given the measurements of internucleosome distance in bulk chromatin of mitotic chromosomes and interphase nuclei unfolded in low-salt buffer (23), we estimated the amount of DNA present at kinetochores in the different cell lines. According to the lengths measured for CENP-A fibers, these values ranged from 8–45 Kbp. The smaller number likely corresponds to fibers that were not completely unfolded, whereas the larger number (from unfolded CENP-COFF and CENP-SKO chromosomes) was remarkably close to the estimated 50–60 kb of DNA in chicken kinetochores determined by quantitative fluorescence microscopy (46) and the ∼40 kb of DNA occupied by CENP-A in chicken nonrepetitive centromeres and neocentromeres determined by ChIP (47).
In the future, it will be important to devise superresolution imaging strategies in which a centrochromatin fiber can be traced in intact mitotic chromosomes. A recent study revealed that kinetochores form large crescents during early prometaphase when they are “searching” for microtubules and become more compact structures once the attachments have matured (48). This raises an extremely interesting fundamental question of whether the underlying chromatin reorganization also changes at this time.
Materials and Methods
Detailed electron microscopy procedures are described in SI Materials and Methods.
Fiber Length Preparation and Length Measurements.
Chromatin fibers were prepared using TEEN buffer (10 mM Triethanolamine:HCl, pH 8.0, 10 mM NaCl, 5 mM EDTA) using an optimized version of a previously described method (49). GFP:CENP-A unfolded centrochromatin was imaged using a CCD camera (CoolSnap HQ, Photometrix) on a wide-field microscope (DeltaVision Spectris; Applied Precision) with a N.A. 1.4 Plan Apochromat 100× lens controlled by DeltaVision SoftWorx (Applied Precision). ImageJ (National Institute of Health) segmented line tool was used to measure centromere chromatin fiber length.
Multipeak Analysis.
Fiber unfolding data were imported in Igor Pro-6.2 (WaveMetrics, Inc.). Data sets were allotted into the appropriate number of histogram bins. The multipeak fitting 2.0 package was used for peak identification using “Auto-Locate Peaks Now.” This automatic peak finding algorithm searches for and identifies subpopulations by finding maxima in the smoothed second derivative of the data. To achieve this, the algorithm estimates both the noise level and optimum smoothing factor of the data. All adjustable parameters were kept within the same range across all samples: noise level, 0.00005–0.06; smooth fraction, 0.05–2.5; minimal fraction, 0.035–0.5. After an initial estimation of the peaks, the fitting algorithm was run and results were summarized in a table containing information about peak location, area, type, amplitude, and residuals. Residuals, or fitting deviations, represent the difference between the observed values with the predicted sample mean and the best fit curve. A positive value of residuals suggests that the measured value is placed above the best fitting curve, whereas a negative one is referred to a value located underneath it. If the best fit curve passes through the value measured, then the residuals equal zero. According to Igor Pro guidelines, good fitting is achieved when deviation of the residuals is less than 0.1.
SI Materials and Methods
Cell Culture.
Chicken B lymphoma DT40 cells were grown in RPMI medium 1640 supplemented with 10% (vol/vol) FBS, 1% chicken serum, and 1% penicillin/streptomycin at 39 °C in 5% CO2. CENP-C (30), CENP-H (27), CENP-I (28), Ndc80 (29), and CENP-TAID (32) conditional knockout cell lines were transfected with GFP:GgCENP-A cloned in pEGFPC1 vector with a 17-amino acid linker (50). Cell lines stably expressing GFP:GgCENP-A were obtained by coelectroporation with puromycin, hygromycin, and geneticin-resistant markers. CENP-N and CENP-W conditional knockout cell lines stably expressing GFP:CENP-A were previously generated (4). The addition to the media of 500 ng/mL of doxycycline or auxin at the final concentration of 125 μM destroyed the expression of the rescuing cDNA. DT40 cells were blocked in mitosis by treating with 500 ng/mL nocodazole for 12 h.
