Significance
The extracellular signals that regulate cell growth and tissue rearrangements during organogenesis are still poorly understood. One large family of secreted signaling molecules, called wingless-type MMTV integration site family member (WNTs), governs many of these processes by controlling cell–cell interactions. Here, we show that two closely related members of the WNT superfamily influence the development of the mammary gland by signaling through different tyrosine kinase receptors. WNT5A enhances the growth of mammary stem/progenitor cells while restricting the process of branching morphogenesis. In contrast, WNT5B represses mammary stem/progenitor cell proliferation through a different receptor. Together, the distinct actions of these highly homologous extracellular cues regulate the developmental processes that supply cells and shape tissues during periods of rapid growth.
Keywords: mammary stem cells, noncanonical Wnt signaling, receptor tyrosine kinase, epithelial morphogenesis
Abstract
The mammary gland consists of an adipose tissue that, in a process called branching morphogenesis, is invaded by a ductal epithelial network comprising basal and luminal epithelial cells. Stem and progenitor cells drive mammary growth, and their proliferation is regulated by multiple extracellular cues. One of the key regulatory pathways for these cells is the β-catenin–dependent, canonical wingless-type MMTV integration site family (WNT) signaling pathway; however, the role of noncanonical WNT signaling within the mammary stem/progenitor system remains elusive. Here, we focused on the noncanonical WNT receptors receptor tyrosine kinase-like orphan receptor 2 (ROR2) and receptor-like tyrosine kinase (RYK) and their activation by WNT5A, one of the hallmark noncanonical WNT ligands, during mammary epithelial growth and branching morphogenesis. We found that WNT5A inhibits mammary branching morphogenesis in vitro and in vivo through the receptor tyrosine kinase ROR2. Unexpectedly, WNT5A was able to enhance mammary epithelial growth, which is in contrast to its next closest relative WNT5B, which potently inhibits mammary stem/progenitor proliferation. We found that RYK, but not ROR2, is necessary for WNT5A-mediated promotion of mammary growth. These findings provide important insight into the biology of noncanonical WNT signaling in adult stem/progenitor cell regulation and development. Future research will determine how these interactions go awry in diseases such as breast cancer.
The mammary gland is composed of a highly dynamic epithelial structure that undergoes multiple rounds of remodeling during puberty, pregnancy, lactation, and involution (1). At puberty, the mammary gland forms a branching ductal network, which connects the nipple to the milk-producing lobuloalveolar structures that arise during pregnancy (2). Development and growth of the mammary gland depends on the function of adult mammary stem cells (MaSCs) (1). These MaSCs are capable of reconstituting a complete mammary epithelial ductal structure when implanted as a single cell into a cleared fat pad in vivo (3–5). The morphogenetic changes of the breast epithelium are closely coordinated within the context of its microenvironment, which consists of a variety of stromal cells such as adipocytes, macrophages, and fibroblasts (6, 7). MaSCs respond to extracellular signals such as wingless-type MMTV integration site family (WNT) ligands provided by stromal cells of the microenvironment. For example, hyperactivation of the canonical WNT/β-catenin signaling pathway in the mammary gland expands the MaSC population by sixfold (4), and WNT ligands are necessary for self-renewal properties of MaSCs (8). Constitutive overexpression of the gene encoding the canonical Wnt1 ligand in this organ ultimately gives rise to tumors, suggesting a direct link between MaSC accumulation and tumor susceptibility (9, 10).
The role of the noncanonical WNT signaling pathway in the regulation of mammary gland development and breast cancer function is obscure. WNT5A and WNT5B represent two noncanonical WNT ligands expressed in the mammary gland (11–14). Within the mammary epithelium, expression of both Wnt5a and Wnt5b is restricted to the more differentiated luminal epithelial cell lineage (14). Several receptors have been implicated in mediating the function of WNT5A and WNT5B. These include the noncanonical receptors, receptor tyrosine kinase-like orphan receptor 1 (ROR1) and ROR2, which are expressed in both the basal and luminal compartments (14, 15), whereas the expression of the receptor RYK remains less well defined. Although several studies have reported inhibitory roles for noncanonical WNT ligands, WNT5A during branching morphogenesis (16) and WNT5B in mammary stem and progenitor outgrowth (16, 17), the receptors mediating these inhibitory functions remain poorly characterized.
Even though the actions of WNT5A and WNT5B have been associated with events occurring during breast cancer initiation and progression, the role of noncanonical WNT signaling in breast cancer remains elusive. Although there is evidence suggesting that secretion of WNT5A by stromal cells may inhibit the actions of tumor-initiating cells in breast cancer (18), other studies show that WNT5A/B may promote epithelial to mesenchymal transition and metastatic progression in breast and other cancers through a noncanonical Frizzled2 pathway (19). Overall, the role of noncanonical WNT signaling in breast cancer appears to be particularly context-dependent, and the diverse effects of noncanonical WNT ligands on cell and developmental pathways remain largely unexplored.
Here, we focused on the role of WNT5A and WNT5B as two of the main mediators of noncanonical WNT signaling in the mammary gland. In particular, we sought to understand how noncanonical WNT signaling is involved in the regulation of MaSC function and branching morphogenesis. Our results show that despite their high degree of similarity, WNT5A and WNT5B may work in a distinct manner involving different receptor molecules. These findings further shed light on our understanding of the intricacies of the WNT signaling pathway and provide crucial insights to better understand their role in diseases such as breast cancer.
Results
WNT5A and WNT5B Differentially Regulate Mammary Growth and Progenitor Cell Proliferation.
