Significance
Higher plants are built from three major tissue types: epidermis, ground tissue, and vascular tissue. Each of these differentiates into several functionally distinct cell types. Although identity switches for the different cell types within the major three tissues have been identified, mechanisms that trigger the initiation of the three tissues themselves have remained obscure. Auxin response, in particular the auxin-dependent transcription factor MONOPTEROS (MP), plays a critical role in Arabidopsis embryonic root initiation. In our study, we identify a set of embryonic MP target genes and show that MP acts as a very first regulator of ground tissue initiation. Moreover, our data provide a framework for the simultaneous formation of multiple cell types by the same transcriptional regulator.
Keywords: plant development, embryogenesis, pattern formation, ground tissue, auxin
Abstract
Plant organs are typically organized into three main tissue layers. The middle ground tissue layer comprises the majority of the plant body and serves a wide range of functions, including photosynthesis, selective nutrient uptake and storage, and gravity sensing. Ground tissue patterning and maintenance in Arabidopsis are controlled by a well-established gene network revolving around the key regulator SHORT-ROOT (SHR). In contrast, it is completely unknown how ground tissue identity is first specified from totipotent precursor cells in the embryo. The plant signaling molecule auxin, acting through AUXIN RESPONSE FACTOR (ARF) transcription factors, is critical for embryo patterning. The auxin effector ARF5/MONOPTEROS (MP) acts both cell-autonomously and noncell-autonomously to control embryonic vascular tissue formation and root initiation, respectively. Here we show that auxin response and ARF activity cell-autonomously control the asymmetric division of the first ground tissue cells. By identifying embryonic target genes, we show that MP transcriptionally initiates the ground tissue lineage and acts upstream of the regulatory network that controls ground tissue patterning and maintenance. Strikingly, whereas the SHR network depends on MP, this MP function is, at least in part, SHR independent. Our study therefore identifies auxin response as a regulator of ground tissue specification in the embryonic root, and reveals that ground tissue initiation and maintenance use different regulators and mechanisms. Moreover, our data provide a framework for the simultaneous formation of multiple cell types by the same transcriptional regulator.
Higher plants are built from three major tissue types: epidermis, ground tissue, and vascular tissue. The ground tissue is the basis for all photosynthetic cells in flowering plants. In addition, it provides a selective barrier for nutrients and acts as a major storage tissue in many plants (1–3). In Arabidopsis, an elaborate regulatory network has been established for the asymmetric divisions within the ground tissue that give rise to the two ground tissue cell types in the root: endodermis and cortex (4–10). This network revolves around the central transcriptional regulator SHORT-ROOT (SHR) that moves from the stele into the ground tissue where it is required in the nucleus to maintain endodermis identity and promote asymmetric division in the daughter cells of the ground tissue stem cells to generate separate endodermis and cortex layers (4–10). The nuclear retention of SHR depends on the activity of SCARECROW (SCR) and the BIRD family of transcription factors that are required to maintain ground tissue identity postembryonically (4, 6, 8–10). In addition, SCR and the heat shock transcription factor SCHIZORIZA (SCZ) regulate asymmetric cell divisions within the ground tissue (11, 12). Open questions, however, are what molecular mechanisms drive establishment of the ground tissue and how this is connected to the regulatory network that controls ground tissue maintenance (13).
The establishment of the ground tissue and the initiation of the root meristem occur at the globular stage of embryogenesis, when the three main tissue identities, and the precursor cell of the organizing center of the root, the hypophysis, are specified from uncommitted precursor cells during a few cell division rounds (13) (Fig. 1A). Our earlier work established a critical role for auxin response, in particular the auxin-dependent and DNA-binding transcription factor AUXIN RESPONSE FACTOR5 (ARF5)/MONOPTEROS (MP), in embryonic root initiation (14). The MP gene is required for root formation at this stage, as evidenced by defects in otherwise stereotypical cell divisions of the first vascular cells and the hypophyseal cell at this stage, and absence of a primary root in the mp mutant (15, 16).
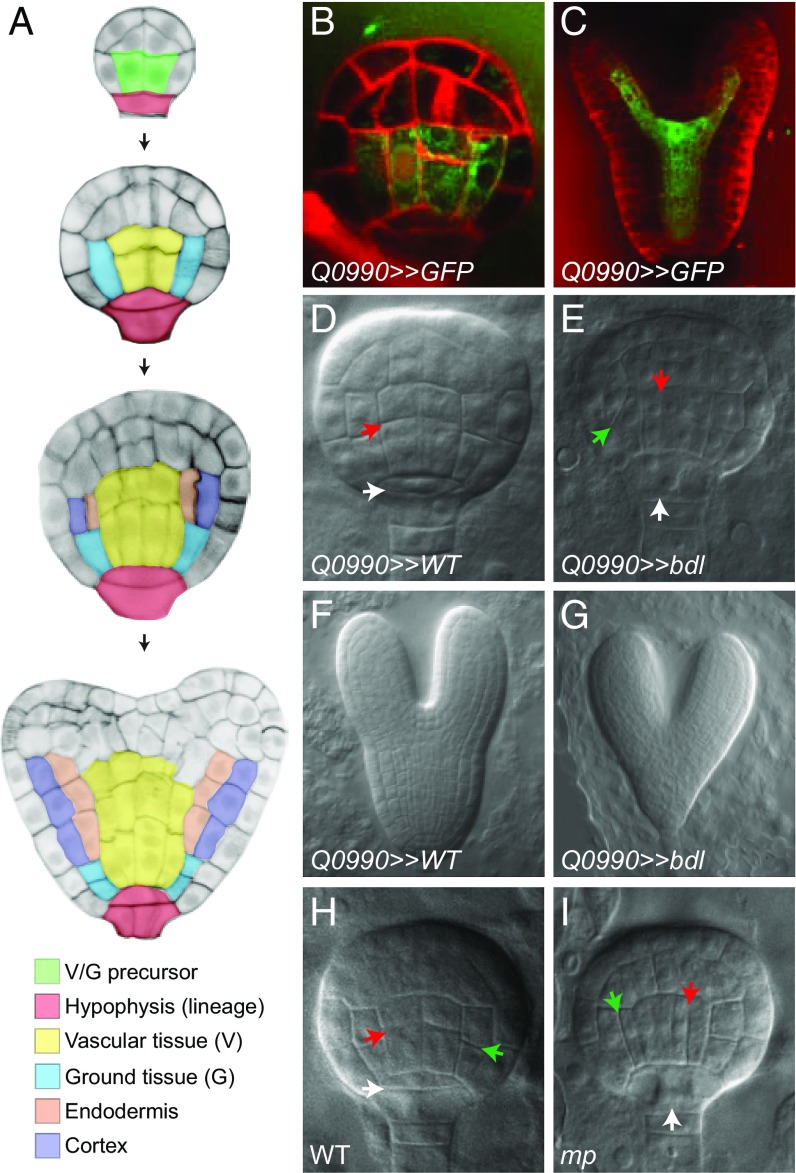
Fig. 1.
