Significance
Nonalcoholic fatty liver disease (NAFLD) is a common chronic hepatic disease, eventually leading to liver fibrosis and cirrhosis. Currently, no effective treatment is available. This study reports the role of cytochrome P450 omega-hydroxylase 4A14 (CYP4A14) in the development and progression of NAFLD. Overexpression of CYP4A14 promotes, whereas gene disruption of CYP4A14 attenuates, high-fat diet (HFD)- and methionine and choline-deficient diet (MCD)-induced liver lipid accumulation. Ablation of CYP4A14 gene markedly attenuates liver damage, inflammation, and fibrosis in MCD-induced nonalcoholic steatohepatitis (NASH). CYP4A14 may represent a therapeutic target for the treatment of NAFLD.
Keywords: NAFLD, arachidonic acid, fatty acid, inflammation, hepatic fibrosis
Abstract
Nonalcoholic fatty liver disease (NAFLD) is characterized by simple hepatic steatosis (SS), nonalcoholic steatohepatitis (NASH), hepatic fibrosis, and cirrhosis. Dysregulated fatty acid metabolism in the liver plays a critical role in the pathogenesis of NAFLD. Cytochrome P450 omega-hydroxylase 4A14 (CYP4A14) is a homolog of human CYP4A hydroxylase that catalyzes omega-hydroxylation of medium-chain fatty acids and arachidonic acid in mice. The goal of this study was to determine the role of CYP4A14 in the development and the progression of NAFLD. Here, we showed that hepatic CYP4A expression was up-regulated in the livers of patients and three murine models of NAFLD. Adenovirus-mediated overexpression of CYP4A14 in the livers of C57BL/6 mice resulted in a fatty liver phenotype with a significant increase in hepatic fatty acid translocase (FAT/CD36) expression. In contrast, CYP4A14 gene-deficient mice fed a high-fat diet or a methionine and choline-deficient (MCD) diet exhibited attenuated liver lipid accumulation and reduced hepatic FAT/CD36 expression. In addition, hepatic inflammation and fibrosis was markedly ameliorated in MCD diet-fed CYP4A14-deficient mice. Collectively, CYP4A14 plays an important role in the pathogenesis of both SS and NASH and may represent a potential therapeutic target for the treatment of NAFLD.
With the increasing incidence of obesity, diabetes, and metabolic syndrome in the general population, nonalcoholic fatty liver disease (NAFLD) is emerging as one of the most common causes of chronic liver disease (1). NAFLD manifests as a histological spectrum of diseases, starting with steatosis (SS) and then possibly progressing to nonalcoholic steatohepatitis (NASH). Hepatic SS is generally thought to be a benign liver lipid accumulation in the absence of histological evidence of hepatocellular injury. However, NASH refers to hepatic steatosis with varying degrees of inflammation, hepatocyte damage, and progressive fibrosis (2). It is generally believed that an imbalance between lipid availability (from circulating lipid uptake or de novo lipogenesis) and lipid disposal (via fatty acid oxidation or triglyceride-rich lipoprotein secretion) initiates hepatic lipid accumulation, which eventually triggers oxidative stress, metabolic inflammation, hepatocyte injury, and extracellular matrix accumulation (3).
The hepatic cytochrome P450 (CYP450) enzyme family possesses NAPDH monooxygenase activity and catalyzes the oxidative metabolism of many exogenous and endogenous chemicals. Increasing evidence demonstrates that some members of the CYP450 family may contribute to the pathogenesis of NAFLD. For example, it has been reported that CYP2E1 plays an important role in promoting the development of NASH by initializing lipid peroxidation due to increased reactive species production (4). The CYP4 family of cytochrome P450s catalyzes omega-hydroxylation of saturated, branched chain, and unsaturated fatty acids. The cytochrome P450 4A (CYP4A) subfamily is one of 18 subfamilies that constitute the CYP4 family and consists of 20 individual forms in nine different mammalian species (5, 6).
In mice, the CYP4A family has four members designated as CYP4A10, CYP4A12a, CYP4A12b, and CYP4A14 (5, 7–9). Mouse CYP4A14 has shown strain-specific expression pattern and is female-predominant in the liver, where its expression is selectively induced by peroxisome proliferator-activated receptor alpha (PPARα) (6, 10, 11). It has also been reported that hepatic CYP4A14 expression levels were significantly increased in ob/ob mice and db/db mice, two murine models of spontaneous nonalcoholic fatty liver disease (12–14). In addition, in two diet-induced mouse models of NAFLD, including high-fat diet (HFD)-induced simple steatosis (SS) or methionine and choline-deficient diet (MCD)-induced NASH, CYP4A14 levels were markedly up-regulated in the liver (7, 8, 15). Inhibition of CYP4A attenuates, whereas induction of CYP4A promotes, hepatic ER stress, insulin resistance, and apoptosis in diabetic mice (16). Moreover, similar to CYP2E1, CYP4A enzymes, especially CYP4A14 and CYP4A10, have been reported to be alternative initiators of oxidative stress in experimental NASH (4). These findings suggest that CYP4A, in particular CYP4A14, may have an important role in the pathogenesis of insulin resistance and NAFLD.
To date, the role of CYP4A in the regulation of hepatic lipid metabolism and in the development of NAFLD remains largely unclear. In this study, we tested the hypothesis that hepatic CYP4A14 plays a critical role in the pathogenesis of SS and NASH. We found that CYP4A14 was markedly up-regulated in both patients and murine models with NAFLD. We then demonstrated that hepatic overexpression of CYP4A14 increased lipid accumulation in the livers of wild-type mice, whereas CYP4A14 gene deficiency prevented the development of SS and NASH in HFD- and MCD-induced NAFLD in mice. Together, our findings provide evidence that CYP4A14 is critical for the development of SS and NASH.
Materials and Methods
Animals and Treatments.