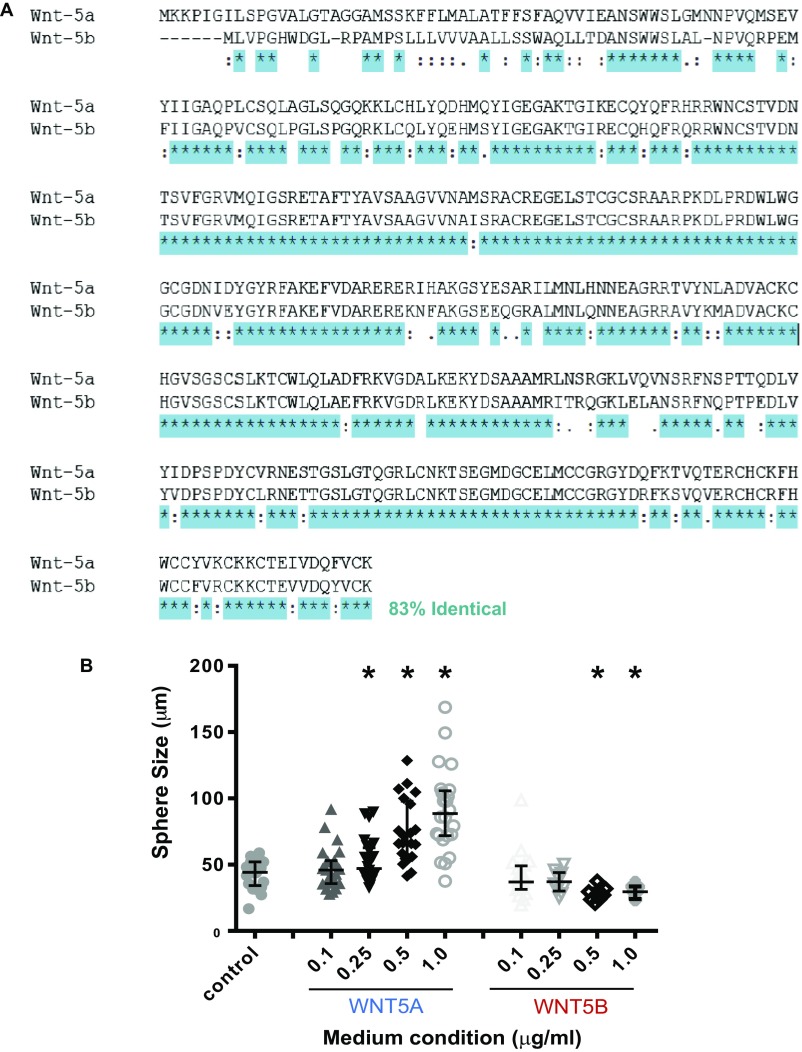
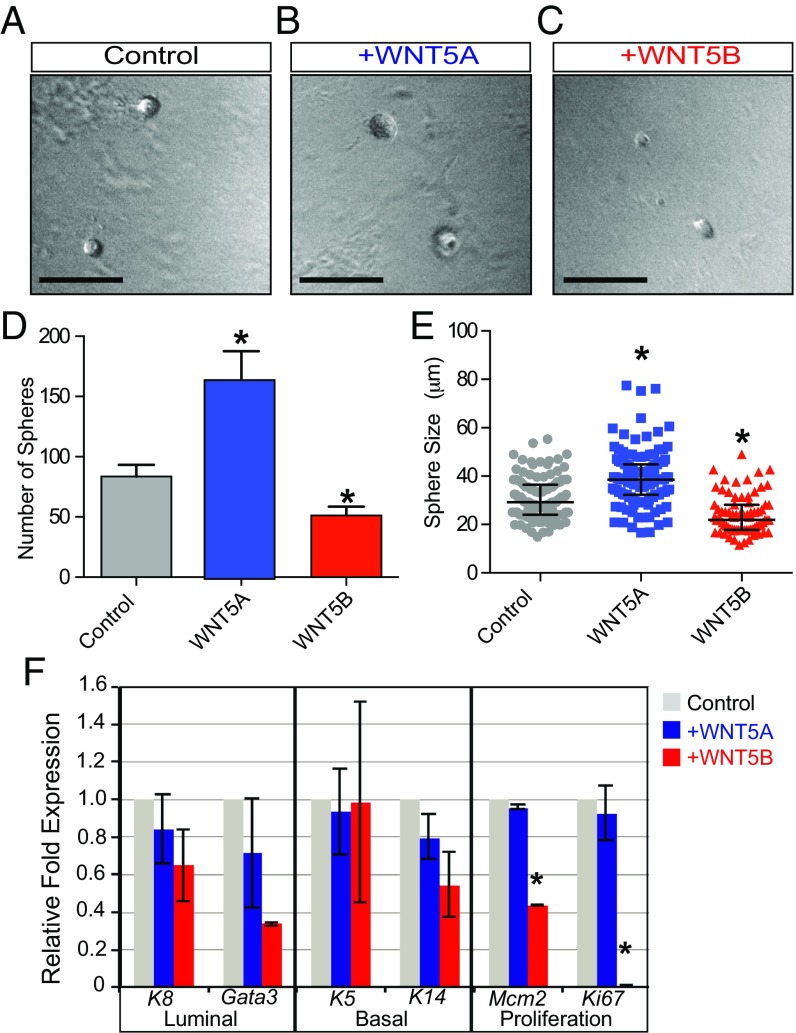
We previously observed that WNT5B is capable of inhibiting mammary epithelial stem and progenitor cell growth capacity in vitro and in vivo, using lentiviral-mediated overexpression in transplanted MaSCs (17). WNT5A and WNT5B show a high degree of amino acid sequence similarity, at 83% (Fig. S1A), which initially suggested that these two WNT ligands may function in a redundant manner. To test whether these two noncanonical WNT ligands are indeed functionally redundant in the context of MaSC/progenitor regulation, we first used the clonal mammosphere assay as a proliferative analysis tool in which single mammary epithelial cells (MECs) are embedded into a 3-dimensional Matrigel-based culture. Using this assay, we then compared the effects of exogenous recombinant WNT5A and WNT5B to vehicle-treated mammosphere formation. Interestingly, we observed a pronounced increase in the number, as well as the size, of mammospheres treated with WNT5A compared with vehicle-treated MECs (Fig. 1 A–E). In contrast, WNT5B demonstrated a strong inhibitory effect on mammosphere formation, which is consistent with our previous findings (17). These distinct effects of WNT5A and WNT5B were dose-dependent in a range from 0.1 to 1 μg/mL (Fig. S1B). Subsequent quantitative PCR (qPCR) analysis revealed that in contrast to WNT5A, WNT5B-treated cells down-regulate expression of genes associated with proliferation and luminal differentiation (Fig. 1F). This suggests that WNT5B blocks mammosphere formation by inducing cell cycle arrest, presumably at an earlier basal MaSC state. Also, despite the high degree of amino acid sequence homology shared by WNT5A and WNT5B (Fig. S1A), these hallmark noncanonical WNT ligands may work in a distinct fashion in the regulation of MEC biology.
Fig. S1.
Wnt5a and Wnt5b sequence and functional characteristics. WNT5A and WNT5B share a high degree of sequence homology, but have distinct functions. Amino acid sequence alignment reveals a high degree (85%) of sequence identity between WNT5A and WNT5B (A). A dose–response assessment using 0.1, 0.25, 0.5, and 1.0 µg/mL recombinant WNT5A/WNT5B protein in the medium of mammosphere assays shows that WNT5A enhances mammosphere size, whereas WNT5B does not. Error bars show median measurement with interquartile range; *P < 0.05, Student’s t test (B).
Fig. 1.
Differential effects of WNT5A and WNT5B on mammosphere outgrowth. Primary WT MECs (2,000 cells per well) were used as single cells in the mammosphere assay and treated with vehicle control or medium containing either WNT5A (0.5 μg/mL) or WNT5B (0.5 μg/mL) recombinant protein. Representative images show mammosphere formation after 7 d. Compared with control conditions (A), WNT5A-treatment promotes mammosphere formation (B), whereas WNT5B treatment inhibits mammosphere formation (C). For each condition, quantification of sphere number (D) and size (E) was carried out. The effects of WNT5A and WNT5B on cell fate and proliferation were measured by qPCR, using cDNA from MECs cultured in a low-adhesion plate (200,000 cells per well) and treated with control medium (gray, Left) or medium containing either WNT5A (blue, Middle) or WNT5B (red, Right) recombinant protein (F). Error bars show SD (n = 3); data representative of three independent experiments. WNT5A-treated cells show an expression profile that was similar to control-treated cells, whereas WNT5B-treated cells showed a significant decrease in the expression of proliferation markers (Mcm2, Ki67) and a trending decrease in luminal differentiation markers (K8, Gata3). Error bars show average with SD (D–F) or median measurement with interquartile range (F). (Scale bars, 100 μm in A–C.) Asterisks represent *P ≤ 0.05, Student’s t test or relative to control.
