Significance
Immune-mediated diseases are prevalent, debilitating, and costly. Unfortunately, current treatments rely on nonspecific immunosuppression, which also shuts down a protective immune response. To circumvent this, we exploited the noninflammatory natural means of clearance of red blood cells (RBCs), in combination with sortase-mediated RBC surface modification to display disease-associated autoantigens as RBCs’ own antigens. We found that this strategy holds promise for prophylaxis and therapy, as shown in a mouse model of multiple sclerosis and of type 1 diabetes.
Keywords: sortase, engineered red blood cells, antigen-specific tolerance, autoimmune diseases
Abstract
Current therapies for autoimmune diseases rely on traditional immunosuppressive medications that expose patients to an increased risk of opportunistic infections and other complications. Immunoregulatory interventions that act prophylactically or therapeutically to induce antigen-specific tolerance might overcome these obstacles. Here we use the transpeptidase sortase to covalently attach disease-associated autoantigens to genetically engineered and to unmodified red blood cells as a means of inducing antigen-specific tolerance. This approach blunts the contribution to immunity of major subsets of immune effector cells (B cells, CD4+ and CD8+ T cells) in an antigen-specific manner. Transfusion of red blood cells expressing self-antigen epitopes can alleviate and even prevent signs of disease in experimental autoimmune encephalomyelitis, as well as maintain normoglycemia in a mouse model of type 1 diabetes.
The incidence of diseases with an immune component continues to increase. Examples include not only ∼80 autoimmune diseases, but also life-threatening conditions caused by immune responses to protein replacement therapies, or by attack of the host’s immune system on transplanted tissues or transferred cells. Treatment of these conditions often depends on prolonged use of immunosuppressants, which lack antigen specificity. Because sustained immunosuppression increases the risk of infection, an important goal remains the development of antigen-specific immune intervention to achieve tolerance, while sparing desirable effector immune responses, such as those directed against pathogens (1). Administration of soluble, disaggregated proteins or peptides, apoptotic cells, or micro/nanoparticles chemically conjugated with antigenic peptide, as well as antibody fusion constructs, have been used with varying degrees of success (2–8). A challenge in the development of antigen-specific immune intervention is the delivery of the antigenic payload to the correct destination for processing, to establish long-lasting peripheral tolerance. Adding to this challenge, the tolerogenic doses of different antigens vary greatly, when tolerance can be achieved at all. Furthermore, the introduction of nonnative materials, such as micro/nanoparticles, might lead to unpredictable adverse effects.
Apoptotic cells are tolerogenic, presumably by displaying self-antigens in a noninflammatory context to antigen-presenting cells, leading to anergy or deletion of immune effector cells (9). Expansion of the regulatory T-cell compartment may also contribute to curtailing autoimmunity (10). Each second, millions of red blood cells (RBCs) are cleared from the circulation by phagocytic cells in the spleen and by the reticuloendothelial system, without obvious signs of inducing an immune response. We exploit this natural route of RBC removal for induction of tolerance. We have found that transfusion of RBCs, covalently modified with an antigenic payload, can induce antigen-specific tolerance in naïve recipients. We use genetically engineered RBCs—as well as their unmodified counterparts—as substrates for sortase, a transpeptidase, to covalently attach peptides from disease-relevant autoantigens. This approach has prophylactic and therapeutic efficacy in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. Similarly, transfusion of RBCs modified with an insulin-derived peptide in the nonobese diabetic (NOD) mouse model of type 1 diabetes (T1D) allows a majority of animals to remain normoglycemic. These results suggest application of this strategy to other autoimmune diseases.
Results
Sortagging Is a Robust, Efficient, and Simple Method to Covalently Modify RBCs Without Compromising Their Biological Properties.
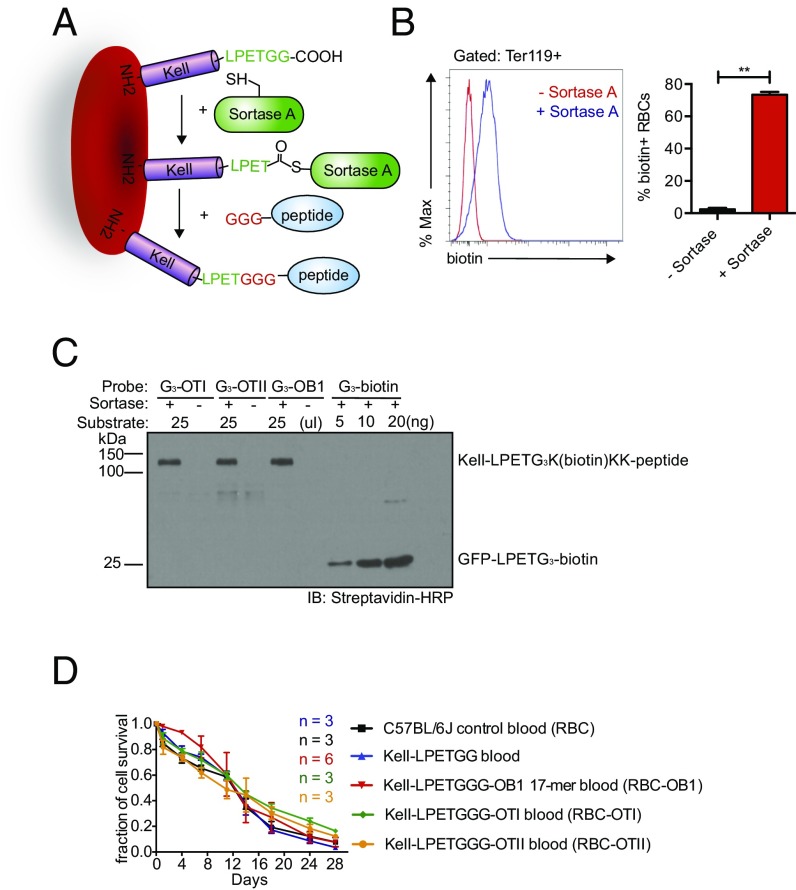
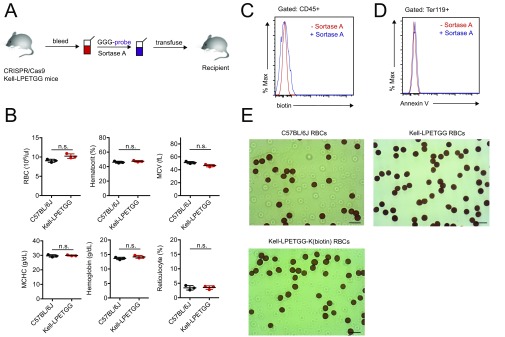
An important aspect of our strategy is to preserve the biological properties of labeled RBCs, so that they remain as close to their native state as possible. We used a sortase A-mediated reaction (“sortagging”) to minimize damage to the RBC membrane (11, 12). Sortase A recognizes an LPXTG motif and cleaves the peptide bond between the threonine and glycine residues in this motif to yield a thioester acyl-enzyme intermediate. A nucleophile that contains a suitably exposed N-terminal glycine, (G)n, can resolve this intermediate, covalently linking the two motifs via a peptide bond (Fig. 1A). We have used retroviral and lentiviral vectors that encode membrane proteins appended with sortase motifs to generate red cells that have variable numbers of sortaggable proteins on their surface (12, 13). Because tolerogenic doses vary among different antigens, it is important to have a source of RBCs that can be modified consistently and reproducibly with a known quantity of antigen. To this end we used CRISPR/Cas9 to generate mice whose RBCs carry the Kell protein extended at its C terminus to include an LPETGG motif, referred to here as Kell-LPETGG mice. Kell-LPETGG mice bred to homozygosity for this modification served as blood donors for the transfusion experiments described below (Fig. S1A). Insertion of the sortase motif does not cause hematological abnormalities, as inferred from complete blood count data (Fig. S1B). Kell-LPETGG is expressed under the control of its endogenous promoter, restricting its expression to the RBC compartment. Neither WT RBCs, nor white blood cells isolated from Kell-LPETGG mice could be labeled with (G)3-K(biotin) in a sortagging reaction, as distinct from Kell-LPETGG RBCs, which were modified with an efficiency of ∼80%, likely an underestimate (Fig. 1B and Fig. S1C). The conditions for the sortagging reaction are mild and no major damage to RBCs was apparent, as assessed by the absence of PtdSer externalization (Annexin V staining) (Fig. S1D). The morphology of sortagged RBCs, regardless of attached payload, was normal (Fig. S1E).
Fig. 1.
Designs and characterization of engineered RBCs. (A) Schematic for Kell C-terminal sortase labeling with GGG-carrying antigens peptides. (B) Evaluation and quantification of mature Kell-LPETGG RBCs for sortase labeling by incubation of RBCs with biotin-containing probes in the presence or absence Sortase A. Cytofluorimetry was performed with anti-TER119, a RBC surface marker, and antibiotin antibodies. (n = 3; **P < 0.01, unpaired t test with Holm–Sidak adjustment). (C) Quantification of sortase labeling of Kell-LPETGG RBCs with different biotin-containing peptides by immunoblotting (IB) using streptavidin-HRP. GFP-biotin carrying a single biotin/mole of GFP was used a reference. (D) CFSE-labeled RBCs from C57BL/6J and Kell-LPETGG mice were transfused into recipient mice. Kell-LPETGG blood samples were also subjected to sortagging with the three different OVA-derived peptides before transfusion. RBC survival in the circulation was tracked via CFSE fluorescence by flow cytometry.
Fig. S1.
(A) Overall experimental scheme. (B) Complete blood count of homozygous Kell-LPETG mice indicate normal RBC number, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration (MCHC), hemoglobin content, and reticulocyte number. (C) Surface labeling of Kell-LPETG RBCs with GGG-biotin as characterized by flow cytometry. Because Kell protein is a RBC-specific protein, none of the other hematopoietic cell subsets, CD45+ cells, were sortagged with GGG-biotin. (D) No increase of Annexin V, a death cell marker, staining upon sortagging. (E) Benzidine staining reveals normal morphology of engineered RBCs. (Scale bars, 10 μm.)
Using three biotinylated peptides of different sequence, we enumerated the number of sortase-modifiable Kell molecules per cell. We performed sortagging reactions on 25 μL of fresh Kell-LPETGG RBCs with GGGK(biotin)KK–OT-I, GGGK(biotin)KK–OT-II, and GGGK(biotin)KK-OB1 peptides as nucleophiles. These peptides represent three different immunodominant peptides of ovalbumin (OVA). They are diverse in length and biophysical properties (Table S1 for the list of antigenic adducts synthesized and attached to Kell-LPETG RBCs). Sortagging yields a consistent number of the various biotinylated payloads attached (Fig. 1C). Using monobiotinylated GFP as a reference, we quantified the number of peptides covalently attached to the surface of Kell-LPETGG RBCs; there were ∼9,000 Kell proteins consistently modified per RBC (Fig. 1C).
