Significance
A major unresolved issue for premenopausal women undergoing chemotherapy is infertility due to the loss of nonrenewable ovarian primordial follicles. We show that pharmacologic down-regulation of the mammalian/mechanistic target of rapamycin (mTOR) pathway during chemotherapy in a mouse model prevents activation of primordial follicles, preserves ovarian function, and maintains normal fertility using clinically available inhibitors of mTOR complex (C)1 and mTORC1/2. These findings represent a feasible pharmacologic approach for preservation of ovarian function and fertility during treatment with conventional chemotherapy.
Keywords: mTOR, fertility, chemotherapy, ovary, ovarian function
Abstract
The ovary contains oocytes within immature (primordial) follicles that are fixed in number at birth. Activation of follicles within this fixed pool causes an irreversible decline in reproductive capacity, known as the ovarian reserve, until menopause. Premenopausal women undergoing commonly used genotoxic (DNA-damaging) chemotherapy experience an accelerated loss of the ovarian reserve, leading to subfertility and infertility. Therefore, there is considerable interest but little effective progress in preserving ovarian function during chemotherapy. Here we show that blocking the kinase mammalian/mechanistic target of rapamycin (mTOR) with clinically available small-molecule inhibitors preserves ovarian function and fertility during chemotherapy. Using a clinically relevant mouse model of chemotherapy-induced gonadotoxicity by cyclophosphamide, and inhibition of mTOR complex 1 (mTORC1) with the clinically approved drug everolimus (RAD001) or inhibition of mTORC1/2 with the experimental drug INK128, we show that mTOR inhibition preserves the ovarian reserve, primordial follicle counts, serum anti-Mullerian hormone levels (a rigorous measure of the ovarian reserve), and fertility. Chemotherapy-treated animals had significantly fewer offspring compared with all other treatment groups, whereas cotreatment with mTOR inhibitors preserved normal fertility. Inhibition of mTORC1 or mTORC1/2 within ovaries was achieved during chemotherapy cotreatment, concomitant with preservation of primordial follicle counts. Importantly, our findings indicate that as little as a two- to fourfold reduction in mTOR activity preserves ovarian function and normal birth numbers. As everolimus is approved for tamoxifen-resistant or relapsing estrogen receptor-positive breast cancer, these findings represent a potentially effective and readily accessible pharmacologic approach to fertility preservation during conventional chemotherapy.
The ovaries have a fixed pool of primordial follicles at birth constituting the ovarian reserve, activation or loss of which is responsible for the irreversible decline in reproductive function culminating in menopause (1). In women undergoing conventional genotoxic chemotherapy as treatment for cancer, the primordial follicle pool is depleted at a rapid pace, resulting in subfertility, infertility, and primary ovarian insufficiency (2). Consequently, for female cancer survivors, the probability of a first child after conventional gonadotoxic chemotherapy is significantly diminished (3). Primary ovarian insufficiency also results in increased morbidity from bone loss, cognitive dysfunction, sexual dysfunction, and cardiovascular disease (2). Apoptosis and vascular effects of chemotherapy are well-described pathways of chemotherapy-mediated ovarian damage, with growing follicles and ovarian stroma particularly susceptible (4, 5). Widely used alkylating chemotherapy agents such as cyclophosphamide (CY), a mainstay of breast cancer chemotherapy, are highly gonadotoxic and induce ovarian damage in part by activation of the PI3K/phosphatase and tensin homolog/protein kinase B (PTEN/AKT) pathway, leading to primordial follicle activation and follicular “burnout” (6, 7).
Ovarian folliculogenesis initiates from the primordial follicle stage, where an oocyte arrested in prophase of meiosis I and surrounded by a single layer of squamous granulosa cells is activated to grow and transition to a primary follicle, secondary follicle, and then ultimately a preovulatory antral follicle (Fig. S1). Most oocytes within the ovary exist in a quiescent state within primordial follicles, relatively resistant to antimitotic and genotoxic agents (8, 9). Follicular burnout refers to the repeated ovarian exposure to chemotherapy and damage to growing follicles, mobilizing dormant primordial follicles to activate and grow to replace damaged antral follicles (6, 8). Premature follicular activation exposes the entire follicular pool to chemotherapy’s genotoxic effects. Interventions designed to promote ovarian quiescence would therefore be expected to reduce the ovarian toxicity of cancer regimens. This is supported by pretreatment suppression of the hypothalamic–pituitary–ovarian axis with gonadotropin releasing hormone (GnRH) analogs that maintain the ovary in a quiescent state, although this approach is controversial presumably because only a fraction of the follicular pool is sensitive to gonadotropins (10, 11). In contrast, we reasoned that pharmacologic suppression of the earliest stages of follicular development should protect the entire follicle pool, thereby greatly expanding pharmacologic fertility preservation. This strategy could complement oocyte and embryo cryopreservation, which provides only a limited supply of gametes or embryos without the benefit of long-term ovarian protection, and which can currently only be used in peri- or postpubertal females (12).
Fig. S1.
Ovarian folliculogenesis and the role of mTOR. A diagram of the sequence of development from primordial follicles to antral follicles is shown. Also shown is the PI3K/AKT/mTOR pathway and the positive effect of activation on stimulation of folliculogenesis. Physiologic ovarian folliculogenesis is shown proceeding from the primordial follicle stage, where an oocyte arrested in prophase of meiosis I and surrounded by a single layer of squamous granulosa cells (in purple) is activated, grows and transitions to a primary follicle, a secondary follicle, and then a preovulatory antral follicle.
Activation of the mammalian/mechanistic target of rapamycin (mTOR) pathway is critical to primordial follicle activation (13–15). mTOR is a serine/threonine kinase and a metabolic sensor that regulates mRNA translation, cell growth, proliferation, autophagy, nutrient signaling, and survival (16). Accelerated mTOR activity in the oocyte by deletion of PTEN- or TSC1-negative regulators simultaneously activates the entire pool of primordial follicles in mice, resulting in primary ovarian insufficiency (13, 14, 17). Thus, mTOR stimulators increase the activation of primordial follicles in animal models and mTOR inhibitors block the primordial-to-primary follicle transition (18), highlighting the critical importance of the PI3K/AKT/mTOR pathway (19).
Results
CY Chemotherapy Reduces Primordial Follicle Counts in a Dose-Dependent Manner and Activates the Transition to Growing Follicles.