Differential Expression of Noncanonical WNT Receptors in Mammary Epithelial Subpopulations.
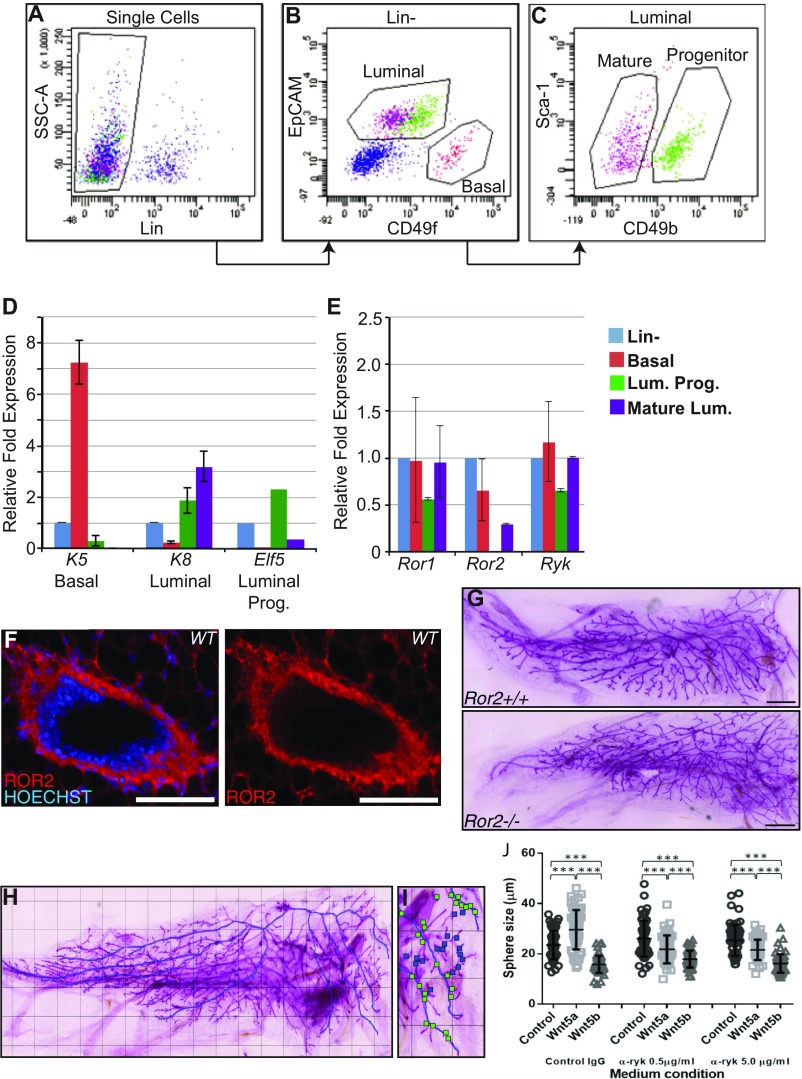
To investigate which noncanonical WNT receptors are involved in mediating WNT5A and WNT5B signaling in breast epithelium, we used qPCR expression analysis on isolated basal and luminal MECs. To this end, we used FACS to separate basal, luminal progenitor, and mature luminal cells harvested from 8-wk-old FvB/N mice (Fig. S2 A–C) and performed qPCR analysis with primers targeting Ror1, Ror2, and Ryk. We also confirmed isolation of mammary subpopulations, using epithelial lineage marker expression (Fig. S2D). We found all three receptors expressed in the mammary gland (Fig. S2E). Although Ror1 and Ryk show expression in all epithelial cell populations, Ror2 showed a differential expression pattern, with high abundance in basal cells, low abundance in mature luminal cells, and undetectable expression in luminal progenitor cells. We also confirmed the basal-specific expression of ROR2 in situ (Fig. S2F) by immunostaining in wild-type (WT) tissue, observing strong staining in the basal compartment (15). Taken together, our results suggest that ROR2 could be mediating a cross-talk between basal and luminal cells by functioning as a receptor on basal cells for luminal-derived WNT5A and WNT5B.
Fig. S2.
Expression of noncanonical WNT receptors in mammary epithelial populations. Mammary populations were isolated using FACS, resulting in the mammary (Linlow), basal (CD49fhi, EpCAMlow), luminal progenitor (CD49flow, EpCAMhigh,CD49bhi), and mature luminal cell (CD49flow, EpCAMhigh, CD49blow) populations (A–C). Sort purity was analyzed by performing RT-qPCR for K5, K8, and ELF5 in each of the following populations (from left to right): Lin- (blue), Basal (red), Luminal progenitors (green), and Mature Luminal (purple) (D). On these MEC populations, a subsequent RT-qPCR was performed for the putative WNT5A and WNT5B receptors Ror1, Ror2, and Ryk (n = 3). Error bars show ± SD of independently isolated, sorted, and RT-qPCR-analyzed cell population (E). Cross-sections through a cryosectioned mammary duct stained with an antibody against ROR2 and the nuclear dye Hoechst (F). Branching analysis was performed on WT and Ror2−/− outgrowths at 12 wk posttransplantation. Whole-mounts of G1 tissue display normal branching patterns (G). Shown here is a stitched image of a Ror2−/− whole-mount collected on a wide-field microscope (Keyence) at 5× resolution with the primary ductal structure outlined in blue (H). Secondary and tertiary branches were marked by green and blue boxes, respectively (I), and then branchpoints were counted. Mammosphere formation was performed in the presence of recombinant WNT5A (0.5 μg/mL) or WNT5B (0.5 μg/mL), and sphere size was measured by microscopy after 7 d (J). Sphere growth is not affected by addition of control IgG, whereas addition of anti-RYK antibody at 0.5 and 5 μg/mL lead to decrease in sphere size in the presence of WNT5A. Error bars show median measurement with interquartile range; ***P < 0.001, Student's t test.
Ror2-Deficient Mammary Glands Show Increased Branching Morphogenesis.