Wild-type 129/SvJ breeders were purchased at 8 weeks of age from the Jackson Laboratory. CYP4A14 gene knockout (cyp4a14−/−) mice were a gift from J. Capdevila at Vanderbilt University, Nashville, TN. Animals were housed under a 12:12-h light/dark cycle and permitted ad libitum consumption of water and diet. Eight-week-old male C57BL/6 mice (Department of Experimental Animals, Peking University) were used to determine CYP4A14 expression in the liver. All procedures were approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center. To overexpress CYP4A14 in the liver, 1.0 × 109 plaque-forming units (pfu) of Ad-CYP4A14 or Ad-GFP were injected into the tail veins of C57BL/6 mice. On the seventh day after viral injection, the animals were killed for further experimental analysis. A 45% high-fat diet (HFD; MD12032) and an otherwise identical diet with 10% fat (control diet; MD12031) were purchased from Medicience Ltd. (Yangzhou, China). Mice were fed either the control diet or the HFD ad libitum for 8 wk. In addition, a methionine and choline-deficient diet MCD diet (MCD; MD12052) and an otherwise identical diet sufficient in methionine and choline (MCD control; MD12051) (Medicience) were obtained to feed C57BL/6 mice ad libitum for 4–8 wk. The mice were not fasted before sample collection. After performing isoflurane anesthesia, blood was collected from the vena cava to isolate serum. Sections of livers from the left lateral lobes were fixed in 4% (wt/vol) paraformaldehyde, then fixed in 20% (wt/vol) sucrose, and finally embedded in paraffin or optimal cutting temperature (OCT) compound. The remaining livers were snap-frozen in liquid nitrogen.
Measurement of Hepatic Triglyceride and Serum Chemistry.
Hepatic triglyceride levels were analyzed as described (17). The activity of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured at the Third Hospital of Peking University.
Analysis of Hepatic Malondialdehyde Levels.
The extent of lipid peroxidation in the liver homogenate was evaluated by measuring the concentration of the thiobarbituric acid-reactive product, malondialdehyde (MDA), using an MDA assay kit (Beyotime Biotechnology).
Immunohistochemistry.
Liver samples from patients with NAFLD were provided by J. Fan at Shanghai Jiaotong University School of Medicine, Shanghai, China. The use of human samples was approved by the Institutional Human Ethical Committee of Xinhua Hospital and informed consent was obtained. Mouse livers were fixed, dehydrated, and embedded in paraffin wax. Fixed liver sections (5 μm) were incubated with an anti-CYP4A antibody (1:100) or an anti–α-SMA antibody (1:400) overnight at 4 °C and then with a polyperoxidase-conjugated goat anti-rabbit IgG (Zhongshan Golden Bridge) for 30 min at 37 °C. The slides were counterstained with hematoxylin.
Quantitative RT-PCR Analysis.
Total RNA was extracted from the frozen liver tissues by using a High Purity Total RNA Extraction Kit (BioTeke). Five micrograms of total RNA from each sample were reverse-transcribed into complementary DNAs (cDNAs). Each cDNA sample was diluted by a factor of 1:100, and 5 μL were used as a template in each PCR (Thermo). The quantitative PCR was performed on an Agilent Mx3000P PCR System (Agilent Technologies) by using the TransStart Top Green qPCR SuperMix (Transgen). Expression levels of the target genes were normalized against an endogenous reference gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For each sample and each gene, PCR was carried out in duplicate and repeated a few times. The specific primer sequences are listed in Table S1.
Table S1.
Sequences of primers used for real-time quantitative PCR
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
| CYP4A14 | TGAATTGCTGCCAGATCCCAC | GTTCAGTGGCTGGTCAGAGTT |
| FAS | GCCATGCCCAGAGGGTGGTT | AGGGTCGACCTGGTCCTCA |
| ACC1 | TGGAGCTAAACCAGCACTCC | GCCAAACCATCCTGTAAGCC |
| CPT1a | ATCGTGGTGGTGGGTGTGATAT | ACGCCACTCACGATGTTCTTC |
| AOX | TGTCATTCCTACCAACTGTC | CCATCTTCTCAACTAACACTC |
| CD36 | GTGCAAAACCCAGATGACGT | TCCAACAGACAGTGAAGGCT |
| LFABP | GCAGAGCCAGGAGAACTTTGAG | TTTGATTTTCTTCCCTTCATGCA |
| apoB | TCACCATTTGCCCTCAACCTAA | GAAGGCTCTTTGGAAGTGTAAAC |
| MTTP | AACTCCTACGAGCCCTCCTT | AGTCCTCCCAGGATCAGCTT |
| TNFα | CACAAGATGCTGGGACAGTGA | TCCTTGATGGTGGTGCATGA |
| MCP1 | AATGAGTAGCAGCAGGTGAGTG | GAAGCCAGCTCTCTCTTCCTC |
| MIP2 | CCCAGACAGAAGTCATAGCCAC | GCCTTGCCTTTGTTCAGTATC |
| IP10 | AAGTGCTGCCGTCATTTTCT | GTGGCAATGATCTCAACACG |
| IL-1α | CGCTTGAGTCGGCAAAGAAA | TGATACTGTCACCCGGCTCT |
| IL-1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| COL1A1 | CAATGCAATGAAGAACTGGACTGT | TCCTACATCTTCTGAGTTTGGTGA |
| COL1A2 | GCAGGGTTCCAACGATGTTG | GCAGCCATCGACTAGGACAGA |
| α-SMA | CTGACAGAGGCACCACTGAA | CATCTCCAGAGTCCAGCACA |
| TGFβ1 | TGACGTCACTGGAGTTGTACGG | GGTTCATGTCATGGATGGTGC |
| GAPDH | AGAACATCATCCCTGCATCC | TTGTCATTGAGAGCAATGCC |
Reaction temperature was 59 °C for all genes.
Western Blot Analysis of Hepatic Proteins.
To determine the expression levels of selected proteins, 80 μg of liver protein was separated by 8% (wt/vol) SDS gel. Western blot analysis was performed as described (17) by using antibodies including anti-EIF5 (1:3,000, SC-282; Santa Cruz), anti-CYP4A14 (1:500, SC-46087; Santa Cruz), anti-CD36 (1:1,000, AB-133625; Abcam), anti-COL1A2 (1:1,000, BS-1530), anti-α-SMA (1:1,000, SIGMA-A2547; Sigma). An immunoblot was performed, and the membrane was developed with enhanced chemiluminescence (ECL).
Statistical Analysis.
The significance of variability was evaluated by unpaired two-tailed Student’s t test. P < 0.05 was considered statistically significant.
Results
Up-Regulation of Hepatic CYP4A14 in Murine Models of NAFLD.