To establish the effects of chemotherapy regimens on primordial follicles, we conducted a dose-finding pilot study of CY exposure. Mice received weekly i.p. injections of vehicle (normal saline), 75 mg/kg CY, or 150 mg/kg CY over 3 wk followed by sacrifice 1 wk following the final dose. No gross abnormalities in the animals were identified at necropsy and by pathohistologic examination. No significant differences in weight at sacrifice were found between treatment groups (Fig. S2A). Because growing follicles may be overrepresented when serial ovarian sections are analyzed and estimations of follicle counts have been shown to be equivalent when whole ovaries are serially sectioned compared with interval sectioning (20), ovaries were sectioned in five 100-μm intervals (interval sectioning). The mean number of primordial follicles per ovarian surface area (mm2), a standard measure of gonadotoxicity, was inversely related to treatment dose, with the untreated control group having the highest average number of primordial follicles per mm2 (3.5 ± 0.5) compared with a conventional dose of 75 mg/kg CY (1.4 ± 0.2) and a sterilizing dose of 150 mg/kg CY (0.2 ± 0.1) (Fig. S2B).
Fig. S2.
Chemotherapy dose finding in the mouse. Mice were treated for 3 wk at the dose levels shown. Mice were killed 1 wk following the final dose of CY. Data are presented as mean ± SEM comparing baseline with 3 wk of treatment as shown at sacrifice. (A) Mouse weights (g) (P > 0.05). (B) Counts of primordial follicles per surface area (**P < 0.001, ***P < 0.0001). Results with SEM shown, five mice per group.
To determine the acute impact of CY exposure on acute ovarian follicle dynamics, we treated mice with one high dose of CY (150 mg/kg) followed by characterization at 24 h postexposure. There were no differences in untreated and treated mean weight, mean ovarian weight, or ovarian surface area. However, CY-treated mice had significantly fewer (60% reduction) primordial follicles than control mice (Fig. 1A). CY treatment also induced rapid follicle activation, demonstrating 2.5 times more growing follicles per mm2 than untreated controls (Fig. 1 B and C).
Fig. 1.
Cytoxan (cyclophosphamide) causes accelerated recruitment of primordial follicles to growing follicles. (A) Primordial follicles per mm2 in ovaries from adult female (8-wk-old) C57BL/6 mice 24 h posttreatment with high-dose CY (150 mg/kg) and untreated controls (***P < 0.0001). Mean ± SEM from five mice per treatment group. (B) Primary follicle counts were significantly greater in CY-treated mice (**P < 0.005). Mean ± SEM from five mice per treatment group. (C) Eight-week-old C57BL/6 mice treated with 150 mg/kg CY had significantly fewer primordial follicles and more primary follicles compared with ovaries from untreated controls. Ovarian sections stained with H&E and analyzed for follicle counts are shown (4× and 40× magnification). Representative growing follicles are marked with arrows.
mTOR Inhibitors Down-Regulate the PI3K/AKT/mTOR Pathway in Ovaries and Preserve Primordial Follicles When Administered CY.
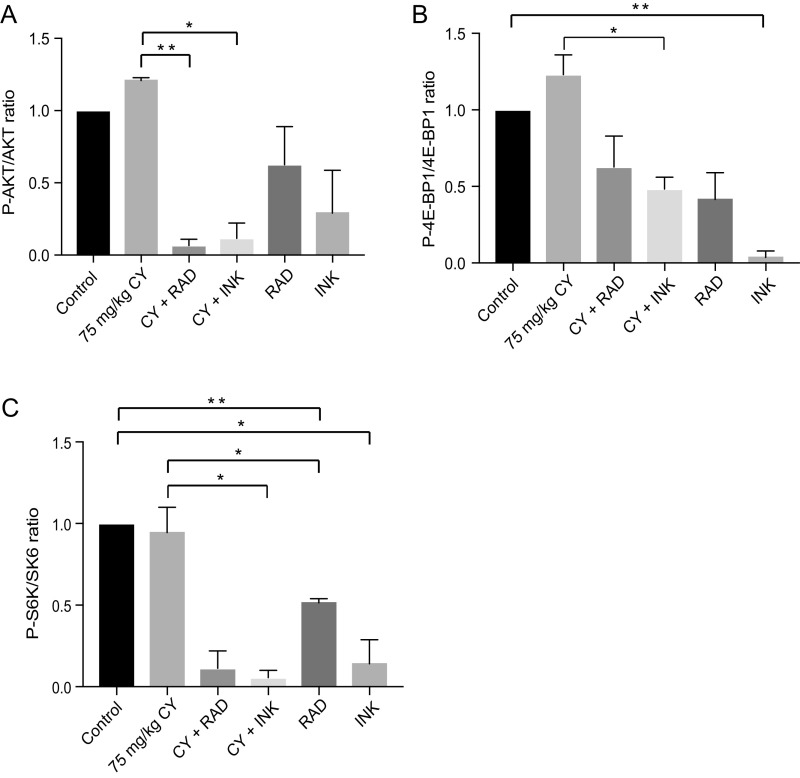
Mice were treated with CY, with or without mTOR inhibitors, as shown in the schema for treatment, and tissues were harvested (Fig. S3). CY treatment moderately increased AKT/mTOR pathway activation, shown by phosphorylation of S473 AKT and S65 4E-BP1 to P-AKT and P-4E-BP1, respectively, compared with untreated controls, measured within 2 h of the last daily mTOR inhibitor treatment (Fig. 2A). Inhibition of mTOR complex 1 (mTORC1) with everolimus (RAD001) or mTORC1/2 with INK reduced phosphorylation of 4E-BP1 and S6 kinase (S6K), with and without CY. Inhibition of AKT phosphorylation was greater in ovaries exposed to INK than RAD. mTORC1/2 inhibition by INK was also more effective in blocking phosphorylation of all downstream targets, consistent with the weaker activity of allosteric RAD (21). Phosphorylation of AKT and S6K was effectively blocked in ovaries exposed to CY with RAD or INK, more so than with mTOR inhibitors alone, suggesting possible synergism (Fig. S4).
Fig. S3.
Experimental schema. C57BL/6 mice at 8 wk of age were treated in groups of five mice. Control was treated with drug carrier (PVP) by gavage, RAD001 at 2.5 mg/kg by gavage, INK128 at 0.3 mg/kg by gavage, and CY 75 mg/kg by i.p. injection.
