Significance
Adaptation to seasonal changes in the environment is critical for survival in all species. In vertebrates, annual oscillations in pituitary hormones underlie the regulation of seasonal physiology. We found that, in sheep, the duration of pineal melatonin output at night controls the production of different forms of the protein vascular endothelial growth factor (VEGF) within a specific pituitary region, the pars tuberalis. Forms that block blood-vessel growth are made in winter, but those that stimulate it are made in summer. Further to the resulting remodelling of the vascular connection between the brain and pituitary, the temporally divergent VEGF-A variants operate as messenger signals on endocrine cells of a different part of the gland, the pars distalis, to regulate seasonal fertility.
Keywords: pituitary gland, angiogenesis, season, VEGF, melatonin
Abstract
Seasonal changes in mammalian physiology, such as those affecting reproduction, hibernation, and metabolism, are controlled by pituitary hormones released in response to annual environmental changes. In temperate zones, the primary environmental cue driving seasonal reproductive cycles is the change in day length (i.e., photoperiod), encoded by the pattern of melatonin secretion from the pineal gland. However, although reproduction relies on hypothalamic gonadotrophin-releasing hormone output, and most cells producing reproductive hormones are in the pars distalis (PD) of the pituitary, melatonin receptors are localized in the pars tuberalis (PT), a physically and functionally separate part of the gland. How melatonin in the PT controls the PD is not understood. Here we show that melatonin time-dependently acts on its receptors in the PT to alter splicing of vascular endothelial growth factor (VEGF). Outside the breeding season (BS), angiogenic VEGF-A stimulates vessel growth in the infundibulum, aiding vascular communication among the PT, PD, and brain. This also acts on VEGF receptor 2 (VEGFR2) expressed in PD prolactin-producing cells known to impair gonadotrophin secretion. In contrast, in the BS, melatonin releases antiangiogenic VEGF-Axxxb from the PT, inhibiting infundibular angiogenesis and diminishing lactotroph (LT) VEGFR2 expression, lifting reproductive axis repression in response to shorter day lengths. The time-dependent, melatonin-induced differential expression of VEGF-A isoforms culminates in alterations in gonadotroph function opposite to those of LTs, with up-regulation and down-regulation of gonadotrophin gene expression during the breeding and nonbreeding seasons, respectively. These results provide a mechanism by which melatonin can control pituitary function in a seasonal manner.
Pituitary hormone secretion regulates multiple functions in the body, including fertility, growth, fluid balance, and the response to stress. This regulation displays annual oscillations in most mammalian species, and is overtly seasonal in animals that have a tightly controlled reproductive window. It is thought that endogenous (i.e., circadian and circannual rhythm generators) and exogenous (i.e., photoperiod) cues can contribute to drive seasonal physiology (1). The duration of nocturnal release of the pineal hormone melatonin underlies the photoperiodic control of seasonality in sheep (2). As the synthesis and release of melatonin is inhibited by light, the longer nights of winter are associated with longer duration of melatonin production, whereas the opposite is true in the summer. Even though the reproductive cycle relies primarily on the secretion of gonadotrophin-releasing hormone (GnRH) from the hypothalamus (3), one of the major sites of melatonin action is the pars tuberalis (PT) of the pituitary gland (4, 5). Indeed, melatonin is a regulator of endocrine function in the pituitary, and specifically inhibits prolactin-producing lactotrophs (LTs), known to be associated with repression of the reproductive cycle, directly within this tissue (6). However, the mechanism through which melatonin exerts this influence remains unclear.
Three functionally distinct regions of the pituitary gland, the PT, pars distalis (PD), and infundibulum, intercommunicate with one another via an elegant portal vascular arrangement. Although melatonin is known to act on its receptors in the PT, LTs—the cells inhibited by melatonin—are exclusively found in the PD (7, 8). The mechanisms through which melatonin, acting in the PT, can control pituitary function in the PD are unresolved. Here we describe how the vascular arrangement in the infundibulum of the pituitary was drastically altered between the breeding season (BS) and nonbreeding season (NBS) in sheep. We therefore tested the hypothesis that melatonin could act to regulate pituitary function through the control of blood vessel growth and communication between the PT and PD.
Blood vessel growth (i.e., angiogenesis) is regulated by vascular endothelial growth factors (VEGFs), a family of peptides of which the most potently angiogenic and widely expressed is VEGF-A. Multiple VEGF-A products can be generated by alternative splicing of a single gene (9, 10). Alternative use of exons 6 and 7 will result in proteins of different length (e.g., 120, 164, or 188 aa in sheep, and 121, 165 or 189 aa in humans). Use of the proximal splice site in exon 8 generates the proangiogenic VEGF-Axxxa isoforms, and use of the distal splice site in exon 8 generates the VEGF-Axxxb isoforms, where xxx denotes the number of amino acids, and a or b denotes the carboxyl-terminal amino acid sequence. The most extensively studied and highly expressed isoforms of each family (VEGF-A165a and VEGF-A165b, respectively) are able to counteract the effects of each other on blood vessel growth (11). When VEGF-A165a binds VEGF receptor 2 (VEGFR2) on endothelial cells, it causes robust autophosphorylation and downstream signaling through phospholipase C, src, ras, and other pathways to induce a multitude of responses, including angiogenesis, vasodilation, increased vascular permeability, and cytoprotection (12). In contrast, although the binding affinity for VEGFR2 is the same as that of VEGF-A165a, VEGF-A165b induces weak phosphorylation (13), does not bind the coreceptor Neuropilin-1 (14), which is responsible for intracellular trafficking and recycling to the membrane (15), and does not activate the full signaling pathway. This means it does not induce angiogenesis or vasodilation (11) or a sustained increase in vascular permeability (16), and results in VEGFR2 degradation, not recycling (15). It does, however, stimulate cytoprotection of endothelial and epithelial cells (17) and neurons (18). These two isoform families therefore have very different physiological consequences (19), but any differential role in seasonal pituitary angiogenesis is unknown.
We found that, whereas total VEGF-A was not altered between the BS and NBS, there was a dramatic switch in splicing in the BS from angiogenic VEGF-Axxxa isoforms to antiangiogenic VEGF-Axxxb isoforms in both the PT and PD. This was mirrored by a reduction in the number of blood vessels in the infundibulum. Melatonin receptor expression in the PT and the infundibulum colocalized with cells expressing VEGF-A. Two potential mechanisms for VEGF-A–mediated regulation of pituitary seasonality are proposed here: (i) VEGF-A acting to regulate blood vessel function, which subsequently controls delivery of other hormones to the PD; and (ii) VEGF-A acting directly on LTs to control prolactin-associated down-regulation of the reproductive axis. We found that VEGFR2 colocalization with prolactin in the PD was increased outside the BS, consistent with a VEGF-Axxxa–mediated repression of fertility. In vitro culture of PT cells from BS sheep showed that duration of melatonin exposure controlled VEGF-A isoform secretion: long exposure induced VEGF-Axxxb production, whereas short exposure induced VEGF-Axxxa production. Culture of PT cells from the NBS revealed that melatonin given at frequencies seen in the winter could switch the expression of VEGF-A isoforms to BS levels. We then showed that PT cells treated with NBS melatonin regimens release VEGF-Axxxa, which directly induced prolactin secretion from PD cells. Finally, the time-dependent, melatonin-induced differential expression of VEGF-A isoforms resulted in alterations of gonadotroph function opposite to those of LTs in each season. Together, these results demonstrate that melatonin-mediated control of VEGF splicing could underlie intrapituitary regulation of seasonal fertility.
Results
Vascular Growth in the Pituitary Gland Is Seasonally Controlled.
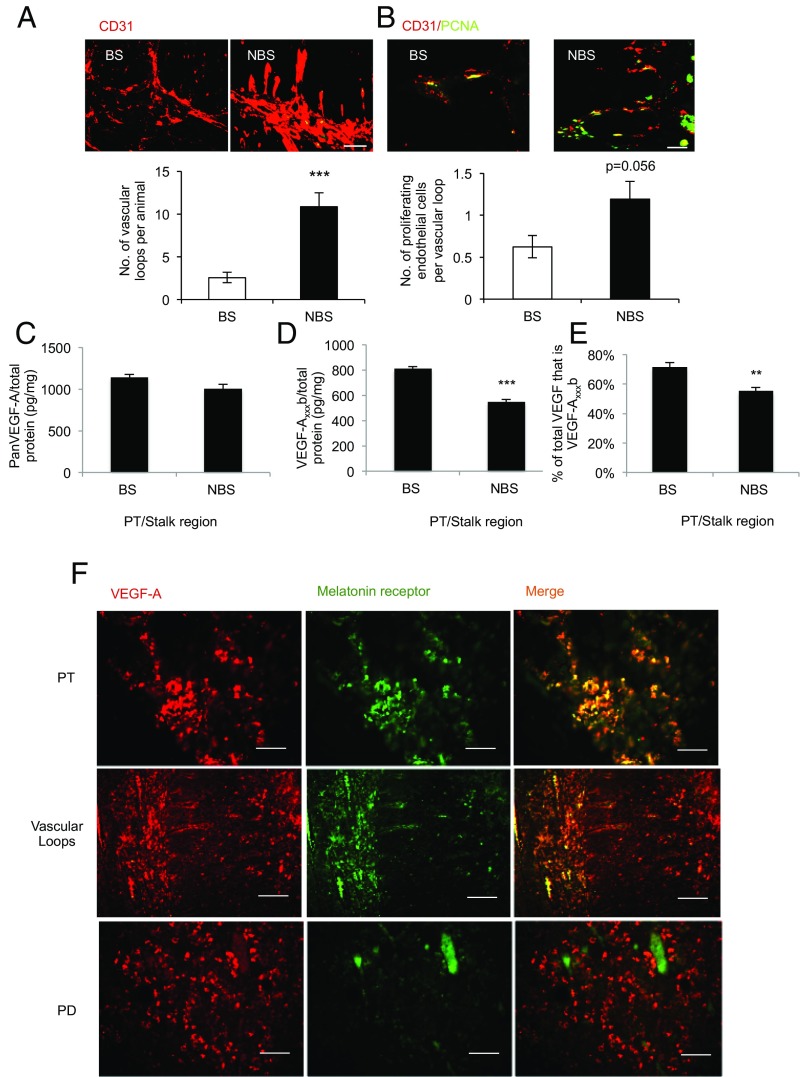
To investigate the vascular architecture of the pituitary in a seasonally breeding mammal, we screened pituitary glands of sheep with the endothelial marker CD31. Staining showed a significant (P < 0.001) increase in the number of vascular loops extending from the PT into the infundibulum in the summer (i.e., NBS) compared with animals culled in the winter (i.e., BS; Fig. 1A). To determine whether this was a result of endothelial proliferation in the NBS, we costained for CD31 and proliferating cell nuclear antigen (PCNA). We detected proliferating endothelial cells in both seasons, but a twofold increase in proliferating endothelial cells in the NBS (Fig. 1B). As angiogenesis is driven by VEGF, we measured VEGF-A in the pars tuberalis/stalk region of the pituitary. There was no difference in VEGF-A as measured by antibodies that detect all isoforms of VEGF-A (i.e., panVEGF; Fig. 1C). However, by using antibodies that specifically detect the exon 8b splice variants (VEGF-Axxxb), the expression of which has been shown to be antiangiogenic in vivo, a reduction in VEGF-Axxxb was measured in the NBS (Fig. 1D). This resulted in a change in the ratio of VEGF-A from 33% excess antiangiogenic isoforms in the BS to 60% excess angiogenic isoforms in the NBS (Fig. 1E). This indicates that the pituitary is in an antiangiogenic state in the BS, and suggests a link between day length and VEGF-A splicing.