Table S1.
Peptide probes: Amino acid sequences of peptide probes
| No. | Type | Sequence (N to C terminus) |
| 1 | GGG-biotin | GGGK(biotin) |
| 2 | GGG–OT-I | GGGK(biotin)KKFRSIINFEKL |
| 3 | GGG–OT-II/GGG-OVA323–339 | GGGK(biotin)KKISQAVHAAHAEINEAGR |
| 4 | GGG-OB1 | GGGK(biotin)KKFDKLPGFGDSIEAQGGK |
| 5 | GGG-MOG35–55 | GGGCKKGSMEVGWYRSPFSRVVHLYRNGK |
| 6 | GGG-InsB9–23 | GGGCKKGSSHLVEALYLVCGERG |
| 7 | biotin-LPETGG | K(biotin)LPETGG |
| 8 | MOG35–55-LPETGG | MEVGWYRSPFSRVVHLYRNGKGSLPETGG |
| 9 | OVA323–339-LPETGG | ISQAVHAAHAEINEAGRGSLPETGG |
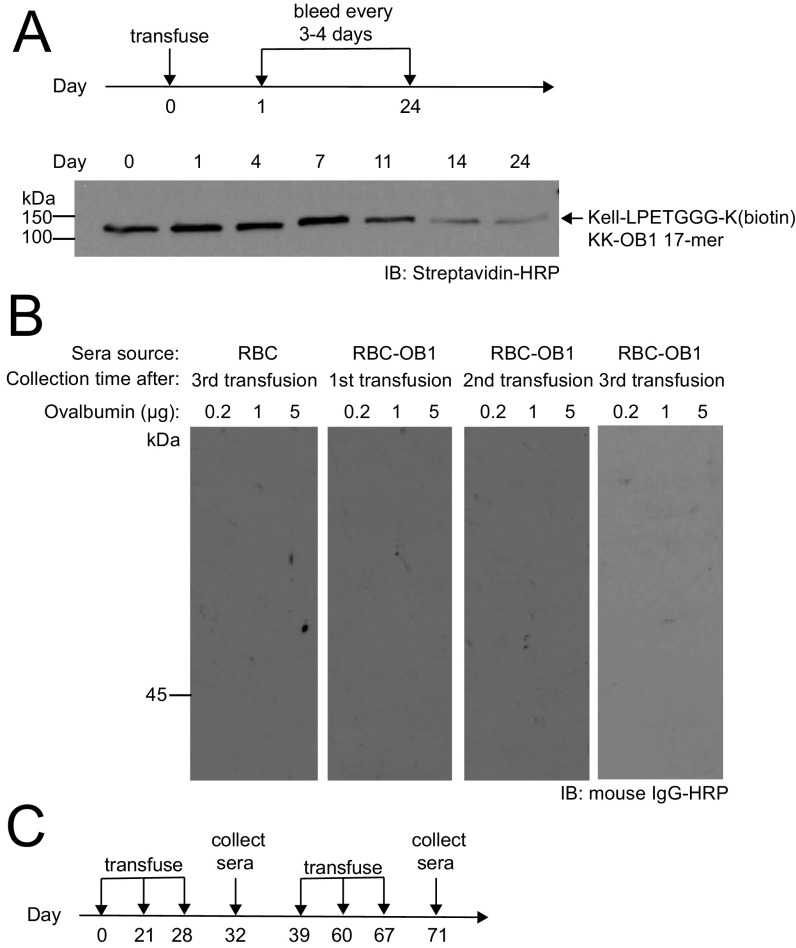
We next characterized the in vivo persistence of sortagged RBCs by assessing their circulatory half-life. We stained the modified RBCs (i.e., RBC–OT-I, RBC–OT-II, and RBC-OB1) with carboxyfluorescein succinimidyl ester (CFSE) before transfusion. Their survival was equal to that of unmodified RBCs, regardless of the identity of the payload attached (Fig. 1D). Because the OB1 peptide linked to Kell is biotinylated, we were able to track its disappearance. Indeed, the loss of the Kell-LPETGGG-K(biotin)KK-OB1 signal obtained by immunoblotting corresponded with the disappearance of CFSE signal (Fig. S2A). Modification by sortase therefore does not accelerate removal of engineered RBCs, which retain the attached peptide while in circulation. We hypothesized that the circulatory persistence (>28 d) of antigen-decorated RBCs creates a window of opportunity for the induction of more complete peripheral tolerance by editing out antigen-specific effector cells.
Fig. S2.
(A) RBC-OB1 blood sample (25 μL) from the consecutive bleeding (as in Fig. 1D) were further characterized by Western blot. Clearance of biotinylated Kell transmembrane proteins are in agreement with the overall RBC clearance rate as indicated by the disappearance of the biotin signal. (B) CFSE-labeled RBCs from C57BL/6J and Kell-LPETGG mice were transfused into recipient mice. One set of Kell-LPETGG blood samples were also subjected to sortagging with OB1 peptides before transfusion. Following the first transfusion (as in Fig. 1D), the same cohort is subjected to two more transfusions with a 1 wk gap between each transfusion (as in Fig. 2A). Western blot against OVA proteins indicates no antibody response upon repeated transfusions of RBC-OB1 into C57BL/6J. (C) Overall RBC and RBC-OB1 transfusion schedule into BALB/c mice in Fig. 2B.
Engineered RBCs Blunt Specific B, CD4, and CD8 T-Cell Responses.
Autoimmunity can result from abnormal behavior of three major immune effectors: B cells, CD4 T cells, CD8 T cells, or a combination thereof. To eliminate potential variables related to diverse T-cell receptor (TCR) or B-cell receptor repertoires, and the potential for self-reactivity, we used the model protein antigen OVA. There are three clonal derivatives of OVA-specific immune effector cells: the CD8 TCR transgenic mouse (OT-I) recognizes the H-2Kb-SIINFEKL complex; the CD4 TCR transgenic mouse (OT-II) recognizes the I-Ab-ISQAVHAAHAEINEAGR complex; and the OVA-specific B-cell transnuclear mouse (OB1) recognizes the FGD-centered epitope contained in the 17-mer FDKLPGFGDSIEAQGGK (14–16).
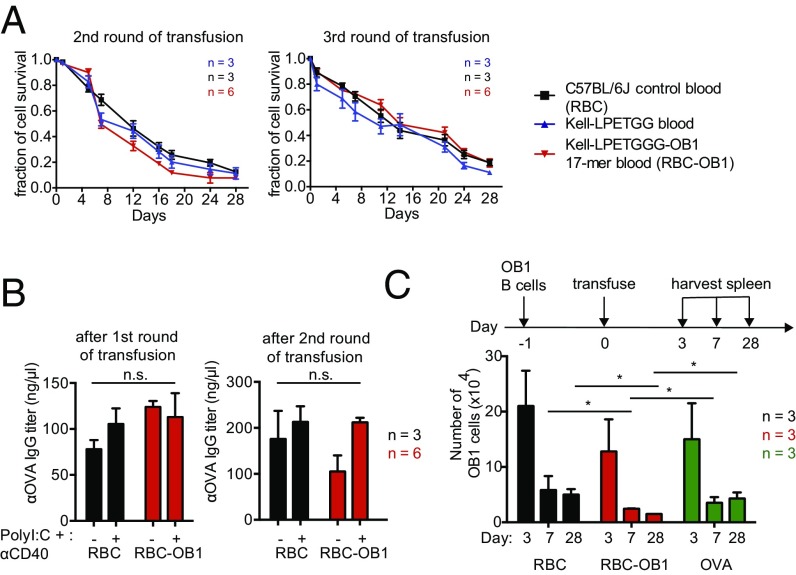
To determine whether a B-cell–specific OVA-derived epitope can be viewed as self when attached to the surface of a RBC, we attached it to Kell-LPETG RBCs using sortase. Repeated transfusions of CFSE-stained RBC-OB1 into a cohort of C57BL/6J recipients did not accelerate the rate of clearance of OB1-modified RBCs (Figs. 1D and 2A). The multiple transfusions did not elicit an antibody response against intact OVA protein (Fig. S2B). To further test the immunogenicity of RBC-OB1, we carried out repeated transfusions of sortagged RBCs into BALB/c mice, which show a Th2-skewed response, favoring IgG1 peoduction in the presence of the adjuvants polyI:C and anti-CD40, administered intraperitoneally (Fig. S2C). Once again, these multiple transfusions of RBC-OB1 did not elicit an antibody response against intact OVA protein (Fig. 2B and Fig. S2C). Finally, we transferred OB1-specific B cells that recognize and respond to the 17-amino acid OB1 peptide. Transferred OB1 B cells disappeared at a faster rate in mice treated with RBC-OB1 than in animals exposed to OVA or to unmodified RBCs, indicating induction of B-cell tolerance (Fig. 2C).
Fig. 2.
OB1 peptide-decorated RBCs blunt responses of OB1-specific B cells. (A) CFSE-labeled RBCs from C57BL/6J and Kell-LPETGG mice were transfused into recipient mice. One set of Kell-LPETGG blood samples were also subjected to sortagging with OB1 peptides before transfusion. Following the first transfusion (as in Fig. 1D), the same cohort is subjected to two more transfusions with a 1-wk gap between each transfusion. RBC survival in the circulation was tracked via CFSE fluorescence by flow cytometry. Repeated transfusions of sortagged RBCs into C57BL/6J mice do not induce faster clearance. (B) A cohort of BALB/c mice was transfused with either C57BL/6J or RBC-OB1. OVA-specific IgG titers at the end of each transfusion were measured by ELISA. (C) Flow cytometry of the total number of adoptively transferred OB1 B cells in spleen, harvested 3, 7, and 28 d after RBC, RBC-OB1, or OVA transfusion. *P < 0.05; ns, not significant.