Fig. 2.
mTORC1 and mTORC1/2 inhibitors down-regulate downstream targets of the PI3K/AKT/mTOR pathway in the ovary. Whole ovaries from five mice per group of 8-wk-old mice treated with CY with or without RAD or INK, RAD or INK alone, or untreated control were lysed for immunoblot or paraffin-embedded, sectioned, and stained for IHC. (A) CY slightly increased phosphorylation of targets of the mTOR pathway P-AKT and P-4E-BP1 compared with control. Inhibition of mTORC1 with RAD and mTORC1/2 with INK decreased phosphorylation of AKT, 4E-BP1, and S6K, with and without CY. Quantification is shown in Fig. S4. (B) mTORC1/2 (INK) inhibition significantly decreased P-4E-BP1 expression compared with control (4.8% vs. 63.2%, ***P < 0.0001). Following CY treatment, primordial follicles were reduced in P-4E-BP1 expression by fourfold by mTORC1/2 (INK) inhibition compared with CY alone (18.2% vs. 71.4%, *P < 0.05). Mean ± SEM. (C) Representative results from P-4E-BP1 IHC are shown. Comparisons made between treated and untreated primordial follicle ooplasms correspond to light ring surrounding nuclei. (D) Representative results from P-S6K IHC are shown. (E) Inhibition of mTORC1 only (RAD) reduced S6K phosphorylation by twofold in follicles of CY-treated animals compared with fourfold for mTORC1/2 inhibition.
Fig. S4.
Immunoblot analysis of whole-ovary lysates assessing downstream targets of mTORC1 and 2. The ratio of phosphorylated to total protein is shown. (A) Ovaries exposed to CY+RAD showed significant inhibition of AKT phosphorylation compared with CY alone (**P < 0.005). Ovaries exposed to CY+INK showed significant inhibition of AKT phosphorylation compared with CY alone (*P < 0.05). Phosphorylation of AKT was decreased in ovaries exposed to CY alongside RAD or INK, more so than with mTOR inhibitors alone. (B) Inhibition of mTORC1/2 with INK decreased phosphorylation of 4E-BP1, with and without CY. Ovaries exposed to INK showed significant inhibition of 4E-BP1 phosphorylation compared with control (**P < 0.005). Ovaries exposed to CY+INK showed significant inhibition of 4E-BP1 phosphorylation compared with CY alone (*P < 0.05). (C) Inhibition of mTORC1 with RAD and mTORC1/2 with INK inhibited phosphorylation of S6 kinase, with and without CY. Phosphorylation of S6K was significantly decreased in RAD compared with control (**P < 0.005) and in CY+RAD compared with CY (*P < 0.05). Phosphorylation of S6K was significantly decreased in INK compared with control (*P < 0.05) and in CY+INK compared with CY (*P < 0.05). Results are quantified from two series of representative immunoblots. A representative immunoblot is shown in Fig. 2A. Data are presented as mean ± SEM.
Following immunoblotting of whole-ovary lysates, immunohistochemistry (IHC) was used to assess the activity of mTORC1 and mTORC1/2 within primordial follicles using 4E-BP1 and S6K phosphorylation within 2 h of the last inhibitor dose. Following treatment with CY and mTORC1/2 (INK) inhibition, primordial follicles averaged fourfold reduced mTOR activity compared with approximately twofold reduction for mTORC1-only inhibition (RAD) (Fig. 2 B–E). Overall, INK was a more effective mTORC1 inhibitor than RAD, even in non–CY-treated controls. RAD efficacy was tested by examining S6K phosphorylation and, in contrast to 4E-BP1 phosphorylation, inhibition of mTORC1-only (RAD) reduced S6K phosphorylation by twofold in follicles of CY-treated animals compared with fourfold for mTORC1/2 inhibition (Fig. 2 D and E).
Using conditions established above, mTOR inhibitors were treated with and without CY to assess the effects of cotreatment on follicle counts (schema, Fig. S3). Female mice were randomized into six groups (n = 5 per group) and treated with 75 mg/kg CY in three weekly doses with or without RAD or INK administered by daily oral gavage, followed by sacrifice 1 wk after the final dose of chemotherapy. Markers of toxicity were compared, including mouse weights (g) pre- and posttreatment, ovarian surface area (mm2), and ovarian weight (μg) (Fig. S5). There were no differences between groups when comparing ovarian surface area at sacrifice or ovarian weight at sacrifice (Fig. S5 A and B). Mouse weight was similar at baseline between all groups with the exception of the RAD+CY group, which had an average weight of 7.9% less than control at baseline (16.4 ± 0.1 vs. 17.7 ± 0.5, P = 0.03) (Fig. S5C). All mice treated with mTOR inhibitors weighed significantly more at sacrifice than they did at baseline, a phenomenon not seen in mice treated with CY or in untreated controls (Fig. S5C). Mice treated with 75 mg/kg CY alone had a 64% reduction in primordial follicles per mm2 compared with untreated controls (Fig. 3 A and B). Mice treated with 75 mg/kg CY together with RAD had twice as many primordial follicles compared with CY alone, and almost three times as many with INK. In animals treated with RAD or INK alone, there was a trend toward greater primordial follicle counts compared with untreated controls, which did not reach statistical significance. There were fewer primary follicles per mm2 with mTORC1 or mTORC1/2 inhibition when cotreated with CY, compared with mTORC1 or mTORC1/2 inhibition alone, supporting a synergistic effect of cotreatment on prevention of follicle activation (Fig. 3C). Secondary follicle counts and antral follicle counts were not statistically different among all treatment groups, despite a trend toward fewer antral follicles in the 75 mg/kg CY group (Fig. 3 D and E). Of note, total follicle counts were lower in all CY-treated mice compared with control, RAD alone, and INK alone, suggesting that mTOR inhibitors incompletely prevented primordial follicle activation and loss. This is likely secondary to the timing of administration (2 d before CY) and time needed to achieve steady-state concentrations, which is ∼7 d (22, 23) (Fig. S6). Strikingly, ovaries of CY-treated mice demonstrated a ratio of growing to primordial follicles more than twice that of ovaries from mice cotreated with CY+RAD or CY+INK (Fig. 3F), supporting our finding that mTOR inhibitors maintain ovarian quiescence and preserve the primordial follicle pool. The histological follicle counts also substantiate the molecular data, indicating that more than twofold, but no more than fourfold, down-regulation of mTORC1 and/or coinhibition of mTORC2 is all that is required to maintain ovarian follicle quiescence.
Fig. S5.
Minimal toxicity is associated with mTOR inhibitor and/or CY treatment in mice. Mice were treated with mTOR inhibitors (RAD, INK) daily for 4 wk with and without CY 75 mg/kg weekly for 3 wk to assess the effects of cotreatment on the ovarian reserve. Markers of toxicity were compared. (A) Ovarian surface area at time of sacrifice (mm2) (P > 0.05). (B) Ovarian weight at time of sacrifice (g) (P > 0.05). (C) Mouse weight at baseline and sacrifice (g). CY+RAD weighed less than control at baseline (*P < 0.05). All RAD- and INK-treated mice gained weight from baseline to sacrifice: CY+RAD (**P > 0.005), CY+INK (**P > 0.005), RAD (**P > 0.005), and INK (*P > 0.05). Data are presented as SEM.
