Significance
General anesthetics are essential to modern medicine, yet their molecular mechanisms remain poorly understood. Whether general anesthetics primarily act by altering lipid bilayer properties or by interacting directly with specific membrane proteins is a longstanding controversy. We now show that diverse classes of general anesthetics do not alter bilayer properties at concentrations that induce clinical anesthesia. Although anesthetics can have bilayer-perturbing effects at supratherapeutic (toxic) concentrations, this has little pharmacological relevance. Our findings exclude indirect ion channel effects due to perturbations of lipid bilayer properties, supporting the notion that general anesthetics interact directly with therapeutically relevant membrane protein targets.
Keywords: anesthetic mechanisms, gramicidin channel, bilayer modification, amphiphiles, isoflurane
Abstract
General anesthetics have revolutionized medicine by facilitating invasive procedures, and have thus become essential drugs. However, detailed understanding of their molecular mechanisms remains elusive. A mechanism proposed over a century ago involving unspecified interactions with the lipid bilayer known as the unitary lipid-based hypothesis of anesthetic action, has been challenged by evidence for direct anesthetic interactions with a range of proteins, including transmembrane ion channels. Anesthetic concentrations in the membrane are high (10–100 mM), however, and there is no experimental evidence ruling out a role for the lipid bilayer in their ion channel effects. A recent hypothesis proposes that anesthetic-induced changes in ion channel function result from changes in bilayer lateral pressure that arise from partitioning of anesthetics into the bilayer. We examined the effects of a broad range of chemically diverse general anesthetics and related nonanesthetics on lipid bilayer properties using an established fluorescence assay that senses drug-induced changes in lipid bilayer properties. None of the compounds tested altered bilayer properties sufficiently to produce meaningful changes in ion channel function at clinically relevant concentrations. Even supra-anesthetic concentrations caused minimal bilayer effects, although much higher (toxic) concentrations of certain anesthetic agents did alter lipid bilayer properties. We conclude that general anesthetics have minimal effects on bilayer properties at clinically relevant concentrations, indicating that anesthetic effects on ion channel function are not bilayer-mediated but rather involve direct protein interactions.
General anesthetics are essential drugs in modern medicine, yet their molecular mechanisms remain poorly understood, as it is unclear whether or not general anesthetics exert their effects by altering lipid bilayer properties. The Meyer-Overton correlation of anesthetic potency with lipophilicity, which does not identify a specific mechanism, led to lipid bilayer-based proposals for the mechanisms of general anesthesia that dominated the field until challenged in the 1970s (1–3). The seminal work of Franks and Lieb led them to conclude that “the lipid bilayer alone is not the anaesthetic site” (2). They subsequently showed that the Meyer-Overton correlation was preserved for inhibition of firefly luciferase, a soluble lipid-free model protein (4). This led to a search for critical protein targets for general anesthetics, which resulted in the identification of a number of plausible voltage-gated and ligand-gated ion channel targets (5, 6). The possible involvement of the lipid bilayer in the effects of lipophilic anesthetics on membrane proteins has not been excluded, however. Anesthetic concentrations in biological membranes are in the 10–100 mM range, making it difficult to exclude bilayer-mediated effects. Indeed, Cantor and colleagues have developed a modern, mechanistic lipid bilayer-based hypothesis, based on changes in the bilayer lateral pressure profile, to explain how general anesthetics could alter the function of membrane proteins (7, 8). An alternative bilayer-based mechanism for general anesthesia has been proposed based on anesthetic-induced changes in membrane domain organization (9, 10).
The concentrations of anesthetics at which effects are observed are critical for interpreting experimental data obtained in vitro. General anesthetics clearly alter lipid bilayer properties at high concentrations, which are irrelevant for clinical anesthesia but could contribute to toxicity at supratherapeutic doses (2, 3). This concentration dependence has clinical implications for the dose-dependent effects of general anesthetics: at low doses, the commonly used volatile anesthetic isoflurane causes only amnesia (11); at medium (therapeutic) doses it produces the desired clinical endpoint of hypnosis and immobility (5, 12); and at even higher supratherapeutic (toxic) doses it causes undesirable side effects such as cardiovascular and respiratory depression (13). Whereas isoflurane at anesthetic concentrations alters ion channel function with no discernible effect on lipid bilayer properties (14), it does alter lipid bilayer properties at supratherapeutic concentrations, supporting the idea that the lipid bilayer is not an important target for its therapeutic effects. The generality of this observation to other anesthetics has not been established, however.
To test the hypothesis that general anesthetics at clinically relevant concentrations do not produce marked changes in lipid bilayer properties, we tested a chemically and pharmacologically diverse panel of representative anesthetics using a functional assay sensitive to alterations in lipid bilayer properties. Our results show that general anesthetics have minimal, if any, effects on lipid bilayer properties at clinical concentrations. Thus, general anesthetics effects on ion channel function involve direct rather than indirect bilayer mediated effects.
Results
The bilayer-modifying potency of anesthetics were examined using a fluorescence quench method (14, 15). Fig. S1 describes the conceptual basis for these experiments, and Fig. S2 shows fluorescence quench traces for a representative experiment with diethyl ether. The results for a group of chemically and pharmacologically diverse anesthetics are summarized in Fig. 1. Inhaled anesthetics, which are delivered as gases, are grouped as ethers or alkanes (Fig. 1 A and B). We also included the nonanesthetic compounds flurothyl and F6, which are predicted by the Meyer-Overton correlation to be anesthetics based on their lipid solubility, yet they do not produce immobilization in response to a painful stimulus (16). We tested inhaled agents at clinically relevant concentrations of 1 MAC (minimum alveolar concentration, defined as the concentration that prevents movement in response to a painful stimulus in 50% of subjects, comparable to EC50) and 2 MAC, as well as a supratherapeutic (toxic) concentration (4 MAC). i.v. anesthetics represent a third group (Fig. 1C), the effects of which were tested at their EC50 for immobilization and multiples thereof.
Fig. S1.
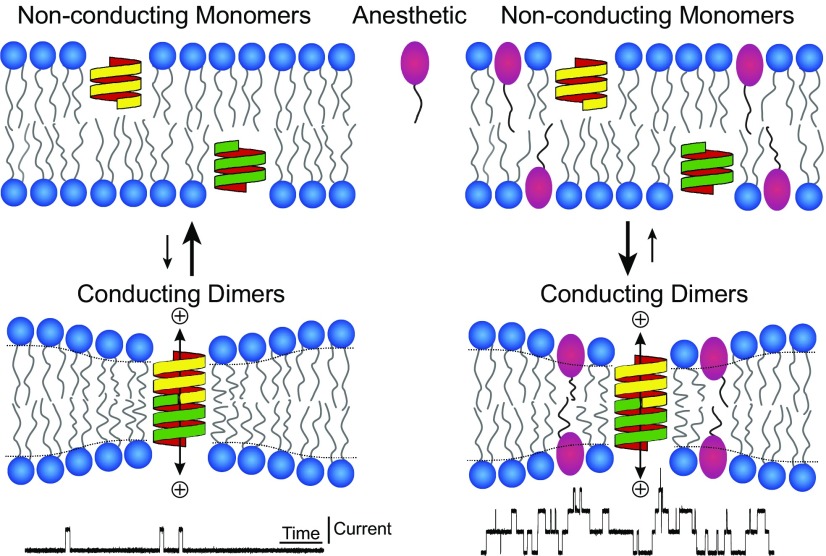
Hydrophobic coupling between gramicidin and a lipid bilayer. When two gramicidin subunits (yellow and green structures) from opposing bilayer leaflets dimerize, they form a cation selective channel. Amphiphilic molecules such as anesthetics (red symbol) affect the conformational changes of membrane bound proteins by altering lipid bilayer properties and gramicidin serves as a probe to track these changes. An increase in gramicidin activity indicates that the monomer ↔ dimer equilibrium has shifted toward the conducting gramicidin dimers (Lower Right).
Fig. S2.