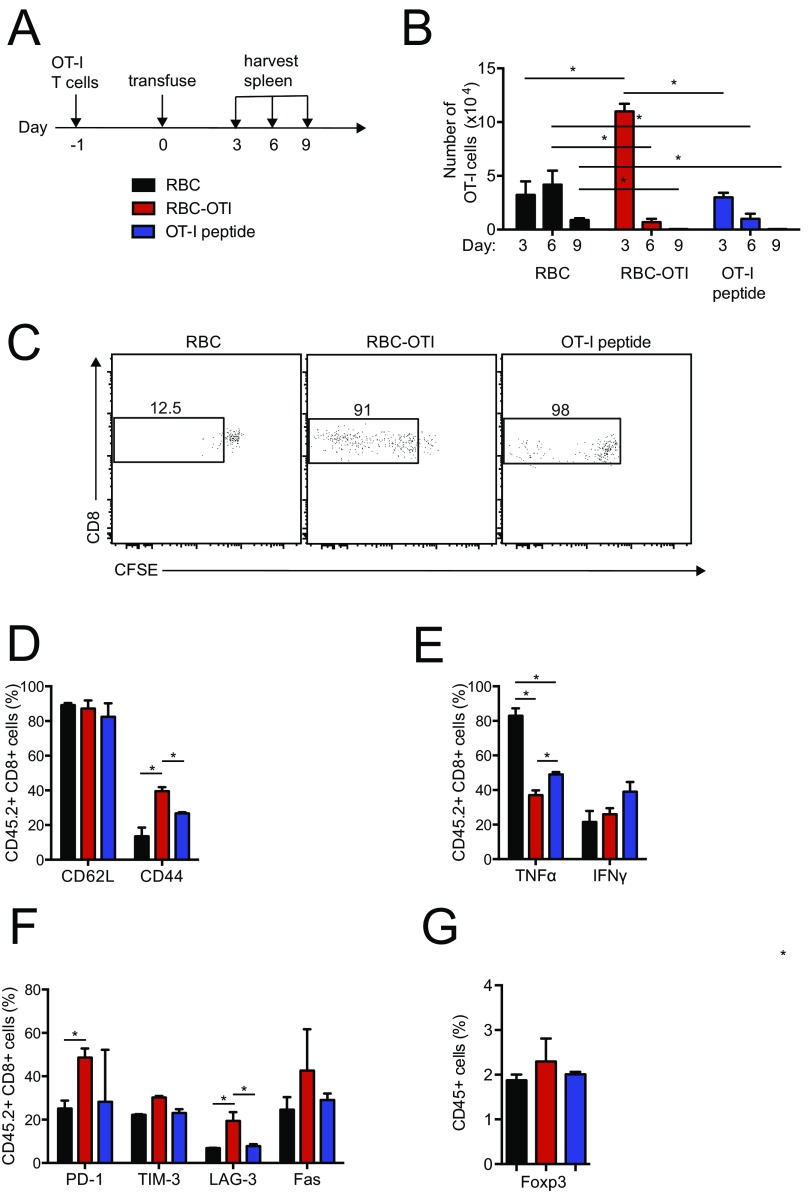
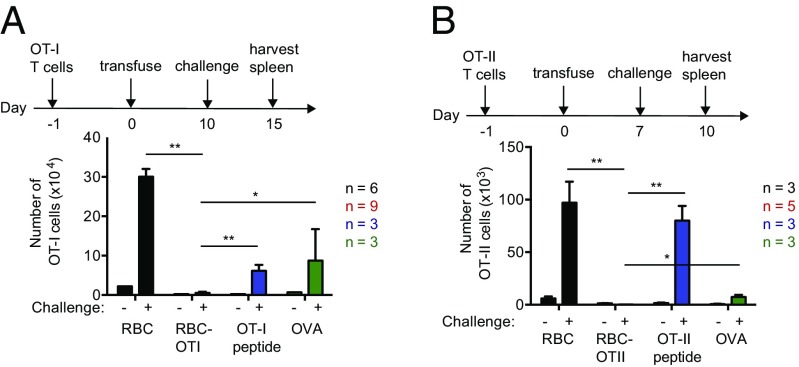
Next, to determine whether a CD8 T-cell–specific epitope can also be viewed as self when attached to the surface of a RBC, we adoptively transferred CFSE-labeled OT-I T cells, followed by transfusion of RBC–OT-I, RBC or free OT-I peptide 1 d later (Fig. S3A). In mice that received RBC–OT-I, transferred OT-I CD8+ T cells showed only modest expansion at first, compared with mice receiving an equivalent number of unmodified RBCs or an equimolar amount of OT-I peptide, as judged from the absolute number of cells recovered from spleen and by CFSE dilution (Fig. S3 B and C). OT-I T cells disappeared after several divisions in both RBC–OT-I and OT-I peptide-treated mice, but at day 3 posttransfer, surviving T cells in RBC–OT-I recipients displayed characteristics of nonresponsive (tolerant) cells: they failed to down-regulate CD62L while remaining CD44+ (Fig. S3D). Upon in vitro restimulation with OT-I peptide, the surviving OT-I T cells produced fewer proinflammatory cytokines, TNF-α, and IFN-γ, than OT-I T cells from mice that received OT-I peptide alone (Fig. S3E). Surviving OT-I T cells showed higher levels of apoptotic and exhaustion markers, such as Fas, PD-1, and LAG-3 (Fig. S3F). Transfusion of RBC–OT-I thus imposes peripheral tolerance in a manner that resembles T-cell exhaustion (17), but may include physical removal as well. By days 6 and 9, far fewer OT-I T cells were detected in RBC–OT-I–transfused mice than in animals that received control RBCs (Fig. S3B). After a subsequent challenge of mice with OT-I peptide in complete Freund’s adjuvant (CFA), a strong adjuvant, the OT-I T cells in mice transfused with RBC–OT-I failed to respond, whereas OT-I T cells in mice injected with an equimolar amount of OT-1 peptide, OVA, or an equal number of control RBCs proliferated as expected (Fig. 3A). In the RBC–OT-I–transfused mice we saw no prominent change in the regulatory T-cell compartment (Fig. S3G).
Fig. S3.
(A) Experimental scheme. (B) The numerical kinetics of splenic OT-I T cells in the adoptive transfer model. RBC–OT-I leads to OT-I T-cell disappearance after an early spurt of proliferation as analyzed by flow cytometry. (C) CFSE-dilution indicates the proliferation of OT-I T cells at day 3 upon treatment administration. Frequency of splenic (D) CD44+ and CD62L+ OT-I T cells, (E) IFN-γ+ and TNF-α+ OT-I T cells, and (F) PD-1+, TIM-3+, LAG-3+, and Fas+ OT-I T cells in the spleen at day 3 upon transfusion. (G) Frequency of splenic Foxp3+ CD4+ regulatory T cells at day 15 after CFA/OT-I peptide challenge. *P < 0.05.
Fig. 3.
Engineered RBCs blunt responses of OVA-specific CD4 and CD8 T-cell responses. (A) Total number of OT-I T cells in spleen following a challenge with saline (−) or OT-I peptide/CFA (+) at day 10. (B) Absolute number of OT-II T cells in spleen, 3 d after challenge with saline (−) or OVA/CFA (+) at day 7. Data are shown as mean ± SD and represent at least two independent experiments. Statistically significant differences are indicated by asterisks: *P < 0.05; **P < 0.01 (unpaired t test with Holm–Sidak adjustment).
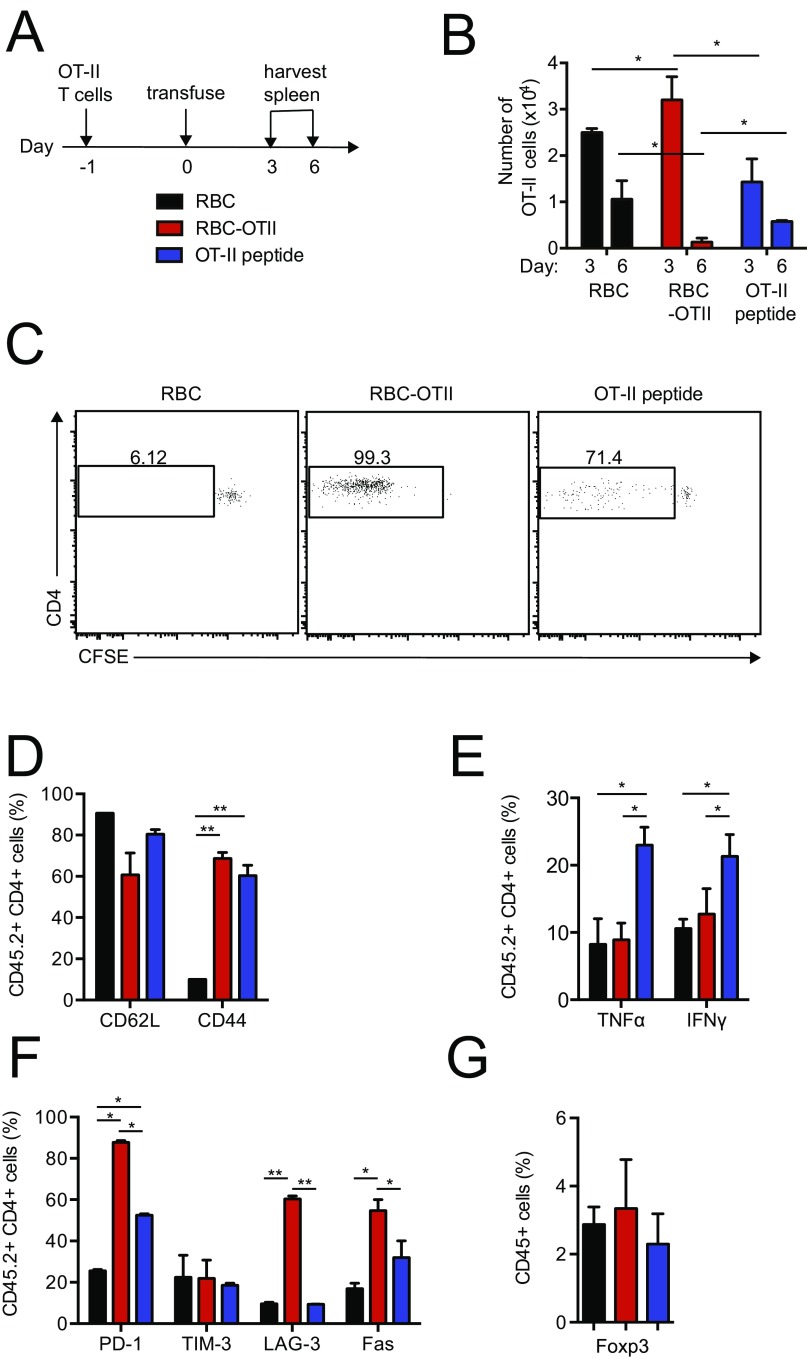
We next assessed whether a similar tolerogenic effect applied to the CD4 T-cell compartment. We adoptively transferred CFSE-labeled OT-II CD4+ T cells, followed the next day by transfusion of RBC, RBC–OT-II, or administration of OT-II peptide. Only transfusion of RBC–OT-II led to rapid division of the transferred OT-II T cells, followed by disappearance of the transferred OT-II T cells by day 6 (Fig. S4 A–C). The surviving OT-II T cells did not respond to a challenge with OVA in CFA (Fig. 3B). There was little change in the regulatory T-cell compartment for mice transfused RBC–OT-I (Fig. S4G). OT-II T cells in mice that received RBC–OT-II also expressed apoptotic markers and resembled anergic T cells (Fig. S4 D–F). Abortive activation or deletion of T and B cells can therefore occur as early as day 3, at a time when >90% of transfused RBCs remain. These results are reminiscent of those obtained through systemic administration of an antigenic payload attached to a peptide adduct designed to bind glycophorin A noncovalently (7, 18). Based on these three OVA models, we conclude that induction of antigen-specific tolerance by modified RBCs can apply to B, CD4 T cells, and CD8 T cells.