Fig. 3.
Cotreatment with mTORC1 and mTORC1/2 inhibitors protects the primordial follicle pool in CY-exposed ovaries. (A and B) Eight-week-old C57BL/6 mice were treated with 75 mg/kg CY i.p. in three weekly doses, ±RAD or INK daily, and killed 1 wk after the final dose of CY. CY-treated mice were 64% reduced in primordial follicles compared with untreated controls. Mice treated with 75 mg/kg CY and RAD had 43% more primordial follicles compared with CY alone (*P < 0.05). Mice treated with CY and INK were 54% increased in primordial follicles compared with CY alone (**P < 0.005). Representative images are shown. (C) Mice cotreated with RAD+CY or INK+CY trended toward fewer primary follicles compared with CY alone (n.s., not significant; P > 0.05). (D and E) Secondary follicle and antral follicle counts were not statistically different among treatment groups despite trending toward fewer antral follicles in the 75 mg/kg CY group (n.s., P > 0.05). (F) Ovaries of CY-treated mice had twice the ratio of growing to primordial follicles compared with all other treatment groups (***P < 0.0001). Ovaries of mice cotreated with CY+RAD or CY+INK had ratios of growing to primordial follicles matching untreated controls. Results are derived from five mice per treatment group with SEM shown.
Fig. S6.
Total follicle counts in treated compared with untreated mice. Total follicle numbers were scored as the sum of all follicles. Results are presented as a scatterplot with spread of counts indicated, with SEM shown.
mTOR Inhibition Prevents Chemotherapy-Mediated Reduction in Serum Anti-Mullerian Hormone in a Dose-Dependent Manner.
Anti-Mullerian hormone (AMH) is produced by the granulosa cells of preantral and small antral follicles, correlates with histological primordial follicle numbers, and is one of the most important measures of ovarian reserve used clinically (24, 25). To investigate the impact of CY treatment on serum AMH, 8-wk-old mice were administered 75 mg/kg CY, 150 mg/kg CY, or vehicle (control) weekly for 3 wk and killed 1 wk following the final treatment. Untreated mice had significantly higher levels of serum AMH compared with 75 mg/kg CY-treated animals, which declined further at 150 mg/kg CY (Fig. 4A), indicative of chemotherapy-driven loss of follicles. Using conditions established above, mTOR inhibitors were treated with and without CY, and effects of cotreatment on serum AMH were assessed. There was no statistically significant difference in circulating AMH levels between untreated controls and RAD- or INK-treated groups in the absence of CY treatment (Fig. 4B). However, mice cotreated for 3 wk with weekly 75 mg/kg CY and daily RAD, and killed at 11 wk of age, showed significantly higher AMH levels compared with mice treated with CY alone (Fig. 4C). Importantly, even a small decrease in AMH levels reflects a clinical reduction in ovarian reserve (26). The same positive trend in AMH levels existed with INK+CY treatment but did not reach statistical significance.
Fig. 4.
Serum AMH decreases after CY treatment whereas mTOR inhibitor cotreatment maintains AMH concentration. (A) AMH serum concentrations were measured in untreated 8-wk-old C57BL/6 mice, mice exposed to three weekly doses of 75 mg/kg CY, and mice exposed to three weekly doses of 150 mg/kg CY. Mice treated with 75 mg/kg CY had significantly lower serum AMH levels compared with control (**P < 0.005), as did mice treated with 150 mg/kg CY (*P < 0.05). (B and C) AMH serum concentrations were measured in 8-wk-old mice that underwent long-term treatment (3 wk) with polyvinylpyrrolidone (PVP) alone daily, 75 mg/kg CY weekly for 3 wk, RAD daily, RAD daily plus 75 mg/kg CY weekly, INK daily, or INK plus 75 mg/kg CY weekly. There were no differences in AMH concentrations in mice treated with RAD or INK alone compared with untreated controls. Mean ± SEM. Mice cotreated for 3 wk with weekly 75 mg/kg CY and daily RAD and killed 1 wk after the final CY treatment had a significantly higher AMH compared with CY alone (12.5 vs. 11.1, *P < 0.05). Mice cotreated for 3 wk with weekly 75 mg/kg CY and daily INK and killed 1 wk after the final CY treatment had a higher AMH level compared with CY alone but this did not reach significance (12.6 vs. 11.1, n.s., P > 0.05). Results are derived from five mice per treatment group with SEM shown.
mTOR Inhibitors Preserve Fertility in Chemotherapy-Treated Mice.
We next investigated whether mTOR inhibition also preserves fertility during chemotherapy. Eight-week-old female mice were treated with a nonsterilizing dose of 75 mg/kg CY in four weekly doses, with or without RAD or INK cotreatment. One additional week of treatment was used to further impact on subfertility, providing even more stringent criteria for the assessment of fertility than used earlier. To ensure two full cycles of primordial follicle activation following treatment, breeding commenced 8 wk following the final treatment. Mice were harem-bred with proven male breeders and given 8 wk to breed (Fig. S3). Studies have shown that rodents are capable of normal mating behavior as early as 14 d following CY exposure (27).
There was no difference in mouse weight following 4 wk of treatment (P > 0.05) (Fig. S7). After an 8-wk delay, all mice had incrementally gained weight and there were no differences in mouse weights between groups, supporting no differences in systemic toxicity between groups (P > 0.05) (Fig. S7). Two out of five female CY-only treated mice were infertile, compared with no infertile females in the RAD+CY and INK+CY groups. The CY-treated animals also had significantly fewer pups per litter compared with controls (3.4 ± 1.7 vs. 8.8 ± 0.5, P < 0.005) (Fig. 5A). Importantly, RAD+CY (7.4 ± 1.2) and INK+CY (7.4 ±0.9) treatment groups produced significantly more pups per litter compared with CY alone (3.4 ± 1.7, P < 0.05). There were no differences in litter size between RAD alone, INK alone, and untreated controls. There were no differences in the percentage of pups born live, pup weight, or pup anomalies among treatment groups (Fig. 5 B and C). The time from the start of breeding (days) to birth was similar between groups, arguing against altered endocrine effects (Fig. 5D). There was a trend toward increased time from male introduction to birth in the CY group compared with untreated controls, which did not reach significance.
Fig. S7.
Mating study mouse weights. (A) There was no difference in mouse weight following 4 wk of treatment. (B) Following an 8-wk delay posttreatment, at the start of breeding, all mice had incrementally gained weight and there were no differences in mouse weights between groups, with SEM shown.
Fig. 5.