Fig. 1.
Angiogenesis in the pituitary is seasonally dependent. (A) Endothelial staining in the PT/infundibulum and quantification of vessel loops in the summer (i.e., NBS) and winter (i.e., BS). (B) Endothelial proliferation (PCNA/CD31 double-positive cells) in the NBS and BS. (C) Total VEGF-A levels in the PT/stalk in the BS and NBS (not significantly different; P > 0.05). (D) VEGF-Axxxb–specific ELISA on protein extracted from pituitaries of animals killed in the BS or NBS. (E) Proportion of VEGF-A that was VEGF-Axxxb in the BS and NBS. (F) Staining of the melatonin receptor (green) and VEGF-A (red) in different regions of the pituitary; costaining (found in PT and vascular loops) is shown as yellow (***P < 0.01 and ***P < 0.001 vs. BS). (Scale bar: 50 µm.)
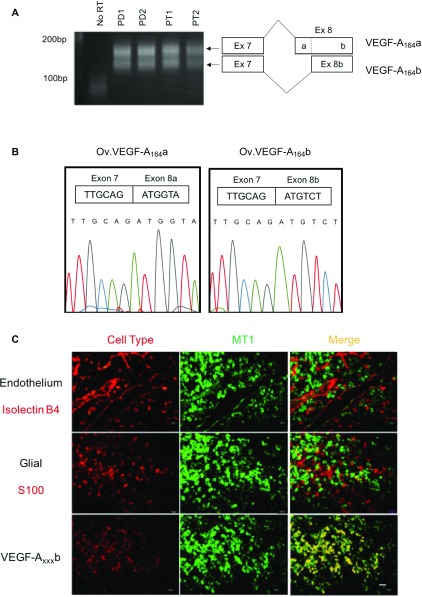
To determine whether VEGF-A was expressed in the pituitary in cells that can respond to day length, we costained for melatonin receptor and VEGF. Fig. 1F shows that MT1 and VEGF-A were colocalized in the PT and, interestingly, also in the vascular loops (Fig. 1F, arrows) that connect the PT with the infundibulum. In contrast, whereas VEGF-A was expressed in the PD, MT1 receptors were not. The antiangiogenic isoforms had not previously been cloned from sheep, so we examined RNA expression by RT-PCR. Both isoforms were detected in pituitaries from sheep in both seasons (PD and PT; Fig. S1A). Cloning and sequencing of the PCR product confirmed that this was sheep VEGF-Axxxb (Fig. S1B).
Fig. S1.
(A) cDNA amplified from the pituitary PD and PT from ewes by primers that detect exon 8a- and exon 8b-containing isoforms (Fig. 1). Both bands were purified and sequenced. (B) Chromatogram of sequence of PCR products. Upper band (Left) shows splicing from exon 7 to exon 8a. Lower band shows splicing from exon7 to exon 8b. (C) MT1 receptor was colocalized with VEGF-Axxxb staining, but not glial cells or endothelium (Scale bar: 20 μm.)
The sheep sequence has a single nucleotide substitution compared with human DNA (a G in sheep, C in humans). This results in a single amino acid difference, with a sequence of SRTRKD instead of SLTRKD in human. Thus, the sheep VEGF-Axxxb isoforms are 1 aa shorter than the human ones. The cell type in which VEGF-Axxxb was expressed in the PT was identified by immunolocalization. Fig. S1C confirms that VEGF-Axxxb is expressed in the MT1-positive cells, which, in the PT, are not endothelial or glial-type folliculostellate (S100+) cells. These results suggested that melatonin could regulate expression of different VEGF-A isoforms in the PT, regulating angiogenesis in the pituitary in a seasonally dependent manner.
VEGF-A Splicing Is Regulated by Duration of Melatonin Exposure in PT Cells.
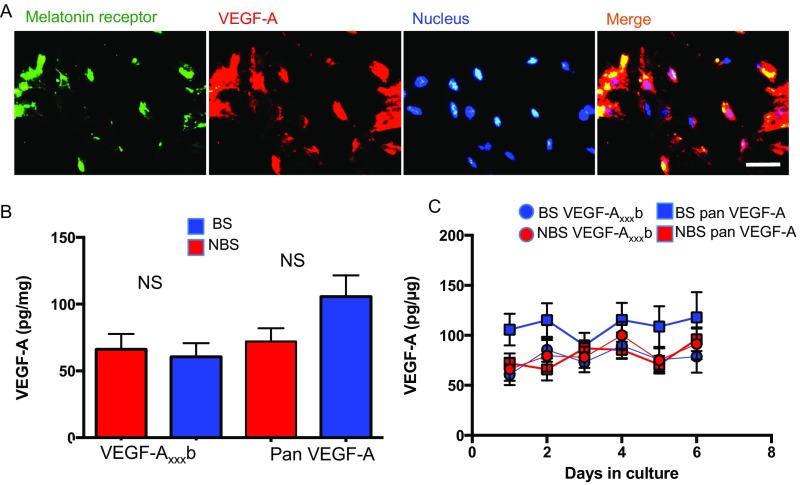
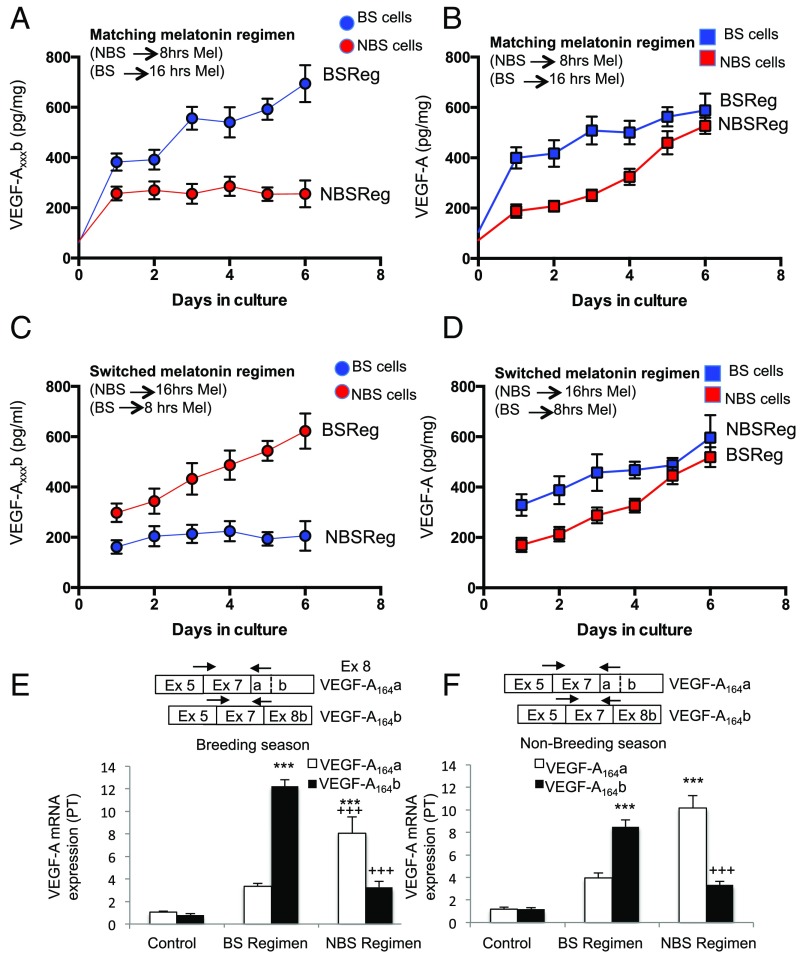
We investigated VEGF-A isoform expression in cells isolated from the PT, which express the melatonin receptor and VEGF-A (Fig. S2A), by isoform family-specific ELISA and real-time PCR. Cells were cultured under conditions without melatonin (control; Fig. S2B) or with melatonin given for 16 h (mimicking winter, i.e., BS regimen), or 8 h (mimicking summer, i.e., NBS regimen). VEGF-Axxxb protein (Fig. 2A) was increased sevenfold by a BS melatonin regimen (Fig. 2A) in cells from BS animals (i.e., given the matching melatonin regimen), whereas panVEGF-A increased only fourfold (Fig. 2B). In contrast, when cells from pituitaries of NBS sheep were given the NBS melatonin regimen, VEGF-Axxxb was increased only twofold (significantly lower than BS; Fig. 2A), but panVEGF-A was increased fivefold (Fig. 2B), although this response took longer than in the BS cells. This suggests that the length of time that the cells are exposed to melatonin on a daily basis controls the expression of the different splice variants of VEGF-A. It also suggests that NBS cells are less prepared to respond to melatonin exposure, as they take longer to increase their VEGF-Axxxa output.
Fig. S2.
(A) PT cells were isolated from sheep in BS and stained for the melatonin receptor, VEGF-A, and Hoechst (Fig. 2). (B) VEGF levels from cells cultured in the absence of melatonin. There were no significant differences between VEGF-A levels from PT cells taken from the winter (BS, blue), or summer (NBS, red) ewes. (C) VEGF levels did not alter over 6 d in primary culture (Scale bar: 20 μm.)