Fig. S4.
(A) Experimental scheme. (B) The numerical kinetics of splenic OT-II T cells in the adoptive transfer model. RBC–OT-II leads to OT-I T-cell disappearance after an early spurt of proliferation as analyzed by flow cytometry. (C) CFSE-dilution indicates the proliferation of OT-II T cells at day 3 upon treatment administration. Frequency of splenic (D) CD44+ and CD62L+ OT-II T cells, (E) IFN-γ+ and TNF-α+ OT-II T cells, and (F) PD-1+, TIM-3+, LAG-3+, and Fas+ OT-II T cells in the spleen at day 3 upon transfusion. (G) Frequency of splenic Foxp3+ CD4+ regulatory T cells at day 10 after CFA/OVA challenge. *P < 0.05; **P < 0.01.
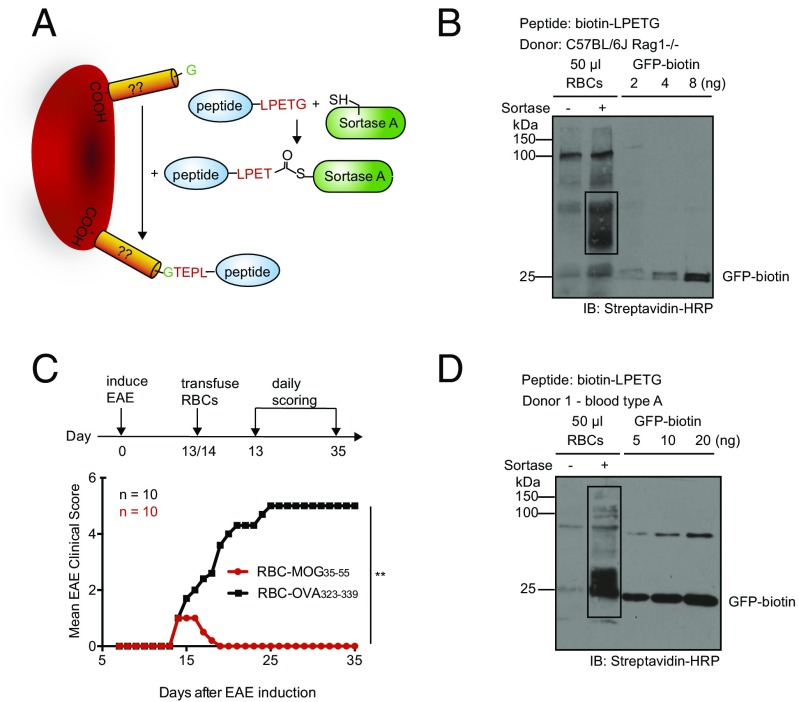
RBCs Carrying MOG35–55 Not Only Confer Protection Against EAE but Can Even Reverse Early Clinical Signs of EAE.
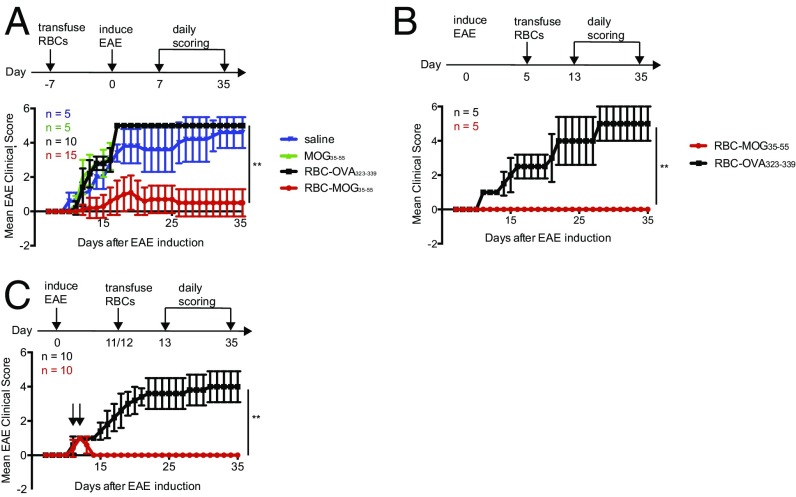
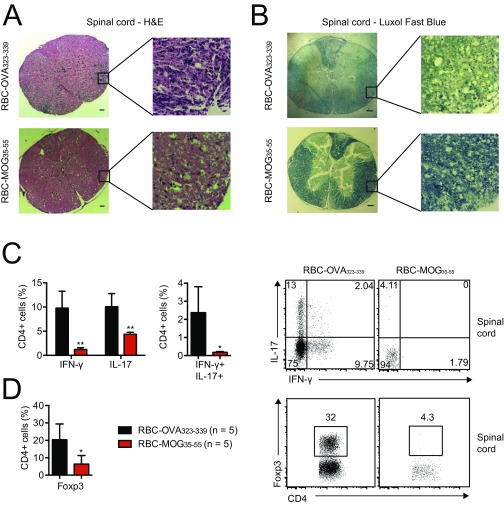
We next tested the ability of RBCs modified with the immunodominant peptide of myelin oligodendrocyte glycoprotein (MOG; residues 35–55), a major central nervous system protein, to affect the course of EAE. Immunization of C57BL/6 mice with MOG35–55 in the presence of CFA and pertussis toxin elicits clinical signs of this multiple sclerosis-like condition within 10–14 d (19). Administration of modified Kell-LPETGG RBCs sortagged with MOG35–55 peptide (RBC-MOG35–55) 7 d before induction of disease delayed onset, if not completely suppressed EAE (Fig. 4A). In contrast, all mice that received Kell-LPETGG RBCs sortagged with an irrelevant peptide (RBC-OVA323–339), unconjugated MOG35–55 peptide, or saline progressed to severe disease (Fig. 4A). Histology of spinal cord sections confirmed the presence of inflammatory nodules and demyelination in mice treated with RBC-OVA323–339 (Fig. S5 A and B). Cells that infiltrate the spinal cord of mice transfused with RBC-OVA323–339 comprise inflammatory Th1 and Th17 CD4 T cells. Although we noted the presence of Foxp3+ regulatory T cells, these failed to suppress disease progression. Spinal cords from RBC-MOG35–55-treated mice lacked both infiltrating inflammatory and regulatory T cells (Fig. S5 C and D).
Fig. 4.
Engineered RBCs in EAE mouse models. (A) Mean EAE clinical scores of mice subjected to transfusion with RBC-MOG35–55, RBC-OVA323–339, unconjugated MOG35–55 peptide, or saline at 7 d before induction of EAE. Mean clinical scores of mice subjected to therapy by transfusion of RBC-MOG35–55 (or RBC-OVA323–339 as control) into (B) mice at preclinical stage and (C) mice with a clinical EAE score of 1; the black arrows indicate time of RBC administration. **P < 0.01, two-way ANOVA with repeated measures. All pair-wise comparisons were performed; RBC-MOG35–55 was shown to be significantly different from other treatment groups.
Fig. S5.
(A) H&E and (B) Luxol fast blue staining of spinal cord sections from MOG35–55-immunized mice that prophylactically received RBC-MOG35–55 or RBC-OVA323–339; these images visualize immune-cell infiltration and demyelination, respectively. (Scale bars, 100 μm.) (C) Flow cytometry analysis of CD4+ lymphocyte infiltrates into the spinal cord of diseased and protected mice at day 15–18 after immunization. (D) Frequency of Foxp3+ CD4+ regulatory T cells in the spinal cord at day 15–18 after immunization. *P < 0.05; **P < 0.01.
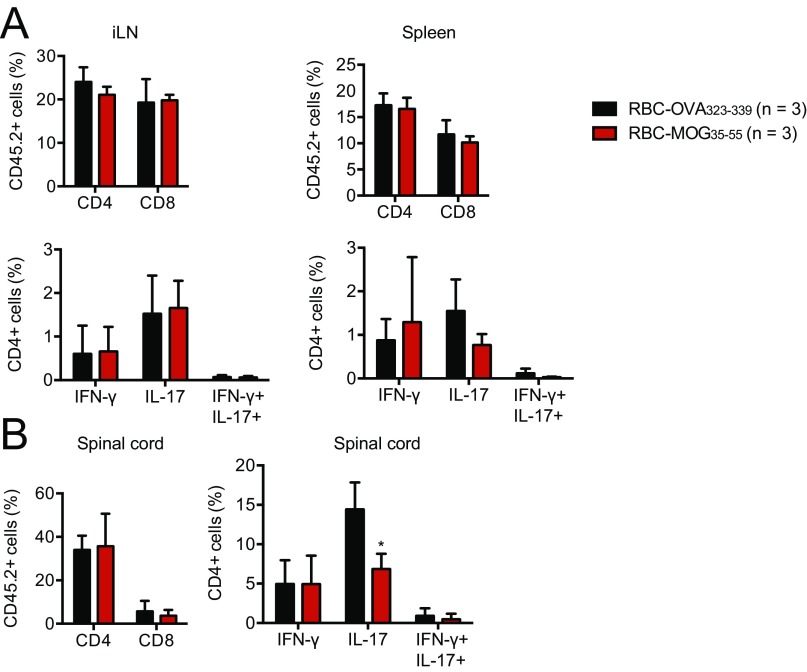
We examined whether RBC-MOG35–55 could interfere in the course of EAE by transfusing mice with RBC-MOG35–55 during the induction period (i.e., at day 5 after disease induction) (Fig. 4B). Although transfusion of RBC-MOG35–55 prevented EAE, its RBC-OVA323–339 counterpart did not (Fig. 4B). To determine whether RBC-MOG35–55 could reverse incipient EAE, we transfused RBC-MOG35–55 into mice that had already developed a limp tail (EAE clinical score of 1). Transfusion of RBC-MOG35–55 into these mice halted progression and alleviated clinical symptoms of EAE (Fig. 4C). This effect—that is, amelioration of EAE symptoms—was rapid. We saw no changes in cellular composition of the inguinal lymph nodes and spleen, but noted a decrease in Th17 cells in spinal cord infiltrates (Fig. S6).