Cell Vital Counts Using Trypan Blue.
For cell count experiments, one part of trypan blue was added to one part of cell suspension at room temperature. DT40 cells were maintained at a concentration of 20 × 104 cells per mL at each dilution time.
CENP-A Total Fluorescence Quantification.
For 50 fibers randomly picked, images were deconvolved and projected into ImageJ (National Institute of Health), and the area occupied by CENP-A signal along each fiber was highlighted by thresholding and then selected with the magic wand. The fluorescence, measured in ImageJ, was annotated. Graphs were produced in Microsoft Excel.
CLEM of Unfolded Fibers.
The CLEM protocol was adapted from a previously established method (51). DT40 cells expressing GFP:CENPA were seeded onto ConA-coated glass-bottomed gridded dishes (MatTeK Corporation, USA) and left to adhere for 1 h. Fibers were prepared using the standard protocol until the point of fixation. Fibers were fixed for 1 h with 3% (vol/vol) glutaraldehyde and 0.5% formaldehyde in 0.2 M sodium cacodylate buffer containing 5 μg/mL Hoechst. Fibers were washed with PBS and imaged in PBS using a DeltaVision microscope (Applied Precision) where GFP:CENP-A centrochromatin was detected. Transmitted light was used to map cell positions via reference coordinates. The reference images allowed for the correlative reidentification of cells of interest by electron microscopy. DeltaVision acquisition was followed by treatment with tannic acid (0.1% in water) for 20 min, followed by osmication (1% osmium tetroxide in PBS) for 1 h. Samples were then washed with PBS, ddH2O, and 30% (vol/vol) ethanol before incubation in uranyl acetate [0.5% in 30% (vol/vol) ethanol] for 1 h. Next, fibers were dehydrated using a graded series of ethanol washes. Following dehydration, samples were infiltrated with ethanol:resin mixtures (2:1 and 1:1) for 20 min each. Finally, cells were embedded in 100% resin (TAAB), with a gelatin capsule of resin covering the cells of interest, before curing at 60 °C for 3 days. Polymerized resin blocks were sectioned and poststained as routine. Samples were viewed using a Phillips CM120 BioTwin transmission electron microscope (FEI) and micrographs acquired using a Gatan Orius CCD camera (Gatan).
Electron Microscopy of Mitotic Nucleosomes.
Chromosomes isolated from colcemid-arrested HeLa cells were centrifuged at 1,400 × g for 20 min at 4 °C onto carbon-coated grids and rinsed in 0.4% Photoflo (Kodak). Grids were fixed in TEEN buffer containing 1% glutaraldehyde for 1–2 h at 4 °C. Grids were consecutively dipped into 1% phosphotungstic acid in 71% (vol/vol) ethanol (15 s), 95% (vol/vol) ethanol (15 s), and 0.4% Photoflo (5 s); blotted dry; and rotary shadowed using platinum:paladium. Images were obtained with a Philips EM-300 at 80 kV (23).
Electron Microscopy of Interphase Nucleosomes.
Size-fractionated chromatin fibers were isolated from chicken erythrocytes and prepared for electron microscopy as previously described (52, 53). Benzylalkyldimethylammonium chloride (BAC) (Sigma) was added to the chromatin to a concentration of 2 × 10–4% (vol/vol). The mixture was incubated at room temperature for 30 min. The chromatin was spread on formvar/carbon-coated copper grids (TAAB). The grids were washed with ddH2O and 90% (vol/vol) ethanol and allowed to dry. For contrast enhancement, the grids were rotary-shadowed by a Leica ACE600 at a pressure of 1–2.5 × 10–5 mbar. Rotating samples were coated with 2 nm platinum (measured by a quartz sensor) at an elevation angle of 7°. The grids were examined by a JEOL JEM-1400 Plus TEM, operated at a magnification of 20K, 80 kV. Electron micrographs were acquired using GATAN OneView camera.