Cotreatment with RAD or INK at the time of CY administration increases murine litter size. The average of five mice per treatment is shown with SEM. (A) Quantification of the number of pups born in first litters. Two mice from the CY treatment group were infertile (0). CY treatment significantly reduces litter size compared with untreated controls (**P < 0.005), and CY+RAD and CY+INK had significantly greater litter sizes than CY alone (*P < 0.05). (B) Quantification of the percentage of live births. Two mice from the CY treatment group are not shown due to infertility (n.s., P > 0.05). (C) Quantification of pup weight. Two mice from the CY treatment group are not shown due to infertility (n.s., P > 0.05). (D) Time (days) from male introduction to birth. CY-treated mice trended toward a longer time to birth, but this did not reach significance (n.s., P > 0.05). Results are derived from five mice per treatment group with SEM shown.
Discussion
Inhibitors of mTORC1 and mTORC1/2 have a growing role in cancer treatment as part of multiagent chemotherapeutic regimens and are being explored for the treatment of a growing list of malignant as well as nonmalignant conditions. Here we further elucidated the critical role of the mTOR pathway in primordial follicle activation and demonstrated that mTOR inhibitors can have a second significant function in promoting follicular quiescence when administered as pretreatment and cotreatment with conventional gonadotoxic chemotherapy. We show that daily administration of mTOR inhibitors that achieve only a two- to fourfold attenuation of mTOR activity can maintain ovarian follicles in their primordial state during chemotherapy treatment, maintain normal serum AMH levels, and preserve normal fertility. Although estrus cyclicity was not assessed, our data do not support an interpretation involving an effect of treatments on estrus cyclicity. The impact of CY on fertility was therefore related to ovarian reserve rather than ovulation. In the mouse, the cycle of follicle maturation requires ∼10 to 12 d from primordial follicle to secondary follicle, and an additional 6 to 12 d for development to the antral follicle (28) (Fig. S8 for follicle classifications). We obtained tissue and serum 1 wk after the third and final treatment dose, so the difference observed in AMH level likely reflects the impact of the first 1 to 2 wk of treatment on the primordial follicle pool. Primordial follicle counts were significantly greater in the RAD+CY group and INK+CY group compared with CY alone, and AMH levels were significantly greater in RAD+CY compared with CY. The actual AMH values were identical between RAD+CY and INK+CY groups, but AMH did not reach significance between the INK+CY and CY groups. This may be attributed to variance but invites further investigation. Whereas primordial follicle counts were significantly greater in mice cotreated with mTOR inhibitors and CY compared with CY alone, total follicle counts were lower among all CY-treated mice compared with mice not exposed to CY. Follicular burnout is likely one of many processes occurring in the ovary during chemotherapy exposure, and growing follicles, vasculature, and ovarian stroma are also susceptible to chemotherapy effects. Importantly, although oral RAD001 is rapidly absorbed, steady-state concentrations are not reached for ∼7 d (22). In our study, mTOR inhibitors were administered for 2 d before the first exposure to chemotherapy, likely insufficient to prevent early recruitment and subsequent loss of primordial follicles during the first CY administration. Future studies will be needed to determine the optimal timing of mTOR inhibitor administration before chemotherapy.
Fig. S8.
Follicle classifications (for SI Materials and Methods). (A) Primordial follicle. Clearly identified oocyte surrounded by a single layer of squamous granulosa cells without a thecal layer. Typically identified within the ovarian cortex and <20 μm. Magnification 40×. (B) Primary follicle. Oocyte surrounded by a single layer of cuboidal granulosa cells. Magnification 40×. (C) Secondary follicle. Oocyte surrounded by two or more layers of cuboidal granulosa cells but no antrum. Magnification 20×. (D) Antral follicle. Many layers of cuboidal granulosa cells with fluid accumulated amid the granulosa cells. Magnification 20×.
Our data suggest that mTOR inhibitors may represent a fertility-sparing pharmacologic therapy that can be administered alongside gonadotoxic chemotherapy. Oocyte or embryo cryopreservation are proven methods of fertility preservation for peri- and postmenarchal females but are time-consuming, costly, may be medically contraindicated, and preserve only a limited number of gametes or embryos with no maintenance of ovarian function. Ovarian tissue cryopreservation is still an experimental technique and requires an initial surgery to remove ovarian tissue followed by subsequent surgery for autotransplantation, and may risk reintroducing malignant cells (29).
Intriguingly, in endometriosis, which contributes to diminished ovarian reserve and an accelerated decline in fertility (1), enhanced follicular recruitment and burnout are observed in the ovarian cortex of ovaries with endometriomas (30). The insulin growth factor system, which regulates signaling through pathways including AKT/mTOR, is altered in endometriotic tissues, suggesting a similar relationship between mTOR activation and follicular burnout in endometriosis (31). Data implicate mTOR up-regulation in the poor reproductive outcomes of overweight and obese patients, who are more likely to be infertile, have higher miscarriage rates, and poorer obstetric outcomes compared with normal-weight peers (32–34). Obese rats demonstrate accelerated ovarian follicle development and follicle loss and activation of the mTOR pathway (35), whereas SIRT1 activators, which suppress mTOR, improve the ovarian reserve (36). Caloric restriction in rats also down-regulates mTOR activity and preserves the ovarian reserve (37). mTOR inhibitors may be attractive for prevention of iatrogenic and noniatrogenic depletion of ovarian reserve (31).
Beyond the important scope of fertility, primary ovarian insufficiency has devastating emotional, psychosocial, and physical consequences, contributing to depression, cognitive dysfunction, bone loss, sexual dysfunction, and even cardiovascular mortality, prevention of which all require maintenance of normal ovarian function (2, 38–40). Moreover, with the continuing rise in average age at first birth in the United States and the reciprocally reduced fertility rate (41, 42), the possibility of extending reproductive potential is attractive. Investigating the relationship between mTOR and the ovarian reserve may provide opportunities for therapeutic intervention. Because the PI3K/AKT/mTOR pathway plays a critical role in primordial follicle activation (13, 43), this relationship has been investigated as a means to promote follicle growth in patients with POI. In murine models, inhibitors of PTEN (a negative regulator of PI3K) and TSC1 and TSC2 (negative regulators of mTOR) have been shown to activate the mTOR pathway and primordial follicles (13, 14, 17). Similarly, AKT stimulators (both PI3K activators and PTEN inhibitors) have been used in both preclinical and experimental clinical models to activate in vitro cultured ovarian cortical strips, retransplant the activated cortical strips, and stimulate follicle growth in vivo (44). In a prospective study of 37 women with primary ovarian insufficiency who underwent in vitro activation of ovarian cortical tissue with an AKT stimulator followed by in vitro fertilization, 24 oocytes were retrieved from six patients, ultimately leading to two successful deliveries (44, 45). Moreover, Rictor/mTORC2 was recently implicated as a key regulator of folliculogenesis, with inactivation of this pathway ubiquitously leading to premature ovarian failure (15).