Fig. S6.
Flow cytometry analyses of (A) CD4+ and CD8+ composition as well as Th1 and Th17 response in the spleen and inguinal lymph nodes a day after RBC-MOG35–55 or RBC-OVA323–339 treatment. (B) Flow cytometry analysis of lymphocyte infiltrates in the spinal cord of diseased and protected mice a day after RBC-MOG35–55 or RBC-OVA323–339 treatment. *P < 0.05.
RBCs Carrying Ins9–23 Confer Protection Against T1D.
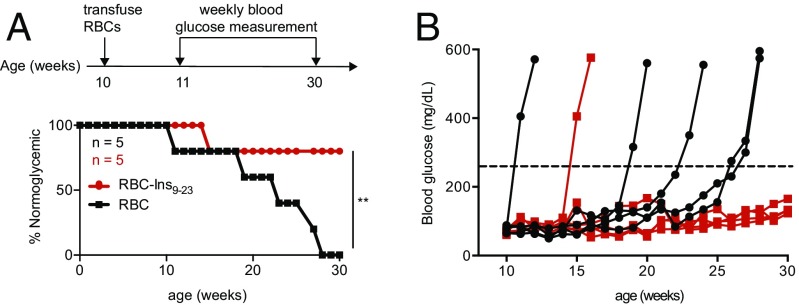
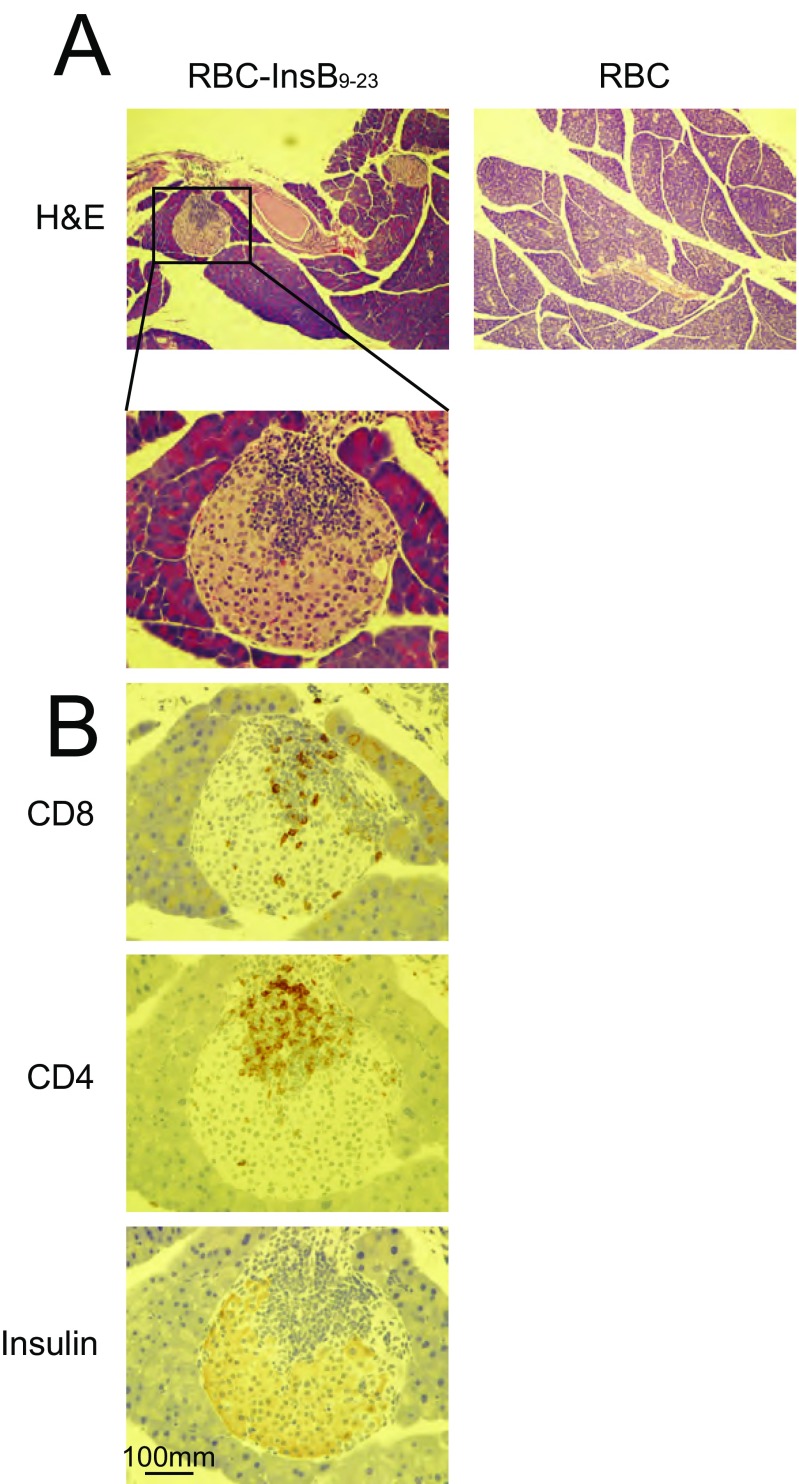
We investigated whether this strategy could also be applied to T1D in the NOD/ShiltJ mouse model. These mice develop T1D as early as 12 wk of age, as manifested by insulitis and low pancreatic insulin content (20). Mice are considered diabetic when their plasma glucose levels rise to >250 mg/dL. Insulin B-chain peptide 9–23 (Ins9–23) is an immunodominant self-antigen, the recognition of which can mediate autoimmune destruction of pancreatic β-cells and impair insulin production and release (21). A single prophylactic transfusion of Kell-LPETGG RBCs sortagged with the Ins9–23 at 10 wk of age protected NOD/ShiltJ from T1D in 80% of animals until week 30, whereas injection of unmodified RBCs did not (Fig. 5). Incomplete protection may be because of epitope spreading (22). The pancreas of mice treated with RBC-InsB9–23, retained insulin-expressing pancreatic islets at 30 wk of age, even with evident infiltration of CD4 and CD8 T cells, whereas in RBC-treated (control) mice, there were no or only very few pancreatic islets that remained (Fig. S7). We attribute this protection to insulin-specific tolerance. The CD8 and CD4 T cells observed in the pancreas most likely are of other specificities. Identification of an expanded set of autoantigens would thus be required to further improve disease outcome.
Fig. 5.
Engineered RBCs in T1D mouse models. (A) Schematic for prophylactic T1D treatment. Blood glucose levels were measured to monitor T1D progression in NOD mice, considered diabetic when glucose levels were >250 mg/dL, **P < 0.01 (log-rank test). (B) Individual blood glucose level measurement in mice treated with RBC or RBC-InsB9–23.
Fig. S7.
(A) H&E and (B) CD4, CD8, and insulin staining of pancreas sections from NOD/ShiltJ mice treated with RBC or RBC-Ins9–23. (Scale bar, 100 μm.) (Magnification: A, 10×; A, Inset, 40×.)
Application of the Sortase Modification Strategy to Human RBCs.
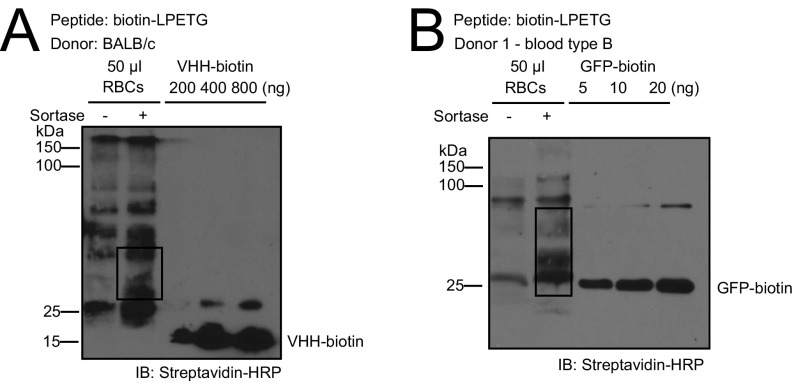
Translation of this approach to human RBCs could rely on the production of RBCs genetically modified to enable a site-specific sortase-catalyzed modification, as we have done for mouse Kell. Unfortunately, production of human RBCs from hematopoietic precursors in vitro, genetically modified to display an LPXTG motif at their surface, remains expensive and time-consuming (12, 23, 24). Because completion of the sortase reaction involves a GGG-containing nucleophile, endogenous proteins on the RBC surface that bear an exposed glycine at their N terminus could serve as a nucleophile, and be used in conjunction with a peptide modified with a C-terminal LPETGG sequence (Fig. 6A) (25). To test this theory, we sortagged mouse C57BL/6 RBCs with various LPETGG-containing biotinylated peptides. Using monobiotinylated GFP as reference, we found that ∼4,500 LPET-containing peptides can be attached to normal C57BL/6J mouse RBCs (Fig. 6B). BALB/c RBCs behave similarly in this reaction (Fig. S8A). Moreover, transfusion with RBC-MOG35–55, C57BL/6 RBCs sortagged with MOG35–55-LPETGG reversed clinical signs of EAE to levels commensurate with RBCs genetically engineered to contain a sortase motif (Fig. 6C).
Fig. 6.
Translatability of engineered RBCs in human system. (A) Schematic for sortagging of endogenous RBC membrane proteins containing a suitably exposed N-terminal glycine with LPETGG-equipped peptide. (B) Installation of biotin-LPETGG peptide onto endogenous sortase substrates on mouse RBCs, analyzed by SDS PAGE followed by immunoblotting using streptavidin-HRP. Endogenous biotinylated substrates are boxed. (C) Mean EAE clinical scores of mice subjected to therapy by transfusion of C57BL6/J RBCs from Rag2−/− mice, sortagged with MOG35–55-LPETGG (or C57BL6/J RBCs Rag2−/− as control) into mice with clinical EAE score of 1; time of RBC administration is indicated by the black arrow. **P < 0.01, two-way ANOVA with repeated measures. (D) Installation of biotin-LPETGG peptide onto endogenous sortase substrates on human RBCs type A, analyzed by SDS PAGE followed by immunoblotting using streptavidin-HRP. Endogenous biotinylated substrates are boxed.
Fig. S8.
Installation of biotin-LPETGG peptide onto endogenous sortase substrates on (A) BALB/c mouse RBCs and (B) human RBCs type B, analyzed by SDS PAGE followed by immunoblotting using Streptavidin-HRP. Endogenous biotinylated substrates are boxed.