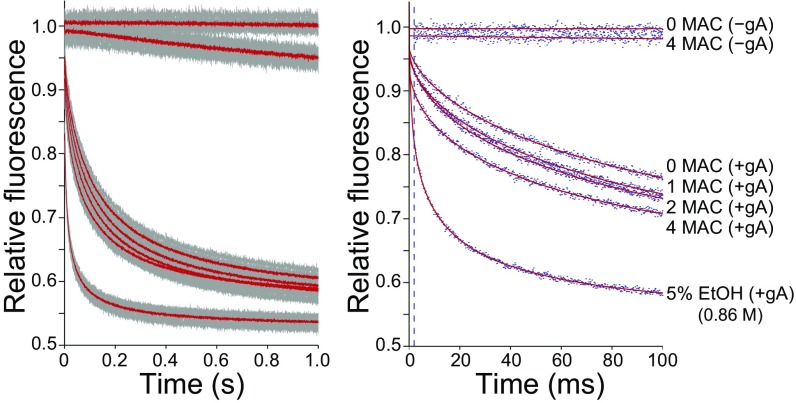
Lipid bilayer-modifying effects of the inhaled anesthetic diethyl ether tested using the gramicidin-based fluorescence assay. (Left) Time course of fluorescence decay of 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS) over 1 s in the absence (−gA) or presence (+gA) of gramicidin and in the absence or presence of diethyl ether (1, 2 and fourfold multiples of the EC50 for anesthesia defined as minimum alveolar concentration, MAC). Gray dots and solid red lines indicate individual data points and the average of all data, respectively. (Right) The first 100 ms of the normalized fluorescence decay from the same experiment as in the left panel. Blue dots are results from one assay for each condition. Because of the unavoidable dispersity in vesicle sizes, the fluorescence quench time course cannot be described by a single exponential decay, but rather by a stretched exponential decay; the stretched exponential fits to the data (2–100 ms) are denoted by red lines; the dashed vertical line at 2 ms marks the time point at which quench rate was determined. Ethanol (EtOH), a strong bilayer modifier at 5% (∼0.86 M) or (in some experiments) butanol at 1% (∼0.11 M) (27) were included as positive controls.
Fig. 1.
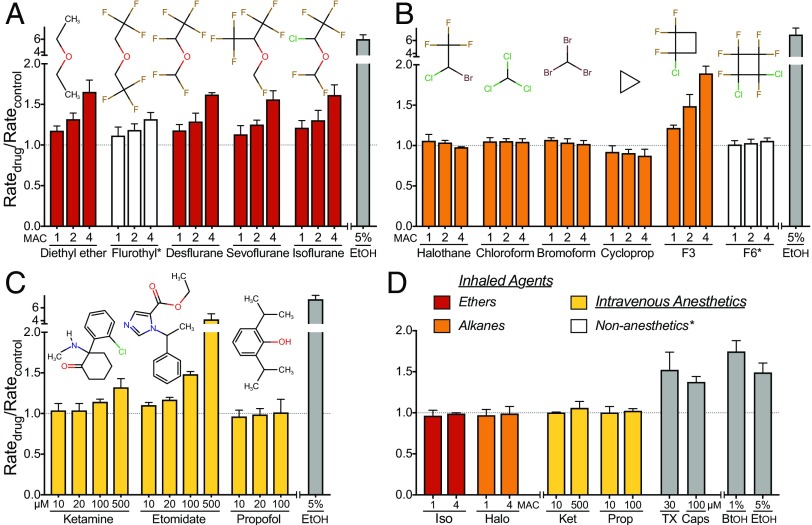
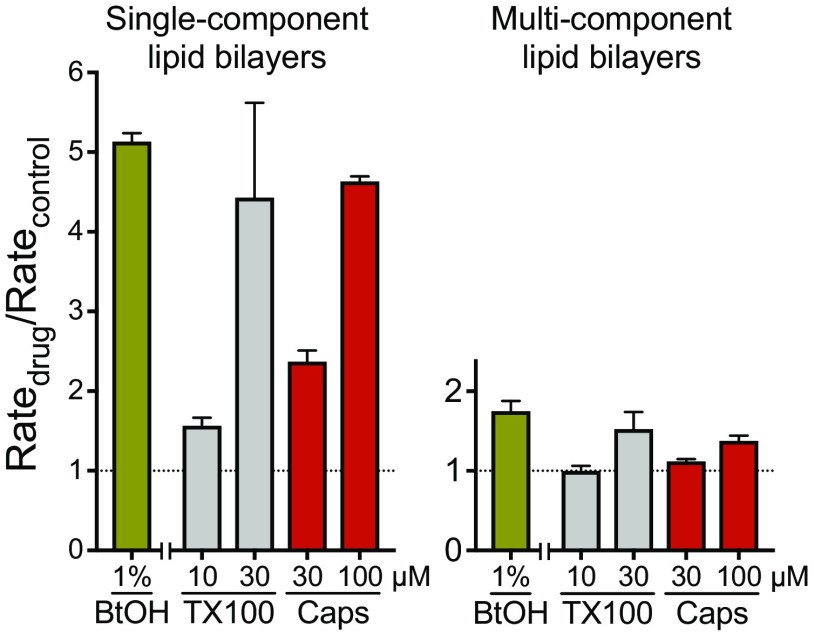
Effects of general anesthetics on lipid bilayer properties. Normalized fluorescence quench rates of inhaled ether (A) and alkane (B) compounds at concentrations of ∼1, 2, and 4 MAC (minimum alveolar concentration, defined as the concentration that prevents movement in response to a painful stimulus in 50% of subjects, comparable to EC50), and of i.v. anesthetics (C) at 10–500 µM, using single-component lipid bilayer vesicles. White columns represent compounds that do not cause immobility (nonanesthetics*) that were tested at concentrations predicted to produce anesthesia based on their lipid solubility. A normalized quench rate (Ratedrug/Ratecontrol) of 1.0 indicates no significant effect on bulk lipid bilayer properties. Ethanol [EtOH] (gray columns), a known bilayer-modifier at 5% (∼0.86 M), was included as a positive control. Data are expressed as mean ± SD, n = 3–5. [10 and 20 µM values for propofol are from (102).]. (D) Effects of anesthetics on lipid bilayer properties in multicomponent bilayer vesicles. Identical experiments (as in A–C) were performed using LUVs composed of an equimolar mixture of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC), cholesterol and brain sphingomyelin. Normalized fluorescence quench rates of select anesthetics (colored columns) representing each group at both low and high concentrations (1 or 4 MAC for isoflurane [Iso] and halothane [Halo]; 10 or 500 µM for ketamine [Ket]; 10 or 100 µM for propofol [Prop]). Conventional bilayer-modifying molecules (gray columns), such as 30 μM Triton X-100 [TX], 100 μM capsaicin [Caps] and alcohols (1% 1-butanol [BtOH] or 5% ethanol [EtOH]), were included as positive controls, which altered lipid bilayer properties even at low concentrations. Data are expressed as mean ± SD, n = 3–5.
At clinical concentrations (1 MAC), most of the anesthetics tested did not produce any effects on lipid bilayer properties detectable in the gramicidin-based fluorescent assay (Fig. 1 B and C). The ethers and F3 altered bulk lipid bilayer properties sufficiently to produce up to a 20% change in quench rate (Fig. 1 A and C), which produces minimal changes in membrane protein function (14), see also Fig. 2. The anesthetic ethers, F3, and the i.v. anesthetic etomidate increased the quench rate by > 50% at higher concentrations, indicating that they can alter lipid bilayer properties at these clinically irrelevant concentrations (see also (2, 3)). The nonanesthetics flurothyl and F6 did not significantly alter lipid bilayer properties.
Fig. 2.