MP controls asymmetric division of the first embryogenic ground tissue cells. (A) Tissue initiation during early Arabidopsis embryogenesis. The 16-cell, globular, transition, and heart stages are shown from Top to Bottom. Central basal cells (green) divide to generate the first vascular (yellow) and ground tissue (blue) cells. These first ground tissue cells divide again to generate endodermis (orange) and cortex (purple) cell types. The extraembryonic suspensor cell adjacent to the embryo is specified as hypophysis (red) and divides asymmetrically to generate root precursors. (B and C) Expression of the Q0990 enhancer trap (in green) in the globular- (B) and heart-stage (C) embryos. Membranes are counterstained by Renaissance RS2200 (red signal). (D–G) Phenotypes of F1 embryos derived from crosses between Q0990 and wild type (D and F) or Q0990 and UAS::bdl (E and G) at globular- (E and F) and heart- (F and G) stages. (H and I) Phenotypes of globular-stage wild-type (H) and mp mutant (I) embryos. Ground tissue divisions are indicated by green arrowheads, vascular divisions by red arrowheads, and hypophysis division by white arrowheads.
So far, only a handful of MP target genes involved in embryonic root initiation have been identified, mostly by inference from postembryonic gene regulation. Nonetheless, we have demonstrated the role of several MP target genes in hypophysis specification (17) and vascular tissue establishment (18, 19) through transcriptome profiling of mp seedlings, as well as seedlings in which ARF activity was inhibited by an inducible version of the dominant mutant ARF inhibitor BODENLOS/IAA12 (BDL) (20, 21). The BDL transcriptional repressor is normally degraded in response to auxin, resulting in ARF-dependent gene expression (22), but as the bdl mutation prevents degradation (21), this version acts as a dominant ARF inhibitor (21). MP function in vascular tissue development is mediated by its target TARGET OF MONOPTEROS 5 (TMO5) (17), whereas MP activity in root initiation is mediated by its target gene TMO7 that encodes a small protein that moves to the neighboring hypophyseal cell to control its division (17).
However, as this previous transcriptome profiling was performed postembryonically, many target genes regulated during root initiation in the embryo may have been missed. In this study, we identify a set of embryonic MP target genes and show that auxin response—and MP—acts as a very first cell-autonomous regulator of ground tissue initiation upstream of the known regulatory network controlling ground tissue maintenance. Strikingly, whereas the SHR network depends on MP, this MP function is, at least in part, SHR independent. Our study therefore reveals that ground tissue initiation and maintenance use different regulators and mechanisms. Moreover, our data provide a framework for the simultaneous formation of multiple cell types by the same transcriptional regulator.
Results
MP Controls Asymmetric Division in the First Embryonic Ground Tissue Cells.
We designed a strategy to identify embryonic MP target genes through local inhibition of MP activity in the Arabidopsis embryo. MP mRNA and protein are initially broadly expressed in the embryo but are absent from the extraembryonic hypophysis. Later, MP expression expands to the hypophysis daughter cells and becomes more restricted to the vascular cells at the heart stage (14, 17, 21). Despite its broad expression pattern, local MP activity in the first vascular and ground tissue cells of the globular-stage embryo (Fig. 1A) alone restores root formation in the mp mutant (14). Furthermore, expression of bdl from the GAL4 driver Q0990 (23) that was expressed specifically in the first putative vascular and ground tissue cells at the globular stage and its daughters (Fig. 1 B and C) (14, 24) induced a perfect phenocopy of the mp mutant (14). The mutant bdl protein cannot be degraded (21) and thus acts as a dominant ARF inhibitor (21) that potentially represses all ARF activity. However, so far, no other ARFs have been shown to be involved in embryonic root meristem formation, suggesting that local bdl misexpression will mainly affect genes normally regulated by MP. We thus locally inhibited MP activity in the putative first vascular and ground tissue cells via GAL4–UAS-based (23) expression of a mutant bdl protein to identify embryonic MP target genes. MP controls the stereotypic division of the first vascular cells and hypophyseal cell in the globular-stage embryo (15, 18). We crossed UAS::bdl plants with Q0990 plants (Q0990>>bdl) and observed that globular-stage embryos showed vascular and hypophysis division defects after 3 d (Fig. 1 D and E). After 6 d, heart-stage embryos showed a completely disorganized embryonic root meristem (Fig. 1 F and G), identical to mp mutants (15, 16).