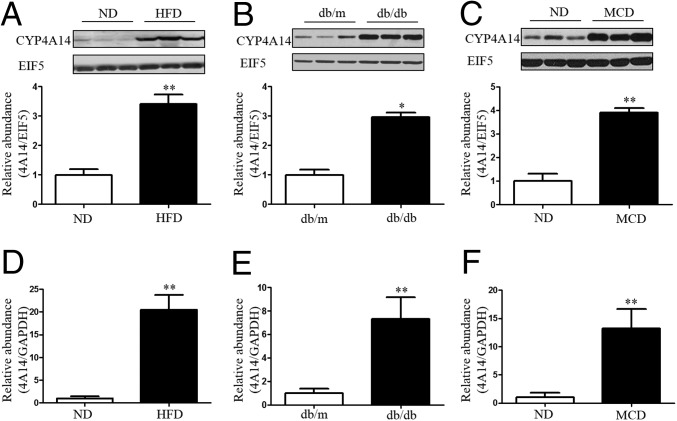
To test the possibility that hepatic CYP4A is involved in the pathogenesis of NAFLD, we measured hepatic CYP4A14 expression levels in three murine models of NAFLD. We first tested CYP4A14 expression in two murine models of simple steatosis (SS), i.e., HFD-fed mice and db/db mice. After feeding normal C57BL/6 mice with a HFD, hepatic CYP4A14 protein levels were increased ∼threefold compared with the mice fed with a normal diet (Fig. 1A). Similarly, in db/db mice, a genetic model of NAFLD, CYP4A14 protein expression was significantly increased in the livers compared with db/m control mice (Fig. 1B). Then, we generated a mouse model of NASH by feeding the mice with a MCD diet (18), which exhibited a marked increase in hepatic CYP4A14 protein levels (Fig. 1C). Furthermore, enhanced hepatic CYP4A14 protein expression was accompanied with a significant increase in CYP4A14 mRNA levels in all three murine models of NAFLD (Fig. 1 D–F). Collectively, these findings support the possibility that increased CYP4A14 expression may contribute to the development of SS and NASH in mice.
Fig. 1.
Up-regulation of CYP4A14 expression in the livers of three murine models of NAFLD. (A–C) Western blot analysis showing a significant increase in hepatic CYP4A14 protein expression in HFD-fed mice (A), db/db mice (B), and MCD-fed mice (C), respectively. *P < 0.05, **P < 0.01, n = 3. (D–F) Real-time PCR assay demonstrating that CYP4A14 mRNA expression was significantly increased in HFD-fed mice (D), db/db mice (E), and MCD-fed mice (F), respectively. **P < 0.01, n = 6. Data are presented as mean ± SEM. ND, normal diet.
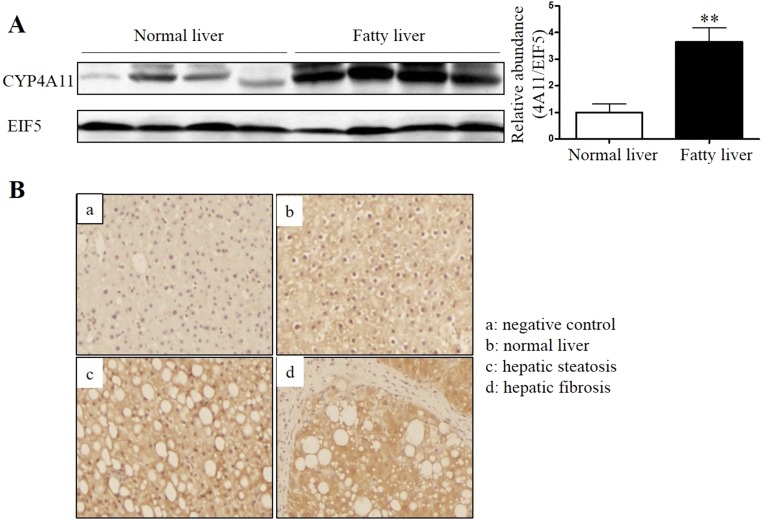
To further investigate the correlation between CYP4A and NAFLD, we also examined expression levels of CYP4A, a homolog of mouse CYP4A14, in liver samples of healthy humans and patients with NAFLD. As shown in Fig. S1, both Western blot (Fig. S1A) and immunohistochemistry (Fig. S1B) assays showed that CYP4A11 expression was significantly up-regulated in the livers of patients with NAFLD. Together, these findings suggest CYP4A family may play an important role in the pathogenesis of NAFLD in both mice and human.
Fig. S1.
Increased CYP4A protein expression in the livers of patients with NAFLD. (A) Western blot assay demonstrating increased CYP4A protein levels in the livers of patients with NAFLD. **P < 0.01, n = 4. (B) Immunohistochemistry analysis showing that NAFLD patients showed stronger hepatic CYP4A immunoreactivity than controls. (Magnification: 200×.) Negative control, immunostaining without primary antibody against CYP4A. Data are presented as mean ± SEM.
Overexpression of Hepatic CYP4A14 Causes Hepatic Lipid Accumulation.
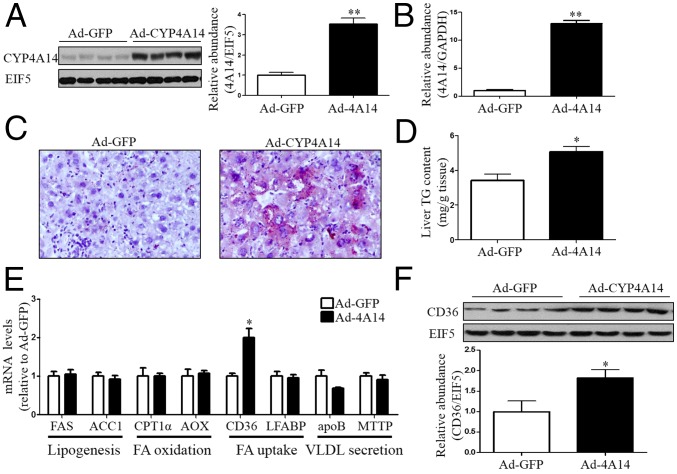
To further demonstrate that CYP4A14 is involved in the development of NAFLD, we overexpressed CYP4A14 in the livers of wild-type mice via an adenovirus-based approach. Hepatic overexpression of CYP4A14 was achieved by tail vein injection of an adenovirus expressing CYP4A14 (Ad-cyp4a14), which was validated by a significant increase in CYP4A14 mRNA and protein levels (Fig. 2 A and B). CYP4A14-overexpressing livers exhibited excessive neutral lipid deposition as assessed by Oil Red O staining (Fig. 2C) and a significant increase in hepatic triglyceride content (Fig. 2D). To elucidate the underlying mechanism responsible for CYP4A14-induced hepatic lipid accumulation, we determined expression levels of genes involved in hepatic lipogenesis (FAS and ACC1), lipid β-oxidation (CPT-1α and AOX), lipid secretion (MTP and apoB), and fatty acid uptake (FAT/CD36 and LFABP). Among these genes, only FAT/CD36 was found to be up-regulated in CYP4A14-overexpressing livers (Fig. 2E). Consistently, protein expression of FAT/CD36 was also significantly increased in the livers with CYP4A14 overexpression (Fig. 2F).
Fig. 2.