Studies are needed to assess the long-term reproductive impact of mTOR inhibitors, and specifically the impact on future pregnancies and offspring. Although few large studies exist regarding the impact of mTOR inhibitors on long-term fertility and pregnancy outcomes, case series and observational studies describe fertility outcomes in patients who have been treated with rapalogs following solid-organ transplant. Following kidney transplant, women treated with long-term sirolimus (13 mo or longer) developed amenorrhea, which resolved with its discontinuation, and men who were azoospermic developed normal semen parameters and fathered children following discontinuation (46). Case reports describe temporary azoospermia in men treated with sirolimus, which resolved after its discontinuation or switch to a different agent, and a rodent study further demonstrated the reversible impact of sirolimus on male fertility (47, 48).
Although our study is limited by the use of a murine model, the mouse and human ovary maintain many functional similarities in that the ovarian reserve in both is maintained in primordial follicles that localize to the ovarian cortex, and follicular development occurs through the same stages (49, 50). It is important to note that the mTOR inhibitor regimens used in clinical oncology practice are based on maximum tolerated doses and are associated with stomatitis, fatigue, gastrointestinal distress, headache, and rash (51). Dosages used in our study were extrapolated from these regimens to mice, and may thus be greater than necessary for fertility preservation. Future studies will assess the effects of mTOR inhibitors and alkylating chemotherapy on tumor dynamics, and determine optimal and minimal dose levels and regimens.
Materials and Methods
In Vivo Murine Model.
Studies were approved by the New York University School of Medicine Institutional Animal Care and Use Committee and conducted in accordance with their guidelines. Female C57BL/6 mice aged 8 wk (Taconic Biosciences) were housed under pathogen-free conditions in autoclaved individually ventilated cages (IVCs) that included autowatering and HEPA-filtered air. Room temperature was maintained at 68 to 69 °F and cages on IVC racks were maintained at 70 to 71 °F. The vivarium light cycle was 12 h on and 12 h off, animals were fed irradiated chow (Purina Lab diet 5053), and water was produced on-site using the reverse-osmosis method with a reserve maintained on-site and delivered to the cage rack via an automatic watering system.
Murine Model Treatment Protocols.
Dose-finding pilot studies were performed to identify the optimal chemotherapy regimen in our murine model. The doses used were CY (Sigma-Aldrich; 6055-19-2) 75 mg/kg, CY 150 mg/kg, RAD001 (Selleckchem.com; S1120; an mTORC1 inhibitor) 2.5 mg/kg, and INK128 (Selleckchem.com; S2811; an mTORC1/2 inhibitor) 0.3 mg/kg. The same dosages of CY, RAD001, and INK128 were maintained for the treatment combinations.
Statistical Analysis.
One-way analysis of variance, Student’s t test, chi-square test, and Fisher’s exact test were used where appropriate. Data are presented as mean ± SEM with significance set at P < 0.05. The ratio of growing to primordial follicles was calculated by dividing the total number of growing follicles (primary, secondary) in each treatment group by the total number of primordial follicles in each treatment group.
Additional Methods and Information.
Additional information can be found in SI Materials and Methods.
SI Materials and Methods
Dose and Treatment Cycle Determination.
The doses and treatment cycles were selected to reflect dosages used in humans. CY dosage is that which would be used by a young reproductive-aged woman pursuing treatment for premenopausal breast cancer. This was calculated by using data extracted from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Breast Cancer (https://www.nccn.org/professionals/physician_gls/f_guidelines.asp). CY is administered at 600 mg/m2 i.v. every 21 d for four cycles. The average body surface area for a 60-kg woman who is 5′4′′ (body mass index 22) is 1.65. At a dose of 600 mg/m2 × 1.65 m2, this would constitute 990 mg per cycle × 4 cycles = 3,960 mg in one round of CY = 3,960/60 kg = 66 mg/kg in a typical round of Cytoxan (cyclophosphamide). The dosage of CY causing amenorrhea in a 60-kg woman is 150 mg/kg in a 30-y-old and 85 mg/kg in a 40-y-old. We thus selected the dosages of CY 150 mg/kg and 75 mg/kg. CY 75 mg/kg is a dosage previously used in rodent studies to produce subfertility.
The dosage of RAD001 used in this study was 2.5 mg⋅kg−1⋅d−1, shown to be an effective dose in xenograft models of human endometrial, pancreatic, and hepatocellular carcinoma, among others. Animal dosages (2.5 mg/kg) were converted to human equivalent doses in mg/kg by multiplying the animal dose by 0.081 (52): 2.5 mg/kg × 0.081 = 0.2 mg/kg adult dosage × 60 kg = 12 mg daily. INK128 was administered at 0.3 mg/kg, a dose showing tumor growth inhibition efficacy in breast cancer xenograft models. The dose range for INK128 in humans in a phase I dose-escalation trial in multiple myeloma and B-cell malignancies ranged from 2 to 7 mg daily. Thus, the dosage of 0.3 mg/kg daily in mice translates to 1.4 mg daily in humans, a similar but slightly lower dose than is currently under investigation for clinical use.
Mice were randomly assigned to one of six treatment groups (n = 5 per group): INK128 0.3 mg/kg (100 μL by oral gavage; i.g.), RAD001 2.5 mg⋅kg−1⋅d−1 (100 μL i.g.), or vehicle (PVP 100 μL i.g.) on days 1 to 5 of weeks 1, 2, and 3, and days 1 to 4 of week 4, with or without CY 75 mg/kg i.p. weekly on day 3 of weeks 1 to 3 (Fig. S3). Mice were killed on day 4 of week 4. At the time of sacrifice, ovaries were harvested and immediately plunged into 4% (vol/vol) paraformaldehyde (PFA) or liquid nitrogen. INK128 was administered at a concentration of 0.3 mg⋅kg−1⋅d−1, a dose showing tumor growth inhibition efficacy in breast cancer xenograft models (53, 54). RAD001 was administered at a dosage of 2.5 mg⋅kg−1⋅d−1, a dose previously used in xenograft models of human pancreatic neuroendocrine tumor metastatic progression and human hepatocellular carcinoma (55, 56).
Systemic Toxicity.
Mean change in animal weight from baseline, mean ovarian weight, and ovarian section area (mm2) were used as proxies for systemic and ovarian toxicity. Ovarian section measurements were taken in two perpendicular diameters, and section area was calculated by multiplying two perpendicular measurements of each section (mm2). All measurements were performed using Digital SlidePath software (Leica Biosystems).
Preparation of Ovarian Sections.