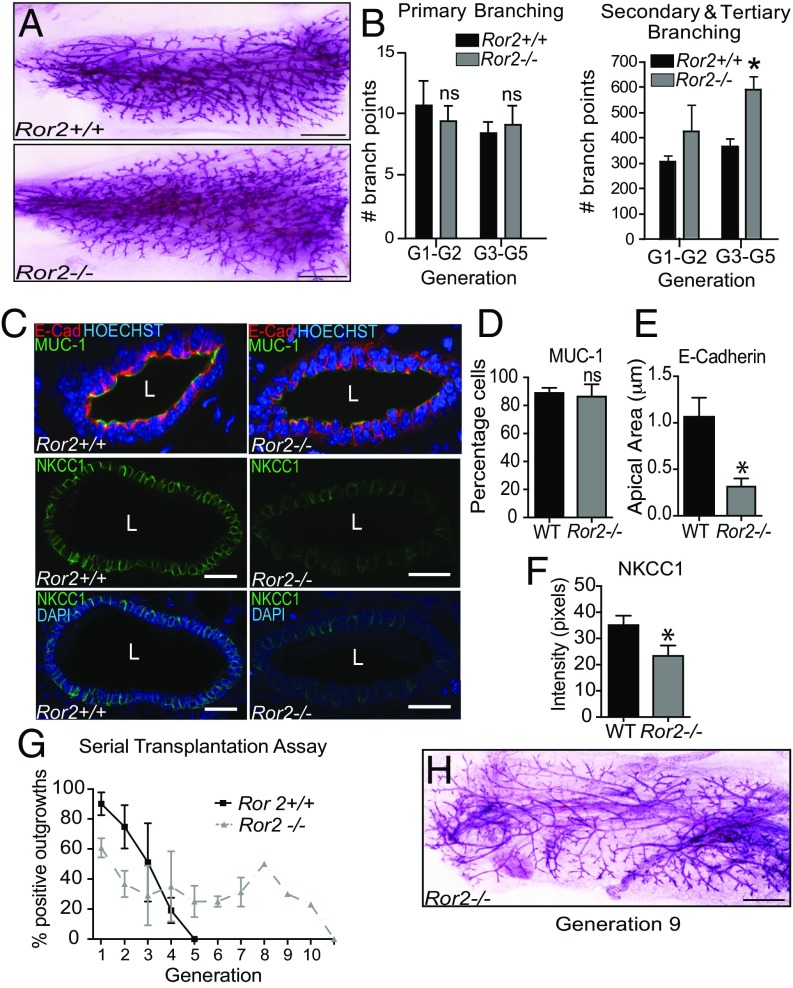
The basal-prominent expression pattern of ROR2 prompted us to study its role in the regulation of mammary gland development in more detail. Ror2-deficient (Ror2−/−) mice have severe developmental defects leading to death of the animal at age embryonic day 18.5 (E18.5) (20). To analyze the postnatal development of the mammary gland, we generated Ror2−/− glands in vivo by using standard protocols to rescue the anlage from E16.5 embryos and transplanted them into precleared fat pads of immunocompromised mice (21). To ensure only epithelial tissue was propagated, we allowed the anlage transplant to develop 12 wk before performing serial transplants with tissue fragments taken from the previous outgrowth. Whole-mounted generation 1 (G1) Ror2−/− outgrowths were morphologically similar to littermate WT outgrowths generated in the contralateral fat pad (Fig. S2G). By G4, however, Ror2−/− outgrowths appeared hyperbranched compared with WT glands (Fig. 2A). We performed branching analysis on four independently derived lines of WT and Ror2−/− tissue (Fig. 2B and Fig. S2 H and I). We found no significant differences in primary branch number between Ror2−/− and WT tissue, even with successive transplantation (G1–G5), indicating that terminal end bud bifurcation is unaffected by the lack of Ror2. In contrast, there was a significant increase in secondary and tertiary branching in Ror2−/− tissue that had been serially transplanted for three to five generations, indicating a progressive misregulation in mechanisms that constrain side-branch formation (Fig. 2B). Cross-sections through WT G4 tissue revealed a normal bilayered epithelial architecture (Fig. 2C), with luminal cells lining up in register to create a smooth apical surface. In contrast, luminal epithelial cells are slightly out of register in the Ror2−/− G4 tissue, but there is no significant difference in apical MUC1 immunostaining, suggesting Ror2−/− luminal cells are properly polarized (Fig. 2 C and D). However, E-cadherin immunostaining in Ror2−/− tissue is reduced at the adherens junctions, which reside just below the tight junctions that demarcate the apical surfaces of luminal cells (Fig. 2 C and E). Instead of the organized belt of E-cadherin staining observed in WT tissue, staining in Ror2−/− tissue extends along the lateral and basal membranes. We also examined the localization of the Na/K/Cl cotransporter, NKCC1, which marks the basolateral surface (22), and found an overall decrease in staining in Ror2−/− tissue (Fig. 2 C and F). Taken together, the data suggest that luminal epithelial cells maintain their proper orientation with regard to the lumen in Ror2−/− tissue, but contacts between cells appear compromised. Previous studies showed that WT tissue fragments can be serially transplanted up to five times before no further outgrowths are obtained (23). In our studies, all three WT lines senesced at G5 (Fig. 2 G and H). In contrast, two of four Ror2−/− transplanted lines displayed increased transplantability, with one senescing at G9 and the second at G11, suggesting that loss of Ror2 regulates either the number of MaSC/progenitor cells or their self-renewal.
Fig. 2.
Ror2-deficient mammary glands show increased secondary and tertiary branching morphogenesis in vivo. Anlage were rescued from E16.5 embryos and transplanted into precleared fat pads of athymic nude mice. After 12 wk, epithelial tissue fragments were used to propagate the line. Whole-mounts of G4 tissue reveal a hyperbranched phenotype (A) that was quantified by tracing the primary and secondary/tertiary branches (B) (n = 4). Immunohistochemistry in cross-sections of G4 Ror2−/− and WT tissue, using E-cadherin (red) MUC-1 (green), NKCC1 (green) antibodies, and Hoechst (blue) for nuclei, reveal disorganization in the Ror2−/− tissue (n = 3) (C). Quantification of MUC-1 (D), E-cadherin (E), and NKCC1 immunostaining (F) in WT and Ror2−/− luminal cells (n = 3). Serial transplantation of tissue fragments show that WT tissue (n = 3) senesces at G5, whereas Ror2−/− tissue displays enhanced transplantability (n = 2). n is the number of independently derived lines, with five contralaterally transplanted mice analyzed per line. (G) Whole-mount of G9 Ror2−/− outgrowth shows epithelial tissue filling the fat pad (H). (Scale bars, 1.5 mm for A and H, 10 μm for C.) *P < 0.05, Student’s t test. Error bars represent ± SE.
WNT5A Inhibits Mammary Epithelial Branching Through ROR2.