Hepatic CYP4A14 overexpression increased hepatic triglycerides accumulation. Male C57BL/6 mice were i.v. injected with 1.5 × 109 pfu control adenovirus (Ad-GFP) (n = 7) or a CYP4A14 (Ad-cyp4a14) expressing adenovirus (n = 7) for 7 d. (A) Western blot analysis of CYP4A14 protein expression in the liver. **P < 0.01, n = 4. (B) Quantitative RT-PCR analysis of CYP4A14 mRNA levels in the liver. **P < 0.01, n = 7. (C) Oil Red O staining showing increased neutral lipid accumulation in Ad-CYP4A14–infected livers. (Magnification: 200×.) (D) Levels of hepatic triglycerides. *P < 0.05, n = 7. (E) Quantitative RT-PCR analysis of mRNA levels of genes involved in hepatic lipid metabolism. *P < 0.05, n = 7. (F) Western blot analysis demonstrating increased CD36 protein expression in Ad-CYP4A14–infected livers. *P < 0.05, n = 4. Data are presented as mean ± SEM. FA, fatty acid; VLDL, very low-density lipoprotein.
CYP4A14 Gene Knockout Mice Are Resistant to HFD-Induced Hepatic Steatosis.
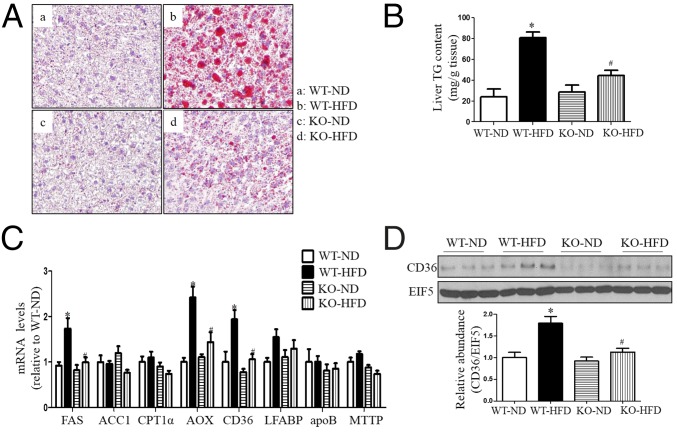
To further determine the role of CYP4A14 in hepatic lipid metabolism, we fed wild-type and CYP4A14 gene knockout (cyp4a14−/−) mice with a high-fat diet for 2 mo. Compared with HFD-fed wild-type mice, cyp4a14−/− mice exhibited a significant reduction in hepatic lipid contents as assessed by Oil Red O staining (Fig. 3A) and liver triglyceride measurements (Fig. 3B). Although mRNA expression of FAS, AOX, and FAT/CD36 was induced in the livers of both genotypes after HFD feeding, the levels were much lower in cyp4a14−/− mice than in wild-type mice (Fig. 3C). Consistently, HFD-induced hepatic FAT/CD36 protein expression was significantly reduced in cyp4a14−/− mice compared with wild-type mice (Fig. 3D). Interestingly, in the livers of cyp4a14−/− mice, mRNA expression of CYP4A10 and CYP4A12 was significantly reduced (Fig. S2).
Fig. 3.
CYP4A14 gene deficiency ameliorated HFD-induced hepatic steatosis. Male wild-type littermates and CYP4A14 gene knockout mice (cyp4a14−/−) were fed a normal diet (ND) or HFD for 8 wk. (A) Oil Red O staining showing a marked attenuation in HFD-induced hepatic steatosis in cyp4a14−/− mice. (Magnification: 200×.) (B) Cyp4a14−/− mice exhibited a significant reduction of hepatic triglycerides levels. n = 6–7. (C) Quantitative RT-PCR analysis demonstrating that CD36 mRNA levels were significantly decreased in the livers of HFD-fed cyp4a14−/− mice. n = 6–7. (D) Western blot analysis showing a reduced hepatic CD36 protein expression in the livers of HFD-fed cyp4a14−/− mice. n = 3. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. WT on ND; #P < 0.05 vs. WT on HFD.
Fig. S2.
Hepatic CYP4A10 and CYP4A12 mRNA levels were down-regulated in CYP4A14 gene-deficient male mice. Quantitative RT-PCR analysis of mRNA levels of hepatic CYP4A10 and CYP4A12 in the livers. Data are presented as mean ± SEM, *P < 0.05, n = 10.
To further test the role of CYP4A14 in hepatic lipid uptake, mouse liver cells were cultured by using wild-type and cyp4a14−/− mice. CYP4A14 gene deficiency significantly reduced the uptake of free fatty acid (Fig. S3 A and B) and the expression of palmitic acid-induced FAT/CD36 mRNA and protein (Fig. S3 C and D). In addition, because it has been reported that CYP4A14 gene is sexually dimorphically expressed and may exhibit phenotypic sex differences (19), we also determined the effect of HFD on hepatic lipid metabolism in female mice. As shown in Fig. S4, female CYP4A14 knockout mice were also resistant to HFD-induced hepatic steatosis.
Fig. S3.
CYP4A14 gene deficiency attenuated palmitic acid-induced lipid accumulation in primary cultured hepatocytes. (A) Fluorescence images of cellular BODIPY-C16 uptake in primary cultured mouse hepatocytes from wild-type and CYP4A14 gene knockout mice. (Magnification: 200×.) The cells were treated with vehicle [control (CON)] or palmitic acid (PA) at 0.5 mmol/L. (B) Quantification of BODIPY-C16 uptake in A. (C) Quantitative RT-PCR analysis of mRNA levels of FAT/CD36. (D) Western blot analysis of protein levels of FAT/CD36. *P < 0.05, n = 3.
Fig. S4.
CYP4A14 gene deficiency ameliorated HFD-induced hepatic steatosis. Female wild-type (fWT) littermates and CYP4A14 gene knockout mice (CYP4A14−/−) were fed a ND or HFD for 8 wk. (A) Oil Red O staining showing a marked attenuation in HFD-induced hepatic steatosis in CYP4A14−/− mice. (Magnification: 200×.) (B) CYP4A14−/− mice exhibited a significant reduction of hepatic triglycerides levels. (C) Quantitative RT-PCR analysis demonstrating that CD36 mRNA levels were significantly decreased in the livers of HFD-fed CYP4A14−/− mice. (D) Western blot analysis showing a reduced hepatic CD36 protein expression in the livers of HFD-fed CYP4A14−/− mice. *P < 0.05, **P < 0.01 vs. fWT-ND; #P < 0.05 vs. fWT-HFD; n = 5–7. Data are presented as mean ± SEM.
Ablation of CYP4A14 Gene Attenuates MCD-Induced Steatohepatitis.