Fig. 2.
VEGF-A isoforms levels are regulated by melatonin periodicity in the PT. (A) PT cells in culture were isolated from pituitaries of sheep and VEGF-Axxxb measured by ELISA. Cells from winter sheep (BS, blue) were treated with melatonin for 16 h each day for 6 d; cells from summer sheep (NBS, red) were treated for 8 h each day with melatonin. (B) Levels of panVEGF-A were also measured from these cells. (C) VEGF-Axxxb levels were measured from sheep PT cells incubated with the incongruous melatonin exposure for the time from which they were harvested (cells from summer sheep were given a winter melatonin regimen; those from winter sheep were given a summer melatonin regimen). (D) Levels of panVEGF-A from cells treated as in C. (E) VEGF-A isoform mRNA expression in PT cells from the BS (winter) after 6 d of treatment with BS and NBS melatonin regimens. (F) VEGF-A isoform mRNA expression in PT cells from the NBS (summer) after 6 d of treatment with BS and NBS melatonin regimens. Boxes show positions of the primers used to amplify the cDNA (***P < 0.001 vs. control, +++P < 0.001 vs. BS regimen).
To determine whether this was dependent on the stage of the annual reproductive cycle, we treated cells with a melatonin regimen that was reversed (i.e., opposite of that of the prevailing photoperiod) and found the same response for VEGF-Axxxb: BS regimen induced VEGF-Axxxb expression, whereas NBS regimen did not (Fig. 2C). The same was true for panVEGF-A (Fig. 2D): expression was induced by both regimens, but NBS cells were slower to respond than BS cells. To confirm that this was the result of a change in the RNA splice isoforms, we measured RNA levels of VEGF-A164b and VEGF-A164a by quantitative RT-PCR using isoform-specific primers. Fig. 2E shows that VEGF-A164a and VEGF-A164b were preferentially up-regulated by the NBS and BS regimens, respectively, in BS cells. In NBS cells, the same effect was induced by switching the melatonin regimen, indicating that this effect is specific to the duration of melatonin exposure, rather than the stage of the annual reproductive cycle from which the cell was sourced. These results indicate that melatonin can control angiogenesis protein production in the PT.
VEGF-A Splice Isoforms and Receptors Are Present in the PD.
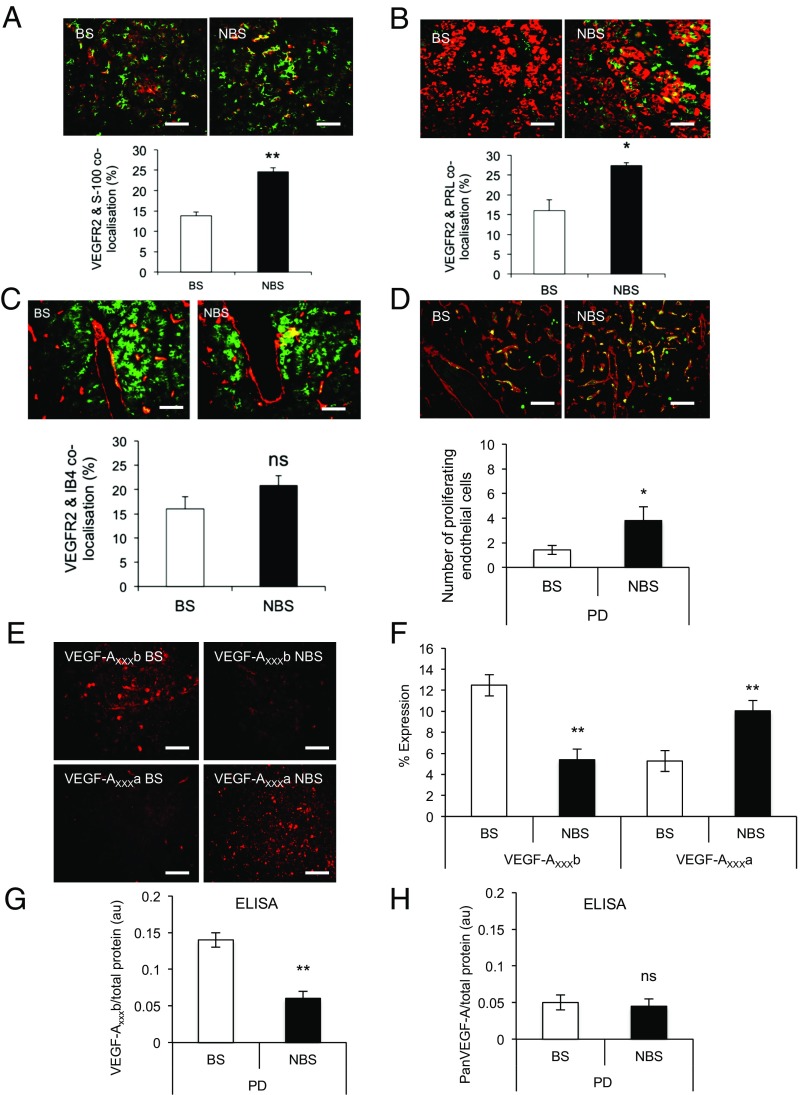
To determine whether VEGF-A could target endocrine and/or nonendocrine cells that are known to display seasonal plasticity, we screened the PD for VEGFR2. Costaining of VEGFR2 with folliculostellate cells (FSCs; Fig. 3A) and LTs (Fig. 3B) showed that VEGFR2 was colocalized with a proportion of FSC and LT, and, critically, that this colocalization increased (P < 0.01 and P < 0.001, respectively) during the NBS, i.e., in the summer. There was also substantial VEGFR2 expression colocalized on endothelial cells in both seasons (Fig. 3C). Screening for proliferating endothelial cells indicated that there was more angiogenesis in the summer (i.e., NBS) in the PD (Fig. 3D), as well as the PT and infundibular stalk (Fig. 1D). Immunofluorescence staining for VEGF-A isoforms indicated that VEGF-Axxxb was significantly down-regulated and VEGF-Axxxa significantly up-regulated in the summer (i.e., NBS; Fig. 3E), providing a rationale for the up-regulation of angiogenesis in the PD. Quantification of the area of staining (Fig. 3F) confirmed this finding, as did quantitative ELISA for VEGF-Axxxb (down-regulation in the summer, i.e., NBS; Fig. 3G) and panVEGF-A (i.e., no change, and hence an implied up-regulation of angiogenic isoforms in the NBS; Fig. 3H).
Fig. 3.
VEGFR2 is up-regulated in the PD during the summer (i.e., NBS). (A) Colocalization of VEGFR2 (green) and glial-type FSCs (red) in the BS (winter) and NBS (summer). (B) VEGFR2 (green) expression in LTs (red) in the BS and NBS. (C) VEGFR2 (green) and endothelial cells stained by isolectin B4 (IB4, red) in both seasons. (D) Proliferating (PCNS, green) endothelial (IB4, red) cells were stained, and colocalization was quantified. (E) VEGF-Axxxb expression was detected in the PD in the winter (BS) but not in the summer (NBS); VEGF-Axxx expression was detected in the summer (NBS) but not in the winter (BS). (F) Quantification of the expression of VEGF-A isoforms. (G) ELISA quantification of the amount of VEGF-Axxxb in the BS and NBS. (H) ELISA quantification of the amount of total VEGF-A in the two seasons (*P < 0.05 and **P < 0.01; ns, nonsignificant at P > 0.05 vs. BS). (Scale bar: 50 µm.)
VEGF-A Isoforms Control Seasonal Endocrine Function.
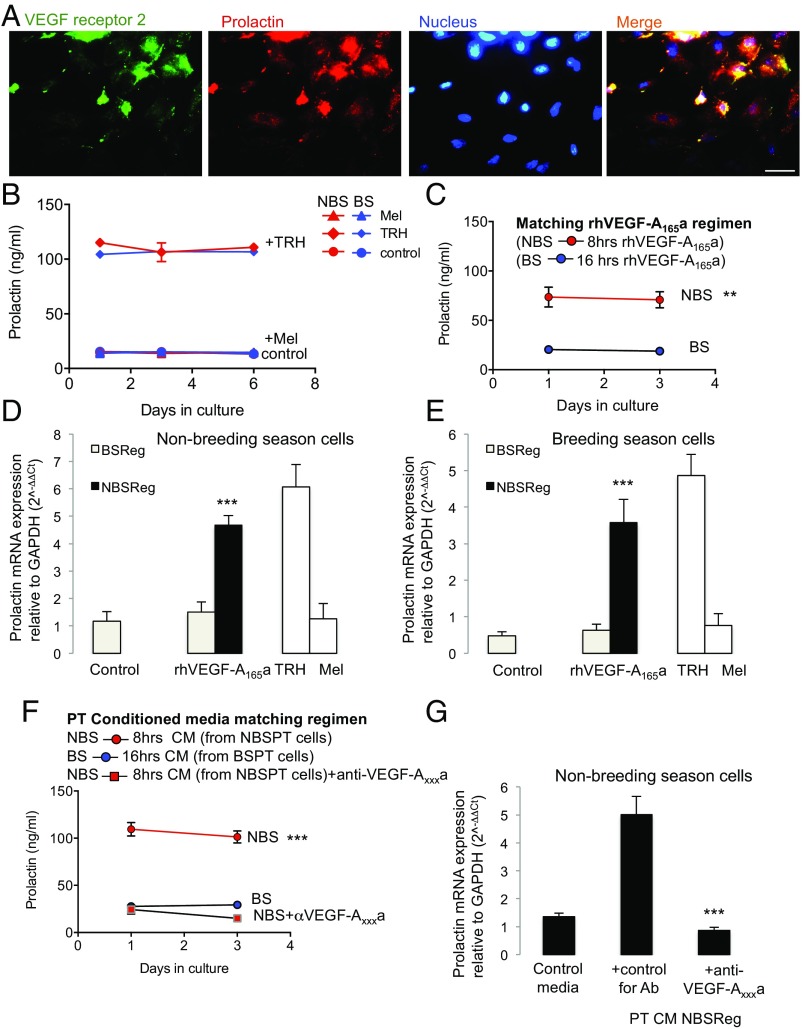
These results led to two hypotheses: (i) that VEGF-A controls angiogenesis and this allows, by increased portal blood flow, an as yet unidentified compound to repress the reproductive axis (presumably, at least in part, by stimulating prolactin); and/or (ii) that VEGF-A itself is a signaling molecule from the PT to the PD, which directly contributes to the inhibitory regulation of the reproductive cycle by releasing prolactin. To test this latter hypothesis, we cultured PD cells from sheep in the NBS or BS and treated them with recombinant human VEGF-A165a (rhVEGF-A165a) or conditioned media from the PT cells taken at the same time of year, and measured prolactin production by RIA. Fig. 4A shows that VEGFR2 and prolactin were both expressed by PD cells in culture. Fig. 4B shows that the cells from both NBS and BS animals could be induced to release prolactin by thyrotrophin-releasing hormone (TRH), but not by melatonin. Fig. 4C shows that rhVEGF-A165a, given for the duration that matches NBS melatonin exposure (i.e., 8 h in the summer), resulted in significant prolactin release from PD cells from NBS animals (P < 0.001) and from cells from the BS (Fig. S3A). It also showed that rhVEGF-A165a, given at BS duration (16 h) did not cause prolactin release from BS (Fig. 4C) or NBS cells (Fig. S3A). We confirmed this at the RNA level (Fig. 4 D and E).