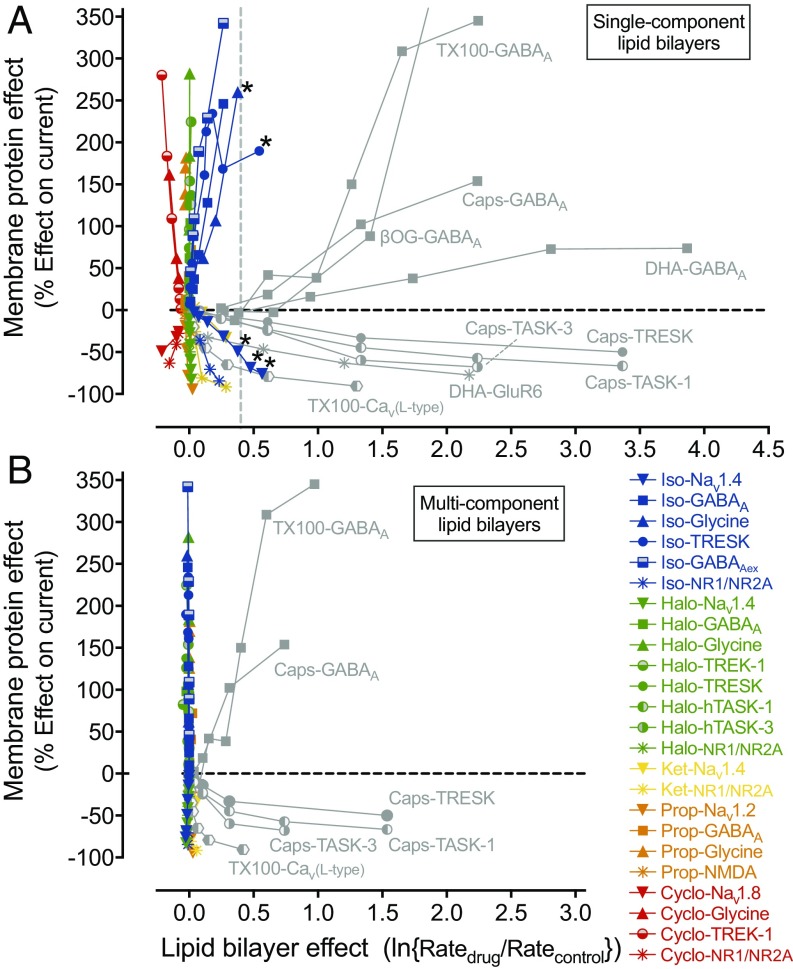
Effects of general anesthetics and other amphiphiles on ion channel function compared with their lipid bilayer modifying properties. (A) Plot of anesthetic- and amphiphile-induced changes in specific ion channel function (as a percentage of potentiation or inhibition of ionic current) against changes in lipid bilayer properties measured (or extrapolated) from the fluorescence quench rate in single component DC22:1PC LUVs. The relation between bilayer-modifying effect and alteration of ion-channel function by five representative anesthetics (isoflurane [Iso], halothane [Halo], ketamine [Ket], propofol [Prop], cyclopropane [Cyclo], colored symbols) and other amphiphiles (Triton X-100 [TX100], β-octyl-glucoside [βOG], capsaicin [Caps], docosahexaenoic acid [DHA], gray symbols) is based on results from this study and from published studies on ion channels (30, 67, 75, 81, 98, 103–122). The horizontal dashed line denotes no change in ion-channel current, and the vertical dashed line shows the threshold for a significant effect on bulk lipid-bilayer properties. All five anesthetics have strong ion channel effects at concentrations at which they have minimal or no bilayer-modifying effects. Gray symbols represent amphiphiles known to strongly alter lipid bilayer properties. A few data points for isoflurane (Iso-Nav1.4, Iso-Glycine and Iso-TRESK, denoted with asterisk) do reach or cross the vertical border, but these bilayer-modifying effects only occur at very high, supratherapeutic concentrations (>4 MAC). (B) Corresponding plot using the changes in fluorescence quench rates for multicomponent lipid bilayer LUVs for isoflurane, halothane, ketamine and propofol (abbreviation and color code as in A), as well as the known bilayer-modifying amphiphiles Triton X-100 and capsaicin (abbreviation as in A; gray symbols). In these multicomponent lipid bilayer experiments, the vertical alignment of the data (corresponding to anesthetics, colored symbols) is much more pronounced compared with the data for single component LUVs (A), confirming that clinical concentrations of anesthetics do not have any lipid bilayer altering effects.
The fluorescence experiments in single-component (1,2-dierucoyl-sn-glycero-3-phosphocholine, DC22:1PC) large unilamellar vesicles (LUVs) are exquisitely sensitive to changes in lipid bilayer elastic properties due to the large hydrophobic mismatch between channel length (∼2.2 nm (17, 18)) and bilayer thickness (∼3.4 nm (19, 20)). Cellular membranes, however, are complex mixtures of many lipid species with lateral domain organization (21–23), and Veatch, Machta and colleagues (9, 10) have proposed that general anesthetics act by altering membrane domain organization. To explore this question, we also performed experiments with LUVs formed using an equimolar mixture of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC), cholesterol and brain sphingomyelin. This mixture is known to form membranes with immiscible liquid domains (24, 25).
None of the anesthetics tested in this system (isoflurane, halothane, ketamine and propofol) affected the quench rate at anesthetic or supratherapeutic concentrations (Fig. 1D), indicating that the anesthetics are unlikely to produce major changes in membrane domain organization under our experimental conditions. Experiments in planar bilayers, formed from the same “raft-forming” lipid mixture as was used here provide evidence for a single type of kinetically homogenous channel (26), which presumably form in the thinner, less ordered Ld phase. “Conventional” bilayer-modifying molecules, like alcohols, Triton X-100 and capsaicin, did produce increases in quench rates (Fig. 1D), albeit only by around 20–35% of the rates observed in DC22:1PC LUVs (14, 27, 28). (Fig. S3 shows amphiphile-induced changes in quench rates in single-component (DC22:1PC) and multicomponent LUVs.) This presumably reflects the smaller hydrophobic thickness of the DC18:1PC-rich liquid disordered (Ld) phase, as experiments with DC18:1PC LUVs show that amphiphiles produce little, if any, changes in fluorescence quench rates (29). We therefore do not observe qualitatively different results from experiments with single- and multicomponent bilayers, confirming the lack of any anesthetic membrane effects at clinical relevant concentrations.
Fig. S3.
Effects of “conventional” bilayer-modifying molecules using single- and multicomponent lipid bilayer vesicles. Comparison of normalized fluorescence quench rates of 1-butanol [BtOH] at 1% (∼0.11 M) or Triton X-100 [TX100] and capsaicin [Caps] at low and high concentrations in LUVs formed with either single-component (DC22:1PC) or multicomponent (equimolar mixture of DC18:1PC; cholesterol and sphingomyelin) lipid bilayers.
Discussion
Our key finding is that a chemically and pharmacologically diverse panel of general anesthetics has no detectable effects on lipid bilayer properties at clinically relevant anesthetic concentrations. These results do not support a lipid bilayer-based mechanism for anesthetic effects on membrane proteins, but rather provide strong support for the hypothesis that general anesthetics exert their desired clinical effects through direct interactions with target membrane proteins.
Power of the Gramicidin-Based Approach.
Our results show that the general anesthetics tested do not alter the gramicidin monomer↔dimer equilibrium. The experiments in single-component bilayers lend themselves to unambiguous interpretation: anesthetics do not alter the energetic cost of the local bilayer deformation, which is the bilayer contribution to the free energy of dimerization (, where M denotes the monomer and D the dimer) associated with forming the bilayer-spanning dimer (30–32). varies as a function changes in bilayer thickness, intrinsic curvature, and the elastic bending and compression moduli, which in turn are determined by lateral interactions among membrane lipids (33–35). Since gramicidin channel function is altered by changes in lateral pressure (36), the lack of effects on gramicidin channel function indicates that general anesthetics have minimal, if any, effects on lipid bilayer elastic properties and lateral pressure.
These results do not exclude alterations in bilayer fluidity, i.e., changes in the lateral or rotational diffusion coefficients in the membrane. Such changes would have equal effects on the rate constants for channel formation and dissociation and therefore would not alter the monomer↔dimer equilibrium (nor the fluorescence quench rate) (37, 38). Similarly, changes in fluidity do not provide a causal mechanism for the bilayer regulation of membrane protein function (37, 39). Nor do our results with single-component DC22:1PC LUVs exclude changes in domain organization of multicomponent membranes, which could alter the lateral organization of channels in cell membranes (40, 41). We therefore did additional experiments using LUVs prepared with an equimolar mixture of DC18:1PC, cholesterol and brain sphingomyelin, a generic raft-forming mixture (24, 25). In this system, the anesthetics tested were as inert as in the single-component LUVs, if not more so, whereas conventional bilayer-modifying compounds (ethanol, 1-butanol, Triton X-100 and capsaicin) increased the quench rate. Ethanol, 1-butanol, Triton X-100 and capsaicin also were less potent than in DC22:1PC LUVs, which we ascribe to a lesser hydrophobic thickness of the Ld domains in LUVs formed by the multicomponent mixture, which also could account for the lesser effect of isoflurane (4 MAC) and ketamine (500 µM) in the that system. The absence of anesthetic effects suggests that anesthetics do not alter domain organization under our experimental conditions.