To test the role of CYP4A14 in the pathogenesis of NASH, wild-type and cyp4a14−/− mice were fed with a MCD diet for 4 wk. Both morphological examination and Oil Red O staining showed excessive lipid accumulation in the livers of wild-type mice. In consistence, hepatic triglyceride contents were also significantly increased in MCD-fed wild-type mice (Fig. 4 A and B). However, ablation of CYP4A14 gene markedly attenuated MCD diet-induced hepatic steatosis (Fig. 4 A and B). Among many genes involved in hepatic lipid metabolism, FAT/CD36 was the gene robustly induced by the MCD diet in both genotypes (Fig. 4 C and D). However, MCD-induced FAT/CD36 mRNA and protein expression was significantly attenuated in cyp4a14−/− mice compared with wild-type mice (Fig. 4 C and D). These findings suggest that attenuated MCD-induced steatosis may be associated with down-regulated FAT/CD36 expression in the livers of cyp4a14−/− mice.
Fig. 4.
CYP4A14 deficiency ameliorated MCD-induced steatohepatitis and liver injury. Male wild-type (WT) littermates and cyp4a14−/− mice were fed with an ND (normal diet) or MCD diet for 4 wk. (A) Oil Red O staining showing that cyp4a14−/− mice were resistant to MCD-induced lipid accumulation in the livers. (Magnification: 200×.) (B) Biochemical assay showing that hepatic triglycerides levels were significantly lower in MCD-fed cyp4a14−/− mice than that in MCD-fed WT mice, n = 8–9. (C) Quantitative RT-PCR analysis showing that hepatic CD36 mRNA levels were significantly reduced in MCD-fed cyp4a14−/− mice compared with MCD-fed WT mice, n = 8–9. (D) Western blot analysis demonstrating that hepatic CD36 protein levels were much lower in MCD-fed cyp4a14−/− mice than that in MCD-fed WT mice, n = 3. (E and F) Cyp4a14−/− mice exhibited reduced serum ALT and AST levels after receiving a MCD diet, n = 8–9. (G) Hepatic levels of malonaldehyde (MDA) in the liver, n = 8–9. (H) Quantitative RT-PCR analysis of mRNA levels of proinflammatory genes in the livers, n = 8–9. *P < 0.05, **P < 0.01 vs. WT on ND, #P < 0.05 vs. WT on MCD. Data are presented as mean ± SEM.
As expected, serum ALT and AST activity was increased in mice fed with an MCD diet compared with mice fed with a control diet (Fig. 4 E and F). Surprisingly, compared with wild-type mice, CYP4A14 gene deficiency significantly decreased serum ALT and AST activity (Fig. 4 E and F), suggesting that ablation of CYP4A14 gene reduced hepatocellular injury in mice fed with an MCD diet. In addition, CYP4A14 gene deficiency markedly ameliorated lipid peroxidation in the livers of mice fed with an MCD diet, as reflected by a marked reduction in hepatic malondialdehyde (MDA) content (Fig. 4G). Importantly, MCD-induced hepatic expression of inflammatory genes including TNFα, MCP1, MIP2, IP10, IL-1α, and IL-1β was also significantly attenuated in cyp4a14−/− mice (Fig. 4H).
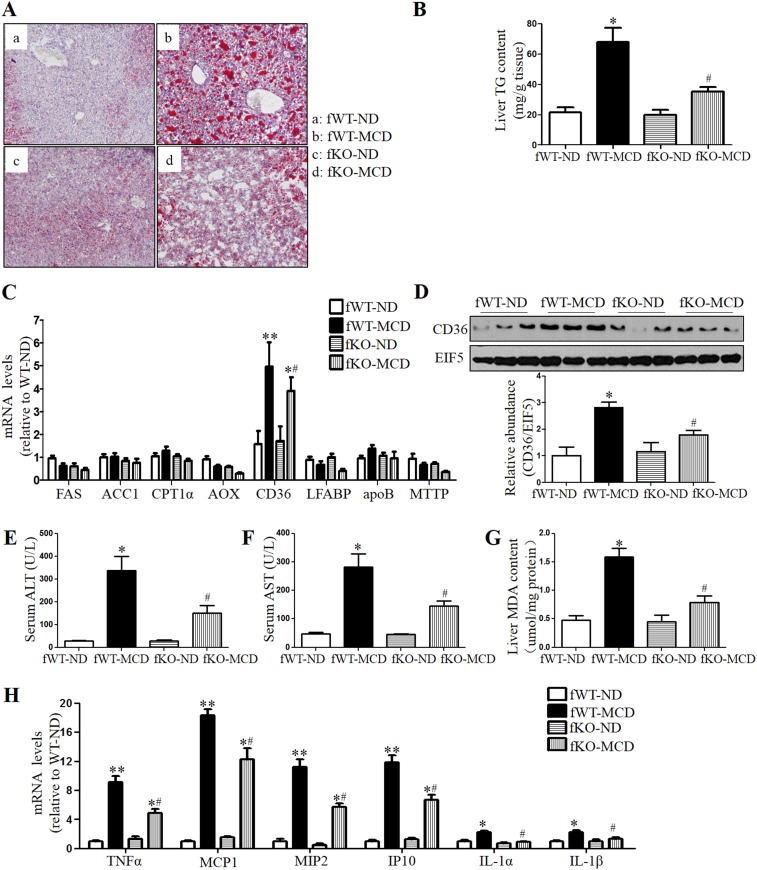
To further test the role of CYP4A in hepatic steatosis, male Sprague–Dawley rats on MCD diet were treated with TS-011, a selective inhibitor of the synthesis of CYP4A metabolite 20-HETE. We found that MCD diet-induced hepatic steatosis and dysfunction were markedly attenuated by TS-011 treatment for 2 wk (Fig. S5). In addition, similar to the effect in male mice, global deletion of CYP4A14 gene also attenuates MCD-induced steatohepatitis in female mice (Fig. S6).
Fig. S5.
Inhibition of CYP4A in Sprague–Dawley rats ameliorated MCD diet-induced steatosis and liver injury. Male Sprague–Dawley rats were fed with MCD diet in the presence or absence of TS-011 for 2 wk. (A) Representative images of Oil Red O staining of livers of mice receiving ND, MCD diet alone, and MCD diet plus TS-011, respectively. (Magnification: 200×.) (B) Levels of hepatic triglycerides. (C) Levels of serum ALT. *P < 0.05, n = 10.
Fig. S6.