Fig. 4.
VEGF-A mediates prolactin release from the PD in an isoform-dependent manner. (A) VEGFR2 expression in cultured prolactin positive cells (i.e., LTs) of the PD. (B) Prolactin secretion following treatment with TRH (positive control), melatonin (Mel, negative control), and medium (control) in PD cells cultured during the NBS (summer) and BS (winter). (C) Prolactin secretion in PD cells from sheep killed in the summer (NBS) after rhVEGF-A165a treatment was greater than that from winter (BS) sheep. (D) Prolactin mRNA expression in PD cells taken from ewes in the summer (NBS) following a summer (NBS) regimen of rhVEGF-A165a (8 h on, 16 h off) was greater than that of cells taken from the same animals following a winter (BS) rhVEGF-A165a regimen (16 h on, 8 h off). (E) In cells taken from ewes in the BS (winter), prolactin was induced only if VEGF-A was given in an NBS (summer) regimen. (F) Conditioned media from summer (NBS) PT cells treated with a summer (NBS) melatonin regimen (red) induced prolactin production. This was blocked by an antibody to VEGF-Axxxa. (G) Prolactin mRNA in summer (NBS) cells was induced in the presence of conditioned media from summer (NBS) regimen PT cells, and this was blocked by an anti-VEGF-Axxxa antibody (**P < 0.01 and ***P < 0.001 vs. BS). (Scale bar: 20 µm.)
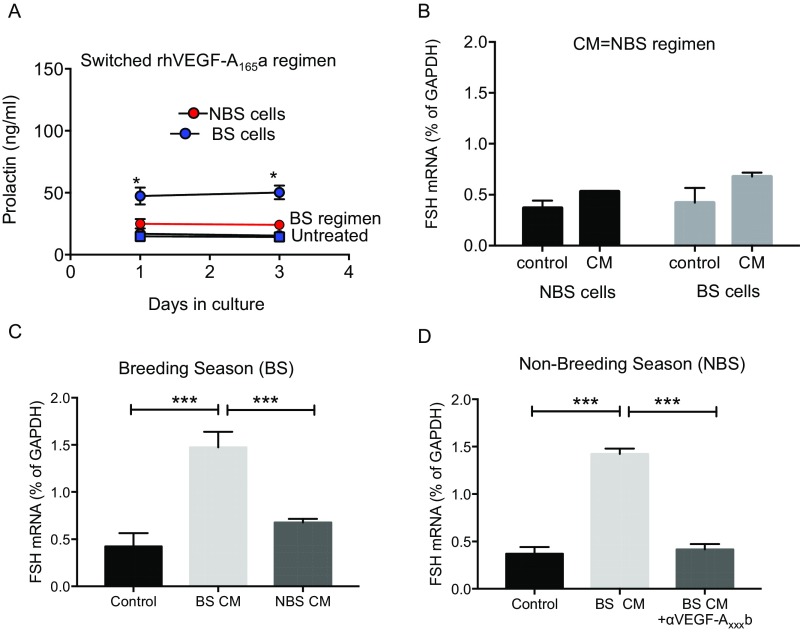
Fig. S3.
(A) Pars distalis cells from NBS treated with rhVEGF-A165a in a BS regimen (red) do not produce prolactin (Fig. 4). In contrast, cells from the BS treated with rhVEGF-A165a in an NBS manner (blue) did produce prolactin. (B) Conditioned media from PT cells treated with an NBS melatonin regimen had no effect on FSH production in PD cells, irrespective of which season the cells were from. (C) BS cells treated with BS, but not with NBS, PT-conditioned media produced FSH mRNA. (D) NBS cells treated with CM from PT cells exposed to a BS regimen of melatonin produced FSH. This was blocked by incubation with an antibody to VEGF-Axxxb (αVEGF-Axxxb; *P < 0.05 vs. untreated).
To determine whether PT cells could generate VEGF-A isoform ratios that induced prolactin, we took conditioned media from the PT cells treated with melatonin and treated the PD cells with this conditioned media to mimic the in vivo situation. Conditioned media from PT cells treated with NBS melatonin regimen significantly stimulated prolactin protein (Fig. 4F) and mRNA (Fig. 4G) in cells from NBS ewes, but did not result in FSH production (Fig. S3B). Critically, this effect was completely blocked by an antibody to VEGF-Axxxa (Fig. 4 F and G). Conditioned media from PT cells treated with BS melatonin regimen had no effect on prolactin production from BS PD cells (Fig. 4F), but did stimulate FSH mRNA production from these cells (Fig. S3C) and from cells from NBS ewes (Fig. S3D), an effect that was inhibited by pretreatment with a VEGF-Axxxb–specific antibody (Fig. S3D). This indicates that melatonin duration-induced differential VEGF-A isoform production by the PT has the potential to regulate the seasonal production of prolactin and FSH by the PD through an intrapituitary paracrine mechanism mediated by VEGF.
Discussion
The rationale for these studies was that dynamic and tightly regulated changes in the vascular communication between the brain and the pituitary gland could underlie seasonal physiology. To that end, we used a highly seasonal animal model, the sheep, with a well-characterized annual reproductive cycle. The results show that the pituitary microvasculature that connects the PT with the neural tissue of the infundibulum, before contacting the PD, displays dramatic seasonal remodeling, and that this could be in response to locally regulated splice variants of VEGF-A in the photoperiodic responsive/melatonin sensitive PT region. Importantly, we found that the melatonin-induced differential expression of VEGF-A isoforms in the PT throughout the annual reproductive cycle not only has the potential to alter the portal microvasculature, but that it could itself operate as a messenger to modify the endocrine output from the PD. We established that the signal from the PT carried to hormone-producing cells in the PD to stimulate prolactin secretion and inhibit FSH during reproductive quiescence is the angiogenic isoform of VEGF, VEGF-A164. Moreover, the results reveal that the splicing of the VEGF-A pre-RNA from antiangiogenic VEGF-A164b in the winter (i.e., BS) to proangiogenic VEGF-A164a in the summer (i.e., NBS) results from the different duration of melatonin exposure, which occurs across seasons, highlighting the existence of a photoperiodically regulated system for the seasonal control of fertility.
The microvasculature of the pituitary gland is key to the regulation of multiple body functions because it controls the blood flow from the hypothalamus, altering the delivery of stimulatory and inhibitory signals to endocrine target cells (20). Here we show that this vascular connection undergoes a remarkable seasonal adaptation throughout the annual reproductive cycle in response to an external cue, namely the changing photoperiod. Such vascular plasticity is manifested in alterations of endothelial cell proliferation that result in timely changes in the vascular loops that connect the PT with the infundibulum before giving rise to the long portal vessels that terminate in the PD. Even though these loops were first described more than six decades ago (21), the possibility that they could alter the connectivity between the brain and the pituitary at certain times of the year was not known. Although no apparent alterations in the ovine pituitary vasculature of male castrates exposed to different photoperiods was described in a recent report, detailed measurements were not undertaken and the vascular loops were not specifically examined (22). The increased number and surface area of these vascular loops during the long days of the NBS, concomitant with an increase in endothelial cell proliferation, is in agreement with the increased number of cells proliferating in the PT shown in the present study and by another group (23) at this time of year. The afferent branches of these loops connect the photoperiodic-responsive PT with the FS cell-rich infundibulum, and efferent branches provide communication between the infundibulum and the PD, where most of the pituitary hormones are produced. Thus, the temporal remodeling of the vascular connection among these three tissues highlights the existence of a control point for seasonal physiology. The importance of this finding is supported by recent studies in rodents showing that the pituitary microvasculature adapts to the needs of pituitary endocrine cells and that it can control endocrine output according to the physiological requirement of the individual (24). This remodeling could substantially increase or decrease the transport of hormones from the PT to the infundibulum and from the infundibulum to the PD, and is therefore able to accentuate or reduce the effect of hypothalamic-derived neuroendocrine signals, such as GnRH, to the hormone-producing cells of the pituitary. It is possible that this regulatory mechanism for the delivery of hypothalamic factors could operate in conjunction with the dynamic retraction/protraction of “endfeet” processes of tanycytes (25), the specialized ependymal cells of the glia, which have been shown to interact with the hypothalamic neuronal terminals and fenestrated capillaries of the median eminence and to play a role in the control of the seasonal reproductive cycle in birds (26). The VEGF isoforms differentially regulate fenestrations of endothelial cells (16), so it is possible that the two mechanisms could work together, although we are unable to use the evidence presented here to differentiate between dependent and independent mechanisms.