Indirect Immunofluorescence.
To analyze GFP:CENP-A localization, an immunostain for CENP-T was performed. Cells were seeded onto Concanavaline A (ConA)-coated coverslips and left to adhere for 1 h in the incubator before fixation. Cells were washed with warm PBS and fixed with prewarmed 4% (vol/vol) formaldehyde/PBS solution for 10 min. Cells were permeabilized by incubating coverslips for 2 min in 0.15% Triton X-100/PBS solution. Cells were blocked in 1% BSA/PBS solution for 1 h at room temperature and subsequently incubated with primary rabbit anti-GgCENP-T antibody diluted 1:1,000 (14) in the blocking solution for 1 h. Before secondary antibody incubation, cells were washed three times in 0.1% Tween20/PBS solution. Fluorophore-conjugated secondary antibody (Alexa Fluor 594; Jackson ImmunoResearch Laboratories, Inc.) was diluted 1:1,000 in the blocking solution and the incubation 45 min long. Several washes followed, and coverslips were finally mounted on slides using Vectashield containing DAPI (Vector Labs) as antifade media. 3D intact cell image stacks were deconvolved, quick projected, and saved as tiff images.
SDS/PAGE and Immunoblotting.
Cell lysates were sonicated and boiled in sample buffer [5% (wt/vol) sucrose, 1% SDS, 16.67 mM Tris-HCl, pH 6.8, 0.67 mM EDTA, 10% (vol/vol) β-Mercaptoethanol, 0.01% bromophenol blue]. Lysates were resolved in SDS/PAGE with 12% (vol/vol) polyacrylamide gels (BioRad electrophiresis apparatus). After transferring the proteins to a nitrocellulose membrane (Amersham, GE), blocking with 3% (wt/vol) low fat milk in 0.05% Tween20/PBS solution for 1 h was performed prior to immunoblotting. Primary antibodies used for immunoblotting included mouse anti–α-tubulin (1:5,000, B512 Sigma), rabbit anti–GgCENP-A (1:1,000), and rabbit anti-GFP (1:1,000, Life Technologies). Membranes were washed in 0.05% Tween20/PBS solution and incubated with secondary antibodies (IRDye 800 or IRDye 680; Li-Cor Biosciences) and abundantly washed before proceeding to the detection using a CCD scanner (Odyssey; Li-Cor Biosciences). Quantification of CENP-A bands was performed using ImageJ and normalized for the tubulin signal.
Acknowledgments
This work was funded by The Wellcome Trust (Grant 107022), of which W.C.E. is a Principal Research Fellow. The Wellcome Trust Centre for Cell Biology is supported by core funding from the Wellcome Trust (203149) and Wellcome Trust Multi User Equipment Grant WT104915MA. This work was supported by Japan Society for Promotion of Science KAKENHI Grants JP25221106 and JP15H05972 (to T.F.). G.V. was supported by a studentship from the Darwin Trust of Edinburgh.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614145114/-/DCSupplemental.