Energetic Coupling Between Membrane Proteins and Host Bilayer.
Membrane proteins are energetically coupled to their host bilayer through hydrophobic interactions (18, 42, 43). Changes in lipid bilayer properties can alter the equilibrium distribution among membrane protein conformational states and thus protein function (44–47) by altering the lipid bilayer contribution to the free energy difference for membrane protein conformational changes (e.g., between conformation I and II, (30)). This energetic coupling forms the conceptual framework for how drug-induced changes in bilayer properties can lead to changes in membrane protein function.
The classic studies of Meyer and Overton (48, 49) on the direct correlation between drug lipophilicity and anesthetic potency in vivo led to early lipid-based hypotheses of mechanisms of anesthesia. A number of compounds, however, do not conform to the predictions of the Meyer-Overton correlation: stereoisomers of anesthetics, for example, can have different anesthetic potencies (50, 51) despite their identical partition coefficients (e.g., ref. 52). The fluorinated nonanesthetics (flurothyl and F6), despite being very lipid soluble and predicted to be anesthetics, do not produce anesthesia in vivo (16) or altered lipid bilayer properties at their predicted anesthetic concentrations (Fig. 1 A and B). The chemically similar anesthetic F3 has minimal bilayer effects at anesthetic concentrations, though it is bilayer-active at higher concentrations. These differences in bilayer-modifying potencies could reflect agent-specific distributions of the different anesthetic and nonanesthetic agents within the bilayer. Anesthetic compounds tend to localize within the hydrocarbon core close to the membrane/solution interface based on NMR (53–55) and molecular dynamics (56) studies in contrast to the nonanesthetics F6 and hexafluoroethane, which localize within the midbilayer hydrocarbon core (53, 55, 57).
Such differential bilayer localizations could in principle explain the different effects of F3 and F6 within the framework of the mechanistic hypothesis of anesthetic effects based on changes in membrane intrinsic curvature (44) or lateral pressure profile (7). This mechanism does not invoke direct drug binding to “target” proteins (8). Rather, it is proposed that compounds accumulate at the aqueous-bilayer interface affecting bilayer properties and thereby modulating membrane protein activity. This hypothesis, however, is difficult to reconcile with the lack of changes in gramicidin channel function at clinically relevant concentrations (14), as well as the observation that the partition coefficient of halothane does not vary as a function of aqueous concentration or mole-fraction in the bilayer (58), a result that excludes large changes in lateral pressure within the bilayer.
Membrane Proteins as Anesthetic Targets.
The notion of the lipid bilayer as the primary target for general anesthetic action was challenged in the late 1970s (2, 3, 59, 60), which stimulated a search for protein targets involved in producing anesthesia. Inhaled general anesthetics were known to bind to globular proteins (61), and alter the function of cytosolic proteins (1, 62), and many ion channels were also found to be affected by anesthetics (63–65). Subsequent studies have identified potential protein targets that include a range of ligand-gated ion channels such as GABAA-receptors (66) and NMDA-type glutamate receptors (67, 68), two-pore domain K+ channels (69, 70), and voltage-gated Ca2+ (71, 72) and Na+ (14, 73) channels.
There is wide consensus that proteins are the most likely targets for i.v. anesthetics. The i.v. anesthetics propofol, thiopental and etomidate produce their effects primarily through potentiation of inhibitory GABAA receptors (74, 75), whereas ketamine inhibits excitatory NMDA-type glutamate receptors and HCN channels (76, 77). Point mutations of GABAA-receptors that result in anesthetic insensitivity markedly reduce propofol and etomidate anesthesia in knock-in mice in vivo (78–80). It remains unclear whether their effects at higher concentrations are due solely to direct effects on non-GABAA proteins targets, or involve contributions from the lipid bilayer, as has been demonstrated for a number of other drugs (26, 28, 30, 81–83). For volatile anesthetics, however, the evidence is not as clear. Halogenated ether anesthetics, for example, fail to produce anesthetic resistance in knock-in mice with GABAA-receptors engineered for resistance to these compounds – suggesting a role for additional or alternate mechanisms, thus raising the question of lipid bilayer contributions. Indeed the range of plausible membrane protein anesthetic targets suggests that a shared (so-called “unitary”) mechanism of action that could include alterated membrane properties might be important for this drug class (84, 85). It is therefore important to know if anesthetics at clinically relevant concentrations alter lipid bilayer properties sufficiently to produce changes in membrane protein function.
Anesthetic Effects on Membrane Protein Function Do Not Correlate with Changes in Bilayer Properties.
Membrane protein function can be modulated by changes in lipid bilayer composition (86–90). Similarly, membrane protein function is regulated by small, membrane-active compounds at concentrations that also alter lipid bilayer properties (26, 28, 30, 81–83, 91–98). It is therefore important to examine changes in membrane protein function in relation to bilayer-modifying effects for a wide range of anesthetics, and thus test the generality of the observation that fluorobenzene anesthetics and isoflurane do not affect bulk membrane properties at clinically relevant concentrations (14).
To highlight the relationship between general anesthetic effects on specific membrane proteins and their lipid bilayer-perturbing effects, we compared our lipid bilayer modification results in LUVs prepared using either single component DC22:1PC or a more complex multicomponent (i.e., ternary) mixture, with published concentration-response studies of anesthetic effects on a variety of anesthetic-sensitive ion channels. We then compared these results to those for conventional bilayer-modifying compounds (Fig. 2).
It is evident that conventional amphiphiles alter ion channel function at concentrations where they perturb lipid bilayer properties (right two quadrants in Fig. 2A), which suggests that they do so by altering lipid bilayer properties. In contrast, anesthetics do not alter lipid bilayer properties at clinically relevant anesthetic concentrations (left two quadrants in Fig. 2A), and there is no clear relation between bilayer-modifying and anesthetic potencies. At supratherapeutic and potentially toxic concentrations, anesthetics can alter lipid bilayer properties, as illustrated by isoflurane effects on Nav1.4, glycine receptors and TRESK (Fig. 2A, denoted with asterisk), in which isoflurane is a bilayer modifier at concentrations above 4 MAC (14). These bilayer effects, however, are unlikely to be relevant for desired anesthetic effects.
The membrane mole-fractions of general anesthetics at 1 MAC (Table S1) are similar to the mole-fractions at which many amphiphiles alter lipid bilayer properties (14, 26, 27, 30, 99, 100). In single-component bilayers, a conventional bilayer modifier, Triton X-100 for example, produces a fourfold increase in the fluorescence quench rate at 30 µM, corresponding to a membrane mole-fraction of ∼0.1 (15). The modest membrane effects of the inhaled anesthetics at 4 MAC, where (except for F3) mole-fractions in the membrane are 0.1 or above, raises the question of why are clinical anesthetics are so inert as lipid bilayer modifiers? Our results do not provide insight into this question, but we note that anesthetics and their nonanesthetic counterparts are located within the bilayer hydrophobic core, with the anesthetics localizing closer to the bilayer/solution interface than the nonanesthetics (53–57), whereas conventional amphiphiles are anchored to the bilayer/solution interface (101).
Table S1.