In other tissues, vascular remodeling and permeability is controlled by VEGF-A (19), so dynamic changes in the pituitary microvasculature were expected to correspond to alterations in VEGF-A expression. Indeed, VEGF-A–mediated changes in endothelial cell proliferation and angiogenesis were communicated in specific regions of the songbird brain across seasons (27). However, VEGF-A had been previously reported to remain unchanged in the pituitary of sheep under different photoperiods (28), and, in the present study, panVEGF-A expression did not differ between BS and NBS animals. Critically, the use of specific antibodies to the pro- and antiangiogenic isoforms of VEGF-A (these distinguish between isoform families, but not between the different length isoforms, hence VEGF-Axxxa and VEGF-Axxxb) revealed differential isoform expression between the long days of the NBS and the short days of the BS, with overexpression of antiangiogenic VEGF-Axxxb variants in the BS and increased expression of proangiogenic VEGF-Axxxa variants in the NBS, providing an explanation for the observed changes in the microvasculature. As the PT is the tissue with the highest density of melatonin receptors (4, 5), and reliably translates the effects of photoperiod on circadian and circannual physiology within the pituitary (1, 29), our results showing the coexpression of VEGF-A and melatonin receptors in PT-specific cells provide compelling evidence that the seasonal regulation of the vascular connection between the brain and the pituitary gland is mediated by a melatonin-induced mechanism within the PT region that leads to differential expression of pro- and antiangiogenic VEGF-A isoforms.
Moreover, our results show that, in addition to the PT-specific cells, melatonin could also act directly on the vascular loops, revealing a target for melatonin action to translate photoperiodic effects on seasonal physiology. As the blood flows from the brain to the pituitary (11), alterations in the vascular loops of the infundibulum that will give rise to the long portal vessels (12) could contribute not only to regulate the transfer of PT products to the PD, but also to alter the delivery of hypothalamic factors; thus, the increased vascular connections during the long days of summer would be expected to favor increased supply of stimulatory and inhibitory hypothalamic signals to the PD at this time of year. Notwithstanding that, the reduction in vascularity during the short days of winter is likely to play a role in the modulation of the gonadotroph response to GnRH by means of preventing desensitization of GnRH receptors (30) and fine-tuning the differential control of gonadotropin secretion (31, 32), which are essential processes to ensure normal fertility.
Photoperiodic information is encoded by the duration of nocturnal melatonin secretion (2), so we used a paradigm whereby ovine PT cells were cultured and exposed daily to summer (i.e., NBS) or winter (i.e., BS) durations of melatonin treatments (8 h vs. 16 h, respectively) over a period of 6 d. PT cells from the same animals were exposed to the matching and nonmatching (i.e., opposite season) melatonin regimens, so we were able to differentiate direct effects of the melatonin signal and those resulting from its interaction with the circannual phase. We show that duration of melatonin exposure induced a striking differential expression of VEGF-A isoforms, with up-regulation of the proangiogenic isoform VEGF-Axxxa by a short-duration regimen (i.e., 8 h, summer; NBS) and up-regulation of the antiangiogenic isoform VEGF-Axxxb by a long-duration regimen (i.e., 16 h, winter; BS). This melatonin duration-dependent differential expression of VEGF-A isoforms was also recorded in cells obtained in the opposite season but at a slower rate, highlighting the requirement of PT cells to be entrained to the new signal. Thus, the results are consistent with the findings ex vivo and demonstrate that pituitary microvascular remodeling is likely to be sensitive to the changing photoperiod and adapts to the physiological requirements of the animal in response to time-dependent melatonin signals acting on VEGF-A. The mechanism through which melatonin switches splicing of the VEGF-A gene is not yet known, but alternative splicing of VEGF has been shown to be regulated by activation of the RNA binding proteins SRSF1, SRSF2, and SRSF6 by the kinases SRPK1 and Clk4 (33).
We then investigated whether the seasonal regulation of VEGF-A in the PT could affect the function of the PD. We show that VEGF-A receptors are expressed in endocrine, endothelial, and FS cells in the PD, and that their colocalization is also under seasonal control, with up-regulation during the long days of the NBS. In addition, there was increased content of proangiogenic VEGF-A isoforms at this time of year and, conversely, increased content of the antiangiogenic isoforms during the short days of the BS. As the seasonal regulation of VEGF-A isoform expression was shown to be melatonin-dependent and, in accordance with previous studies (34), the PD was shown not to contain melatonin receptors, the varying content of VEGF-A isoforms in the PD is likely to rely on a paracrine mechanism (35). The physiological significance of this was first revealed in the PD microvasculature, with increased endothelial cell proliferation demonstrated during the NBS. We show that this increase in angiogenesis at this time of the annual reproductive cycle is concomitant with an increase in the prevalence of FS cells containing VEGF receptors. FS cells are glial-like, nonendocrine cells that, via gap junctions, generate a 3D network throughout the pituitary to coordinate its function (36, 37). These cells secrete an array of paracrine factors known to influence endocrine cells such as gonadotrophs and LTs, and are a primary source of VEGF-A (35). In seasonal breeders, FS cells are distributed throughout the PD and PT (38) and respond to photoperiodic changes with a high degree of plasticity (39, 40). In sheep, significant ultrastructural changes, together with enhanced number of intercellular adherens junctions and increased number of elongated processes surrounding endocrine cell clusters, were reported during the long days of the NBS (41). As FS cells do not contain melatonin receptors (42), our findings revealing up-regulation of VEGF receptor content in these cells at this time of year provide evidence for a role of VEGF-A in the dynamic changes of the FS cell network to control vascular plasticity via the regulation of its own production during the annual reproductive cycle. The seasonally regulated differential expression of VEGF-A isoforms in the pituitary gland of a short-day breeder unraveled here could also operate in long-day breeders, such as hamsters and horses, as part of the mechanisms controlling their annual physiology. Indeed, preliminary results have provided evidence that, in thoroughbred horses, VEGF-A isoform expression in the PT and PD regions of the pituitary is also seasonally regulated (43), suggesting that this is a conserved mechanism for seasonal adaptation in photoperiodic mammals.
Notably, we show that, in addition to its actions on the pituitary vasculature and FS cell population, VEGF-A has a potent prolactin releasing effect, and that this stimulation depends on time of exposure of the ligand and density of VEGF receptors in LTs, which is increased during the long days of the NBS. Moreover, these stimulatory effects of VEGF-A on prolactin synthesis and release were accompanied by suppression of the gonadotrophic axis, as revealed by inhibition of FSH gene expression. Melatonin was shown to mediate the photoperiodic regulation of prolactin secretion through a direct action within the pituitary gland (6). Because MT1 melatonin receptors are selectively expressed in the PT, and this region is deprived of LT cells (7, 8), a paracrine mechanism for the control of prolactin secretion from the PD is warranted. Activation of MT1 melatonin receptors in the PT is known to inhibit adenylyl cyclase, and pharmacological studies in sheep have shown that melatonin impairs forskolin-induced hypersecretion of cAMP, with inhibition of prolactin from the PD through the reduction of a paracrine signal (44). However, although several compounds such as tachykinins, substance P, and neurokinin A are produced by the PT and can stimulate prolactin release (45–47), characterization of the chemical identity of that signal has been elusive. Here we show that the stimulatory effects of VEGF-A on prolactin were mimicked by conditioned media from PT cultures exposed to an NBS regimen of melatonin, and that these actions of PT media were blocked by a specific VEGF-Axxxa antibody, demonstrating that VEGF-A is a potential paracrine signal, and that melatonin-induced differential VEGF-A isoform production by the PT can regulate the seasonal production of prolactin and FSH.
Because these effects were also recorded in PD cells obtained in the opposite season (i.e., BS), albeit with a 3-d lag required for adaptation, our results show that the photoperiodically induced paracrine mechanism mediated by VEGF-A can ultimately override the circannual phase of the PD target cells, and entrain it to the new photoperiod. The increased VEGF receptor content in the PD during the NBS plays a major role in mediating this process, and thus in the biological adaptation to a summer physiology, because VEGF-A treatments mimicking an NBS melatonin regimen showed a delayed response in BS cultures in which the VEGF receptor content was reduced. Entrainment of the PD cells to a specific phase of the circannual cycle explains why NBS cells failed to secrete prolactin in response to the first 8 h of a BS (16 h) VEGF-A regimen or PT-conditioned media from the BS.
In rodents, melatonin-induced suppression of cAMP is followed by sensitization of adenosine A2b receptor signaling, leading to subsequent increase in cAMP and cAMP response element binding protein (CREB) phosphorylation (28). Disruption of this signaling pathway in MT1 melatonin receptor-KO mice resulted in altered prolactin secretion, implicating cAMP and adenosine in this biological response to melatonin. Our results indicate that VEGF-A is likely to be downstream of that pathway to bring about the biological response. Indeed, cAMP signaling, CREB phosphorylation, and adenosine are associated with angiogenesis (48, 49) via stimulation of VEGF-A (50), and, whereas pharmacologically induced cAMP up-regulation and treatment with adenosine stimulated VEGF-A expression in smooth muscle cells (50), the selective knockdown of all VEGF-A isoforms blocked the actions of elevated cAMP on hippocampal neurons (51). The melatonin-induced VEGF-A regulation of prolactin secretion shown in this study will have an impact on the gonadotrophic axis in addition to its direct inhibition of FSH, because, when combined with dopamine, prolactin impairs the gonadotroph response to GnRH in a seasonally dependent manner in long- and short-day breeders (52–54).