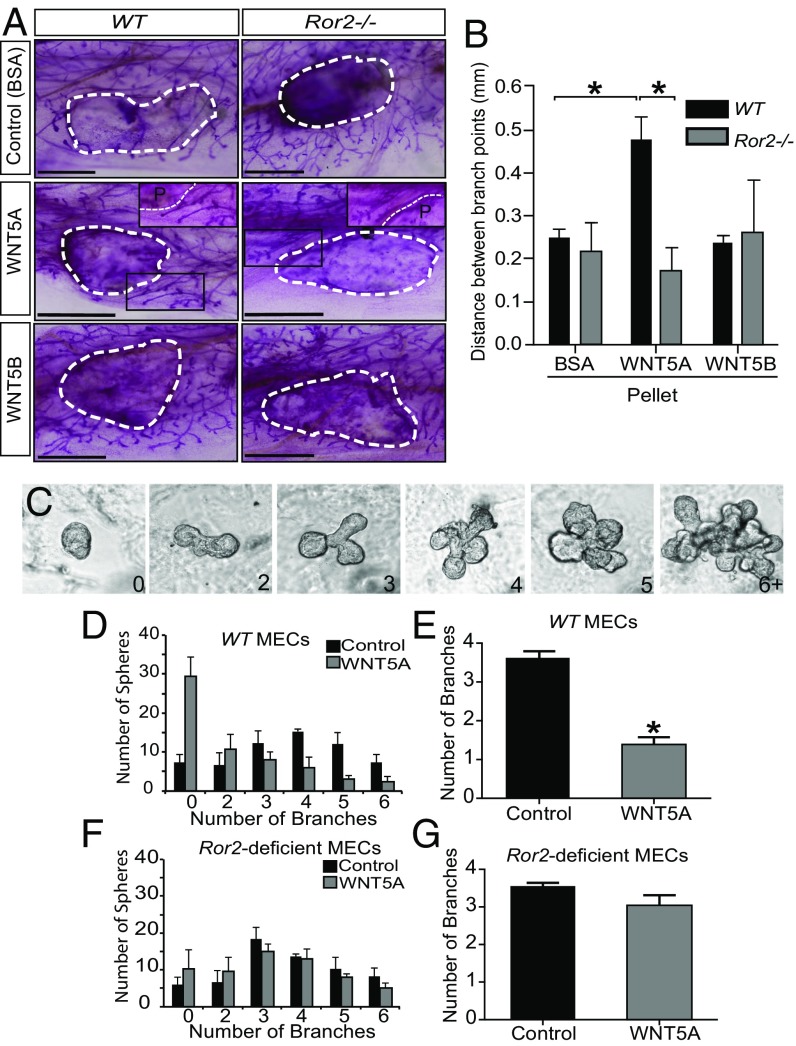
Next, we focused on the interaction of ROR2 with noncanonical WNT ligands in the context of mammary branching morphogenesis. Both WNT5A and WNT5B have previously been shown to inhibit mammary branching morphogenesis in vivo (16, 17). To test whether ROR2 is involved in this process, we used our Ror2−/− transplantation model in combination with Elvax slow-release pellets to locally deliver WNT ligands into the tissue adjacent to the epithelial outgrowths. To quantify the effect of WNT5A and WNT5B on branching morphogenesis in vivo, we measured the distance between branch points in close proximity to the transplanted pellets. We observed significantly increased distance between branches near the WNT5A pellets, an indication of reduced branching compared with BSA-containing control pellets. In contrast, WNT5B had no measurable effect on mammary side branching under these conditions, which further underlines that WNT5A and WNT5B may possess different functional properties. Importantly, in the absence of Ror2, the inhibitory effects of WNT5A on side branching were completely abolished (Fig. 3 A and B), indicating that ROR2 functions as the primary receptor mediating this regulatory role of WNT5A during mammary branching morphogenesis. We further corroborated these findings using an established ex vivo surrogate assay for mammary branching morphogenesis (Fig. 3C). After 7 d of WNT5A treatment, WT mammary epithelial organoids showed significantly fewer branches compared with mock treated samples (Fig. 3 D and E). In contrast, Ror2−/− organoids treated with WNT5A showed no change in branching after 7 d (Fig. 3 F and G), demonstrating that WNT5A inhibits mammary branching morphogenesis through ROR2.
Fig. 3.
WNT5A inhibits mammary branching morphogenesis through ROR2. WT and Ror2−/− fragments were contralaterally transplanted into precleared mammary fat pads. Elvax slow-release pellets containing BSA, WNT5A, or WNT5B were implanted bilaterally 3 wk posttransplant. The tissue was harvested and carmine stained for whole-mount analysis 1.5 wk after the Elvax implantation (A). The distance between branch points was quantified by tracing the ductal structure in proximity of the pellet and measuring the length between branch points (n = 3 lines per five contralaterally transplanted mice per line) (B). Ex vivo branching morphogenesis of reaggregated primary MECs in the presence of FGF2 was carried out over the course of 7 d, and the number of branches per organoid was determined. Example images for organoids with 0–6+ branches are shown (C). WT MECs show significant reduction of organoid branching in the presence of WNT5A compared with control conditions (D and E) (n = 3). Organoids derived from Ror2-deficient MECs show no decrease in the number of branches per organoid when treated with WNT5A (F and G) (n = 3). *P < 0.05, Student’s t test. Error bars represent ± SE. (Scale bars, 1.5 mm for A.)
WNT5A Promotes Mammosphere Formation Through RYK.
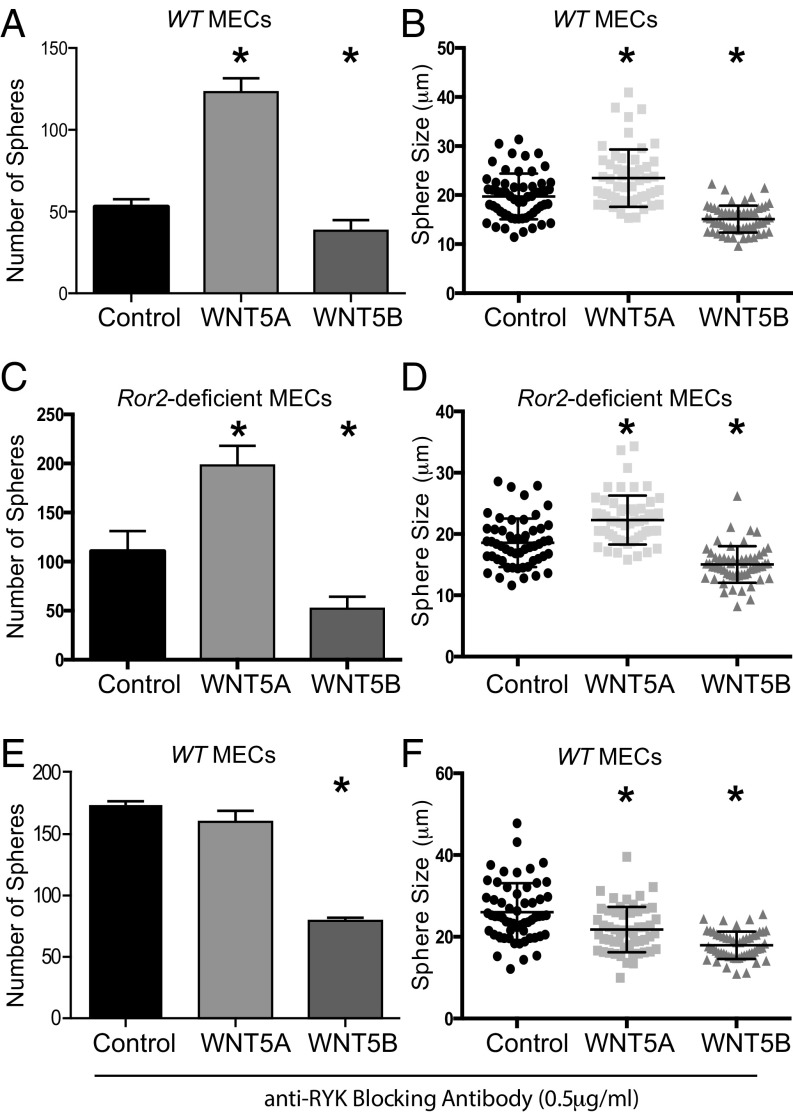
We initially observed distinct effects of WNT5A and WNT5B on mammosphere formation, which may be driven primarily by different basal stem and progenitor cell functions, rather than the process of branching morphogenesis. We therefore explored the role of ROR2 as it relates to its basal epithelial expression pattern, and our results suggest that the loss of Ror2 promotes the longevity of stem cells and their potential to serially undergo mammary morphogenesis in vivo (Figs. 1 and 2). To this end, we isolated MECs from WT and Ror2−/− outgrowths harvested from contralaterally transplanted animals and subjected these to the mammosphere formation assay in the presence or absence of WNT5A and WNT5B (Fig. 4). We observed no difference in the effects of WNT5A or WNT5B on sphere forming capacity in WT and ROR2−/− samples (Fig. 4 A–D), demonstrating these Wnt ligands act independent of ROR2 in this context. In treated samples, we found that WNT5A promoted and WNT5B reduced the number and size of mammospheres independent of ROR2 (Fig. 4 A–D), indicating that a different noncanonical receptor is governing the effects of WNT5A and WNT5B on mammosphere formation.