References
- 1.Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- 2.Palmer DK, O’Day K, Trong HL, Charbonneau H, Margolis RL. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci USA. 1991;88(9):3734–3738. doi: 10.1073/pnas.88.9.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blower MD, Sullivan BA, Karpen GH. Conserved organization of centromeric chromatin in flies and humans. Dev Cell. 2002;2(3):319–330. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro SA, et al. A super-resolution map of the vertebrate kinetochore. Proc Natl Acad Sci USA. 2010;107(23):10484–10489. doi: 10.1073/pnas.1002325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11(11):1076–1083. doi: 10.1038/nsmb845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foltz DR, et al. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8(5):458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- 7.Okada M, et al. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8(5):446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- 8.Izuta H, Ikeno M, Suzuki N, Tomonaga T, Nozaki N, et al. Comprehensive analysis of the ICEN (Interphase Centromere Complex) components enriched in the CENP-A chromatin of human cells. Genes Cells. 2006;11(6):673–684. doi: 10.1111/j.1365-2443.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw SM, Harrison SC. An Iml3-Chl4 heterodimer links the core centromere to factors required for accurate chromosome segregation. Cell Reports. 2013;5(1):29–36. doi: 10.1016/j.celrep.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basilico F, et al. The pseudo GTPase CENP-M drives human kinetochore assembly. eLife. 2014;3:e02978. doi: 10.7554/eLife.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskat A, et al. Step-wise assembly, maturation and dynamic behavior of the human CENP-P/O/R/Q/U kinetochore sub-complex. PLoS One. 2012;7(9):e44717. doi: 10.1371/journal.pone.0044717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino T, et al. CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell. 2012;148(3):487–501. doi: 10.1016/j.cell.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleiffer A, et al. CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol. 2012;14(6):604–613. doi: 10.1038/ncb2493. [DOI] [PubMed] [Google Scholar]
- 14.Hori T, et al. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008;135(6):1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Dornblut C, et al. A CENP-S/X complex assembles at the centromere in S and G2 phases of the human cell cycle. Open Biol. 2014;4:130229. doi: 10.1098/rsob.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke CA, Bernat RL, Earnshaw WC. CENP-B: A major human centromere protein located beneath the kinetochore. J Cell Biol. 1990;110(5):1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh H, et al. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70(1):115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki A, et al. Spindle microtubules generate tension-dependent changes in the distribution of inner kinetochore proteins. J Cell Biol. 2011;193(1):125–140. doi: 10.1083/jcb.201012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joglekar AP, Bloom K, Salmon ED. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr Biol. 2009;19(8):694–699. doi: 10.1016/j.cub.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan X, et al. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137(4):672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, Badger BL, Wan X, DeLuca JG, Salmon ED. The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch. Dev Cell. 2014;30(6):717–730. doi: 10.1016/j.devcel.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinkowski RP, Meyne J, Brinkley BR. The centromere-kinetochore complex: A repeat subunit model. J Cell Biol. 1991;113(5):1091–1110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earnshaw WC, Laemmli UK. Architecture of metaphase chromosomes and chromosome scaffolds. J Cell Biol. 1983;96(1):84–93. doi: 10.1083/jcb.96.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodor DL, et al. The quantitative architecture of centromeric chromatin. eLife. 2014;3:e02137. doi: 10.7554/eLife.02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finch JT, Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci USA. 1976;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amano M, et al. The CENP-S complex is essential for the stable assembly of outer kinetochore structure. J Cell Biol. 2009;186(2):173–182. doi: 10.1083/jcb.200903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukagawa T, et al. CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J. 2001;20(16):4603–4617. doi: 10.1093/emboj/20.16.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishihashi A, et al. CENP-I is essential for centromere function in vertebrate cells. Dev Cell. 2002;2(4):463–476. doi: 10.1016/s1534-5807(02)00144-2. [DOI] [PubMed] [Google Scholar]
- 29.Hori T, Haraguchi T, Hiraoka Y, Kimura H, Fukagawa T. Dynamic behavior of Nuf2-Hec1 complex that localizes to the centrosome and centromere and is essential for mitotic progression in vertebrate cells. J Cell Sci. 2003;116(Pt 16):3347–3362. doi: 10.1242/jcs.00645. [DOI] [PubMed] [Google Scholar]