Surprisingly, in Q0990>>bdl globular-stage embryos, we also observed division defects in the first ground tissue cells (Fig. 1E). Therefore, we reexamined mp mutant embryos. During normal embryo development, the first vascular and ground tissue cells divide anticlinally to produce daughter cells for these tissues that will be incorporated into the root (Fig. 1A). We examined early mp mutant embryos that were recognizable through aberrant hypophysis division and frequent aberrant division of the first vascular cells (Fig. 1 H and I) (18). Remarkably, although this aspect of the mp mutant phenotype has remained unnoticed despite ongoing investigation of the mp mutant since its isolation 25 y ago, we observed abnormally oriented division planes in the first ground tissue cells in ∼50% of mp mutant embryos, in two independent mp alleles (46.9% division defects in at least one of the two cells in median view, n = 49 embryos for mpB4149; 57.1% division defects, n = 49 for mpS319; 0% division defects in Col-0, n = 57; 1.9% defects in Utrecht ecotype, n = 53; mpB4149 is in Utrecht background). In most cases, the first ground tissue cells divided periclinally instead of anticlinally (Fig. 1 H and I), but oblique divisions were also observed. These data indicate that MP controls the earliest asymmetric division in embryonic ground tissue that generates the ground tissue daughter cells (Fig. 1A). These daughter cells in turn will engage in another asymmetric division that gives rise to the two ground tissue cell types in the root: the endodermis and cortex (Fig. 1A). Thus, auxin response and MP are involved in the earliest asymmetric division of embryonic ground tissue cells.
Identification of Embryonic MP Targets.
We next asked whether MP might promote gene expression specifically in the first embryonic ground tissue cells of the globular-stage embryo. The strong pleiotropic effect of the mp mutation (15–18) creates a very brief window during early embryogenesis after MP activation and before visible phenotype occurrence, during which transcriptional targets can be identified. However, given that the homozygous mp mutant is sterile, and that mutant embryos are thus surrounded by wild-type seed and fruit tissues, isolation of embryos will be required to detect the effect of mp mutation on gene expression. Furthermore, ubiquitous MP expression, connected to multiple functions in the globular-stage embryo (14, 17, 21), pose challenges to finding gene expression changes that are related to individual MP functions. We therefore adopted an elaborate strategy to locally inhibit MP and identify only embryonic MP target genes. We locally inhibited MP activity specifically in the first embryonic vascular and ground tissue cells via expression of the mutant bdl protein from the GAL4 driver Q0990 (Fig. 1 B–E). For transcriptome profiling, we manually dissected globular-stage (3 d) and heart-stage (6 d) embryos from ovules (25) from Q0990 × UAS::bdl crosses and included Q0990 × wild type as a control. From four biological replicates each, RNA was processed and hybridized to Arabidopsis 70-mer oligo arrays as previously described (25). Initial analysis confirmed that BDL expression was ∼2.6-fold up-regulated in both globular- and heart-stage embryos (Fig. 2A). Therefore, we performed statistical analysis for differential expression and selected genes based on an arbitrary threshold of a twofold change in gene expression and significance at q ≤ 0.05 (Student’s t test; false discovery rate corrected for multiple testing). This analysis identified 145 down-regulated genes and 412 up-regulated genes at the globular stage, and 382 down-regulated genes and 147 up-regulated genes at the heart stage (Dataset S1; available at NCBI Gene Expression Omnibus; https://www.ncbi.nlm.nih.gov/geo/; accession no. GSE78695). We aimed to identify novel MP target genes involved in the earliest events of embryonic root meristem initiation in the globular-stage embryo. As most MP target genes are expected to be activated by MP (17, 26–29), we focused our analysis on the 145 down-regulated genes in the globular-stage embryo (SI Appendix, Table S1). Among these genes, ∼25% are transcription factors (36 of 145 genes) and 27 were also down-regulated in heart-stage embryos (Fig. 2B). This relatively low overlap in gene expression between globular- and heart-stage embryos reflects the strong pleiotropic effect of the mp mutation on the heart-stage embryo phenotype and gene expression, and hinders the identification of transcriptional MP targets at this stage. As anticipated, we identified several genes previously shown to be auxin dependent (Fig. 2A). Strikingly, the most down-regulated gene was the previously identified MP target TMO7, and TMO5-LIKE2 and TMO6-LIKE2 were also among the down-regulated genes (Fig. 2A). In addition, we observed strong down-regulation of the hypophysis-expressed WUSCHEL RELATED HOMEOBOX 5 (WOX5) gene (30) (Fig. 2A), indicating that MP inhibition in the inner basal embryo cells also results in noncell-autonomous effects on gene expression in the hypophysis.
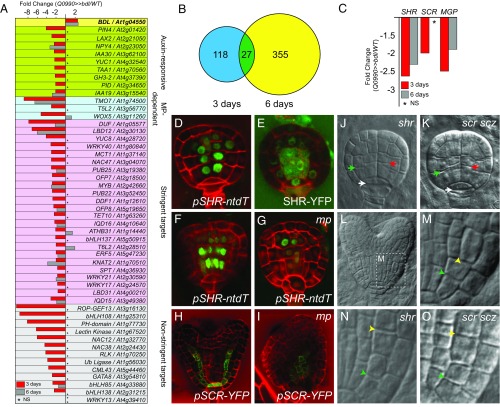
Fig. 2.