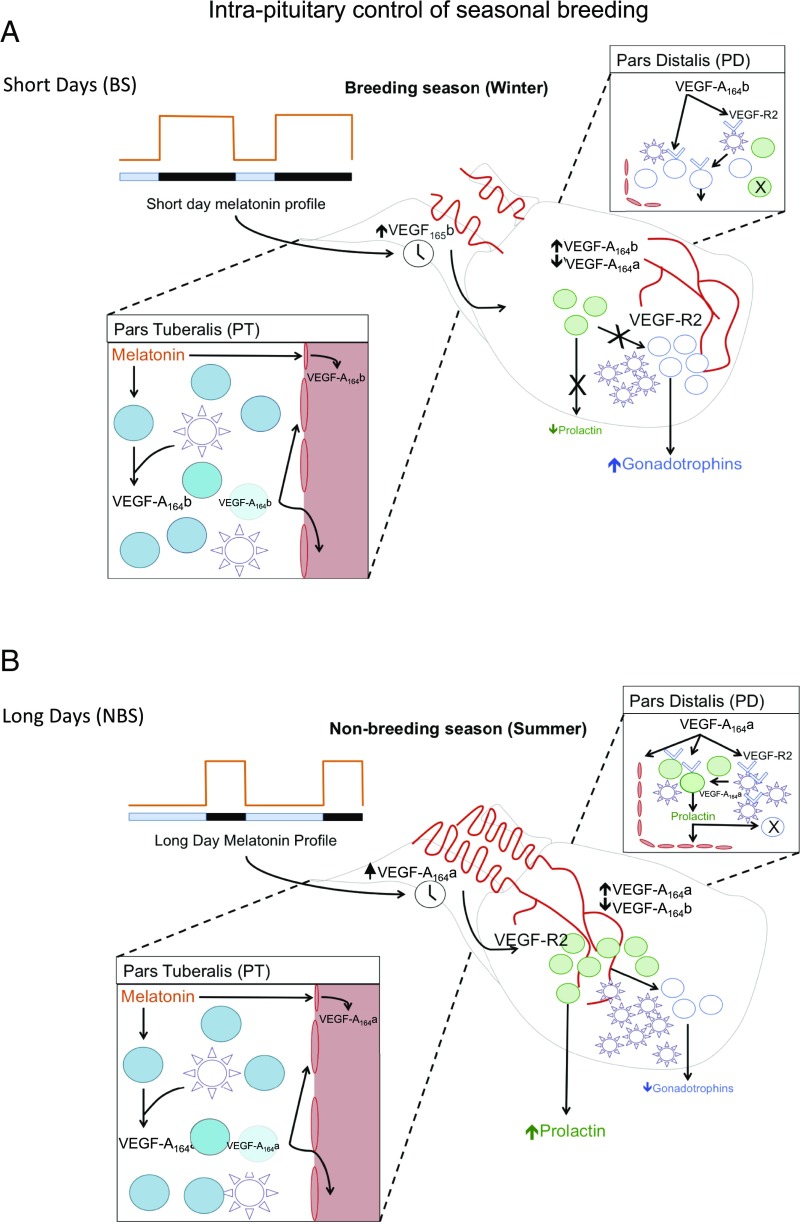
Our results provide evidence for an intrapituitary mechanism that responds to an external independent signal to regulate seasonal physiology. We propose a model whereby the duration of nocturnal melatonin secretion promotes alternative splicing of the VEGF-A gene, leading to differential synthesis and release of proangiogenic and antiangiogenic isoforms of VEGF-A within the PT region of the pituitary gland and in the vascular loops that connect this tissue with the infundibulum (Fig. 5). The resulting output of VEGF-A isoforms will have two complementary effects: (i) it alters the temporal vascular connection between the brain and the pituitary gland and (ii) it can be used as a paracrine signal to modify the seasonal activity of endocrine cells in the PD that control reproduction. In this model, the long duration of nocturnal melatonin exposure during the winter up-regulates the secretion of antiangiogenic isoforms VEGF-Axxxb at the expense of proangiogenic isoforms VEGF-Axxxa, resulting in reduced angiogenesis, reduced density of VEGF receptors in endocrine and FS cells, suppression of prolactin secretion, and no inhibition of the gonadotrophic axis characteristic of the BS. Conversely, the short duration of nocturnal melatonin exposure during the summer will up-regulate the secretion of proangiogenic isoforms VEGF-Axxxa at the expense of antiangiogenic isoforms VEGF-Axxxb, leading to increased angiogenesis, increased density of VEGF receptors in endocrine and FS cells, stimulation of prolactin secretion, and inhibition of the gonadotrophic axis, characteristic of the NBS. Thus, the model permits a physiological adaptation to the seasonal requirements of the species by means of an angiogenesis-dependent intercommunication between two regions of the pituitary.
Fig. 5.
Working model for a melatonin-induced, VEGF-A isoform-dependent intrapituitary regulation of seasonal physiology. In this model, the duration of nocturnal melatonin secretion induces differential synthesis and release of proangiogenic and antiangiogenic isoforms of VEGF-A in the PT region of the ovine pituitary and in the vascular loops that connect this tissue with the infundibulum. (A) In the short days of winter (BS), the long duration of nocturnal melatonin exposure up-regulates the secretion of the antiangiogenic isoform VEGF-A164b at the expense of the proangiogenic isoform VEGF-A164a, resulting in reduced angiogenesis, reduced density of VEGF receptors in endocrine and FS cells of the PD, suppression of prolactin secretion, and no inhibition of the gonadotrophic axis. (B) In contrast, during the long days of summer (NBS), the short duration of nocturnal melatonin exposure up-regulates the secretion of the proangiogenic isoform VEGF-A164a at the expense of the antiangiogenic isoform VEGF-A164b, leading to increased angiogenesis, increased density of VEGF receptors in endocrine and FS cells of the PD, stimulation of prolactin secretion, and inhibition of the gonadotrophic axis.
Materials and Methods
Details of standard protocols are given in SI Materials and Methods. Ovine pituitary glands were obtained from ovary-intact females during the BS (i.e., December/January) and the NBS (i.e., June/July). Animals were killed for commercial reasons at an abattoir, and pituitaries were removed immediately after death. During the BS, ewes were confirmed to be sexually active on the basis of a recently formed corpus luteum (CL) together with the presence of a large follicle (>2 cm). By contrast, in the NBS, ewes were considered to be anestrus when no CL but a corpus albicans was observed in the gonad, and follicles present were < 2 mm in diameter.
Expression Studies.
Pituitaries were stained and RNA was extracted (55) by using standard procedures (antibodies are detailed in Tables S1 and S2 and primers are shown in Table S3). The term “VEGF-Axxxb” is used because the antibodies do not distinguish between the different VEGF-Axxxb isoforms (e.g., VEGF-A121b, VEGF-A165b, VEGF-A189b). The term “VEGF-A164b” or “VEGF-A165b” is used when the methodology specifically describes the sheep 164-aa isoform (isoform-specific RT-PCR, as the forward primers cross exon 5 and exon 7, or the human 165-aa isoform when recombinant protein is used).
Table S1.
Primary antibodies
| Peptide target | Antigen sequence | Species raised in | Manufacturer and catalog code or reference | Working concentration |
| VEGF-Axxxa | Raised against human proangiogenic isoforms (exon 8a) | Rabbit polyclonal | Produced in house (Microvascular Research Laboratories, University of Bristol) | 1 mg/mL diluted 1:1,000 |
| VEGF-Axxxb | Against human anti-angiogenic isoforms (exon 8b; 56/8) | Mouse monoclonal | Produced in house (Microvascular Research Laboratories, University of Bristol) | 2.5 mg/mL diluted 1:100 |
| Pan VEGF-A | Against all isoforms of human VEGF-A (A20) | Rabbit polyclonal | Santa Cruz Biotechnology; sc-152 | 200 µg/mL diluted 1:100 |
| VEGF receptor 2 | Soluble extracellular human VEGF-R2 | Mouse monoclonal | Abcam; ab9530 | 1 mg/mL diluted 1:20 |
| FSCs | Recombinant full length bovine S-100 protein | Rabbit polyclonal | Abcam; ab868 | Concentration undetermined by manufacturer; dilution 1:1,000 |
| Melatonin receptor | C terminus of human MT1-R | Goat polyclonal | Santa Cruz; sc-13186 | 200 µg/mL diluted 1:100–2 µg/mL |
| Prolactin | Raised against full length ovine prolactin | Rabbit polyclonal | Lifespan Biosciences; LS-C124425 | Unknown manufacturer concentration; 1:5,000 dilution |
| Proliferation marker | PCNA | Mouse monoclonal | Invitrogen; 08–0110 | 2 μg/mL |
| Endothelial cell marker | Isolectin b4 against using human blood group B erythrocytes | Lectin from Bandeiraea simplicifolia; isolectin B4 (BSI-B4), peroxidase conjugate, lyophilized powder | Sigma Aldrich; L5391 | 200 µg/mL |
| Endothelial cell marker | Raised against murine CD31 | Rabbit polyclonal | Abcam; ab28364 | 2 µg/mL |
Table S2.
Secondary antibodies
| Species raised | Species against | Color (wavelength, nm) | Manufacturer | Cat. no. |
| Goat | Mouse | Green (488) | Life Technologies | A-10680 |
| Donkey | Rabbit | Red (555) | Life Technologies | A-31572 |
| Streptavidin | NA | Green (488) | Life Technologies | S11223 |
| Streptavidin | NA | Red (555) | Life Technologies | S21381 |
Table S3.
Primers
| Name | Sequence |
| VEGF-A FWD 1 | CAAATGTGAATGCAGACCAAAG |
| VEGF-A REV 1 | TGTGTCAGTCTTTCCTGGTGA |
| VEGF-A FWD 2 | CTCACCAAAGCCAGCACATAG |
| VEGF-A REV 2 | GACACAGAACTACCCATAGCCG |
| VEGF-A FWD 3 | CTCACCAAAGCCAGCACATAG |
| VEGF-A REV 3 | ACACAGAACTACCCATAGCCG |
| VEGF-A exon 1 FWD | CGG TGGTACTTGAAAGAC |
| VEGF-A exon 8b REV | CAGAGTGGTCCTTTCTGACTGTGTCTTGCTGGGTATCGGCGGC |
| VEGF-A exon 7 FWD | ATAAAGCAAGGCAAGAAATCCCTG |
| VEGF-A exon 7 FWD2 | GAAATCCCTGTGGGCCTTGCTAGA |
Primary Cell Cultures.
Ovine primary pituitary cultures were produced by careful dissection and dissociation of the PD and PT of three or four pituitaries as previously described (52). Previous studies have demonstrated the validity of this method for producing a reliable hormone output in response to exogenous hormone releasing secretagogues in vitro (52, 56).
Both ELISA methods have been previously described (11, 57, 58). A rhVEGF165b-positive control was included in triplicate for the human VEGF-A ELISA, allowing calculation of VEGF-Atotal concentration to compensate for reduced VEGF-Axxxb affinity of ∼42% as previously published (59). Prolactin was measured by RIA using purified ovine prolactin for standards. A linear relationship was detected when the measured hormone concentration (in nanograms per milliliter) was plotted against the concentration of diluted serum samples.
Statistical Analysis.
In the BS and NBS cultures, a total of five separate experimental treatments were applied to PT cells, and nine experimental treatments were applied to the PD cells. For each treatment, six wells were assigned, and the experiments were repeated independently three times in both seasons with reproducible results. The reported values represent the mean ± SEM. The effects of season and experimental treatment and their interaction on the secretion of VEGF-A and prolactin from ovine primary pituitary cell cultures were examined by using ANOVA followed by Fisher’s post hoc test. Because a season by treatment interaction was observed for each compound, separate ANOVAs were then used to examine the effects of experimental treatment within season. For all other variables, one-way ANOVA was applied. All data were confirmed to be normally distributed by D’Agostino and Pearson omnibus normality test. Data were considered to be statistically significant at P < 0.05; however, wherever detected, smaller log value (P < 0.01, P < 0.001) probabilities are reported.