Fig. 4.
The role of ROR2 and RYK in WNT5A- and WNT5B-mediated regulation of mammosphere formation. MECs from contralateral WT (A and B) or Ror2−/− outgrowths (C and D) (2,000 cells per well) were used as single cells in mammosphere assays and treated with control medium or medium containing either WNT5A (0.5 μg/mL) or WNT5B (0.5 μg/mL) recombinant protein (n = 3). The experiment was also performed in the presence of anti-RYK blocking antibody, using WT cells (E and F) (n = 3). After 7 d in WNT containing culture medium, sphere number (A, C, and E) and size (B, D, and F) were determined for the WT, Ror2−/−, and anti-RYK-treated spheres. Error bar shows SD (A, C, and E) or median measurement with interquartile range (B, D, and F). *P ≤ 0.05, Student’s t test.
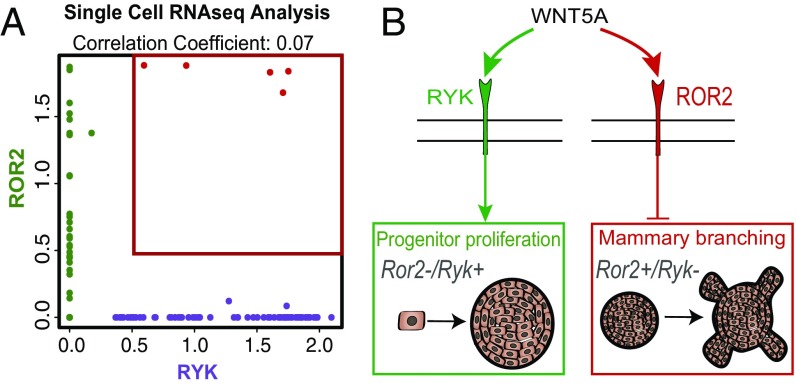
We next focused on the receptor tyrosine kinase RYK, which has been implicated as a receptor for WNT5A (24). To interfere with RYK function in the presence or absence of WNT ligand, we used a blocking antibody approach that has been previously used to inhibit RYK function during neuron specification (25). Although WNT5B inhibited mammosphere formation independent of RYK blockade, we found that the increase in number and size of mammospheres treated with WNT5A could be abolished by the presence of anti-RYK blocking antibody (Fig. 4 E and F), demonstrating that WNT5A may increase MaSC capacity and proliferation by activating RYK-dependent, noncanonical WNT signaling. Importantly, control experiments under the same conditions, but treated with nonspecific IgG instead of anti-RYK treatment, did not alter mammosphere capacity (Fig. S2J). Interestingly, we noticed that in the presence of RYK-blocking antibody, WNT5A changed its function from promoting to inhibiting mammosphere growth, as measured by sphere size, whereas the number of spheres remained comparable to control (Fig. 4 E and F). This could suggest that in the absence of RYK, WNT5A engages different WNT coreceptors, leading to inhibition of progenitor cell proliferation or promotion of cell differentiation. Together, these findings show that WNT5A may activate the receptor tyrosine kinases ROR2 and RYK, presumably on different stem or progenitor populations that contribute to distinct morphogenetic processes during mammary branching and MaSC outgrowth (Figs. 3 and 4). To further corroborate this notion, we analyzed the expression pattern for ROR2 and RYK on a single-cell level, using a single-cell RNAseq dataset generated in a currently ongoing study, which revealed that ROR2 and RYK are indeed expressed in a mutually exclusive manner in the vast majority of MECs (Fig. 5 A and B).
Fig. 5.
Single-cell analysis and functional model summarizing the role of WNT5A in mammary development. Scatter plot of single-cell correlation analysis of ROR2 and RYK expression in mouse MECs shows very low correlation and almost mutually exclusive expression (A), demonstrating that ROR2 and RYK are expressed in different populations of cells. Schematic illustrating the differential expression patterns of noncanonical WNT receptors in MEC populations and the signaling capacities of WNT5A acting either through ROR2 to block branching morphogenesis or through RYK to promote stem/progenitor proliferation (B).
Discussion
Although canonical WNT signaling has been extensively studied in the context of mammary gland biology, MaSC regulation, and cancer, the noncanonical WNT pathway remains elusive. To learn more about the role of noncanonical WNT signaling in mammary development, we focused here on the hallmark noncanonical ligands WNT5A and WNT5B because they are prominently expressed in the mammary gland. By investigating the alternate WNT receptors, ROR2 and RYK receptor tyrosine kinases, our studies provide insight into noncanonical WNT signaling pathways during mammary development. Whereas previous studies have shown that loss of either Wnt5a or Ror2 results in a hyperbranching phenotype (15), we demonstrate a ligand/receptor relationship by showing that ROR2 is required for the inhibitory action of WNT5A on branch formation. Despite being highly homologous, WNT5B has no such effect. We further distinguished the activities of these two WNTs in mammosphere assays by demonstrating that WNT5A enhances mammosphere formation through the RYK receptor. This stimulatory activity is surprising in light of studies on human tumors that showed tumor-suppressive activity of WNT5A, with loss of Wnt5a expression correlating with more aggressive tumor subtypes, earlier relapse, and significantly shorter overall patient survival (26, 27). WNT5A was shown to induce the formation of a RYK/TGFβR1 complex in MCF10A cells and activate SMAD2 in mammary epithelial cells harvested from preneoplastic MMTV-ErbB2/Wnt5a+/− mice (27). However, in studies on nonimmortalized cells, we and others (28) find that WNT5A promotes mammosphere formation, a positive effect on stem/progenitors consistent with WNT5A’s ability to support the self-renewal of mouse spermatogonial stem cells by inhibiting apoptosis (29). In two studies, pharmacological inhibition of the Jun N-terminal kinase abrogated the WNT5A-mediated outcome (28, 29), and in the studies on mammospheres, the effect was also reversed by knocking down Ror2 (28). In contrast, our studies show that WNT5A enhances the formation of mammospheres through the RYK receptor, independent of ROR2, perhaps reflecting the difference between knocking down and knocking out a receptor. Our studies also demonstrate that WNT5B blocks mammosphere formation, possibly by inhibiting cell cycle progression because markers for cell cycle entry, Mcm-2 and Ki67, are significantly down-regulated in response to WNT5B. These results are consistent with our previous studies demonstrating an inhibitory affect associated with WNT5B treatment on MaSC proliferation in vitro and in vivo (17). Although we have not yet identified the receptor mediating this effect, we have ruled out ROR2. Taken together, our data show that noncanonical WNT5A and WNT5B have distinct functions despite a high degree of sequence homology. Our study reveals that WNT5A signals through two different receptors: ROR2 to inhibit branching morphogenesis and RYK to promote mammary MaSC/progenitor proliferation. ROR2 also has a separate function in enhancing MaSC/progenitor self-renewal, but the ligand mediating this effect is unknown.