Following sacrifice, both ovaries from each mouse were isolated. One ovary was weighed and transferred to a cryogenic vial in liquid nitrogen for further analysis. The other ovary was fixed in 4% PFA at 4 °C overnight, transferred to 70% ethanol, dehydrated in a series of ethanol concentrations, cleared in xylene, and embedded in paraffin. Ovarian sectioning was performed on all ovaries from killed animals in 5-μm sections taken from five intervals 100-μm apart throughout each ovary. At each consecutive interval, six sections were cut serially, resulting in five sets with nearly identical ovarian sections.
Hematoxylin and Eosin Staining and Follicle Classification.
The first slide of each 100-μm interval was stained with H&E, and five consecutive blank slides were cut at 5-μm intervals. Performing follicle counts by sectioning at five 100-μm intervals was deemed equivalent to whole-ovary sectioning in a pilot study, as described (20). Blinded follicle counts were conducted by one investigator, and follicle counts were independently confirmed by a second blinded reviewer. Where discordant, sections were rereviewed and a score arrived at. Examination of primordial follicles was performed at 40× or 80× magnification, primary follicles at 40× or 20×, secondary at 20× or 10×, and antral follicles at 10× or 4×. Primordial follicles were primarily identified close to the ovarian surface within the ovarian cortex, as reported (57). Differential follicle counts were performed and follicle stage was classified according to accepted published definitions (58). A follicle was defined as primordial if the oocyte was surrounded by a single layer of squamous granulosa cells (Fig. S8A). A primary follicle was defined as an oocyte surrounded by a single layer of cuboidal granulosa cells. A secondary follicle was defined by two or more layers of cuboidal granulosa cells with no antrum (greater than or equal to one cuboidal granulosa cell in the second layer was defined as a secondary follicle) (Fig. S8 B and C). An early antral follicle was defined by two or more layers of cuboidal granulosa cells with more than one small antral cavity (identified by an antrum measuring larger than the size of a single granulosa cell). An antral follicle was defined by two or more layers of cuboidal granulosa cells with one large antrum (Fig. S8D). Only follicles containing an oocyte were counted, and atretic follicles were excluded. All primary and secondary follicles were grouped together as “growing” follicles for the purposes of analysis. Follicle counts were reported as the average number of follicles per section as well as the average number of follicles per ovarian surface area (mm2) to account for intraovarian and interovarian differences in section area. All measurements of ovarian surface area were performed using Digital SlidePath software (Leica Biosystems).
Immunohistochemistry.
Ovarian sections prepared as described above were stained to demonstrate the effect of treatment on the target tissue. Paraffin-embedded ovarian sections were warmed and serially deparaffinized in xylene and ethanol, introduced into an antigen unmasking solution, and blocked, and sections were incubated overnight at 4 °C with antibodies to S65 phospho-4E-BP1 (9451) and T389 phospho-S6 kinase (9205) from Cell Signaling Technology. All antibodies were used at 1:1,000 dilution and compared with isotype control antibodies. Antibody to eIF4A was a gift from W. Merrick, Case Western Reserve University, Cleveland, OH. Primordial follicles showing diffuse ooplasmic staining were considered positive. Primordial follicles were scored in a binary manner as either 0 (negative) or 1 (positive).
Serum Measurements.
Blood was extracted by terminal cardiac puncture from each mouse at the time of sacrifice, collected into serum separator tubes (BD; Microtainer 365956), and separated by centrifugation at 1,500 × g for 10 min at 4 °C. Serum was cryopreserved at −80 °C. Serum AMH concentrations were quantified by mouse-specific AMH ELISA (MyBioSource) according to the manufacturer’s instructions (conducted by DS Biotech).
Immunoblot Analyses of mTORC1/2 Pathway Activity.
Mouse ovaries (n = 30) were placed in liquid nitrogen at the time of sacrifice. A minimum of three ovaries from each treatment group were individually homogenized in a buffer of 150 mM NaCl, 50 mM Tris⋅HCl (pH 8), 1 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 25 mM β-glycerophosphate, 2 mM Na3VO4, and Protease Inhibitor (EDTA-free) tablets (Thermo Fisher Scientific). Protein concentration was determined by the bicinchoninic acid assay. To determine the total levels and phosphorylation status of specific proteins, equal amounts of protein were resolved by SDS/PAGE in 8%, 10%, or 12% gels as indicated and transferred to 0.45-μm PVDF membranes (Millipore) for immunoblotting. The following Cell Signaling antibodies were used: mTOR [rabbit monoclonal antibody (mAb) 2972], S2448 phospho-mTOR (rabbit mAb 2971), AKT (rabbit mAb 9272), S473 phospho-AKT (rabbit mAb 9271), S6 (rabbit mAb 2217), phospho-S6 (rabbit mAb 2211), 4E-BP1 (rabbit mAb 9452), S65 phospho-4E-BP1 (rabbit mAb 9451), and anti-rabbit eEF2 or eIF4A to verify equal loading, all used at 1:1,000 dilution. The Enhanced Chemiluminescence (ECL; GE Healthcare) or SuperSignal (Thermo Scientific) procedure was used to visualize protein signals as described by the manufacturers. The light intensity of each band was quantified with ImageJ software (NIH).
Mating Protocol.
Mice were randomly assigned to one of six treatment groups (n = 5 per group): INK128 0.3 mg/kg, RAD001 2.5 mg⋅kg−1⋅d−1, or vehicle (PVP 100 μL) on days 1 to 5 of weeks 1, 2, 3, and 4, with or without CY 75 mg/kg i.p. weekly on day 3 of weeks 1 to 4. Eight weeks following treatment, mice were housed with proven male breeders (C57BL/6, 3- to 5-mo-old; Charles River). Harem mating of two females per one male was used where applicable. In total, each treatment maintained two harem-breeding cages and one one-on-one breeding cage. Female mice were removed from the breeding cage at confirmed signs of pregnancy, including a weight >31g. Separated females were monitored every 24 to 36 h for pups. Following birth, pups were counted and weighed. Data recorded following birth included female weight postbirth, number of pups, percent of living pups, weight of pups, pup anomalies, and days from male interaction to birth. Mice were given up to 8 wk for breeding and successful birth of a first litter. Infertility was defined as no sign of pregnancy by 8 wk in a trio-breeding cage with a fertile female partner.
Acknowledgments
We gratefully acknowledge the technical assistance provided by B. Dinardo, L. Larkin, the NYU Experimental Pathology Histology Core Lab, and the intellectual contributions of D. Silvera and A. Alard. This work was supported by the American Society for Reproductive Medicine (K.N.G.), Foundation for Women’s Wellness (K.N.G.), Center for Reproductive Health After Disease Grant P50 HD076188 from the National Centers for Translational Research in Reproduction and Infertility (to F.E.D.), Avon Foundation (R.J.S.), and Breast Cancer Research Foundation (R.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617233114/-/DCSupplemental.