MP controls ground tissue initiation upstream and independently of SHR. (A) Differential gene expression (in fold change Q0990>>bdl/Q0990>>WT) in Q0990>>bdl embryos at 3 d (red columns) and 6 d (gray columns) after pollination. Genes are grouped by category (known auxin responsive; known MP dependent; and novel targets either at high or low stringency). NS, not significant. (B) Venn diagram of genes significantly (fold change < -2; P value <0.05) down-regulated in Q0990>>bdl embryos relative to Q0990>>WT embryos isolated 3 or 6 d after pollination. (C) Differential expression of SHR, SCR, and MGP in microarray analysis of Q0990>>bdl embryos at 3 d (red) and 6 d (gray) after pollination. NS, not significant. (D) Expression of pSHR-ntdTomato (green) in a wild-type globular-stage embryo. (E) SHR-YFP protein localization (green) in a wild-type globular-stage embryo. (F and G) Expression of pSHR-ntdTomato (green) in wild-type (F) and mp mutant (G) late globular-stage embryos. (H and I) Expression of pSCR-YFP (green) in wild-type (H) and mp mutant (I) heart-stage embryos. Images in F–I were taken at identical settings. (J and K) Globular-stage shr (J) and scr scz (K) embryos. Ground tissue divisions are indicated by green arrowheads, vascular divisions by red arrowheads, and hypophysis division by white arrowheads. (L) Wild-type heart-stage embryo indicating the area magnified in M that encompasses early ground tissue divisions. (M–O) Ground tissue divisions in wild-type (M), shr (N), and scr scz (O) heart-stage embryos. Initial ground tissue division is indicated by green arrowhead and subsequent division by a yellow arrowhead.
MP Transcriptionally Controls the Regulatory Network for Ground Tissue Maintenance.
Because our transcriptome dataset (Dataset S1 and Fig. 2A) shows differential expression of the few known embryonic MP target genes, we analyzed the expression of known ground tissue regulators in our microarray data. We found that SHR expression was significantly down-regulated (Fig. 2C). Moreover, the direct SHR target SCARECROW (SCR) (5, 7, 31) and the direct SHR target and BIRD family gene MAGPIE (MGP) (31) were also down-regulated already in globular-stage embryos (Fig. 2C). This finding suggests that MP is required to activate SHR expression in the early embryo. Indeed, SHR is expressed in the first vascular cells of globular-stage embryos (Fig. 2D), and SHR protein moved into the first ground tissue cells and hypophyseal cell of globular-stage embryos (Fig. 2E). We analyzed SHR and SCR expression in mp mutant embryos and observed severely decreased expression of both genes in the basal embryo domain (Fig. 2 F–I) (n = 15 for SHR, n = 18 for SCR). In contrast, SHR expression was still detected in the apical embryo domain (Fig. 2G). These results indicate that MP activity is locally required for SHR and SCR expression in the embryonic root meristem and acts upstream of these well-known regulators of ground tissue patterning.
The SHR Network Is Dispensable for Ground Tissue Initiation.
SHR is required to pattern the ground tissue and maintain ground tissue identity postembryonically (4, 5, 7, 31, 32). So far, a role for SHR in ground tissue establishment has not been reported, but it is unknown whether SHR is not involved in this process or whether the globular-stage-embryo phenotype of the shr mutant has not been investigated in sufficient detail to detect a specific defect in the first division. Therefore, we analyzed whether the first ground tissue division was affected in shr-2 mutant embryos. We found these cells to divide normally in globular-stage shr-2 mutant embryos (2.4% division defects, n = 41) (Fig. 2J). Previously, a double mutant between scr and a mutation in the SCHIZORIZA gene, also involved in SHR-dependent asymmetric divisions in the root meristem and embryonic ground tissue (11, 12), was shown to not form ground tissue stem cells (11). We therefore also included the scr scz double mutant in our analysis and found the first ground tissue divisions to be normal (Fig. 2K). Both shr (as shown previously in ref. 33) and scr scz mutants displayed a defect in the later, periclinal division of the daughter cells of the first ground tissue cells, which gives rise to endodermis and cortex in wild type (Fig. 2 L–O). These results suggest that either SHR acts at a later step in ground tissue development, or alternatively, SHR action in ground tissue establishment might be masked due to functional redundancy with other GRAS family transcription factors (34–37). We investigated the expression pattern of other SHR/SCR-related GRAS family genes that were down-regulated at the globular stage in our microarray (SI Appendix, Fig. S1). The much broader expression domains of these genes (SI Appendix, Fig. S1) suggest that they may not have a function specific to ground tissue formation. In addition, higher order mutant combinations within the BIRD family were recently shown to develop embryonic ground tissue, although the activity of the BIRDs is crucial to maintain ground tissue identity postembryonically (10). Therefore, ground tissue network genes appear not to be involved in the establishment of the ground tissue.
MP Transcriptionally Initiates the Embryonic Ground Tissue.
Our results suggest that in contrast to SHR network mutants, MP is involved in the establishment of the ground tissue. To determine whether such a role is reflected by the MP-dependent embryonic transcriptome, we investigated the expression pattern of 37 down-regulated genes that were not previously reported to have a role in embryo development (SI Appendix, Table S2 and Dataset S1). We generated transcriptional reporters, consisting of a 2-kb fragment upstream of the ATG start codon, and a sensitive nuclear triple GFP reporter (38), and performed expression analysis in the embryo and postembryonic root. Our data reveal gene expression patterns reflecting the known roles of auxin response in vascular tissue establishment (two genes), root initiation (three genes), and hypophysis formation (four genes) (Fig. 3 A–F, SI Appendix, Fig. S2 and Table S2, and Dataset S1). The expression of many of these genes was strongly decreased in mp mutant embryos (SI Appendix, Fig. S3 and Dataset S1). Therefore, our approach identified several known and many previously unidentified ARF-regulated genes in the early embryo, many of which likely are output of MP activity.