- 30.Kwon MS, Hori T, Okada M, Fukagawa T. CENP-C is involved in chromosome segregation, mitotic checkpoint function, and kinetochore assembly. Mol Biol Cell. 2007;18(6):2155–2168. doi: 10.1091/mbc.E07-01-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori T, Okada M, Maenaka K, Fukagawa T. CENP-O class proteins form a stable complex and are required for proper kinetochore function. Mol Biol Cell. 2008;19(3):843–854. doi: 10.1091/mbc.E07-06-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood L, et al. Auxin/AID versus conventional knockouts: Distinguishing the roles of CENP-T/W in mitotic kinetochore assembly and stability. Open Biol. 2016;6(1):150230. doi: 10.1098/rsob.150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomkiel J, Cooke CA, Saitoh H, Bernat RL, Earnshaw WC. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J Cell Biol. 1994;125(3):531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori T, Shang WH, Takeuchi K, Fukagawa T. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol. 2013;200(1):45–60. doi: 10.1083/jcb.201210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagpal H, et al. Dynamic changes in CCAN organization through CENP-C during cell-cycle progression. Mol Biol Cell. 2015;26(21):3768–3776. doi: 10.1091/mbc.E15-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klare K, et al. CENP-C is a blueprint for constitutive centromere-associated network assembly within human kinetochores. J Cell Biol. 2015;210(1):11–22. doi: 10.1083/jcb.201412028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samejima I, et al. Whole-proteome genetic analysis of dependencies in assembly of a vertebrate kinetochore. J Cell Biol. 2015;211(6):1141–1156. doi: 10.1083/jcb.201508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Régnier V, et al. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol. 2005;25(10):3967–3981. doi: 10.1128/MCB.25.10.3967-3981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh TR, et al. MHF1-MHF2, a histone-fold-containing protein complex, participates in the Fanconi anemia pathway via FANCM. Mol Cell. 2010;37(6):879–886. doi: 10.1016/j.molcel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Z, et al. A histone-fold complex and FANCM form a conserved DNA-remodeling complex to maintain genome stability. Mol Cell. 2010;37(6):865–878. doi: 10.1016/j.molcel.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinley KL, et al. The CENP-L-N complex forms a critical node in an integrated meshwork of interactions at the centromere-kinetochore interface. Mol Cell. 2015;60(6):886–898. doi: 10.1016/j.molcel.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przewloka MR, et al. CENP-C is a structural platform for kinetochore assembly. Curr Biol. 2011;21(5):399–405. doi: 10.1016/j.cub.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Nishino T, et al. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 2013;32(3):424–436. doi: 10.1038/emboj.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynne DJ, Funabiki H. Kinetochore function is controlled by a phospho-dependent coexpansion of inner and outer components. J Cell Biol. 2015;210(6):899–916. doi: 10.1083/jcb.201506020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson M, Haase J, Yeh E, Bloom K. Function and assembly of DNA looping, clustering, and microtubule attachment complexes within a eukaryotic kinetochore. Mol Biol Cell. 2009;20(19):4131–4139. doi: 10.1091/mbc.E09-05-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribeiro SA, Vagnarelli P, Earnshaw WC. DNA content of a functioning chicken kinetochore. Chromosome Res. 2014;22(1):7–13. doi: 10.1007/s10577-014-9410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang WH, et al. Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell. 2013;24(6):635–648. doi: 10.1016/j.devcel.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magidson V, et al. Adaptive changes in the kinetochore architecture facilitate proper spindle assembly. Nat Cell Biol. 2015;17(9):1134–1144. doi: 10.1038/ncb3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell. 2003;5(2):323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro SA, et al. Condensin regulates the stiffness of vertebrate centromeres. Mol Biol Cell. 2009;20(9):2371–2380. doi: 10.1091/mbc.E08-11-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Booth DG, et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife. 2014;3:e01641. doi: 10.7554/eLife.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allan J, Staynov DZ, Gould H. Reversible dissociation of linker histone from chromatin with preservation of internucleosomal repeat. Proc Natl Acad Sci USA. 1980;77(2):885–889. doi: 10.1073/pnas.77.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allan J, Harborne N, Rau DC, Gould H. Participation of core histone “tails” in the stabilization of the chromatin solenoid. J Cell Biol. 1982;93(2):285–297. doi: 10.1083/jcb.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]