Human RBCs likewise possess endogenous proteins that can serve as nucleophiles and resolve the LPET-sortase covalent intermediate. RBCs from unrelated donors sortagged with LPETGG- biotin yielded very similar labeling profiles; ∼3,000 peptides were attached to each human RBC (Fig. 6D and Fig. S8B). As assessed in an immunoblot, sortagging of endogenous RBC proteins yields a similar banding profile for two unrelated donors. In principle, this strategy allows the transfusion of enzymatically modified autologous RBCs within 1 h of obtaining the RBC population.
Discussion
We use sortase to modify the surface of human and mouse RBCs by covalently attaching peptides and other payloads. In one line of experiment, we used CRISPR/Cas9 to introduce into the murine germ line the LPETGG sortase motif at the C terminus of the Kell protein. Fresh red cells from these mice can be incubated with sortase and any payload bearing an N-terminal (G)n sequence, allowing attachment of ∼9,000 payloads per cell. The entire process from bleeding to transfusion takes no more than 60 min.
Our second application uses unmodified mouse or human red cells, and presents a more viable option for a clinical setting. Instead of relying on an added nucleophile equipped with N-terminal glycines, we performed the sortagging reaction by relying on endogenous RBC surface proteins with one or more exposed N-terminal glycines. We provided the antigenic payload as a peptide equipped with an LPXTG extension at its C terminus. Both Kell-LPETGG and unmodified RBCs yield RBCs decorated with several thousand copies of the desired antigen per cell. These red cells confer tolerance not only against OVA, but also against MOG and the immunodominant peptide of insulin. For both EAE and T1D, we achieved prophylaxis, as well as amelioration of clinical signs of disease. Both can confer protection with a single administration.
Several alternative approaches have been used to achieve antigen-specific tolerance in an autoimmune disease setting, with varying degrees of success. DNA vaccination usually requires multiple dosing, at times requiring coadministration of immunosuppressants (26–29). Although efficacious prophylactically, therapeutic efforts present more of a challenge (30). Inadvertent activation of innate immunity caused by the delivery vector, as well as antivector immunity, are additional confounding factors (31).
To achieve tolerance, intravenous peptide delivery necessitates the administration of multiple doses, depending on the disease model examined (32–34). Peptides, proteins, or conjugated peptides (e.g., peptides conjugated to anti-DEC205) delivered systemically do not benefit from specific targeting, as in the case of our sortase-modified RBCs. Oral tolerance likewise requires administration of large amounts of antigen and multiple doses (35–38), but orally administered peptide treatments have so far failed in human clinical trials (39).
Dying cells—including aged RBCs—are phagocytosed by macrophages or dendritic cells, often at specific anatomical locations. The identity and context of phagocytes that ingest the antigen-loaded RBCs could lead to different outcomes, in terms of both antigen presentation and stimulation of an immune response (40–42). Phagocytosis triggers elaborate signals that might either induce tolerance or an immune response (9, 10). Splenocytes or peripheral blood mononuclear cells chemically modified with peptides have been explored as tolerogens, but these require the use of isogeneic cells (6, 43, 44). Chemical modification using carbodiimide- or maleimide-based coupling strategies shows considerable variation in conjugation efficiency, and modify surface proteins without necessarily leading to the formation of the desired adducts (43, 44). Using modifiers that target RBCs noncovalently, such as a module that recognizes glycophorin A, can lead to uneven distribution of the payload by dissociation (7). Cell types other than the intended phagocytes may acquire the antigen, leading to uncertain outcomes (9, 45, 46). When using nanoparticles/microparticles as a vehicle for the delivery of autoantigens (46–49), one must consider delivery to many different sites depending on size and other biophysical properties of these preparations.
In comparing our method to other means of tolerance induction, ours specifically addresses the issue of autoimmunity in a polyclonal setting using enzymatically modified RBCs. An advantage of using Kell-LPETGG RBCs lies in the amount of antigen that can be attached covalently to a well-defined target on the RBC surface, and in a reproducible and controlled manner. Rh-negative, blood group O RBCs could be stockpiled as a source of universal donor RBCs. Given the broad acceptance and safety profile of RBC transfusions, this antigen-specific tolerance strategy promises a lack of adverse effects. Furthermore, our approach offers the use of a wide breadth of antigens, because both natural and synthetic payloads can be attached simply by attaching the necessary sortase motifs. Antigen-decorated RBCs may thus provide a simple means to treat autoimmune disorders without compromising systemic immunity, and we suggest that such modified RBCs deserve further study as possible therapeutic agents. Nonetheless, the very existence of blood-group antigens, such as Kell, underscores the fact that RBCs are not always immunologically inert and that attempts at tolerance induction must be approached on a case-by-case basis.
Materials and Methods
Details of the mouse strains, RBC sortagging protocols, in vivo experimental set-up, and other methods (flow cytometry, ELISA, and Western blotting) are provided in the SI Materials and Methods. All mice were maintained according to protocols approved by the Massachusetts Institute of Technology Committee on Animal Care.
SI Materials and Methods
Mouse Strains.
C57BL/6J (CD45.2+), B6.SJL-Ptprc (CD45.1+), BALB/c, NOD/ShiltJ, C57BL/6 Rag2−/−, OT-I Rag2−/−, and OT-II Rag2−/− mice were purchased from the Jackson Laboratory or bred in the animal facility of the Whitehead Institute for Biomedical Research (Cambridge, MA). Generation of Kell-LPETGG and OB1 mice has been described elsewhere; these mice were crossed into C57BL/6 for six generations (16, 50). All mice were maintained according to protocols approved by the Massachusetts Institute of Technology Committee on Animal Care. The number of animals/group used to detect the efficacy of treatments is determined according to previous studies (48, 51). Furthermore, all animals used were included in the analyses.
Sortagging of Kell-LPETGG, C57BL/6J Rag2−/−, BALB/c, and Human RBCs.
All sortagging reactions were carried out using sortase A (Staphylococcus aureus) lacking the first 59 residues and containing the seven mutations described earlier that enhance activity and confer calcium independence: E105K/E108A/P94R/D160N/D165A/K190E/K196T (52, 53). This version of Sortase A was produced and purified as described elsewhere (11). All peptides used in the sortagging reactions (as described in Table S1) were produced by the Massachusetts Institute of Technology Koch Institute Biopolymer Core Facility through standard solid phase peptide synthesis. For sortagging, 200 μL RBCs were collected into heparinized tubes, washed once with PBS, and resuspended in ∼300 μL PBS or DMEM media. Next, a sortagging mixture of sortase A, LPETG (or GGG)-containing substrates, and 10× Sortase buffer (500 mM Tris⋅HCl pH7.5, 1.5 M NaCl) were added to the RBC suspension to a final concentration of 40 μM sortase A and 200 μM substrates. The reaction was carried out in a total volume of 500 μL. The RBC sortagging mixture was incubated for 30 min at 4 °C. Sortagged RBCs were then washed three times with PBS before transfusion or further analyses.
Flow Cytometry Analyses on Engineered RBCs.
RBCs were washed once with PBS and resuspended in FACS buffer [2 mM EDTA and 5% (vol/vol) FBS in PBS]. To detect the specificity of RBC surface labeling, the RBCs were stained with anti–CD45.2-APCeFluor780 (eBioscience, 47-0454-82), anti–TER119-APC (eBioscience, 17-5921-83), and anti–Biotin-PE (eBioscience, 12-9895-82) for 30 min at 4 °C. Samples were washed twice with FACS buffer before further analysis. For apoptosis assays, Annexin V staining was carried out according to the manufacturer’s protocol (Life Technologies, A35136). All flow data were acquired on a FACS Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Cytospin and Benzidine Staining.
Approximately 100,000 WT, Kell-LPETGG, and sortagged RBCs in PBS were centrifuged onto poly-l-lysine–coated slides for 5 min at 400 rpm (Thermo Shandon, Cytospin 3). Samples were air-dried, fixed in cold methanol for 15 min at room temperature, and stained with 3,3′-Diaminobenzidine (DAB) according to the manufacturer’s protocol (Sigma). Stained sections were imaged using a Zeiss Axioskop at 40× magnification.
Western Blotting for RBC Sortagging Quantification.
For Western blotting, 25 μL or 50 μL sortagged-RBCs were lysed with NH4Cl solution (Stemcell Technologies). Lysed RBC remnants were washed once with PBS and the membrane pelle- solubilized in 25 mM Tris·HCl (pH7.5), 0.5% Nonidet P-40, 5 mM MgCl2, 150 mM NaCl supplemented with a Complete Mini Protease Inhibitor mixture tablet (Roche). The soluble supernatant was collected and incubated with SDS sample buffer and boiled. Samples were resolved by SDS/PAGE and transferred onto PVDF membranes for immunoblotting against biotin, using streptavidin-HRP (GE Healthcare). The resulting blot intensity was then quantified using ImageJ software. Monobiotinylated GFP and nanobodies generated by means of sortagging were used as a reference for quantitation. In brief, a linear plot was generated from the reference protein intensities and the number of RBC proteins labeled was then derived by cross-referencing its intensity.
Transfusion and in Vivo Survival of Engineered RBCs.
After 200 μL RBCs were subjected to sortagging, they were washed twice in PBS and resuspended in PBS. Labeling of RBCs was carried out using 5 μM CFSE according to the manufacturer’s instructions (Life Technologies). RBCs were then washed, counted, and resuspended in sterile PBS for intravenous injection into C57BL/6J recipient mice. An equal volume of unmodified RBCs, similarly stained, were used as a control. Blood samples (∼20 μL) were collected by retro-orbital bleeding 1 h after transfusion at day 0 and every 3–4 d for 1 mo as indicated in the text. These blood samples were washed once with FACS buffer and then subjected to staining with anti–TER119-APC for 30 min on ice. Samples were washed twice with FACS buffer before analysis on a FACS Fortessa flow cytometer for CFSE and TER119 signals. Subsequent transfusions (second and third) were carried out in similar fashion, using the same cohort of recipient mice a week after the last bleeding. We also transfused a separate cohort of C57BL/6J recipient mice with 200 μL Kell-LPETGG RBCs that sortagged with OB1 peptide. Blood samples (∼25 μL) were collected for each time point as described in Fig. S2A. These samples were lysed and subjected to Western blotting as described above to identify biotinylated Kell proteins remaining from sortagged RBCs.
Immunoblotting for Antibody Response Against OVA.