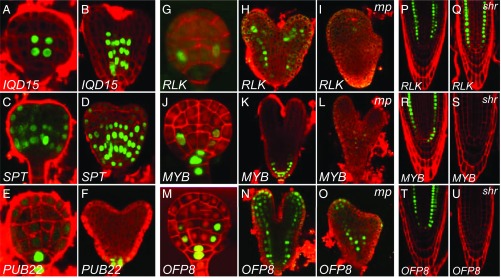
Fig. 3.
MP transcriptionally initiates the embryonic ground tissue in a SHR-independent manner. (A–F) Expression of promoter-n3GFP reporters for IQD15/At3g49380 (A and B), SPT/At4g36930 (C and D), and PUB22/At3g52450 (E and F) genes. Images in A–F show expression in globular and heart stage. (G–O) Expression of promoter-n3GFP reporters for RLK/At1g70250 (G–I), MYB/At2g42660 (J–L), and OFP8/At5g19650 (M–O) genes. Images in G, J, and M show expression in globular stage. Images in H, I, K, L, N, and O show expression in wild-type (H, K, and N) and mp mutant embryos (I, L, and O) of comparable age and were taken at identical imaging settings. (P–U) Expression of promoter-n3GFP reporters for RLK (P and Q), MYB (R and S), and OFP8 (T and U) in wild-type (P, R, and T) and shr mutant (Q, S, and U) root tips. Red counterstaining in A–F, H, I, K, L, and N–U is FM4-64 and in G, J, and M it is Renaissance RS2200.
In addition, we observed three genes that were expressed in the ground tissue of the embryonic root. A thaumatin-like Receptor-Like Kinase (RLK; At1g70250) was expressed specifically in the first ground tissue cells of the globular-stage embryo and retained expression in all ground tissue cells of the root throughout embryo (Fig. 3 G and H) and postembryonic development (Fig. 3P). A MYB domain-like gene of the SHAQKYF class (here referred to as MYB; At2g42660) showed a similar embryo expression pattern, but was additionally expressed in both hypophysis daughter cells (Fig. 3 J and K). In the postembryonic root, MYB was expressed in the QC and cortex layer (Fig. 3R). Finally, OVATE FAMILY PROTEIN 8 (OFP8; At5g19650) expression was initially observed in the hypophysis, whereas the earliest ground tissue expression was detected in the daughter cells of the first ground tissue cells in the globular-stage embryo (Fig. 3M). In the postembryonic root, OFP8 was expressed in the QC and both ground tissue layers (Fig. 3T).
We next tested whether these genes are regulated by MP. The bdl inhibitor can inhibit ARFs other than MP (39), and it is therefore possible that differential gene expression upon local bdl expression is caused by inhibition of other ARFs, coexpressed with MP (40). To test whether these genes represent MP output, we analyzed the expression of these genes in mp mutant embryos. MP was required for normal expression of both RLK (Fig. 3 H and I and SI Appendix, Fig. S4; n = 12) and MYB (Fig. 3 K and L and SI Appendix, Fig. S4; n = 16). In contrast, the OFP8 gene that in wild type is expressed in a broader domain, was not down-regulated in mp mutant embryos (Fig. 3 N and O and SI Appendix, Fig. S4; n = 14). These results demonstrate that the first dedicated ground tissue cells have a distinct transcriptional program, and reveal auxin response, in particular MP, as a critical regulator of this program.
MP Controls Ground Tissue Initiation in a SHR-Independent Manner.
The MP-dependent gene expression in the first ground tissue cells and the division defects in the first ground tissue cells in the mp mutant strongly suggest that MP activity is required to specify the first embryonic ground tissue cells. The loss of SHR and SCR gene expression in the basal embryo domain of the mp mutant suggests that MP acts before and upstream of SHR in this process, and the shr mutant suggests that MP acts independently of SHR to specify the first ground tissue cells. To further test whether the specification of the first ground tissue cells requires SHR activity, we introduced the MP-dependent ground tissue reporters into the shr mutant background. We observed strong RLK expression in the root meristem of the shr mutant (Fig. 3 P and Q; n = 24). In contrast, expression of the MYB and OFP8 reporters was lost in shr roots (Fig. 3 R–U; n = 24 for both reporters). We conclude that, whereas some of its activity is mediated by downstream SHR action, MP activates ground tissue-specific gene expression and ground tissue initiation, at least in part independently of SHR.
ARF Activity Acts Cell-Autonomously to Specify the First Ground Tissue Cells.
Whereas ground tissue patterning and maintenance require intercellular transport of SHR (4, 8, 32), MP acts both cell-autonomously and noncell-autonomously in the early embryo to specify distinct cell types (17, 18). A critical question is whether the role for MP in ground tissue initiation is a cell-autonomous output, or rather follows from its activity in vascular cells, such as is the case for hypophysis specification (14, 17). To first determine whether ground tissue cells accumulate auxin and feature a transcriptional response, we made use of two reporters with improved sensitivity to measure auxin response and auxin accumulation (41). The R2D2 reporter consists of auxin-degradable (DII) and auxin nondegradable (mDII) fluorescent proteins, whose ratio is a proxy for the level of auxin. Using the R2D2 reporter, we observed auxin accumulation in the first vascular cells as well as in the first ground tissue cells and the protoderm, hypophysis, and suspensor (Fig. 4A). Quantification of the mDII/DII ratio (n = 14 embryos) showed that auxin activity is lowest in protoderm cells, increased in ground tissue, and highest in vascular cells (Fig. 4B). The auxin accumulation detected in ground tissue cells also induces gene expression, as the generic ARF-dependent DR5v2 reporter (41) not only detected an auxin response in the first vascular cells, hypophysis, and suspensor, but also in the first ground tissue cells (42) (Fig. 4 C and D).