SI Materials and Methods
Ovine pituitary glands were obtained from ovary-intact females during the BS (December/January) and the NBS (June/July). Animals were killed for commercial reasons at an abattoir (University of Bristol Abattoir, Langford, United Kingdom), and pituitaries were removed immediately after death. During the BS, ewes were confirmed to be sexually active on the basis of a recently formed CL together with the presence of a large follicle (>2 cm). By contrast, in the NBS, ewes were considered to be anestrus when no CL but a corpus albicans was observed in the gonad, and follicles present were <2 mm in diameter.
Immunofluorescent Staining.
Pituitaries assigned for immunofluorescent staining (BS, n = 6; NBS, n = 6) were fixed in Bouin’s solution for 24 h and then moved to 70% (vol/vol) ethanol, and sectioned at 5 μm. Following sequential dehydration, sections were submerged in PBS solution with 0.1% Triton-X (PBS-T) and then 0.01 M sodium citrate buffer (pH 6; Sigma) and heated for 3 min at full power and 12 min at subboiling temperature. Sections were then washed in PBS-T (three times, 5 min each) and blocked in 5% goat serum diluted in 1% BSA PBS-T (0.01%) for 2 h at room temperature. A range of primary antibodies were used for double fluorescent immunohistochemistry, each diluted to a concentration determined during preliminary investigations (Table S1). Secondary antibodies were diluted as outlined in Table S2 and left to incubate on the section for 2 h at room temperature.
cDNA Synthesis and RT-PCR and Quantitative RT-PCR.
Pituitaries assigned for DNA analysis of VEGF-A expression were flash-frozen in liquid nitrogen following dissection. RNA extraction was carried out by TRI reagent method, itself a modification from the original phenol/chloroform extraction developed by Chomczynski and Sacchi (55). Multiple pairs of primers were used to amplify the various VEGF-A isoforms (Table S3). To generate cDNA, 2 μg of RNA and 2 U of RNase-Free DNase (RQ1, M6101; Promega) were incubated in a 1× reaction buffer solution for 1 h at 37 °C, before 1 μL of DNase stop solution was added to terminate the reaction and the sample was heat-inactivated for 10 min at 65 °C. The DNase-treated RNA sample was requantified by using a NanoDrop ND-1000 spectrophotometer, and 1 μg of RNA was resuspended to a total volume of 10 μL. To this, 2 μL of Oligo (dT)15 primers (C1101; Promega) and 1 μL of hexamers (random primers, C1181; Promega) were added. The reaction mix was then incubated at 70 °C for 10 min before immediately being quenched on ice for 5 min. With a final reverse transcription reaction volume of 50 μL, 400 U of MMLV reverse transcriptase (M5301; Promega), 40 U of RNasin ribonuclease inhibitor (N2611; Promega), and 0.5 mM dNTPs (BIO-39049; Bioline) were added to the RNA/primer mix. The reaction was incubated for 1.5 h at 37 °C with a final 70 °C inactivation step. Final concentration of fresh cDNA was determined by spectrophotometry.
For RT-PCR, forward and reverse primer (1 μM; Table S3) were added each with 1.2 mM MgCl2, 200 μM deoxynucleotide triphosphates, and 1 U of Taq polymerase (Abgene/Thermo Fisher). PCR was undertaken for 35 cycles at 95 °C for 1 min, 60 °C for 5 min, and 72 °C for 5 min, with a 2-min, 95 °C denaturing step at the beginning and a 72 °C extension step at the end. PCR products were run on 3% agarose gels containing 0.5 μg/mL ethidium bromide and visualized under a UV transilluminator.
For quantitative RT-PCR, cDNA was added to 5.5 μL of SYBRG fast track with Rox (Kappa Biosystems), with 1 µM of each primer in a total of 18 µL. Samples were loaded in triplicate, and a negative control of water was added. GAPDH was used as a reference gene.
Primary Cell Cultures.
Ovine primary pituitary cultures were produced by using a method previously described (52). Briefly, the PD and PT of three or four pituitaries were carefully dissected and incubated in a 0.1% collagenase D (Boehringer Mannheim) and hyaluronidase (Sigma-Aldrich) solution in a shaking water bath at 37 °C for 75 min. The tissue was then manually dispersed in PBS solution (Sigma-Aldrich), and the mixed pituitary cells were resuspended in M199 medium (Invitrogen) containing 10 mg/mL of insulin, 50 mg/mL of gentamicin, 100 IU/mL of penicillin-streptomycin (Sigma-Aldrich), and 10% steroid-free lamb serum (Invitrogen), before being plated at a density of 200,000 cells/well in 24-well plates. The experiment was repeated three times each in the BS and NBS, totalling 9–12 animals per season. During BS and NBS, cells were maintained in culture for 6 d. M199 media was changed at each time point outlined later. Previous studies have demonstrated the validity of this method for producing a reliable hormone output in response to exogenous hormone releasing secretagogues in vitro (52, 56).
PT cells were assigned one of the following treatments: (i) control, M199 media alone, changed daily at 5 PM and 9 AM; (ii) BS regimen, melatonin (1 µM) administered at 5 PM, removed at 9 AM, media alone administered from 9 AM until 5 PM; or (iii) NBS regimen, melatonin administered at 9 PM, removed at 5 AM, media from 5 AM until 9 PM. Three experimental groups of PT cells per season were used. Six wells were assigned per treatment, three wells used for RNA-based assays, and three wells for protein-based assays. In one repeat of each experiment per season, one well per treatment was used for immunofluorescence. PD cells were treated with the following treatments: (i) control (C), M199 media alone, changed every day at 5 PM and 9 AM; (ii) BS regimen, recombinant human VEGF-A165a (1 nM rhVEGF-A165a), 1 µM melatonin, 0.1 µM TRH, or conditioned media from PT cells treated as described earlier administered at 5 PM, removed at 9 AM, and media alone administered from 9 AM until 5 PM; or (iii) NBS regimen, rhVEGF-A165a melatonin, TRH, or conditioned media from PT cells treated as described earlier, administered at 9 PM, removed at 5 AM, media alone administered from 5 AM until 9 PM. rhVEGF-A165a was administered at 1 nM based on preliminary investigations that produced a VEGF-A dose response to melatonin from PT primary cells in culture.
Total Protein Quantification.
Measurement of total protein was determined using the BioRad Protein Assay. Each sample was measured in triplicate by adding 10 μL per well (from 1:10 to 1:200 dilution, dependent on extract source) to a 96-well protein assay plate (BD Falcon). Serial dilutions of BSA (1,000, 500, 250, 125, 62.5, 31.25, 15.125, and 0 ng/mL) were used to generate a standard curve. BioRad Protein Assay Dye Reagent (BioRad) was diluted 1:5 in PBS solution, and 200 μL per well was added to samples. Finally, the concentration of total protein was measured by using the Opsys MR plate reader (Dynex) at a wavelength of 595 nm and 490 nm.
Protein Extraction and VEGF-A/Hormone Assays.
Protein extraction from cell cultures was carried out using RIPA buffer [50 mM Tris⋅HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal CA-630 (Nonidet P-40), 0.5% sodium deoxycholate, and 0.1% SDS; Sigma] with an additional mixture of proteinase inhibitors and stabilizers (1:50 protease inhibitor mixture; Sigma). For protein extracted from tissue and protein extracted from media, total VEGF-A and VEGF-Axxxb concentrations were determined by VEGF-Axxxb (DY3045; R&D Systems) and human VEGF-A (DY293B; R&D Systems) ELISAs. Protein was extracted from the media following the TCA precipitation procedure. Protein was extracted from tissue by homogenizing the tissue and adding a protease inhibitor mixture and RIPA buffer.
Both ELISA methods have been previously described (11, 57, 58). An rhVEGF-Axxxb–positive control was included in triplicate for the human VEGF ELISA, allowing calculation of VEGF-Atotal concentration to compensate for the reduced VEGF-Axxxb affinity of ∼42% as previously published (59). The concentration of endogenous prolactin in culture wells following the application of PD treatments was measured by RIA using purified ovine prolactin for standards and iodination provided by A. F. Parlow, University of California, Los Angeles, and the National Hormone and Peptide Program, and an anti-ovine PRL antibody (ASMcN R 50) provided by A. S. McNeilly, Medical Research Council Human Reproductive Sciences Unit, Edinburgh, United Kingdom, as previously described (52). A linear relationship was detected when the measured hormone concentration (in nanograms per milliliter) was plotted against the concentration of diluted serum samples.
Acknowledgments
The authors thank the National Hormone and Peptide Program and Dr. A. F. Parlow (University of California, Los Angeles) for prolactin radioimmunoassay standards, and Prof. A. S. McNeilly (Medical Research Council Human Reproductive Sciences Unit, Edinburgh, United Kingdom) for ovine prolactin antibody. This work was supported by the Biotechnology and Biological Sciences Research Council (D.J.T., J.C.-M.), the British Society for Neuroendocrinology (D.J.T.), and the British Heart Foundation (D.O.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618917114/-/DCSupplemental.