CYP4A14 deficiency ameliorated MCD-induced steatohepatitis and liver injury. Female wild-type (fWT) littermates and CYP4A14−/− mice were fed with an ND or MCD diet for 4 wk. (A) Oil Red O staining showing that CYP4A14−/− mice were resistant to MCD-induced lipid accumulation in the livers. (Magnification: 200×.) (B) Biochemical assay showing that hepatic triglycerides levels were significantly lower in MCD-fed CYP4A14−/− mice than that in MCD-fed WT mice. (C) Quantitative RT-PCR analysis showing that hepatic CD36 mRNA levels were significantly reduced in MCD-fed CYP4A14−/− mice compared with MCD-fed WT mice. (D) Western blot analysis demonstrating that hepatic CD36 protein levels were much lower in MCD-fed CYP4A14−/− mice than that in MCD-fed WT mice. (E and F) CYP4A14−/− mice exhibited reduced serum ALT and AST levels after receiving an MCD diet. (G) Hepatic levels of malonaldehyde (MDA) in the liver. (H) Quantitative RT-PCR analysis of mRNA levels of proinflammatory genes in the livers. *P < 0.05, **P < 0.01 vs. fWT-ND; #P < 0.05 vs. fWT-MCD; n = 5–7. Data are presented as mean ± SEM. TG, triglyceride.
Loss of CYP4A14 Function Reduces Hepatic Fibrosis in Mice Fed an MCD Diet.
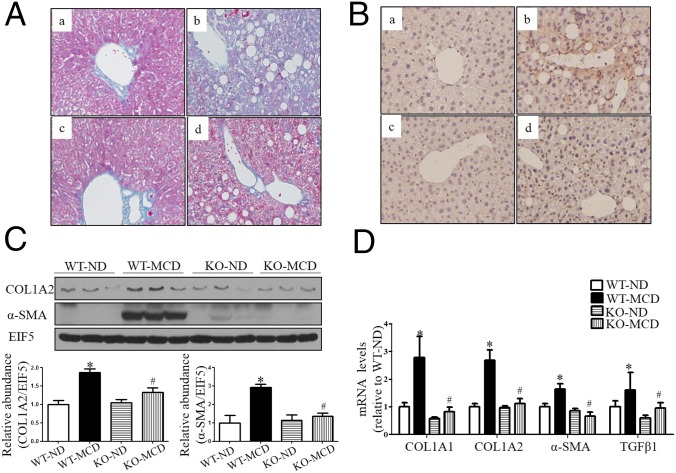
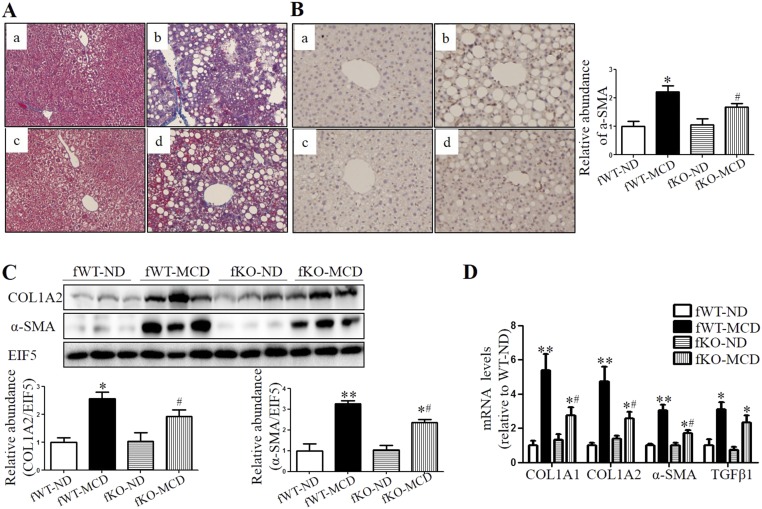
As revealed by Masson’s staining, wild-type mice fed with MCD diet for 8 wk exhibited portal and perisinusoidal fibrosis (Fig. 5A). However, CYP4A14 gene deficiency significantly attenuated hepatic fibrosis (Fig. 5A). To evaluate the involvement of CYP4A14 in the activation of hepatic stellate cells (HSC), the expression of α-SMA (smooth muscle actin), an activated HSC marker, was examined via an immunohistochemical assay and Western blot analysis. Protein expression of α-SMA was markedly increased in wild-type mice, however, only a slight increase was observed in cyp4a14−/− mice (Fig. 5 B and C). Because of a key role of activated HSC in hepatic fibrosis, this finding suggests that CYP4A14 gene deficiency may suppress hepatic fibrosis by blocking HSC activation. In support, MCD diet-induced gene expression of profibrotic and fibrosis-related genes (COL1A1, COL1A2, α-SMA, and TGFβ1) was markedly attenuated in cyp4a14−/− mice (Fig. 5 C and D). Similar to the effect in male mice, global deletion of CYP4A14 gene also attenuates MCD-induced hepatic fibrosis in female mice (Fig. S7).
Fig. 5.
CYP4A14 deficiency ameliorated MCD-induced hepatic fibrosis. Male wild-type (WT) and cyp4a14−/− mice were fed with an ND (normal diet) or MCD diet for 8 wk. (A) Masson’s staining indicating that MCD-induced hepatic fibrosis was significantly attenuated in cyp4a14−/− mice. a, WT on ND; b, WT on MCD; c, CYP4A14−/− on ND; d, CYP4A14−/− on MCD. (Magnification: 200×.) (B) CYP4A14 gene deficiency markedly attenuated MCD-induced α-SMA protein expression in the livers as assessed by an immunostaining analysis. n = 5–7. a, WT on ND; b, WT on MCD; c, CYP4A14−/− on ND; d, CYP4A14−/− on MCD. (Magnification: 200×.) (C) Western blot assay demonstrating reduced protein levels of COL1A2 and αSMA in the livers of cyp4a14−/− mice. n = 3. (D) Quantitative RT-PCR analysis showing reduced mRNA levels of collagen 1a1, collagen 1a2, α-SMA, and TGFβ1 in the livers of cyp4a14−/− mice. mRNA levels of related genes of hepatic fibrosis, n = 5–7. *P < 0.05 vs. WT on ND; #P < 0.05 vs. WT on MCD. Data are presented as mean ± SEM.
Fig. S7.