References
- 1.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Practice Committee of the American Society for Reproductive Medicine Female age-related fertility decline. Committee opinion no. 589. Obstet Gynecol. 2014;123(3):719–721. doi: 10.1097/01.AOG.0000444440.96486.61. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Magelssen H, Melve KK, Skjaerven R, Fosså SD. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum Reprod. 2008;23(1):178–186. doi: 10.1093/humrep/dem362. [DOI] [PubMed] [Google Scholar]
- 4.Utsunomiya T, Tanaka T, Utsunomiya H, Umesaki N. A novel molecular mechanism for anticancer drug-induced ovarian failure: Irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. Int J Oncol. 2008;32(5):991–1000. [PubMed] [Google Scholar]
- 5.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3(8):782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalich-Philosoph L, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 7.Chang EM, et al. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS One. 2015;10(12):e0144245. doi: 10.1371/journal.pone.0144245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 9.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, et al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: A meta-analysis of randomized controlled trials. Breast. 2013;22(2):150–157. doi: 10.1016/j.breast.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Moore HC, et al. POEMS/S0230 Investigators Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franasiak JM, et al. Endometrial CXCL13 expression is cycle regulated in humans and aberrantly expressed in humans and rhesus macaques with endometriosis. Reprod Sci. 2014;22(4):442–451. doi: 10.1177/1933719114542011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari D, et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15(12):765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- 14.Adhikari D, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, et al. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J Biol Chem. 2015;290(10):6387–6396. doi: 10.1074/jbc.M114.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, et al. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle. 2015;14(5):721–731. doi: 10.1080/15384101.2014.995496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107(22):10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith BJ, Plowchalk DR, Sipes IG, Mattison DR. Comparison of random and serial sections in assessment of ovarian toxicity. Reprod Toxicol. 1991;5(4):379–383. doi: 10.1016/0890-6238(91)90097-y. [DOI] [PubMed] [Google Scholar]
- 21.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 22.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Tardif S, et al. Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015;70(5):577–587. doi: 10.1093/gerona/glu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.van Rooij IA, et al. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 26.Dewailly D, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 27.Jarrell JF, Bodo L, YoungLai EV, Barr RD, O’Connell GJ. The short-term reproductive toxicity of cyclophosphamide in the female rat. Reprod Toxicol. 1991;5(6):481–485. doi: 10.1016/0890-6238(91)90019-c. [DOI] [PubMed] [Google Scholar]
- 28.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA. 2002;99(5):2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Practice Committee of the American Society for Reproductive Medicine Ovarian tissue cryopreservation: A committee opinion. Fertil Steril. 2014;101(5):1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Kitajima M, et al. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014;101(4):1031–1037. doi: 10.1016/j.fertnstert.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum Reprod Update. 2016;22(3):dmv060. doi: 10.1093/humupd/dmv060. [DOI] [PubMed] [Google Scholar]
- 32.Bellver J. Obesity and poor reproductive outcome: Female and male body weight matter. Fertil Steril. 2013;99(6):1558–1559. doi: 10.1016/j.fertnstert.2013.01.142. [DOI] [PubMed] [Google Scholar]
- 33.van der Steeg JW, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–328. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- 34.Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. 2011;118(1):63–70. doi: 10.1097/AOG.0b013e31821fd360. [DOI] [PubMed] [Google Scholar]
- 35.Wang N, et al. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63(1):94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhou XL, et al. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res. 2014;7:97. doi: 10.1186/s13048-014-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, et al. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015;22(1):60–67. doi: 10.1177/1933719114542016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterling EW, Nelson LM. From victim to survivor to thriver: Helping women with primary ovarian insufficiency integrate recovery, self-management, and wellness. Semin Reprod Med. 2011;29(4):353–361. doi: 10.1055/s-0031-1280920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allshouse AA, Semple AL, Santoro NF. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015;22(2):166–174. doi: 10.1097/GME.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 40.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 41.Mills M, Rindfuss RR, McDonald P, te Velde E. ESHRE Reproduction and Society Task Force Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9):1–68. [PubMed] [Google Scholar]
- 43.Zhang H, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24(21):2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura K, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki N, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 46.Boobes Y, Bernieh B, Saadi H, Raafat Al Hakim M, Abouchacra S. Gonadal dysfunction and infertility in kidney transplant patients receiving sirolimus. Int Urol Nephrol. 2010;42(2):493–498. doi: 10.1007/s11255-009-9644-8. [DOI] [PubMed] [Google Scholar]
- 47.Deutsch MA, et al. Sirolimus-associated infertility: Case report and literature review of possible mechanisms. Am J Transplant. 2007;7(10):2414–2421. doi: 10.1111/j.1600-6143.2007.01929.x. [DOI] [PubMed] [Google Scholar]
- 48.Rovira J, et al. Sirolimus-associated testicular toxicity: Detrimental but reversible. Transplantation. 2012;93(9):874–879. doi: 10.1097/TP.0b013e31824bf1f0. [DOI] [PubMed] [Google Scholar]
- 49.Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh) 1969;62(1):98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- 50.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50(2):225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 51.Paplomata E, Zelnak A, O’Regan R. Everolimus: Side effect profile and management of toxicities in breast cancer. Breast Cancer Res Treat. 2013;140(3):453–462. doi: 10.1007/s10549-013-2630-y. [DOI] [PubMed] [Google Scholar]
- 52.Gokmen-Polar Y, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136(3):673–682. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg. 2009;6(2):47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Djukom C, et al. Dual inhibition of PI3K and mTOR signaling pathways decreases human pancreatic neuroendocrine tumor metastatic progression. Pancreas. 2014;43(1):88–92. doi: 10.1097/MPA.0b013e3182a44ab4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huynh H, et al. Loss of tuberous sclerosis complex 2 (TSC2) is frequent in hepatocellular carcinoma and predicts response to mTORC1 inhibitor everolimus. Mol Cancer Ther. 2015;14(5):1224–1235. doi: 10.1158/1535-7163.MCT-14-0768. [DOI] [PubMed] [Google Scholar]
- 56.Smith BJ, Plowchalk DR, Sipes IG, Mattison DR. Comparison of random and serial sections in assessment of ovarian toxicity. Reprod Toxicol. 1991;5(4):379–383. doi: 10.1016/0890-6238(91)90097-y. [DOI] [PubMed] [Google Scholar]
- 57.Kerr JB, et al. Quantification of healthy follicles in the neonatal and adult mouse ovary: Evidence for maintenance of primordial follicle supply. Reproduction. 2006;132(1):95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]