Multiple potential noncanonical WNT receptors are expressed in the mammary gland. Similar to a previous study (16), our results show that Ror2 is predominantly expressed in the basal population with little or no expression in luminal progenitors and low expression in mature luminal cells. In contrast, the expression of Ryk and Ror1 is more evenly distributed across populations. Because the basal compartment contains a subpopulation of MaSCs (1), we further explored the function of ROR2 in this compartment by examining the phenotype of Ror2−/− glands. Our immunostaining data revealed strong basal expression of ROR2 in myoepithelial cells along the duct (15). We further explored ROR2 function by examining its knock-out phenotype, using the technique of transplantation to circumvent the perinatal lethality of the mutation. Loss of either Wnt5a or Ror2 has been associated with a hyperbranching phenotype in the mammary gland, but the molecular mechanism behind this phenotype remains elusive. Although Ror2−/− mammary glands exhibited no defects in primary branch formation, excessive secondary and tertiary branching was observed after three successive rounds of transplantation. In contrast, others have demonstrated an initial branching defect in mammary outgrowths generated from Ror2 knockdown cells (15), suggesting there may be activation of compensatory signaling pathways caused by the germline deletion of this tyrosine kinase receptor, which complicates the detection of branching defects in Ror2−/− tissue. By implanting Elvax pellets in vivo into fat pads containing WT and Ror2−/− outgrowths, we demonstrate that WNT5A, but not WNT5B, has the capacity to reduce branch formation in a Ror2-dependent manner. Histological analysis revealed disorganized epithelial architecture that is consistent with a low level of ROR2 expression in mature luminal cells and may be a result of defects in the actin cytoskeleton, which is disrupted in the absence of ROR2 (15). Using an in vitro branching assay, our studies further revealed that WNT5A negatively regulates branch formation through the ROR2 axis, supporting a crucial developmental role for WNT5A/ROR2 signaling in restricting mammary branching morphogenesis.
Despite their high degree of sequence similarity, WNT5A showed substantially distinct functional properties compared with WNT5B, resulting in enhanced MaSC/progenitor-mediated mammosphere formation in a RYK-dependent manner. By using an anti-RYK, function-blocking antibody, we determined that the RYK pathway is responsible for the WNT5A-mediated increase in mammosphere formation. It is tempting to speculate that high local levels of WNT5A may be important during the formation of terminal end buds invading into the mammary fat pad, when enhanced proliferation is required and premature formation of side branches has to be suppressed. The distinct effects of WNT5A inhibiting branching morphogenesis through ROR2, and activation of MaSC/progenitor proliferation through RYK, also indicate that there are distinct stem and progenitor cell populations associated with these aspects of mammary gland development.
Taken together, our studies provide important insight into the biology of noncanonical WNT signaling in adult stem cell regulation and development. We discovered that WNT5A and WNT5B may have biologically distinct functions during mammary development. Although WNT5B blocks MaSC growth and has no measurable effect on branching morphogenesis, WNT5A enhances MaSC outgrowth and negatively regulates mammary branching. These effects are mediated through diverse engagement of RYK and ROR2, which highlights the complexity and context dependency of WNT signaling in general. Future research will determine how these interactions go awry in diseases such as breast cancer.
Experimental Procedures
Mice.
FVB/N mice were purchased from Charles River Laboratories. KO mice were generated and genotyped as described; Ror2−/− (20). Mice were maintained in a pathogen-free facility. All mouse procedures were approved by the University of California, San Francisco, or University of California, Santa Cruz, Institutional Animal Care and Use committees.
Recombinant Proteins and Antibodies.
The list of recombinant proteins and antibodies is provided in the SI Experimental Procedures.
Mammosphere Formation and Branching Assay.
Mammosphere assays were carried out as described previously (4, 5), and as described in SI Experimental Procedures.
Mammary Gland Transplantation, in Vivo Branch Quantification, and Elvax Implantation.
Mammary anlage were rescued from Ror2−/− embryos and transplanted into precleared fat pads of Foxn1nu mice, as previously reported (21) and described in the SI Experimental Procedures.
Immunohistochemistry.
Immunohistochemistry was performed as previously described with ref. 30. Images were collected on a Leica SP5 confocal microscope. Brightfield imaging was performed on a Biorevo BZ-9000 Digital Microscope (Keyence).
Statistical Analysis.
All statistical analyses (Student’s t test) were conducted on Graphpad Prism; P values of less than 0.05 were considered significant.
SI Experimental Procedures
SI Recombinant Proteins and Antibodies.
The list of recombinant proteins and antibodies is provided here. Recombinant mouse WNT5A and WNT5B were purchased from R&D Systems. Ryk antibody was purchased from Abgent (AP7677a). Cryosections were immunostained with rabbit anti MUC-1 (Abcam, GR77880-2), mouse anti E-cadherin (BD Biosciences, 70177), goat anti-NKCC1 (Santa Cruz Biotechnology), mouse anti-ROR2 (Developmental Studies Hybridoma Bank, Nt 2535–2835), and Hoechst (Anaspec, 85239).
SI Mammosphere Formation and Branching Assay.
Briefly, a single-cell suspension of MECs was prepared by mechanical dissociation of the tissue followed by digestion in media for 1 h at 37 °C with 300 U/mL collagenase (Sigma) and 100 U/mL hyaluronidase (Sigma). Before filtering through 40-mm mesh and resuspending in media, the resultant organoid suspension was sequentially resuspended in 0.25% trypsin-EGTA for 2 min, 0.1 mg/mL DNase (Worthington) for 5 min, and 0.64% NH4Cl for 3 min.
MECs (2,000) were diluted in 100 μL of 50% mammosphere growth medium and 50% Matrigel (354234; BD Biosciences), which was then plated out in 12-well plates. MEC growth medium consisted of Epicult-B Mouse Medium Kit (05610; Stem Cell Technologies) supplemented with 5% FBS to promote sphere outgrowth, and was added to the cells after the MEC-containing Matrigel layer had solidified. After 24 h, the medium was changed to serum-free Epicult-B Medium to the experimental medium containing WNT proteins and/or antibodies. Experimental conditions were performed in triplicates and sphere formation was analyzed after 7 d by counting the number of spheres per well and measuring sphere size on microscopic images using Image J software.
For mammary branching analysis, 1 × 106 single cells from primary mouse mammary preparations were allowed to form aggregates in 500 μL primary MEC growth medium by incubation overnight at 37 °C with 5% (vol/vol) CO2 in a 24-well ultra-low-attachment tissue culture plate. The next day, aggregates were harvested, washed, and plated out in 50 µL Matrigel in a 24-well plate at about 200 aggregates per well. We then added FGF2 media [DMEM/F12, 1% vol/vol insulin, transferrin, selenium (Sigma) and 1% vol/vol penicillin/streptomycin] or FGF2/branching medium [minimal medium + 2.5 nM FGF2 [Sigma F0291]) with or without WNT ligand, as recently described for the induction of branching morphogenesis (31). At least 50 aggregates/well were scored and branches were quantified with branching counted as three ducts or more, as established previously in organoid culture models. At least three independent samples per condition were averaged for each experiment.