OVA protein (Sigma, 0.2, 1, and 5 μg) was incubated with SDS sample buffer, boiled, resolved by SDS/PAGE, and transferred onto a PVDF membrane. The resulting membrane was blocked with 5% (wt/vol) BSA for 3 h at room temperature before overnight incubation with 1:3,000 dilution of serum samples (from C57BL/6J recipient mice treated as described above) in PBS-T (PBS plus 0.1% Tween20) at 4 °C. Membranes were washed with PBS-T 3 × 5 min with agitation and then incubated with goat anti-mouse IgG-HRP (SouthernBiotech) at 1:10,000 in PBS-T for 1 h at room temperature before developing with enhanced chemiluminescence substrate (Perkin-Elmer).
Repeated Transfusions of Engineered RBCs.
After 200 μL Kell-LPETGG RBCs were sortagged with OB1 peptide, RBCs were washed twice in 1× PBS and resuspended in PBS. RBCs were then washed again, counted, and resuspended in sterile PBS for intravenous injection into BALB/c recipient mice. An equal volume of unmodified RBCs served as a control. The two groups of experimental mice were further divided into two different subgroups: one group was injected intraperitoneally with 100 μL saline and the other was injected intraperitoneally with 50 μg PolyI:C (Sigma, P0913) and 25 μg anti-CD40 (SouthernBiotech, 1645-01). Subsequent boosts were carried out on day 21 and day 28. These three injections are the first round of transfusion and serum samples were collected 4 d after the last boost. The second round of transfusion was then carried out in similar fashion, starting 7 d after the first serum collection.
OVA-Specific ELISA.
For OVA-specific ELISA, 96-well plates were coated with 10 μg/mL OVA in PBS overnight at 4 °C and blocked in blocking buffer [0.05% Tween20 + 2% (wt/vol) BSA in PBS] before addition of 10-μL serum samples (from BALB/c mice experimentally treated as described above) in 90 μL PBS. Incubation with test serum was for 3 h at room temperature. Plates were washed four times with PBS, incubated with goat anti-mouse IgG-HRP (SouthernBiotech) at 1:10,000 in blocking buffer for 1 h, and developed with 3,3′,5,5′-Tetramethylbenzidine (TMB) liquid substrate reagent (Sigma). The reaction was stopped with 1 N HCl and absorbance was read at 450 nm. Serially diluted OB1-specific IgG1 antibody was used as the standard for comparison. OB1-specific IgG1 was purified from OB1 hybridoma supernatants using Protein G, eluted using 0.1 M glycine, pH 2.7, on 1:20 volume Tris⋅HCl, pH 8.5, to neutralize the pH and then dialyzed against PBS (54).
Flow Cytometry Analyses.
All flow data were acquired on a FACS Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). All FACS stainings for surface receptors were carried out in FACS buffer for 30 min on ice. The following is the list of antibodies used: anti–CD45.1-PeCy7 (eBioscience, 25-0453-82), anti–CD45.1-APC (BioLegend, 110714), anti–CD45.2-APC eFluor780 (eBioscience, 47-0454-82), anti-CD45.2-Alexa 700 (BioLegend, 109822), anti–CD19-APC (eBioscience, 17-0193-82), anti–CD3e-PE (eBioscience, 12-0031-83), anti–CD8α-eFluor450 (eBioscience, 48-0081-82), anti–CD4-eFluor450 (eBioscience, 48-0041-82), anti–CD4-APC (BioLegend, 100516), anti–CD62L-APC (eBioscience, 17-0621-83), anti–CD44-PE (eBioscience, 12-0441-83), anti–TNF-α–APC (eBioscience, 17-7321-82), anti–IFN-γ–PE (eBioscience, 12-7311-82), anti–IFN-γ–FITC (BioLegend, 505806), anti–PD1-PeCy7 (BioLegend, 109110), anti–TIM3-APC (BioLegend, 119706), anti–LAG3-PE (BioLegend, 125208), anti–Fas-PeCy7 (BioLegend, 305622), anti–Foxp3-PE (eBioscience, 12-5883-82), and anti–IL17-PE (BioLegend, 506904).
OB1 B-Cell Adoptive Transfer.
B cells from OB1 (CD45.2) mice were isolated by negative selection using CD43 Dynabeads, according to the manufacturer’s protocol (Life Technologies). Purity was ∼90%. OB1 B cells were then stained with 5 μM CFSE according to the manufacturer's instructions (Life Technologies), washed three times, counted and resuspended in sterile PBS. Five million CFSE-labeled OB1 cells were injected intravenously into congenically marked B6.SJL-Ptprc (CD45.1+) recipient mice. A day later, 200 μL Kell-LPETGG RBCs sortagged with OB1 peptide, 200 μL unmodified RBCs, or an equimolar amount of OVA were administered intravenously. At days 3, 7, and 28 postinjection, spleens were harvested and splenocytes suspensions were stained and evaluated by flow cytometry.
OT-I Adoptive Transfer and Challenge.
Splenic CD8+ T cells from OT-I Rag2−/− were enriched by negative selection using magnetic beads (Miltenyi Biotec, 130-095-236) and labeled with CFSE as described above. Next, 500,000 CD8+ T cells were transferred intravenously into congenically marked B6.SJL-Ptprc (CD45.1+) mice. Transfusions of 200 μL RBC, 200 μL RBC–OT-I, an equimolar amount of OVA protein, or an equimolar amount of OT-I peptide (InvivoGen) were carried out the day after adoptive transfer. At day 3, 6, or 9, mice were killed, spleens were collected, and splenocytes were subjected to flow cytometry. After adoptive transfer and transfusion, where indicated, mice were challenged on day 10 with 25 μg OT-I peptide in CFA (Sigma). Mice were killed 5 d later. Spleens were harvested and splenocytes were analyzed by flow cytometry as described above.
OT-II T-Cell Adoptive Transfer and Challenge.
Splenic CD4+ T cells from OT-II Rag2−/− were enriched by negative selection using magnetic beads (Miltenyi Biotec, 130-104-453) and labeled with CFSE as described above. Next, 500,000 CD4+ T cells were transferred intravenously into B6.SJL-Ptprc (CD45.1+) mice. Transfusion of 200 μL RBC, 200 μL RBC–OT-II, an equimolar amount of OVA protein, or an equimolar amount of OT-II peptide (InvivoGen) was carried out the day after adoptive transfer. At day 3 or 6, mice were killed and spleens were collected and splenocytes were analyzed by flow cytometry. After adoptive transfer and transfusion, mice were challenged on day 7 with 100 μg OVA protein in CFA. Mice were killed 3 d later. Spleens were harvested and splenocytes were analyzed by flow cytometry, as described above.
Intracellular Cytokine Staining.
Two million splenocytes were plated in 96-well round-bottomed plates and treated with Cell Stimulation Mixtures (eBioscience) and Brefeldin A (eBioscience) for 4 h at 37 °C in complete RPMI [RPMI 1640, 10% (vol/vol) heat-inactivated FBS, 50 μM β-mecaptoethanol, 100 U/mL Pen/Strep, 1× Gibco MEM NonEssential Amino Acids Solution (Life Technologies), 1 mM Sodium pyruvate, 1 mM Hepes]. Cells were then surface-stained, fixed, and permeabilized using BD Cytofix/Cytoperm (BD Biosciences), according to the manufacturer’s protocol. Intracellular staining was then performed according to the manufacturer’s protocols. Samples were then analyzed by flow cytometry.
Staining for Regulatory T Cells.
Freshly isolated splenocytes were first surface-stained, before Foxp3 intracellular staining protocols, using Foxp3/Transcription Factor Staining Buffer Set (eBioscience), performed according to the manufacturer’s protocols.
Active EAE Induction and Clinical Evaluation.
Female C57BL/6 mice (10–12 wk of age) were immunized with Hooke kits: an emulsion of MOG35–55 in CFA and pertussis toxin in PBS according to the manufacturer’s instructions (Hooke laboratories). Mice were scored daily, starting on day 10 postimmunization by an investigator blinded to the experimental treatment of individual mice. Mice were assigned to different experimental treatments randomly and housed together to eliminate intercage variability. All treatments were carried out on at least five mice and in at least two independent experiments, as indicated in the figure legends. All animals were included in the analyses. Clinical score is defined as follows: 1, limp tail; 2, partial hind leg paralysis; 3, complete hind leg paralysis; 4, complete hind and partial front leg paralysis; and 5, moribund. Easy access to wet food and water was provided for the experimental mice throughout the disease progression.
Prophylactic, Preclinical, and Therapeutic Treatment on EAE Mice.
For prophylactic treatment, 200 μL Kell-LPETGG RBCs sortagged with MOG35–55 or OVA323–339 peptides (RBC-MOG35–55 or RBC-OVA323–339, respectively), an equal volume of PBS, and an equimolar amount of MOG35–55 peptide were transfused 7 d before induction of EAE. For the preclinical treatment, transfusion of 200 μL RBC–MOG35–55 or RBC-OVA323–339 was carried out on day 5 after active EAE induction. Finally, for therapeutic treatment, 200 μL RBC-MOG35–55 or RBC-OVA323–339 were administered on the day of onset of EAE. Similarly, for the experiment in Fig. 6C, 200 μL native RBCs from C57BL/6J Rag2−/−, were subjected to sortagging with MOG35–55 or OVA323–339 peptides and the resulting sortagged RBCs were administered on the day of EAE onset. No animals were excluded from the analyses.
Spinal Cord Histology.
Mice were killed by CO2 inhalation and then perfused with 5 mM EDTA in PBS. Spinal cords were isolated and fixed in 10% (wt/vol) formalin solution (Sigma), embedded in paraffin, sectioned at 20 μm, and stained with H&E or Luxol Fast Blue (Massachusettes Institute of Technology Koch Institute Histology Core Facility). Stained sections were imaged using Zeiss Axioskop at 2.5× and 20× magnification.
Pancreas Histology and Immunohistochemistry.
To prepare formalin-fixed sections, freshly excised samples of pancreas tissue were fixed in 15 mL 10% (wt/vol) neutral buffered formalin solution (Sigma-Aldrich # HT5014) with gentle agitation for at least 24 h. After embedding the tissues in paraffin, 5-mm sections were prepared and stained with H&E following established protocols (55). Immunohistochemistry of formalin-fixed tissue was performed on a Leica Bond III autostaining platform using the Bond Polymer Refine Detection kit (Leica Biosystems). For CD8 staining, antigen retrieval was performed using Bond Epitope Retrieval Solution 1 (Leica Biosystems) for 30 min at 100 °C. CD4 staining antigen retrieval was performed using Bond Epitope Retrieval Solution 2 (Leica Biosystems) for 20 min at 100 °C. Slides were then incubated with anti-CD8 primary antibody (eBioscience, clone 4SM15) or anti-CD4 primary antibody (eBioscience, clone 4SM95), respectively, for 30 min at room temperature and detected with goat anti-rat IgG, HRP-conjugated (Millipore, #AP136A) and visualized with DAB and Gill’s Hematoxylin, components of the Bond Polymer Refine Detection Kit (Leica Biosystems). Antigen retrieval for insulin staining was carried out using Bond Epitope Retrieval Solution 1 (Leica Biosystems) for 30 min at 100 °C. Slides were then incubated with anti-Insulin antibody (Dako, #N1542) for 30 min at room temperature and directly detected with Bond Polymer Refine Detection Kit (Leica Biosystems).