Properties of general anesthetics studied
| Anesthetic | MAC or EC50 at 25 °C, mM | cLogP | KP | Predicted membrane concentration at 1 MAC, mM | Mole fraction |
| Inhaled anesthetics | |||||
| Ethers | |||||
| Diethyl ether | 12.7 (134) | 1.0 | 10 | 121 | 0.093 |
| Flurothyl* | 0.30 (135) | 1.5 | 32 | 9 | 0.007 |
| Desflurane | 0.65 (134) | 1.9 | 79 | 47 | 0.038 |
| Sevoflurane | 0.41 (134) | 2.5 | 316 | 115 | 0.089 |
| Isoflurane | 0.30 (134) | 2.3 | 200 | 57 | 0.049 |
| Alkanes | |||||
| Halothane | 0.30 (134) | 2.3 | 200 | 57 | 0.051 |
| Chloroform | 1.10 (126) | 1.8 | 63 | 61 | 0.049 |
| Bromoform | 0.19 (136) | 2.3 | 200 | 36 | 0.031 |
| Cyclopropane | 1.20 (134) | 1.7 | 50 | 64 | 0.052 |
| F3 | 0.84 (16) | 0.5 | 3 | 3 | 0.002 |
| F6* | 0.02 (16) | 1.7 | 50 | 1 | 0.001 |
| Intravenous anesthetics | |||||
| Ketamine | 0.009 (137) | 2.2 | 158 | 1 | 0.001 |
| Etomidate | 0.003 (138) | 3.3 | 1,995 | 4 | 0.004 |
| Propofol | 0.001 (122) | 4.2 | 15,848 | 4 | 0.004 |
EC50, free plasma concentration for immobilization; MAC, minimum alveolar concentration for immobilization. Membrane concentrations and mole fraction were estimated following ref. 99 using 1.5 cm3 and 2.72 × 10−4 cm3 for the volume of aqueous solution (Vaq) and lipids (Vlip), respectively.
Compounds that do not cause immobility (nonanesthetics) were tested at concentrations that are predicted to produce anesthesia based on their lipid solubility.
Conclusions
We used a well-characterized model system to explore the bilayer-modifying potencies of general anesthetics. Anesthetics are weak bilayer modifiers at clinically relevant anesthetic concentrations, whether tested in bilayers formed by a single phospholipid or by a multicomponent lipid mixture that is known to exhibit domain immiscibility. Clinical anesthesia is therefore unlikely to involve alterations in lipid bilayer properties. Our results support the notion that general anesthesia involves specific anesthetic-membrane protein interactions, which in turn alter central nervous system properties to produce the characteristic features of general anesthesia.
Materials and Methods
Details are in SI Materials and Methods. Anesthetic and nonanesthetic solutions were prepared as described (14), and their lipid bilayer perturbing effects were tested using a gramicidin-based fluorescence assay (GBFA). Single component large unilamellar vesicles (LUVs) composed of 1,2-dierucoyl-sn-glycero-3-phosphocholine (DC22:1PC) or multicomponent LUVs composed of equimolar amounts of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC), cholesterol and brain sphingomyelin were filled with the Tl+ quenchable fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS). Changes in fluorescence decay were measured using a stopped-flow spectrophotometer followed by offline analysis as described (14, 15).
SI Materials and Methods
Materials.
Isoflurane and sevoflurane were from Abbott Laboratories; thymol-free halothane from Halocarbon Laboratories; desflurane from Baxter Healthcare Corporation; F3 (1-chloro-1,2,2-trifluorocyclobutane) and the nonimmobilizer F6 (1,2-dichlorohexafluorocyclobutane) from Matrix Scientific. All other anesthetics (diethyl ether, chloroform, bromoform, cyclopropane, flurothyl, propofol, ketamine and etomidate) were from Sigma-Aldrich. The sodium salt of 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS) was from Thermo Fisher, 1,2-dierucoyl-sn-glycero-3-phosphocholine (DC22:1PC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC), cholesterol and porcine brain sphingomyelin were from Avanti Polar Lipids; all other chemicals and gramicidin from Bacillus brevis were from Sigma-Aldrich.
Anesthetics.
The mole-fraction of anesthetics in the lipid bilayer was estimated according to Bruno et al. (2007) (99). Membrane concentration of anesthetic was determined as:
| [S1] |
where KP is the octanol/water partition coefficient, [Anes]nom the nominal aqueous anesthetic concentration in the lipid-vesicle system, and Vaq and Vlip the aqueous and lipid volumes in the system; the second term on the right accounts for depletion due to drug redistribution between aqueous and membrane phases. The mole-fraction of anesthetic, mAnes, was determined as:
| [S2] |
where [Lipid]m denotes the lipid concentration in the membrane phase (∼1 M). Because logP (logarithm of the partition coefficient into bilayer) for ether anesthetics is 2–3, and the volume of the aqueous phase is ∼104 times higher than the volume of the membrane phase, anesthetic depletion from the aqueous phase is minimal and the aqueous concentrations are close to nominal concentrations. KP was estimated as 10cLogP, where cLogP was estimated using the ACD/Labs LogP algorithm at www.chemspider.com. For drugs for which experimental data are available (58, 123, 124), estimated partition coefficients agree reasonably well with measured bilayer/electrolyte partition coefficients. Anesthetics were tested at concentrations that were close to 1, 2 and fourfold multiples of the EC50 for anesthesia defined as minimum alveolar concentration (MAC) for inhaled agents (125). Because so many studies on anesthetic mechanisms have been done on rodents, in particular rats, and we use rat data to construct Fig. 1, we chose MAC values for rodents (except for i.v. anesthetics, where human values are available and cyclopropane, for which we also chose the human value, which is lower than the rat value and allowed us to display data at 4 MAC; and for bromoform, for which only tadpole data exists) and adjusted anesthetic concentrations to our experimental conditions of 25 °C using Eq. 4 of Franks & Lieb (126). Saturated solutions of inhaled anesthetics were prepared as previously described (127). Gas-tight glass syringes were used to further dilute saturated stock solutions to working concentrations, which were then equilibrated in a temperature controlled enclosure before each experiment. The stopped-flow spectrophotometer is a gas-tight closed system extending from the injection ports to the outlet. Final solution concentrations were confirmed using gas chromatography as described (127).
Measurements of Changes in Lipid Bilayer Properties.
The bilayer-modifying effects of anesthetics were measured using the gramicidin-based fluorescence assay (GBFA). This assay uses the channel-forming antibiotic gramicidin, which forms monovalent 5cation-conducting channels following dimerization of nonconducting monomeric subunits in the bilayer (15, 30, 128, 129). The transmembrane length of the gramicidin channel is less than the thickness of the bilayer, which imposes a bilayer deformation around the gramicidin channel as it dimerizes to form a conducting channel (Fig. S1). The monomer↔dimer equilibrium is closely coupled to the energy required to deform the bilayer adjacent to the channel, such that changes in gramicidin channel activity becomes a measure of changes in lipid bilayer properties (130).
Results from a representative gramicidin-based fluorescence assay (GBFA) of the effects of the inhaled anesthetic diethyl ether are shown in Fig. S2. The fluorescence decay is due to Tl+ entering through dimeric, bilayer-spanning gramicidin channels (Fig. S1) in large unilamellar vesicles (mean diameter 128–130 nm), thereby quenching the fluorescence of the intravesicular reporter ANTS (15, 128, 131). Low ether concentrations produced little change in the fluorescence quench rate, relative to control; higher (supratherapeutic) concentrations accelerated fluorescence decay by facilitating dimerization of gramicidin monomers, which led to increased Tl+ flux into the vesicles.
Single-component large unilamellar vesicles (LUVs) were formed using a four-step process of sonication, freeze-thawing, extrusion and elution. Briefly, DC22:1PC without or with gramicidin (molar ratio 1000:1) was dried under nitrogen and desiccated overnight, to ensure complete removal of organic solvent. The LUVs were rehydrated in a solution of 25 mM ANTS, 100 mM NaNO3 and 10 mM Hepes (pH 7.0). The resulting multilamellar vesicles were passed 21 times through a 100 nm polycarbonate membrane using a miniextruder (Avanti Polar Lipids); extravesicular ANTS was removed by filtration through a desalting column.
Multicomponent LUVs similarly were prepared using a three-step process of freeze-thawing, extrusion, and elution. Equimolar amounts of DC18:1PC, cholesterol and brain sphingomyelin were combined with gramicidin (molar ratio 200:1), dried under nitrogen, and desiccated under vacuum for 3 h to ensure complete removal of organic solvent. The LUVs were rehydrated in a prewarmed (50 °C) solution consisting of 25 mM ANTS, 100 mM NaNO3 and 10 mM Hepes (pH 7.0). Five cycles of freeze-thawing were performed by flash-freezing in liquid nitrogen and subsequent thawing in a 50 °C water bath. The samples were vortexed after each thaw cycle. The temperature likewise was maintained at 50 °C during extrusion through a 100 nm polycarbonate membrane using a miniextruder (Avanti Polar Lipids) mounted on a heating block. After extrusion, vesicles were cooled to room temperature and filtered through a desalting column to remove extravesicular ANTS.