For qPCR expression analysis, 200,000 cells per well were plated in a low-adhesion plate and treated with control medium or medium containing 0.5 μg/mL recombinant WNT5A or WNT5B for 24 h, followed by RNA isolation using a Qiagen minikit (74104). cDNA synthesis was performed using the Invitrogen SuperScript III system (18080-051), and quantitative reverse transcription-PCR was done via the Sybrgreen (Applied Biosystems, 4309155) method and an Eppendorf Realplex Mastercycler. Primers were purchased from SABiosciences. Relative quantification of gene expression was calculated according the Pfaffl method (32). Target gene expression was normalized to GAPDH reference gene expression.
Primers for qPCR with Sequence 5′->3′ (Forward; Reverse): Mouse Ror1: AACCCTTGATGAGCCGATGAA; CAGCGGATACTGGGAGGTG; Mouse Ror2: ATCGACACCTTGGGACAACC; AGTGCAGGATTGCCGTCTG; and Mouse Ryk T2: GTCACTACGCTCTGTCCTTTAAC; GCTCGACCCGAAACACTGATAA.
SI Mammary Gland Transplantation, in Vivo Branch Quantification, and Elvax Implantation.
Female athymic mice (Foxn1nu/nu) were obtained at 21 d of age, and both #4 fat pads were cleared for use as transplant host. Mammary anlage were rescued from Ror2−/− embryos and transplanted into these precleared fat pads. Contralateral outgrowths were harvested 12 wk posttransplant and subjected to whole-mount carmine staining. For serial studies, epithelial fragments were harvested and transplanted into a new host. For Elvax studies, pellets were generated by lyophilizing 250 ng of WNT5A or WNT5B and 1 mg of BSA, or 1 mg of BSA (control), and then mixing the material with 20% solution of ethylene vinyl acetate copolymer (Dupont) in dichloromethane; the mixture was quick-frozen in acetone-dry ice; and the resulting pellet was dried (33). Pellets of the same weight were contralaterally implanted at the forefront of the growing ductal tree in athymic mice transplanted with WT or Ror2−/− epithelia and harvested after 10 d (34). Primary branches were defined as ducts extending from the site of transplantation and terminating in an end bud. Secondary and tertiary branches were defined as bifurcating from primary ducts or secondary branches, respectively. Branch number was quantified by tracing the primary ductal structure and counting the number of primary bifurcated (C) and secondary/tertiary branches in Fiji (35).
Acknowledgments
We thank Ying Yu for technical assistance and animal handling and Devon A. Lawson for comments and technical contributions. We also thank Santa Cruz Biotechnology for the NKCC1 antibody. This work was supported by California Institute for Regenerative Medicine Awards FA1-00617-1 and CL1-00506-1.2 (facilities); NIH Awards R01 GM098897 (to L.H.), R01 CA057621 (to Z.W.), and K99/R00 CA181490 (to K.K.); National Human Genome Research Institute NHGRI-R25HG006836 predoctoral support (to P.S.); NIH National Cancer Institute Training Grant 5T32EB009418 (to N.P.); and Department of Defense Congressionally Directed Medical Research Program Award W81XWH-12-1-0272 (to A.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701464114/-/DCSupplemental.
References
- 1.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23(22):2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternlicht MD. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8(1):201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plaks V, et al. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Reports. 2013;3(1):70–78. doi: 10.1016/j.celrep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 5.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322(5907):1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296(5570):1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6(6):568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100(26):15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA. 2004;101(12):4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235(12):3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim E, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12(2):R21. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendrick H, et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591. doi: 10.1186/1471-2164-9-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji H, et al. Proteomic profiling of secretome and adherent plasma membranes from distinct mammary epithelial cell subpopulations. Proteomics. 2011;11(20):4029–4039. doi: 10.1002/pmic.201100102. [DOI] [PubMed] [Google Scholar]
- 15.Roarty K, Shore AN, Creighton CJ, Rosen JM. Ror2 regulates branching, differentiation, and actin-cytoskeletal dynamics within the mammary epithelium. J Cell Biol. 2015;208(3):351–366. doi: 10.1083/jcb.201408058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134(21):3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 17.Kessenbrock K, et al. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 2013;13(3):300–313. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borcherding N, Bormann N, Kusner D, Kolb R, Zhang W. Transcriptome analysis of basal and luminal tumor-initiating cells in ErbB2-driven breast cancer. Genom Data. 2015;4:119–122. doi: 10.1016/j.gdata.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gujral TS, et al. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159(4):844–856. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi S, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5(1):71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 21.Young LJT. The Cleared Mammary Fat Pad and the Transplantation of Mammary Gland Morphological Structures and Cells. Kluwer Academic/Plenum Press; New York: 2000. pp. 67–74. [Google Scholar]
- 22.Shillingford JM, Miyoshi K, Flagella M, Shull GE, Hennighausen L. Mouse mammary epithelial cells express the Na-K-Cl cotransporter, NKCC1: Characterization, localization, and involvement in ductal development and morphogenesis. Mol Endocrinol. 2002;16(6):1309–1321. doi: 10.1210/mend.16.6.0857. [DOI] [PubMed] [Google Scholar]
- 23.Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: A serial transplantation study. Proc Natl Acad Sci USA. 1968;61(1):53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Povinelli BJ, Nemeth MJ. Wnt5a regulates hematopoietic stem cell proliferation and repopulation through the Ryk receptor. Stem Cells. 2014;32(1):105–115. doi: 10.1002/stem.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong J, et al. The Wnt receptor Ryk controls specification of GABAergic neurons versus oligodendrocytes during telencephalon development. Development. 2011;138(3):409–419. doi: 10.1242/dev.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jönsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62(2):409–416. [PubMed] [Google Scholar]
- 27.Borcherding N, et al. Paracrine WNT5A signaling inhibits expansion of tumor-initiating cells. Cancer Res. 2015;75(10):1972–1982. doi: 10.1158/0008-5472.CAN-14-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Many AM, Brown AM. Both canonical and non-canonical Wnt signaling independently promote stem cell growth in mammospheres. PLoS One. 2014;9(7):e101800. doi: 10.1371/journal.pone.0101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124(Pt 14):2357–2366. doi: 10.1242/jcs.080903. [DOI] [PubMed] [Google Scholar]
- 30.Strickland P, Shin GC, Plump A, Tessier-Lavigne M, Hinck L. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development. 2006;133(5):823–832. doi: 10.1242/dev.02261. [DOI] [PubMed] [Google Scholar]
- 31.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14(4):570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silberstein GB, Daniel CW. Elvax 40P implants: Sustained, local release of bioactive molecules influencing mammary ductal development. Dev Biol. 1982;93(1):272–278. doi: 10.1016/0012-1606(82)90259-7. [DOI] [PubMed] [Google Scholar]
- 34.Marlow R, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res. 2008;68(19):7819–7827. doi: 10.1158/0008-5472.CAN-08-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]