Analyses of Th1 and Th17 Response of the Spinal Cord Infiltrates, Spleens, and Lymph Nodes.
Isolation of the immune cells that infiltrate the spinal cord was carried out by homogenizing the spinal cord, followed by 38% Percoll (Sigma) gradient separation (100% Percoll is 1.123 g/mL). Isolated cells were plated in 48-well plates and treated with 50 ng/mL phorbol myristate acetate/PMA (Sigma) and 500 ng/mL ionomycin (Sigma) for 2 h at 37 °C in complete RPMI media, followed by the addition of 10 μg/mL monensin (Sigma) and incubated for 2 more hours. Cells were then surface stained, fixed, and permeabilized using Foxp3/Transcription Factor Staining Buffer Set according to the manufacturer’s protocol. Intracellular and Foxp3 staining were performed according to the manufacturer’s protocols and cell samples were then used for flow cytometry. Single cell suspensions were prepared from spleens and lymph nodes. The resulting cells were incubated in vitro as above and analyzed as for the spinal cord infiltrates described above.
T1D Model and Blood Glucose Measurement.
Ten female NOD/ShiltJ mice at 8 wk of age were housed under specific-pathogen free conditions. At 10-wk of age, 200 μL unmodified RBC or RBC-InsB9–23 were transfused intravenously. Blood glucose measurements were carried out weekly, starting on week 10 by an investigator blinded to the experimental treatment of individual mice. Mice were assigned to different experimental treatments randomly and cohoused together to eliminate intercage variability. Mice were considered diabetic when their blood glucose level exceeded 260 mg/dL as measured by Bayer Contour Diabetes Meter/Glucose Test Strip. All animals were included in the analyses.
Statistical Methods.
All data are presented as mean ± SD and represent at least two independent experiments. All statistical analyses were performed using Prism 6. Statistically significant differences are indicated by asterisks as follows: *P < 0.05; **P < 0.01. Statistical methods used are indicated in the corresponding legend of each figure.
Acknowledgments
We thank members of the H.F.L. and H.L.P. laboratories, especially Lenka Kundrat and Jiahai Shi, for discussions; Jessica R. Ingram and Yushu Xie for critical reading of the manuscript; Marko Knoll and Leif S. Ludwig for providing reagents; John Jackson, Tony Chavarria, and Ferenc Reinhardt for mouse husbandry; Tom DiCesare for assistance with illustrations; and Prathapan Thiru and George W. Bell in Bioinformatics and Research Computing (Whitehead Institute) for assistance with statistical analyses. This work was supported by Defense Advanced Research Projects Agency Contract HR0011-12-2-0015 (to H.L.P. and H.F.L.) and grants from the Schlumberger Foundation Faculty for the Future, the Howard Hughes Medical Institute International Student Research Fellowship, and the Siebel Scholarship (to N.P.).
Footnotes
Conflict of interest statement: H.L.P. serves as a paid consultant and owns equity in Rubies, a company that seeks to apply modified red blood cells for treatment of disease. H.F.L. and H.L.P. serve as advisors and have equity in Rubius, a biotechnology company that seeks to exploit but does not provide financial support for the technology described in this paper.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701746114/-/DCSupplemental.
References
- 1.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435(7042):612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 2.Liblau RS, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci USA. 1996;93(7):3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7(9):665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 4.Luo X, Miller SD, Shea LD. Immune tolerance for autoimmune disease and cell transplantation. Annu Rev Biomed Eng. 2016;18:181–205. doi: 10.1146/annurev-bioeng-110315-020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cremel M, Guérin N, Horand F, Banz A, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013;443(1-2):39–49. doi: 10.1016/j.ijpharm.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Lutterotti A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: A phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(188):188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci USA. 2013;110(1):E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorentz KM, et al. Engineered binding to erythrocytes induces immunological tolerance to E. coli aspariganise. Sci Adv. 2015;1(6):e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith TS, Ferguson TA. Cell death in the maintenance and abrogation of tolerance: The five Ws of dying cells. Immunity. 2011;35(4):456–466. doi: 10.1016/j.immuni.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Sortagging: A versatile method for protein labeling. Nat Chem Biol. 2007;3(11):707–708. doi: 10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci USA. 2014;111(28):10131–10136. doi: 10.1073/pnas.1409861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strijbis K, Spooner E, Ploegh HL. Protein ligation in living cells using sortase. Traffic. 2012;13(6):780–789. doi: 10.1111/j.1600-0854.2012.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterry SJ, Kelly JM, Turner SJ, Carbone FR. T cell receptor V alpha bias can be determined by TCR-contact residues within an MHC-bound peptide. Immunol Cell Biol. 1995;73(1):89–94. doi: 10.1038/icb.1995.14. [DOI] [PubMed] [Google Scholar]
- 15.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 16.Dougan SK, et al. IgG1+ ovalbumin-specific B-cell transnuclear mice show class switch recombination in rare allelically included B cells. Proc Natl Acad Sci USA. 2012;109(34):13739–13744. doi: 10.1073/pnas.1210273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci Rep. 2015;5:15907. doi: 10.1038/srep15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181(6):3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino S, et al. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 21.Eisenbarth GS. Insulin autoimmunity: immunogenetics/immunopathogenesis of type 1A diabetes. Ann N Y Acad Sci. 2003;1005:109–118. doi: 10.1196/annals.1288.012. [DOI] [PubMed] [Google Scholar]
- 22.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121(16):3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giarratana MC, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118(19):5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swee LK, Lourido S, Bell GW, Ingram JR, Ploegh HL. One-step enzymatic modification of the cell surface redirects cellular cytotoxicity and parasite tropism. ACS Chem Biol. 2015;10(2):460–465. doi: 10.1021/cb500462t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz PJ, et al. Suppressive immunization with DNA encoding a self-peptide prevents autoimmune disease: Modulation of T cell costimulation. J Immunol. 1999;162(6):3336–3341. [PubMed] [Google Scholar]
- 27.Garren H, et al. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity. 2001;15(1):15–22. doi: 10.1016/s1074-7613(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y, et al. Treg cell resistance to apoptosis in DNA vaccination for experimental autoimmune encephalomyelitis treatment. PLoS One. 2012;7(11):e49994. doi: 10.1371/journal.pone.0049994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrera F, et al. Gene vaccination for the induction of immune tolerance. Ann N Y Acad Sci. 2007;1110:99–111. doi: 10.1196/annals.1423.012. [DOI] [PubMed] [Google Scholar]
- 30.Fissolo N, et al. Treatment with MOG-DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up-regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:139. doi: 10.1186/1742-2094-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker D, Hankey DJ. Gene therapy in autoimmune, demyelinating disease of the central nervous system. Gene Ther. 2003;10(10):844–853. doi: 10.1038/sj.gt.3302025. [DOI] [PubMed] [Google Scholar]
- 32.Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191(6):2938–2947. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- 33.Wadwa M, Klopfleisch R, Buer J, Westendorf AM. Targeting antigens to Dec-205 on dendritic cells induces immune protection in experimental colitis in mice. Eur J Microbiol Immunol (Bp) 2016;6(1):1–8. doi: 10.1556/1886.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aichele P, et al. Peptide-induced T-cell tolerance to prevent autoimmune diabetes in a transgenic mouse model. Proc Natl Acad Sci USA. 1994;91(2):444–448. doi: 10.1073/pnas.91.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.al-Sabbagh A, Miller A, Santos LM, Weiner HL. Antigen-driven tissue-specific suppression following oral tolerance: Orally administered myelin basic protein suppresses proteolipid protein-induced experimental autoimmune encephalomyelitis in the SJL mouse. Eur J Immunol. 1994;24(9):2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 36.Park MJ, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+CD25+ regulatory T cells in Peyer’s patches in an orally tolerized, collagen-induced arthritis mouse model. Arthritis Res Ther. 2008;10(1):R11. doi: 10.1186/ar2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer 1. Proc Natl Acad Sci USA. 1999;96(7):3842–3847. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood. 2015;125(15):2418–2427. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci. 2004;1029:211–224. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- 40.Banz A, Cremel M, Rembert A, Godfrin Y. In situ targeting of dendritic cells by antigen-loaded red blood cells: A novel approach to cancer immunotherapy. Vaccine. 2010;28(17):2965–2972. doi: 10.1016/j.vaccine.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Calabro S, et al. Bridging channel dendritic cells induce immunity to transfused red blood cells. J Exp Med. 2016;213(6):887–896. doi: 10.1084/jem.20151720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards AL, Hendrickson JE, Zimring JC, Hudson KE. Erythrophagocytosis by plasmacytoid dendritic cells and monocytes is enhanced during inflammation. Transfusion. 2016;56(4):905–916. doi: 10.1111/trf.13497. [DOI] [PubMed] [Google Scholar]
- 43.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(4):2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 44.Getts DR, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187(5):2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyake Y, et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117(8):2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy CL, et al. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J Immunol. 2013;191(10):5278–5290. doi: 10.4049/jimmunol.1203131. [DOI] [PubMed] [Google Scholar]
- 47.Getts DR, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30(12):1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappellano G, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine. 2014;32(43):5681–5689. doi: 10.1016/j.vaccine.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Hunter Z, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8(3):2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruyama T, et al. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33(5):538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akbarpour M, et al. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci Transl Med. 2015;7(289):289ra81. doi: 10.1126/scitranslmed.aaa3032. [DOI] [PubMed] [Google Scholar]
- 52.Chen I, Dorr BM, Liu DR. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc Natl Acad Sci USA. 2011;108(28):11399–11404. doi: 10.1073/pnas.1101046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirakawa H, Ishikawa S, Nagamune T. Design of Ca2+-independent Staphylococcus aureus sortase A mutants. Biotechnol Bioeng. 2012;109(12):2955–2961. doi: 10.1002/bit.24585. [DOI] [PubMed] [Google Scholar]
- 54.Avalos AM, et al. Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J Exp Med. 2014;211(2):365–379. doi: 10.1084/jem.20131603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikel UV, editor. Advanced Laboratory Methods in Histology and Pathology. Armed Forces Institute of Pathology, American Registry of Pathology; Washington, DC: 1994. [Google Scholar]