CYP4A14 deficiency ameliorated MCD-induced hepatic fibrosis. Female wild-type (fWT) and CYP4A14−/− mice (fCYP4A14−/−) were fed with an ND or MCD diet for 8 wk. (A) Masson’s staining indicating that MCD-induced hepatic fibrosis was significantly attenuated in CYP4A14−/− mice. a, fWT on ND; b, fWT on MCD; c, fCYP4A14−/− on ND; d, fCYP4A14−/− on MCD. (Magnification: 200×.) (B) CYP4A14 gene deficiency markedly attenuated MCD-induced α-SMA protein expression in the livers as assessed by an immunostaining analysis. a, fWT on ND; b, fWT on MCD; c, fCYP4A14−/− on ND; d, fCYP4A14−/− on MCD; Semiquantitative analysis was shown in the bar graph (Right). (Magnification: 200×.) (C) Western blot assay demonstrating reduced protein levels of COL1A2 and α-SMA in the livers of CYP4A14−/− mice. (D) Quantitative RT-PCR analysis showing reduced mRNA levels of profibrotic genes, collagen 1a1, collagen 1a2, α-SMA, and TGFβ1 in the livers of CYP4A14−/− mice. *P < 0.05, **P < 0.01 vs. fWT-ND; #P < 0.05 vs. fWT-MCD; n = 5–7. Data are presented as mean ± SEM.
Discussion
CYP4A14 is a hydroxylase that catalyzes omega-hydroxylation of medium-chain fatty acids and arachidonic acid in mice. It is highly expressed in the liver and kidneys. Increasing evidence has suggested that CYP4A14 plays an important role in the pathogenesis of hypertension (19). However, its biological function in the liver remains largely unknown. In this study, we tested the hypothesis that hepatic CYP4A14 plays a critical role in the pathogenesis of NAFLD. We found that CYP4A14 was markedly up-regulated in both patients and murine models with NAFLD. We then demonstrated that hepatic overexpression of CYP4A14 increased lipid accumulation in wild-type mouse livers. Finally, we provided evidence that CYP4A14 gene deficiency prevented the development of HFD-induced SS and MCD-induced NASH in mice. Collectively, these findings demonstrate a critical role of CYP4A14 in the development of SS and NASH.
CYP4A14 is abundantly expressed in the liver. Consistent with previous reports (7, 8, 15), the present study also showed that hepatic CYP4A14 expression was markedly up-regulated in three murine models of NAFLD, including db/db mice and HFD- and MCD-induced fatty liver disease. Importantly, we found that patients with NAFLD exhibited a marked increase in CYP4A protein expression, which is in line with a previous finding that CYP4A levels were significantly increased in hepatic lipid droplets of patients with NAFLD (20). Together, these studies indicate an important role of CYP4A in the pathogenesis of simple steatosis and NASH.
The goal of the present study was to determine the role of CYP4A14 in the development and the progress of NAFLD. By using an adenovirus-based approach, we found that overexpression of CYP4A14 in the livers of C57BL/6 mice resulted in a fatty liver phenotype, with increased hepatic triglyceride content. In contrast, CYP4A14 gene-deficient mice fed with an HFD exhibited markedly attenuated liver lipid accumulation. In addition, hepatic inflammation and fibrosis was markedly ameliorated in CYP4A14 gene-deficient mice on an MCD diet. These findings provide clear evidence that CYP4A14 plays an important role in the liver lipid homeostasis regulation and in the pathogenesis of both SS and NASH. Therefore, CYP4A14 may represent a potential therapeutic target for the treatment of NAFLD.
One of the most striking findings in the present study is that overexpression of CYP4A14 resulted in a significant increase in hepatic FAT/CD36 expression at both the mRNA and protein levels. Liver FAT/CD36 expression was significantly increased in mice fed with an HFD or MCD diet. However, hepatic HFD- and MCD-induced FAT/CD36 expression was almost completely abolished in mice deficient for the CYP4A14 gene. These results demonstrate that CYP4A14-induced liver fat accumulation may be mediated via increased expression of FAT/CD36 (21). FAT/CD36 is a membrane glycoprotein mediating cellular long chain fatty acid uptake and a receptor for various ligands including collagens type I and IV, VLDL, and oxidized LDL (3, 22). Recent studies indicate that increased FAT/CD36 localization/stabilization at the plasma membrane may be a key to enhancing hepatic fat uptake and, thus, play an important role in the progression of NAFLD with age (23). In addition, hepatic FAT/CD36 up-regulation is associated with insulin resistance, hyperinsulinaemia, and increased steatosis in patients with NASH (24). More importantly, forced expression of hepatic CD36 increases hepatic triglyceride storage in wild-type mice (25), whereas hepatocyte-specific disruption of CD36 attenuates fatty liver in mice on an HFD (26). Therefore, induced FAT/CD36 expression is likely responsible for CYP4A14-associated hepatic steatosis. However, the underlying mechanism by which CYP4A14 enhances hepatic FAT/CD36 expression remains unclear and warrants further investigation.
The present study also unveils the important role of CYP4A14 in the development of NASH. In MCD-induced NASH mice, CYP4A14 gene deficiency markedly attenuated not only lipid accumulation, but also hepatic inflammation and fibrosis. In both male and female mice receiving MCD diet, CYP4A14 gene deficiency resulted in a marked reduction in mRNA levels of inflammatory genes in the livers including IL1, TNFα, MCP1, MIP2, and IP10. In addition, hepatic gene expression of collagen I and α-SMA was also significantly attenuated in MCD diet-fed CYP4A14 gene knockout mice compared with their wild-type littermates. Although the mechanisms by which CYP4A14 promotes the development of NASH remain unclear, a previous report that CYP4A14 may represent an alternative initiator of oxidative stress in experimental NASH suggests oxidative stress may be involved in this process (4). Oxidative stress has been considered as an important “secondary hit” for NASH development, which can promote the deterioration of liver function in NAFLD (27). In the present study, we found that hepatic MDA levels were significantly increased in MCD-fed wild-type mice but were markedly attenuated in CYP4A14 gene-deficient mice. Therefore, suppressed oxidative stress may be responsible for improved liver function, inflammation, and fibrosis in CYP4A14 gene-deficient mice. In addition, reduced hepatic FAT/CD36 expression may also contribute to the improvement of NASH in CYP4A14 gene knockout mice, because up-regulation of FAT/CD36 has been reported to be associated with liver damage and fibrosis in patients with NASH (28).
It has been reported that the CYP4A14 gene is sexually dimorphically expressed and may exhibit phenotypic sex differences (19). In the present study, we determined the effect of an HFD and MCD on hepatic lipid accumulation, inflammatory, and fibrotic processes in both genders and found almost identical phenotypic changes in the liver. Therefore, CYP4A14 may play a similar role in hepatic lipid homeostasis regulation and in the pathogenesis of NAFLD in both sexes.
In summary, this study provides a link between CYP4A14 and NAFLD. Overexpression of CYP4A14 promotes, whereas gene disruption of CYP4A14 attenuates, HFD- and MCD-induced liver lipid accumulation. Ablation of CYP4A14 gene markedly attenuates liver damage, inflammation, and fibrosis in MCD-induced NASH. Thus, CYP4A14 may represent a potential therapeutic target for the treatment of fatty liver, especially NASH.