References
- 1.Lincoln GA, Clarke IJ, Hut RA, Hazlerigg DG. Characterizing a mammalian circannual pacemaker. Science. 2006;314(5807):1941–1944. doi: 10.1126/science.1132009. [DOI] [PubMed] [Google Scholar]
- 2.Bittman EL, Karsch FJ. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol Reprod. 1984;30(3):585–593. doi: 10.1095/biolreprod30.3.585. [DOI] [PubMed] [Google Scholar]
- 3.Clarke IJ, Campbell R, Smith JT, Prevot V, Wray S. Neuroendocrine control of reproduction. In: Fink G, Pfaff DW, Levine JE, editors. Handbook of Neuroendocrinology. Elsevier; Amsterdam: 2012. pp. 197–236. [Google Scholar]
- 4.de Reviers MM, Ravault JP, Tillet Y, Pelletier J. Melatonin binding sites in the sheep pars tuberalis. Neurosci Lett. 1989;100(1-3):89–93. doi: 10.1016/0304-3940(89)90665-4. [DOI] [PubMed] [Google Scholar]
- 5.Morgan PJ, Williams LM, Davidson G, Lawson W, Howell E. Melatonin receptors on ovine pars tuberalis: Characterization and autoradiographicai localization. J Neuroendocrinol. 1989;1(1):1–4. doi: 10.1111/j.1365-2826.1989.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Lincoln GA, Clarke IJ. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo-pituitary disconnected rams: Evidence for translation of the melatonin signal in the pituitary gland. J Neuroendocrinol. 1994;6(3):251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 7.Gross DS. The mammalian hypophysial pars tuberalis: A comparative immunocytochemical study. Gen Comp Endocrinol. 1984;56(2):283–298. doi: 10.1016/0016-6480(84)90043-1. [DOI] [PubMed] [Google Scholar]
- 8.Tortonese DJ, Brooks J, Ingleton PM, McNeilly AS. Detection of prolactin receptor gene expression in the sheep pituitary gland and visualization of the specific translation of the signal in gonadotrophs. Endocrinology. 1998;139(12):5215–5223. doi: 10.1210/endo.139.12.6365. [DOI] [PubMed] [Google Scholar]
- 9.Bates DO, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–4131. [PubMed] [Google Scholar]
- 10.Houck KA, et al. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 11.Woolard J, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: Mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 12.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437(2):169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68(12):4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 14.Cébe Suarez S, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63(17):2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood. 2011;118(3):816–826. doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- 16.Oltean S, et al. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J Am Soc Nephrol. 2015;26(8):1889–1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnussen AL, et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Invest Ophthalmol Vis Sci. 2010;51(8):4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beazley-Long N, Gaston K, Harper SJ, Orlando A, Bates DO. Novel mechanisms of resistance to vemurafenib in melanoma - V600E B-Raf reversion and switching VEGF-A splice isoform expression. Am J Cancer Res. 2014;5(1):433–441. [PMC free article] [PubMed] [Google Scholar]
- 19.Harper SJ, Bates DO. VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houssay BA, Biasotti A, Sammartino R. Modifications fonctionnelles de l'hypophyse apres les lesions infundibulo-tuberiennes chez le crapaud. C R Seances Soc Biol. 1935;120:725–727. [Google Scholar]
- 21.Daniel PM, Prichard MM. The vascular arrangements of the pituitary gland. Q J Exp Physiol Cogn Med Sci. 1957;42(3):237–248. doi: 10.1113/expphysiol.1957.sp001259. [DOI] [PubMed] [Google Scholar]
- 22.Wood SH, et al. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals. Curr Biol. 2015;25(20):2651–2662. doi: 10.1016/j.cub.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migaud M, Batailler M, Pillon D, Franceschini I, Malpaux B. Seasonal changes in cell proliferation in the adult sheep brain and pars tuberalis. J Biol Rhythms. 2011;26(6):486–496. doi: 10.1177/0748730411420062. [DOI] [PubMed] [Google Scholar]
- 24.Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23(6):261–269. doi: 10.1016/j.tem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Prevot V, et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: Implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94(3):809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145(9):4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Ye R, Goldman SA. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain. Neuroscience. 2013;239(3):139–148. doi: 10.1016/j.neuroscience.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabbour HN, Boddy SC, Lincoln GA. Pattern and localisation of expression of vascular endothelial growth factor and its receptor flt-1 in the ovine pituitary gland: Expression is independent of hypothalamic control. Mol Cell Endocrinol. 1997;134(2):91–100. doi: 10.1016/s0303-7207(97)00158-5. [DOI] [PubMed] [Google Scholar]
- 29.von Gall C, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5(3):234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- 30.McArdle CA, Franklin J, Green L, Hislop JN. Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors. J Endocrinol. 2002;173(1):1–11. doi: 10.1677/joe.0.1730001. [DOI] [PubMed] [Google Scholar]
- 31.Clarke IJ, Burman KJ, Doughton BW, Cummins JT. Effects of constant infusion of gonadotrophin-releasing hormone in ovariectomized ewes with hypothalamo-pituitary disconnection: Further evidence for differential control of LH and FSH secretion and the lack of a priming effect. J Endocrinol. 1986;111(1):43–49. doi: 10.1677/joe.0.1110043. [DOI] [PubMed] [Google Scholar]
- 32.McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: Regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463–476. [PubMed] [Google Scholar]
- 33.Nowak DG, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121(pt 20):3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams LM. Melatonin-binding sites in the rat brain and pituitary mapped by in-vitro autoradiography. J Mol Endocrinol. 1989;3(1):71–75. doi: 10.1677/jme.0.0030071. [DOI] [PubMed] [Google Scholar]
- 35.Denef C. Paracrinicity: The story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20(1):1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fauquier T, Guérineau NC, McKinney RA, Bauer K, Mollard P. Folliculostellate cell network: A route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci USA. 2001;98(15):8891–8896. doi: 10.1073/pnas.151339598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila-Porcile E. Le réseau des cellules folliculo-stellaireset les follicules de l’adénohypophyse du rat (Pars distalis) Cell Tissue Res. 1972;129(3):328–369. [PubMed] [Google Scholar]
- 38.Henderson HL, Hodson DJ, Gregory SJ, Townsend J, Tortonese DJ. Gonadotropin-releasing hormone stimulates prolactin release from lactotrophs in photoperiodic species through a gonadotropin-independent mechanism. Biol Reprod. 2008;78(2):370–377. doi: 10.1095/biolreprod.107.064063. [DOI] [PubMed] [Google Scholar]
- 39.Acosta M, Mohamed F. Effect of the photoperiod and administration of melatonin on folliculostellate cells of the pituitary pars distalis of adult male viscacha (Lagostomus maximus maximus) Acta Histochem. 2011;113(6):640–646. doi: 10.1016/j.acthis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Vitale ML, Cardin J, Gilula NB, Carbajal ME, Pelletier RM. Dynamics of connexin 43 levels and distribution in the mink (Mustela vison) anterior pituitary are associated with seasonal changes in anterior pituitary prolactin content. Biol Reprod. 2001;64(2):625–633. doi: 10.1095/biolreprod64.2.625. [DOI] [PubMed] [Google Scholar]
- 41.Christian HC, Imirtziadis L, Tortonese D. Ultrastructural changes in lactotrophs and folliculo-stellate cells in the ovine pituitary during the annual reproductive cycle. J Neuroendocrinol. 2015;27(4):277–284. doi: 10.1111/jne.12261. [DOI] [PubMed] [Google Scholar]
- 42.Klosen P, et al. The mt1 melatonin receptor and RORbeta receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J Histochem Cytochem. 2002;50(12):1647–1657. doi: 10.1177/002215540205001209. [DOI] [PubMed] [Google Scholar]
- 43.Yeomans A, Thompson N, Castle-Miller J, Bates DO, Tortonese DJ. Mechanisms underlying pituitary microvasculature remodelling in Thoroughbred horses during the annual reproductive cycle. Reprod Abstracts. 2014;1:P342. [Google Scholar]
- 44.Morgan PJ, et al. The ovine pars tuberalis secretes a factor(s) that regulates gene expression in both lactotropic and nonlactotropic pituitary cells. Endocrinology. 1996;137(9):4018–4026. doi: 10.1210/endo.137.9.8756579. [DOI] [PubMed] [Google Scholar]
- 45.Dupré SM, et al. Identification of Eya3 and TAC1 as long-day signals in the sheep pituitary. Curr Biol. 2010;20(9):829–835. doi: 10.1016/j.cub.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckstein N, et al. Effects of substance P on anterior pituitary secretion in the female rhesus monkey. Neuroendocrinology. 1980;31(5):338–342. doi: 10.1159/000123098. [DOI] [PubMed] [Google Scholar]
- 47.Skinner DC. Rethinking the stalk effect: A new hypothesis explaining suprasellar tumor-induced hyperprolactinemia. Med Hypotheses. 2009;72(3):309–310. doi: 10.1016/j.mehy.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant S, Melan M, Latimer J, Witt-Enderby P. Melatonin and breast cancer: Cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med. 2009;11:e5. doi: 10.1017/S1462399409000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meininger CJ, Schelling ME, Granger HJ. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am J Physiol. 1988;255(3 pt 2):H554–H562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- 50.Pueyo ME, Chen Y, D’Angelo G, Michel JB. Regulation of vascular endothelial growth factor expression by cAMP in rat aortic smooth muscle cells. Exp Cell Res. 1998;238(2):354–358. doi: 10.1006/excr.1997.3864. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, et al. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci. 2009;29(26):8493–8505. doi: 10.1523/JNEUROSCI.1321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory SJ, Townsend J, McNeilly AS, Tortonese DJ. Effects of prolactin on the luteinizing hormone response to gonadotropin- releasing hormone in primary pituitary cell cultures during the ovine annual reproductive cycle. Biol Reprod. 2004;70(5):1299–1305. doi: 10.1095/biolreprod.103.022806. [DOI] [PubMed] [Google Scholar]
- 53.Hodson DJ, Henderson HL, Townsend J, Tortonese DJ. Photoperiodic modulation of the suppressive actions of prolactin and dopamine on the pituitary gonadotropin responses to gonadotropin-releasing hormone in sheep. Biol Reprod. 2012;86(4):122–130. doi: 10.1095/biolreprod.111.096909. [DOI] [PubMed] [Google Scholar]
- 54.Hodson DJ, Townsend J, Gregory SJ, Walters C, Tortonese DJ. Role of prolactin in the gonadotroph responsiveness to gonadotrophin-releasing hormone during the equine annual reproductive cycle. J Neuroendocrinol. 2010;22(6):509–517. doi: 10.1111/j.1365-2826.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 55.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 56.Tortonese DJ, Lincoln GA. Effects of melatonin in the mediobasal hypothalamus on the secretion of gonadotrophins in sheep: Role of dopaminergic pathways. J Endocrinol. 1995;146(3):543–552. doi: 10.1677/joe.0.1460543. [DOI] [PubMed] [Google Scholar]
- 57.Bills VL, et al. Failure to up-regulate VEGF165b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin Sci (Lond) 2009;116(3):265–272. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perrin RM, et al. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48(11):2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 59.Varey AH, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: Balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98(8):1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]