The size distributions of LUV preparations were determined using dynamic light scattering (Malvern Zetasizer Nano ZS). Except for one determination on one DC22:1PC preparation, the distributions were unimodal with Z-averaged diameters and polydispersity indices ranging between 128 and 140 nm and 0.03 and 0.15 (n = 6), respectively, for DC22:1PC LUVs (e.g., ref. 132); the corresponding values for the LUVs formed from the ternary mixture were: Z-averaged diameters ranged between 128 and 140 nm, and the polydispersity indices ranged between 0.12 and 0.14.
Changes in the gramicidin monomer↔dimer equilibrium were estimated from the rate of ANTS fluorescence quenching by Tl+ using a stopped-flow spectrofluorometer (SX.20; Applied Photophysics) with dead time of ∼1.5 ms. The experiments were performed at 25 °C, and the samples were excited at 352 nm, and fluorescence emission above 455 nm was recorded. Vesicles were incubated with test compounds for 10 min at 25 °C before measuring the fluorescence quenching rate.
Because of unavoidable LUV size dispersion, fluorescence quench curves cannot be described by single exponential decays; results were analyzed using a stretched exponential, a mathematically efficient way to express large sums of similar exponential decays (133). The fluorescence quench time courses were analyzed using MATLAB (v.7.9, The MathWorks), and the fluorescence quench rates for the first 2–100 ms of the individual quench curves were fitted by the stretched exponential:
| [S3] |
where F(t) denotes the fluorescence intensity as a function of time t, τ0 is a parameter with units of time, and β (0 < β ≤ 1) is a measure of sample dispersion. The quench rate at 2 ms was determined as (133):
| [S4] |
Data are reported as means normalized to the control rate in the absence of anesthetic (n ≥ 3).
Acknowledgments
We thank Dr. Daniel A. Heller (Memorial Sloan Kettering Cancer Center) for use of the dynamic light-scattering apparatus, Milka Doktorova for invaluable advice on how to prepare the mixed-lipid LUVs, and Thasin A. Peyear for assistance with the dynamic light-scattering measurements. This work was supported by National Institutes of Health Grants GM058055 (to H.C.H.) and GM021347 (to O.S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611717114/-/DCSupplemental.
References
- 1.Ueda I, Kamaya H. Kinetic and thermodynamic aspects of the mechanism of general anesthesia in a model system of firefly luminescence in vitro. Anesthesiology. 1973;38(5):425–436. doi: 10.1097/00000542-197305000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Franks NP, Lieb WR. Where do general anaesthetics act? Nature. 1978;274(5669):339–342. doi: 10.1038/274339a0. [DOI] [PubMed] [Google Scholar]
- 3.Richards CD, et al. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- 4.Franks NP, Lieb WR. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- 5.Hemmings HC, Jr, et al. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26(10):503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5(9):709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 7.Cantor RS. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Biochemistry. 1997;36(9):2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 8.Sonner JM, Cantor RS. Molecular mechanisms of drug action: An emerging view. Annu Rev Biophys. 2013;42:143–167. doi: 10.1146/annurev-biophys-083012-130341. [DOI] [PubMed] [Google Scholar]
- 9.Gray E, Karslake J, Machta BB, Veatch SL. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys J. 2013;105(12):2751–2759. doi: 10.1016/j.bpj.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machta BB, et al. Conditions that stabilize membrane domains also antagonize n-alcohol anesthesia. Biophys J. 2016;111(3):537–545. doi: 10.1016/j.bpj.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer R, Bennett HL, Eger EI, 2nd, Heilbron D. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology. 1992;77(5):888–898. doi: 10.1097/00000542-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348(21):2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 13.Alagesan K, Nunn JF, Feeley TW, Heneghan CP. Comparison of the respiratory depressant effects of halothane and isoflurane in routine surgery. Br J Anaesth. 1987;59(9):1070–1079. doi: 10.1093/bja/59.9.1070. [DOI] [PubMed] [Google Scholar]
- 14.Herold KF, et al. Volatile anesthetics inhibit sodium channels without altering bulk lipid bilayer properties. J Gen Physiol. 2014;144(6):545–560. doi: 10.1085/jgp.201411172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingólfsson HI, Andersen OS. Screening for small molecules’ bilayer-modifying potential using a gramicidin-based fluorescence assay. Assay Drug Dev Technol. 2010;8(4):427–436. doi: 10.1089/adt.2009.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koblin DD, et al. Polyhalogenated and perfluorinated compounds that disobey the Meyer-Overton hypothesis. Anesth Analg. 1994;79(6):1043–1048. doi: 10.1213/00000539-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Elliott JR, Needham D, Dilger JP, Haydon DA. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983;735(1):95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang HW. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986;50(6):1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caffrey M, Feigenson GW. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981;20(7):1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BA, Engelman DM. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, Maxfield FR. Membrane domains. Annu Rev Cell Dev Biol. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]
- 22.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3(10):a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Meer G, de Kroon AI. Lipid map of the mammalian cell. J Cell Sci. 2011;124(Pt 1):5–8. doi: 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- 24.Gandhavadi M, Allende D, Vidal A, Simon SA, McIntosh TJ. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys J. 2002;82(3):1469–1482. doi: 10.1016/S0006-3495(02)75501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85(5):3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusinova R, Koeppe RE, 2nd, Andersen OS. A general mechanism for drug promiscuity: Studies with amiodarone and other antiarrhythmics. J Gen Physiol. 2015;146(6):463–475. doi: 10.1085/jgp.201511470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingólfsson HI, Andersen OS. Alcohol’s effects on lipid bilayer properties. Biophys J. 2011;101(4):847–855. doi: 10.1016/j.bpj.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingólfsson HI, et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem Biol. 2014;9(8):1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Gross RW. Potassium flux through gramicidin ion channels is augmented in vesicles comprised of plasmenylcholine: Correlations between gramicidin conformation and function in chemically distinct host bilayer matrices. Biochemistry. 1995;34(22):7356–7364. doi: 10.1021/bi00022a008. [DOI] [PubMed] [Google Scholar]
- 30.Rusinova R, et al. Thiazolidinedione insulin sensitizers alter lipid bilayer properties and voltage-dependent sodium channel function: Implications for drug discovery. J Gen Physiol. 2011;138(2):249–270. doi: 10.1085/jgp.201010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen C, Goulian M, Andersen OS. Energetics of inclusion-induced bilayer deformations. Biophys J. 1998;74(4):1966–1983. doi: 10.1016/S0006-3495(98)77904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundbaek JA, Andersen OS. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys J. 1999;76(2):889–895. doi: 10.1016/S0006-3495(99)77252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helfrich W. Amphiphilic mesophases made of defects. In: Balian R, Kléman M, Poirier JP, editors. Physics of Defects. North-Holland Publishing; New York: 1981. pp. 716–755. [Google Scholar]
- 34.Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys J. 2007;93(11):3884–3899. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szleifer I, Kramer D, Ben‐Shaul A, Gelbart WM, Safran SA. Molecular theory of curvature elasticity in surfactant films. J Chem Phys. 1990;92(11):6800–6817. [Google Scholar]