SI Materials and Methods
Primary Culture of Mouse Hepatocytes.
Primary mouse hepatocytes were cultured as described (29). In brief, 5- to 6-wk-old male wild-type or CYP4A14 gene deficiency mice were anesthetized with 10% (wt/vol) chloral hydrate, and a catheter was placed in the inferior vena cava. The livers were perfused with 1 mL of heparin (320 m/mL), 40 mL of solution I (Krebs’s solution and 0.1 mmol/L EGTA), and 30 mL of solution II (Krebs’s solution, 2.74 mmol/L CaCl2, and 0.05% collagenase I), respectively. The perfused livers were passed through a 400-mm screening size filter by flushing with RPMI medium 1640. The hepatocytes were collected by centrifuge at 50 × g for 2 min. Hepatocytes were resuspended with RPMI medium 1640 and plated in six-well plates for experiments after three washes with RPMI medium 1640.
Fatty Acid Uptake Experiments.
Measurement of fatty acid uptake was performed by incubating cells with 4,4-difluoro-5,7-dimethyl-4-bora-3α,4α-diaza-s-indacene-3-hexadecanoic acid (BODIPY-C16) from Invitrogen. Hepatocytes from WT and CYP4A14 gene knockout mice were cultured on collagen-coated microscope coverslips, the cells treated with 0.5 mmol/L normal control (CON) or 0.5 mmol/L palmitate acid (PA) for 24 h, then were serum-starved for 3 h and rinsed with 1× PBS. Hepatocytes were incubated for 3 min in 1× PBS supplemented with BODIPY-C16 to a final concentration of 200 nmol/L, rinsed three times with ice-cold 1× PBS, and fixed in ice-cold 4% (wt/vol) paraformaldehyde for 15 min. The fluorescence intensity was quantified by using the NIH ImageJ software.
Treatment of Sprague–Dawley rats with TS-011.
Sprague–Dawley (SD) rats (male, ∼200 g of body weight) from Department of Experiment Animals, Peking University were randomly divided into three groups (10 rats per group) as follows: ND (normal diet) plus vehicle administered via s.c. minipump; MCD diet plus vehicle administered via s.c. minipump; MCD diet plus TS-011 (10 mg/kg per d) given via s.c. minipump for 2 wk. TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], a selective inhibitor of the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE), was provided by AstraZeneca (Shanghai, China). MCD diet was purchased from Medicience Ltd. The rats were housed under a 12:12-h light/dark cycle and permitted ad libitum consumption of water and diet. The mice were not fasted before sample collection. Under isoflurane anesthesia, blood was collected from the vena cava into an empty syringe for the collection of serum. Sections of the left lateral lobes of livers were fixed in 4% (wt/vol) paraformaldehyde for 3 h, and then in 20% (wt/vol) sucrose, and finally embedded in paraffin or optimal cutting temperature. The remaining livers were snap-frozen in liquid nitrogen.
Acknowledgments
This study was supported by National Natural Science Foundation Grant 81570636/81500538/81390351/91639201/81100611, China Postdoctoral Science Foundation Grant 2015M570724, Shenzhen Peacock Program Grant KQTD20140630100746562, Natural Science Foundation of Shenzhen University Grant 201412, Robert A Welch Foundation Grant E-0004, the Swedish Science Council, and Center for Medical Innovations.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700172114/-/DCSupplemental.
References
- 1.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 2.Mishra A, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. J Clin Exp Hepatol. 2012;2(2):135–144. doi: 10.1016/S0973-6883(12)60102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48(1):1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Leclercq IA, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75(12):2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Simpson AE. The cytochrome P450 4 (CYP4) family. Gen Pharmacol. 1997;28(3):351–359. doi: 10.1016/s0306-3623(96)00246-7. [DOI] [PubMed] [Google Scholar]
- 7.Gyamfi MA, Damjanov I, French S, Wan YJ. The pathogenesis of ethanol versus methionine and choline deficient diet-induced liver injury. Biochem Pharmacol. 2008;75(4):981–995. doi: 10.1016/j.bcp.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyamfi MA, Tanaka Y, He L, Klaassen CD, Wan YJ. Hepatic effects of a methionine-choline-deficient diet in hepatocyte RXRalpha-null mice. Toxicol Appl Pharmacol. 2009;234(2):166–178. doi: 10.1016/j.taap.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YK, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245(3):326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Klaassen CD. Hormonal regulation of Cyp4a isoforms in mouse liver and kidney. Xenobiotica. 2013;43(12):1055–1063. doi: 10.3109/00498254.2013.797622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos. 2012;40(7):1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatuphonprasert W, Nemoto N, Sakuma T, Jarukamjorn K. Modulations of cytochrome P450 expression in diabetic mice by berberine. Chem Biol Interact. 2012;196(1-2):23–29. doi: 10.1016/j.cbi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Yoshinari K, Takagi S, Sugatani J, Miwa M. Changes in the expression of cytochromes P450 and nuclear receptors in the liver of genetically diabetic db/db mice. Biol Pharm Bull. 2006;29(8):1634–1638. doi: 10.1248/bpb.29.1634. [DOI] [PubMed] [Google Scholar]
- 14.Enriquez A, Leclercq I, Farrell GC, Robertson G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun. 1999;255(2):300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- 15.Patsouris D, Reddy JK, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147(3):1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- 16.Park EC, et al. Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology. 2014;147(4):860–869. doi: 10.1053/j.gastro.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, et al. FAM3A activates PI3K p110α/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology. 2014;59(5):1779–1790. doi: 10.1002/hep.26945. [DOI] [PubMed] [Google Scholar]
- 18.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111(6):1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 19.Holla VR, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA. 2001;98(9):5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su W, et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2014;111(31):11437–11442. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin C, et al. CD36 as a lipid sensor. Physiol Behav. 2011;105(1):36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Sheedfar F, et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging (Albany NY) 2014;6(4):281–295. doi: 10.18632/aging.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miquilena-Colina ME, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 25.Koonen DP, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CG, et al. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed Mice. Endocrinology. 2016;157(2):570–585. doi: 10.1210/en.2015-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 28.Bechmann LP, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30(6):850–859. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, et al. Hepatic overexpression of ATP synthase β subunit activates PI3K/Akt pathway to ameliorate hyperglycemia of diabetic mice. Diabetes. 2014;63(3):947–959. doi: 10.2337/db13-1096. [DOI] [PubMed] [Google Scholar]