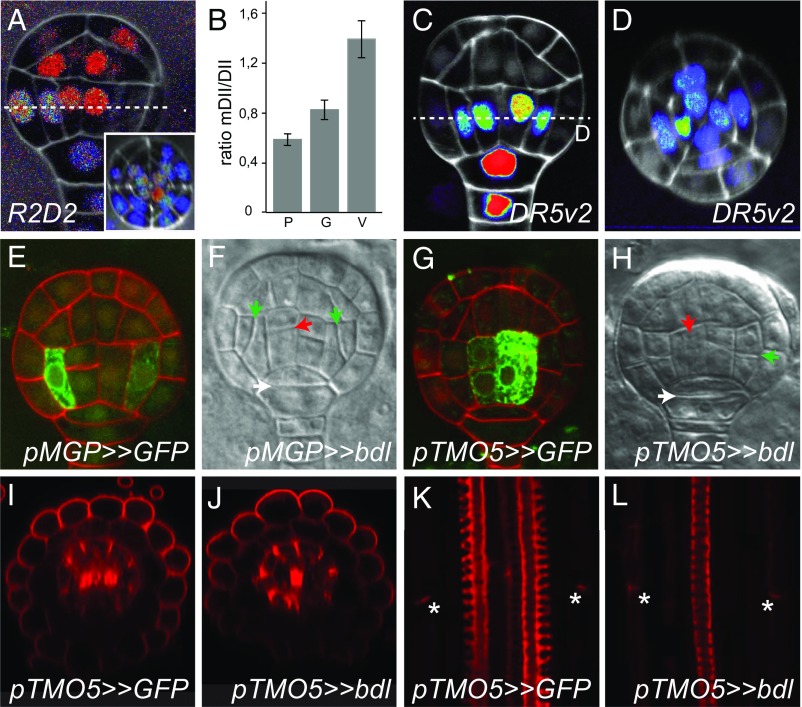
Fig. 4.
Auxin response cell-autonomously controls ground and vascular tissue initiation. (A) Ratio of DII-3xYFP and mDII-tdTomato proteins in globular-stage R2D2 embryo in longitudinal (A) or transverse (Inset in A) cross-section. Ratio (tdTomato/YFP) is shown on a false color scale with red indicating high and blue, low ratio. (B) Quantification of the mDII/DII fluorescence intensity ratio in nuclei of protoderm (P), ground tissue (G), and vascular tissue (V) cells in globular-stage embryos (n = 14 embryos; error bars are SEM). (C and D) Expression of DR5v2-ntdTomato in globular-stage embryo in longitudinal (C) and transverse (D) cross-sections. False color scale is red for high fluorescence signal and blue for low signal. White counterstain is Renaissance RS2200 signal. (E) Expression of GFP in a globular-stage embryo of the pMGP>>GFP driver line. (F) Divisions in a globular-stage pMGP>>bdl F1 embryo. (G) Expression of GFP in a globular-stage embryo of the pTMO5>>GFP driver line. (H) Divisions in a globular-stage pTMO5>>bdl F1 embryo. Red counterstaining in E and G is Renaissance RS2200 signal. Ground tissue divisions are indicated by green arrowheads, vascular divisions by red arrowheads, and hypophysis division by white arrowheads in F and H. (I and J) Radial and (K and L) longitudinal cross-sections of basic fuchsin-stained pTMO5>>GFP (I and K) and pTMO5>>bdl (J and L) roots. Intense central signal in I and J represents protoxylem, and patches surrounding the vasculature represent Casparian strips. Lignified Casparian strips are indicated by asterisks in K and L.
We next locally inhibited ARF activity specifically in the globular-stage embryo using the GAL4–UAS-based transactivation system driven by early tissue-specific promoters. To drive bdl expression in the early ground tissue, we generated a GAL4 driver line with the MGP promoter that is expressed specifically in the first ground tissue cells (Fig. 4E and SI Appendix, Fig. S5) (8). Consistent with a cell-autonomous role of ARFs in the first ground tissue cells, pMGP>>bdl showed striking oblique division defects (85% of embryos showing defective division in at least one of the two cells in median view; n = 68 embryos; Fig. 4F and SI Appendix, Fig. S5), very similar to those observed in Q0990>>bdl embryos (Fig. 1E) and in the mp mutant (Fig. 1I). Importantly, neither vascular nor hypophysis cell divisions were affected in pMGP>>bdl embryos (vascular cells: 0%, n = 68; hypophysis: 1.5%; n = 68; Fig. 4F). Later during embryogenesis, the MGP promoter was expressed more broadly (8) (SI Appendix, Fig. S5), and pMGP>>bdl seedlings did not develop an organized primary root (SI Appendix, Fig. S5). To exclude that the ground tissue defect is induced irrespectively of where ARF activity is inhibited, and depends both on cell-autonomous and noncell-autonomous ARF function, we next expressed bdl using a driver based on the TMO5 promoter. This driver reproduced reported (17) TMO5 expression in the first vascular cells of the globular-stage embryo (Fig. 4G) and postembryonic root (SI Appendix, Fig. S5), and induced abnormal divisions in the first vascular cells (Fig. 4H). However, ground tissue divisions in TMO5>>bdl embryos were normal (Fig. 4H), and postembryonic roots showed well-organized ground tissue with endodermis marked by Casparian strips and a separate cortex layer (Fig. 4 I–L). Activity of the bdl inhibitor was evident from the reduced vascular bundle with monarch symmetry having a single xylem pole (Fig. 4 I–L). Identical vascular patterning defects correspond to the previously described tmo5 t5l1 double mutants that have been shown to act downstream of MP (17, 18). In summary, both auxin response reporters and local inhibition of auxin response demonstrate that ARFs control ground tissue initiation cell-autonomously.