- 36.Goulian M, et al. Gramicidin channel kinetics under tension. Biophys J. 1998;74(1):328–337. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AG. Lipids and their effects on membrane proteins: Evidence against a role for fluidity. Prog Lipid Res. 1991;30(4):323–348. doi: 10.1016/0163-7827(91)90002-m. [DOI] [PubMed] [Google Scholar]
- 38.Schurr JM. The role of diffusion in enzyme kinetics. Biophys J. 1970;10(8):717–727. doi: 10.1016/S0006-3495(70)86331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carruthers A, Melchior DL. Human erythrocyte hexose transporter activity is governed by bilayer lipid composition in reconstituted vesicles. Biochemistry. 1984;23(26):6901–6911. doi: 10.1021/bi00321a096. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell KM, Martens JR, Tamkun MM. Localization of ion channels to lipid raft domains within the cardiovascular system. Trends Cardiovasc Med. 2004;14(2):37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83(5):2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouritsen OG, Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruner SM. Intrinsic curvature hypothesis for biomembrane lipid composition: A role for nonbilayer lipids. Proc Natl Acad Sci USA. 1985;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruner SM, Shyamsunder E. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Ann N Y Acad Sci. 1991;625:685–697. doi: 10.1111/j.1749-6632.1991.tb33902.x. [DOI] [PubMed] [Google Scholar]
- 45.Sackmann E. Physical basis of trigger processes and membrane structures. In: Chapman D, editor. Biological Membranes. Academic; London: 1984. pp. 105–143. [Google Scholar]
- 46.Keller SL, et al. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993;65(1):23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundbaek JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994;104(4):645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer H. Zur theorie der alkoholnarkose. Arch Exp Pathol Pharmakol. 1899;42(2-4):109–118. [Google Scholar]
- 49.Overton C. Studien über die Narkose Zugleich ein Beitrag zur Allgemeinen Pharmakologie. Verlag von Gustav Fischer; Jena, Germany: 1901. [Google Scholar]
- 50.Brosnan R, et al. Chirality in anesthesia II: Stereoselective modulation of ion channel function by secondary alcohol enantiomers. Anesth Analg. 2006;103(1):86–91. doi: 10.1213/01.ane.0000221437.87338.af. [DOI] [PubMed] [Google Scholar]
- 51.Franks NP, Lieb WR. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991;254(5030):427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- 52.Dickinson R, Franks NP, Lieb WR. Can the stereoselective effects of the anesthetic isoflurane be accounted for by lipid solubility? Biophys J. 1994;66(6):2019–2023. doi: 10.1016/S0006-3495(94)80994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.North C, Cafiso DS. Contrasting membrane localization and behavior of halogenated cyclobutanes that follow or violate the Meyer-Overton hypothesis of general anesthetic potency. Biophys J. 1997;72(4):1754–1761. doi: 10.1016/S0006-3495(97)78821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baber J, Ellena JF, Cafiso DS. Distribution of general anesthetics in phospholipid bilayers determined using 2H NMR and 1H-1H NOE spectroscopy. Biochemistry. 1995;34(19):6533–6539. doi: 10.1021/bi00019a035. [DOI] [PubMed] [Google Scholar]
- 55.Tang P, Yan B, Xu Y. Different distribution of fluorinated anesthetics and nonanesthetics in model membrane: A 19F NMR study. Biophys J. 1997;72(4):1676–1682. doi: 10.1016/S0006-3495(97)78813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arcario MJ, Mayne CG, Tajkhorshid E. Atomistic models of general anesthetics for use in in silico biological studies. J Phys Chem B. 2014;118(42):12075–12086. doi: 10.1021/jp502716m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu R, Loll PJ, Eckenhoff RG. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 2005;19(6):567–576. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- 58.Simon SA, McIntosh TJ, Bennett PB, Shrivastav BB. Interaction of halothane with lipid bilayers. Mol Pharmacol. 1979;16(1):163–170. [PubMed] [Google Scholar]
- 59.Franks NP, Lieb WR. The structure of lipid bilayers and the effects of general anaesthetics. An x-ray and neutron diffraction study. J Mol Biol. 1979;133(4):469–500. doi: 10.1016/0022-2836(79)90403-0. [DOI] [PubMed] [Google Scholar]
- 60.Franks NP, Lieb WR. Mechanisms of general anesthesia. Environ Health Perspect. 1990;87:199–205. doi: 10.1289/ehp.9087199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoenborn BP, Featherstone RM. Molecular forces in anesthesia. Adv Pharmacol. 1967;5:1–17. doi: 10.1016/s1054-3589(08)60652-3. [DOI] [PubMed] [Google Scholar]
- 62.Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- 63.Macdonald RL, Barker JL. Different actions of anticonvulsant and anesthetic barbiturates revealed by use of cultured mammalian neurons. Science. 1978;200(4343):775–777. doi: 10.1126/science.205953. [DOI] [PubMed] [Google Scholar]
- 64.Haydon DA, Simon AJ. Excitation of the squid giant axon by general anaesthetics. J Physiol. 1988;402:375–389. doi: 10.1113/jphysiol.1988.sp017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicoll RA. Pentobarbital: Differential postsynaptic actions on sympathetic ganglion cells. Science. 1978;199(4327):451–452. doi: 10.1126/science.202032. [DOI] [PubMed] [Google Scholar]
- 66.Zimmerman SA, Jones MV, Harrison NL. Potentiation of gamma-aminobutyric acid A receptor Cl− current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther. 1994;270(3):987–991. [PubMed] [Google Scholar]
- 67.Dickinson R, et al. Competitive inhibition at the glycine site of the N-methyl-d-aspartate receptor by the anesthetics xenon and isoflurane: Evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107(5):756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 68.Haseneder R, et al. Xenon reduces N-methyl-d-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology. 2008;109(6):998–1006. doi: 10.1097/ALN.0b013e31818d6aee. [DOI] [PubMed] [Google Scholar]
- 69.Patel AJ, Honoré E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95(4):1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- 70.Sirois JE, Lynch C, 3rd, Bayliss DA. Convergent and reciprocal modulation of a leak K+ current and Ih by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. J Physiol. 2002;541(Pt 3):717–729. doi: 10.1113/jphysiol.2002.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nikonorov IM, Blanck TJ, Recio-Pinto E. The effects of halothane on single human neuronal L-type calcium channels. Anesth Analg. 1998;86(4):885–895. doi: 10.1097/00000539-199804000-00038. [DOI] [PubMed] [Google Scholar]
- 72.Study RE. Isoflurane inhibits multiple voltage-gated calcium currents in hippocampal pyramidal neurons. Anesthesiology. 1994;81(1):104–116. doi: 10.1097/00000542-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Herold KF, Hemmings HC., Jr Sodium channels as targets for volatile anesthetics. Front Pharmacol. 2012;3:50. doi: 10.3389/fphar.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Concas A, et al. The general anesthetic propofol enhances the function of gamma-aminobutyric acid-coupled chloride channel in the rat cerebral cortex. J Neurochem. 1990;55(6):2135–2138. doi: 10.1111/j.1471-4159.1990.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 75.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104(3):619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci. 2009;29(3):600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: A site of action of ketamine? Nature. 1985;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 78.Werner DF, et al. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring α2(S270H/L277A) GABAA receptor subunits that are resistant to isoflurane. J Pharmacol Exp Ther. 2011;336(1):134–144. doi: 10.1124/jpet.110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeller A, et al. 2008. Inhibitory ligand-gated ion channels as substrates for general anesthetic actions. Modern Anesthetics, eds Schüttler J, Schwilden H, Handbook of Experimental Pharmacology (Springer, Berlin), Vol 182, pp 31–51.