Discussion
The three main tissue identities: epidermis, ground tissue, and vascular tissue, are specified early during Arabidopsis embryo development, and auxin is a prominent regulator of this stage of development (13, 43). Auxin mainly acts through the activity of the key auxin effector ARF5/MP at the globular stage of embryo development, and the mp mutant is impaired in multiple cell specification events at this stage. The pleiotropic nature and phenotypic severity of the mp mutant so far obscured the identification of MP-controlled tissue specification beyond vascular tissue initiation. Here, we have taken a local inhibition strategy coupled to genome-wide transcript profiling on isolated embryos. This strategy allowed the identification of a relatively small set of genes controlled by auxin in a small subset of cells giving rise to the vascular and ground tissue. Validation experiments showed that most of these genes are indeed expressed in the embryonic root domain and depend on the key auxin effector ARF5/MP. In addition to identifying MP-dependent genes that are activated in vascular tissue, the embryonic root meristem or the root cap precursors, this analysis surprisingly also led to the identification of a set of auxin-dependent genes that mark the first ground tissue cells. Despite many efforts in dissecting the gene regulatory network that controls ground tissue development (5–8, 35–42), so far no factors have been identified that regulate ground tissue establishment. We now identify auxin response, and its effector MP, as regulators of this critical first step. Genetic and expression analysis shows that MP acts before and upstream of the well-known SHR network. Importantly, our work suggests that embryonic tissue specification on one hand, and the subsequent tissue patterning into endodermis and cortex cell layers and postembryonic tissue maintenance on the other hand, use different regulators and different mechanisms. This observation is apparent at several levels. First, MP transcriptionally initiates the first ground tissue cells in a SHR-independent manner (this study). Second, mutant analysis suggests that SHR network genes are not required for tissue initiation (this study) (10, 44), and vice versa, expression of the MP inhibitor bdl from the SCR promoter that is expressed in the ground tissue from late globular stage of embryogenesis onward did not induce developmental defects in embryonic and postembryonic root patterning (14). Third, whereas ground tissue patterning and maintenance require intercellular transport of SHR from the vascular tissue to the ground tissue, the initiation step is cell-autonomously controlled by MP in the first ground tissue cells. Therefore, this study provides an entry point to study tissue initiation. We expect that further functional analysis of the newly identified MP-dependent ground tissue-specific genes in the embryo will help to further define the mechanism driving ground tissue initiation.
Our work also demonstrated that, at the scale of a few cell layers, auxin promotes the initiation of multiple cell types. Interestingly, whereas root initiation involves noncell-autonomous MP action (14, 17), both vascular and ground tissue initiation are cell-autonomously controlled by ARF activity. In the mp mutant, cells in the position of ground tissue or vascular tissue precursors fail to properly express markers for either of these identities. We have only seen loss of marker expression in inner cells in mp mutant embryos, and it is therefore unclear what the identity of these cells is. It was previously shown that a protoderm-specific marker is restricted to the L1 layer in mp mutant embryos (38), which suggests that there is no ectopic protoderm identity.
Our work offers the opportunity to investigate dosage-dependent auxin responses. An important future question is whether vascular and ground tissue represent two quantitatively different outputs of auxin and MP activity. Analysis of R2D2 and DR5v2 reporters (Fig. 4 A–D) suggests that the first vascular cells accumulate more auxin and respond more avidly to auxin than ground tissue initials. It is however questionable whether this quantitative difference translates to differential expression of endogenous MP target genes or whether this difference can by itself be sufficient to activate distinct sets of genes. Alternatively, cells in these two positions differ in more than only the intensity of auxin response, and these differences direct different auxin responses. Manipulation of auxin response in these cells should help to resolve this question.
This study therefore provides a framework for the simultaneous formation of tissue types by the same transcriptional regulator, and further research might reveal important insights in the mechanisms of tissue specification. Furthermore, this study reveals a previously unidentified regulator of the embryonic ground tissue and reveals that ground tissue initiation and maintenance use different regulators and mechanisms.
Supplementary Material
Acknowledgments
We are indebted to Ikram Blilou, Liam Dolan, and the Nottingham Arabidopsis Stock Centre for sharing seeds. This work was funded by the European Research Council (Starting Grant CELLPATTERN, Contract 281573 to D.W.), The Netherlands Organisation for Scientific Research (NWO; ALW-VIDI-864.06.012 and ALW-820.02.019 to D.W. and ALW-VENI-863.12.010 to C.A.t.H. and ALW-831.14.003 to M.S. and D.W.), and the National Research Council Canada Genomics and Health Initiative grant (to D.X. and R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE78695).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616493114/-/DCSupplemental.