- 80.Jurd R, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor beta3 subunit. FASEB J. 2003;17(2):250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 81.Lundbaek JA, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68(3):680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 82.Søgaard R, Ebert B, Klaerke D, Werge T. Triton X-100 inhibits agonist-induced currents and suppresses benzodiazepine modulation of GABAA receptors in Xenopus oocytes. Biochim Biophys Acta. 2009;1788(5):1073–1080. doi: 10.1016/j.bbamem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Søgaard R, et al. GABAA receptor function is regulated by lipid bilayer elasticity. Biochemistry. 2006;45(43):13118–13129. doi: 10.1021/bi060734+. [DOI] [PubMed] [Google Scholar]
- 84.Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107(3):832–848. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urban BW. The site of anesthetic action. Handb Exp Pharmacol. 2008;2008(182):3–29. doi: 10.1007/978-3-540-74806-9_1. [DOI] [PubMed] [Google Scholar]
- 86.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666(1-2):62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: An energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 88.Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim Biophys Acta. 2008;1778(7-8):1545–1575. doi: 10.1016/j.bbamem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 89.Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73(1-2):159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 90.Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res. 1985;26(9):1015–1035. [PubMed] [Google Scholar]
- 91.Lundbaek JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35(12):3825–3830. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- 92.Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: Importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42(46):13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 93.Lundbaek JA, et al. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123(5):599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Artigas P, et al. 2,3-Butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: The role of bilayer material properties. Mol Pharmacol. 2006;70(6):2015–2026. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- 95.Barrantes FJ. Structural-functional correlates of the nicotinic acetylcholine receptor and its lipid microenvironment. FASEB J. 1993;7(15):1460–1467. doi: 10.1096/fasebj.7.15.8262330. [DOI] [PubMed] [Google Scholar]
- 96.Nie SQ, Majarais I, Kwan CY, Epand RM. Analogues of tetramethylpyrazine affect membrane fluidity of liposomes: Relationship to their biological activities. Eur J Pharmacol. 1994;266(1):11–18. doi: 10.1016/0922-4106(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 97.Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972;24(4):583–655. [PubMed] [Google Scholar]
- 98.Chisari M, et al. Structurally diverse amphiphiles exhibit biphasic modulation of GABAA receptors: Similarities and differences with neurosteroid actions. Br J Pharmacol. 2010;160(1):130–141. doi: 10.1111/j.1476-5381.2010.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruno MJ, Koeppe RE, 2nd, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci USA. 2007;104(23):9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawyer DB, Koeppe RE, 2nd, Andersen OS. Induction of conductance heterogeneity in gramicidin channels. Biochemistry. 1989;28(16):6571–6583. doi: 10.1021/bi00442a007. [DOI] [PubMed] [Google Scholar]
- 101.Nazari M, Kurdi M, Heerklotz H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys J. 2012;102(3):498–506. doi: 10.1016/j.bpj.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tibbs GR, et al. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J Pharmacol Exp Ther. 2013;345(3):363–373. doi: 10.1124/jpet.113.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jia F, et al. Isoflurane is a potent modulator of extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;324(3):1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- 104.Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996;119(7):1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patel AJ, et al. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2(5):422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 106.Andres-Enguix I, et al. Determinants of the anesthetic sensitivity of two-pore domain acid-sensitive potassium channels: Molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J Biol Chem. 2007;282(29):20977–20990. doi: 10.1074/jbc.M610692200. [DOI] [PubMed] [Google Scholar]
- 107.Liu C, Au JD, Zou HL, Cotten JF, Yost CS. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth Analg. 2004;99(6):1715–1722. doi: 10.1213/01.ANE.0000136849.07384.44. [DOI] [PubMed] [Google Scholar]
- 108.Beltrán LR, et al. The pungent substances piperine, capsaicin, 6-gingerol and polygodial inhibit the human two-pore domain potassium channels TASK-1, TASK-3 and TRESK. Front Pharmacol. 2013;4:141. doi: 10.3389/fphar.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hollmann MW, Liu HT, Hoenemann CW, Liu WH, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium. Part II: Interactions with volatile anesthetics. Anesth Analg. 2001;92(5):1182–1191. doi: 10.1097/00000539-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 110.Ogata J, et al. Effects of anesthetics on mutant N-methyl-d-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318(1):434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 111.Narang D, et al. Triton X-100 inhibits L-type voltage-operated calcium channels. Can J Physiol Pharmacol. 2013;91(4):316–324. doi: 10.1139/cjpp-2012-0257. [DOI] [PubMed] [Google Scholar]
- 112.Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med. 2006;2(2):S12–S18. [PubMed] [Google Scholar]
- 113.Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-d-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol. 1995;116(2):1761–1768. doi: 10.1111/j.1476-5381.1995.tb16660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-d-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475(1):83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilding TJ, Chai YH, Huettner JE. Inhibition of rat neuronal kainate receptors by cis-unsaturated fatty acids. J Physiol. 1998;513(Pt 2):331–339. doi: 10.1111/j.1469-7793.1998.331bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ouyang W, Herold KF, Hemmings HC., Jr Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110(3):582–590. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rehberg B, Duch DS. Suppression of central nervous system sodium channels by propofol. Anesthesiology. 1999;91(2):512–520. doi: 10.1097/00000542-199908000-00026. [DOI] [PubMed] [Google Scholar]
- 118.Gruss M, et al. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65(2):443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 119.Hara K, Eger EI, 2nd, Laster MJ, Harris RA. Nonhalogenated alkanes cyclopropane and butane affect neurotransmitter-gated ion channel and G-protein-coupled receptors: Differential actions on GABAA and glycine receptors. Anesthesiology. 2002;97(6):1512–1520. doi: 10.1097/00000542-200212000-00025. [DOI] [PubMed] [Google Scholar]
- 120.Wagner LE, 2nd, Gingrich KJ, Kulli JC, Yang J. Ketamine blockade of voltage-gated sodium channels: Evidence for a shared receptor site with local anesthetics. Anesthesiology. 2001;95(6):1406–1413. doi: 10.1097/00000542-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 121.Liu HT, Hollmann MW, Liu WH, Hoenemann CW, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium: Part I. Anesth Analg. 2001;92(5):1173–1181. doi: 10.1097/00000539-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 122.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 123.Hill MW. Partition coefficients of some anaesthetic-like molecules between water and smectic mesophases of dipalmitoyl phosphatidylcholine. Biochem Soc Trans. 1975;3(1):149–152. doi: 10.1042/bst0030149. [DOI] [PubMed] [Google Scholar]
- 124.Smith RA, Porter EG, Miller KW. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta. 1981;645(2):327–338. doi: 10.1016/0005-2736(81)90204-2. [DOI] [PubMed] [Google Scholar]
- 125.Eger EI, 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: A standard of anesthetic potency. Anesthesiology. 1965;26(6):756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 126.Franks NP, Lieb WR. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br J Anaesth. 1993;71(1):65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- 127.Herold KF, Nau C, Ouyang W, Hemmings HC., Jr Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009;111(3):591–599. doi: 10.1097/ALN.0b013e3181af64d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ingólfsson HI, Sanford RL, Kapoor R, Andersen OS. Gramicidin-based fluorescence assay; for determining small molecules potential for modifying lipid bilayer properties. J Vis Exp. 2010;2010(44):e2131. doi: 10.3791/2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Connell AM, Koeppe RE, 2nd, Andersen OS. Kinetics of gramicidin channel formation in lipid bilayers: Transmembrane monomer association. Science. 1990;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- 130.Lundbaek JA, Koeppe RE, 2nd, Andersen OS. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc Natl Acad Sci USA. 2010;107(35):15427–15430. doi: 10.1073/pnas.1007455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moore HP, Raftery MA. Direct spectroscopic studies of cation translocation by Torpedo acetylcholine receptor on a time scale of physiological relevance. Proc Natl Acad Sci USA. 1980;77(8):4509–4513. doi: 10.1073/pnas.77.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hackley VA, Clogston JD. Measuring the hydrodynamic size of nanoparticles in aqueous media using batch-mode dynamic light scattering. Methods Mol Biol. 2011;697:35–52. doi: 10.1007/978-1-60327-198-1_4. [DOI] [PubMed] [Google Scholar]
- 133.Berberan-Santos MN, Bodunov EN, Valeur B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential) Chem Phys. 2005;315(1–2):171–182. [Google Scholar]
- 134.Taheri S, et al. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg. 1991;72(5):627–634. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 135.Koblin DD, et al. Are convulsant gases also anesthetics? Anesth Analg. 1981;60(7):464–470. [PubMed] [Google Scholar]
- 136.Franks NP, Jenkins A, Conti E, Lieb WR, Brick P. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys J. 1998;75(5):2205–2211. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Idvall J, Ahlgren I, Aronsen KR, Stenberg P. Ketamine infusions: Pharmacokinetics and clinical effects. Br J Anaesth. 1979;51(12):1167–1173. doi: 10.1093/bja/51.12.1167. [DOI] [PubMed] [Google Scholar]
- 138.Giese JL, Stanley TH. Etomidate: A new intravenous anesthetic induction agent. Pharmacotherapy. 1983;3(5):251–258. doi: 10.1002/j.1875-9114.1983.tb03266.x. [DOI] [PubMed] [Google Scholar]