References
- 1.Evert RF, Esau K. Esau’s Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. 3rd Ed Wiley-Interscience; Hoboken, N.J: 2006. [Google Scholar]
- 2.Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64(1):531–558. doi: 10.1146/annurev-arplant-050312-120050. [DOI] [PubMed] [Google Scholar]
- 3.Dinneny JR. A gateway with a guard: How the endodermis regulates growth through hormone signaling. Plant Sci. 2014;214:14–19. doi: 10.1016/j.plantsci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Cui H, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316(5823):421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 5.Helariutta Y, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101(5):555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- 6.Long Y, et al. Arabidopsis BIRD zinc finger proteins jointly stabilize tissue boundaries by confining the cell fate regulator SHORT-ROOT and contributing to fate specification. Plant Cell. 2015;27(4):1185–1199. doi: 10.1105/tpc.114.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sozzani R, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466(7302):128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch D, et al. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007;21(17):2196–2204. doi: 10.1101/gad.440307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Y, et al. SCARECROW-LIKE23 and SCARECROW jointly specify endodermal cell fate but distinctly control SHORT-ROOT movement. Plant J. 2015;84(4):773–784. doi: 10.1111/tpj.13038. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Risueno MA, et al. Transcriptional control of tissue formation throughout root development. Science. 2015;350(6259):426–430. doi: 10.1126/science.aad1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pernas M, Ryan E, Dolan L. SCHIZORIZA controls tissue system complexity in plants. Curr Biol. 2010;20(9):818–823. doi: 10.1016/j.cub.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 12.ten Hove CA, et al. SCHIZORIZA encodes a nuclear factor regulating asymmetry of stem cell divisions in the Arabidopsis root. Curr Biol. 2010;20(5):452–457. doi: 10.1016/j.cub.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 13.ten Hove CA, Lu K-J, Weijers D. Building a plant: Cell fate specification in the early Arabidopsis embryo. Development. 2015;142(3):420–430. doi: 10.1242/dev.111500. [DOI] [PubMed] [Google Scholar]
- 14.Weijers D, et al. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell. 2006;10(2):265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Berleth T, Jurgens G. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development. 1993;118(2):575–587. [Google Scholar]
- 16.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17(5):1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlereth A, et al. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464(7290):913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- 18.De Rybel B, et al. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev Cell. 2013;24(4):426–437. doi: 10.1016/j.devcel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 19.De Rybel B, et al. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science. 2014;345(6197):1255215. doi: 10.1126/science.1255215. [DOI] [PubMed] [Google Scholar]
- 20.Hamann T, Mayer U, Jürgens G. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development. 1999;126(7):1387–1395. doi: 10.1242/dev.126.7.1387. [DOI] [PubMed] [Google Scholar]
- 21.Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002;16(13):1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmasiri N, et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9(1):109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Haseloff J, Dormand EL, Brand AH. Live imaging with green fluorescent protein. Methods Mol Biol. 1999;122:241–259. doi: 10.1385/1-59259-722-x:241. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel CL, Marrison J, Mattsson J, Haseloff J, Bougourd SM. Ectopic divisions in vascular and ground tissues of Arabidopsis thaliana result in distinct leaf venation defects. J Exp Bot. 2012;63(14):5351–5364. doi: 10.1093/jxb/ers196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang D, et al. Genome-wide analysis reveals gene expression and metabolic network dynamics during embryo development in Arabidopsis. Plant Physiol. 2011;156(1):346–356. doi: 10.1104/pp.110.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole M, et al. DORNROSCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development. 2009;136(10):1643–1651. doi: 10.1242/dev.032177. [DOI] [PubMed] [Google Scholar]
- 27.Donner TJ, Sherr I, Scarpella E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development. 2009;136(19):3235–3246. doi: 10.1242/dev.037028. [DOI] [PubMed] [Google Scholar]
- 28.Konishi M, Donner TJ, Scarpella E, Yanagisawa S. MONOPTEROS directly activates the auxin-inducible promoter of the Dof5.8 transcription factor gene in Arabidopsis thaliana leaf provascular cells. J Exp Bot. 2015;66(1):283–291. doi: 10.1093/jxb/eru418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krogan NT, Yin X, Ckurshumova W, Berleth T. Distinct subclades of Aux/IAA genes are direct targets of ARF5/MP transcriptional regulation. New Phytol. 2014;204(3):474–483. doi: 10.1111/nph.12994. [DOI] [PubMed] [Google Scholar]
- 30.Haecker A, et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131(3):657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 31.Levesque MP, et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006;4(5):e143. doi: 10.1371/journal.pbio.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413(6853):307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 33.Scheres B, et al. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121(1):53–62. [Google Scholar]
- 34.Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18(1):111–119. doi: 10.1046/j.1365-313x.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 35.Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218(5):683–692. doi: 10.1007/s00425-004-1203-z. [DOI] [PubMed] [Google Scholar]
- 36.Lee M-H, et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol Biol. 2008;67(6):659–670. doi: 10.1007/s11103-008-9345-1. [DOI] [PubMed] [Google Scholar]
- 37.Engstrom EM. Phylogenetic analysis of GRAS proteins from moss, lycophyte and vascular plant lineages reveals that GRAS genes arose and underwent substantial diversification in the ancestral lineage common to bryophytes and vascular plants. Plant Signal Behav. 2011;6(6):850–854. doi: 10.4161/psb.6.6.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada S, Jürgens G. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development. 2007;134(6):1141–1150. doi: 10.1242/dev.02803. [DOI] [PubMed] [Google Scholar]
- 39.Weijers D, et al. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005;24(10):1874–1885. doi: 10.1038/sj.emboj.7600659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rademacher EH, et al. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 2011;68(4):597–606. doi: 10.1111/j.1365-313X.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 41.Liao C-Y, et al. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods. 2015;12(3):207–210, 2, 210. doi: 10.1038/nmeth.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smit ME, Weijers D. The role of auxin signaling in early embryo pattern formation. Curr Opin Plant Biol. 2015;28:99–105. doi: 10.1016/j.pbi.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Möller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. 2009;1(5):a001545. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development. 2000;127(3):595–603. doi: 10.1242/dev.127.3.595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.