Abstract
Self-reactive B cells are tolerized at various stages of B-cell development and differentiation, including the immature B-cell stage (central tolerance) and the germinal center (GC) B-cell stage, and B-cell tolerance involves various mechanisms such as deletion, anergy, and receptor editing. Self-reactive B cells generated by random immunoglobulin variable gene rearrangements are tolerized by central tolerance and anergy in the periphery, and these processes involve apoptosis regulated by Bim, a pro-apoptotic member of the Bcl-2 family, and regulation of B-cell signaling by various phosphatases, including SHIP-1 and SHP-1. Self-reactive B cells generated by somatic mutations during GC reaction are also eliminated. Fas is not directly involved in this process but prevents persistence of GC reaction that allows generation of less stringently regulated B cells, including self-reactive B cells. Defects in self-tolerance preferentially cause lupus-like disease with production of anti-nuclear antibodies, probably due to the presence of a large potential B-cell repertoire reactive to nucleic acids and the presence of nucleic acid-induced activation mechanisms in various immune cells, including B cells and dendritic cells. A feed-forward loop composed of anti-nuclear antibodies produced by B cells and type 1 interferons secreted from nucleic acid-activated dendritic cells plays a crucial role in the development of systemic lupus erythematosus.
Keywords: b cell, b cell tolerance, autoimmunity, self-reactive b cells
Introduction
Studies on autoantibody-transgenic mice and analyses of the repertoire of various B-cell subsets in humans and mice have demonstrated that self-reactive B cells are negatively regulated at various stages of B-cell development and maturation, including the immature B-cell stage in bone marrow, transitional B-cell stage, and germinal center (GC) B-cell stage 1, 2. There are multiple mechanisms for B-cell tolerance, such as deletion, functional inactivation (anergy), and alteration of antigen specificity by replacement of immunoglobulin (Ig) variable (V) gene segments (receptor editing) 3, 4. Some self-reactive B cells emerge in the peripheral lymphoid organs without being deleted or functionally inactivated but do not differentiate to plasma cells even in the presence of interaction with self-antigens as if they are ignored (clonal ignorance) 2. In some autoantibody-transgenic mice, self-reactive B cells are accumulated in marginal zone B cells 5– 7, suggesting that these self-reactive B cells are positively selected to differentiate to marginal zone B cells. However, self-reactive marginal zone B cells are also tolerized 8.
Autoantibodies to nuclear antigens are characteristically produced in patients with systemic lupus erythematosus (SLE) and its animal models and play a pathogenic role in the development of this disease. Production of these autoantibodies requires a break of B-cell tolerance because B cells reactive to nuclear antigens have been shown to be tolerized 1, 9. Genes expressed in B cells are enriched in SLE-associated genes whereas those expressed in CD4 + T cells are enriched in genes associated with rheumatoid arthritis 10, suggesting that defects in B cells play a central role in the development of SLE. In mice, SLE-like disease is the most common autoimmune disease developed by genetic defects in B cells 11. These defects include those that break B-cell tolerance by regulating B-cell activation and survival irrespective of antigen specificity 12, 13. Thus, general (antigen-non-specific) defects in B-cell tolerance induce production of autoantibodies to nuclear antigens, suggesting the presence of mechanisms for preferential production of these autoantibodies. One of the mechanisms appears to be a large potential B-cell repertoire reactive to nuclear antigens 1. In both humans and mice, reactivity to nuclear antigens is demonstrated in more than half of immature B cells in which the B-cell repertoire is formed by random recombination of Ig V gene segments but not yet selected by antigens. Another mechanism involves nucleic acid (NA) sensors that activate various cell types, including B cells, upon interaction with NAs 14– 16. NA sensors play a crucial role in the defense against microbes, especially viruses, through recognition of microbial DNA and RNA but also are involved in the activation of B cells reactive to nuclear antigens containing NAs, leading to production of autoantibodies to nuclear antigens. Thus, development of SLE involves both functional defects in B cells and NA-induced activation of immune cells. Crucial roles of these mechanisms are supported by the findings that SLE-associated genes in humans contain a number of genes involved in the regulation of B-cell signaling, NA degradation, or sensing of NAs, including the NA sensors TLR7 and TLR9 17, 18. In this review, I discuss mechanisms for B-cell tolerance and its break in SLE with a focus on regulation of B-cell signaling and NA-mediated immune cell activation. I also discuss tolerance of self-reactive B cells generated by somatic mutations of Ig V genes in GC reaction and the contribution of GC reaction in autoimmunity.
Nucleic acid-induced B-cell activation and interferons
TLR7 and TLR9 are endosome-localizing innate NA sensors recognizing RNA and DNA, respectively, and are involved in immune responses to microbes, especially viruses, by recognizing microbial NAs 14. They are also involved in autoantibody production to NA-related self-antigens 15, 16. Patients with SLE produce autoantibodies to the complexes of NAs and nuclear proteins such as nucleosomes and Sm/RNP. Nucleosomes and Sm/RNP contain DNA and RNA, respectively, and thus are recognized by TLR9 and TLR7, respectively 19, 20, both of which are expressed in B cells as well as innate immune cells. When these nuclear self-antigens are released from dead cells, they interact with B cells reactive to these self-antigens through B-cell antigen receptor (BCR) and are translocated together with BCR to endosomes where they are recognized by TLR7 and TLR9 ( Figure 1). In these self-reactive B cells, the combination of signaling through BCR and co-stimulatory signaling through TLRs induces cell activation, leading to production of autoantibodies to the nuclear self-antigens. Complexes of NAs and nuclear proteins are more immunogenic than NAs alone and this is probably due to resistance to degradation by nucleases. Deficiency in TLR7 and TLR9 markedly reduces autoantibody to Sm/RNP and DNA, respectively, in lupus-prone mice 21, clearly indicating that B-cell activation mediated by NA sensors facilitates production of autoantibodies to nuclear antigens. Although both TLR7 and TLR9 are involved in autoantibody production to nuclear self-antigens, TLR7 but not TLR9 is required for the development of autoimmune disease in a mouse model 21. TLR9 rather ameliorates TLR7-dependent development of lupus-like disease 22, 23 by competing endosomal transport with TLR7 24. On the basis of these findings, together with the genetic findings, demonstrating the association of RNA-sensing pathways with lupus, a dominant role of RNA-related antigens in the development of lupus is suggested 16, although the mechanism is not yet understood.
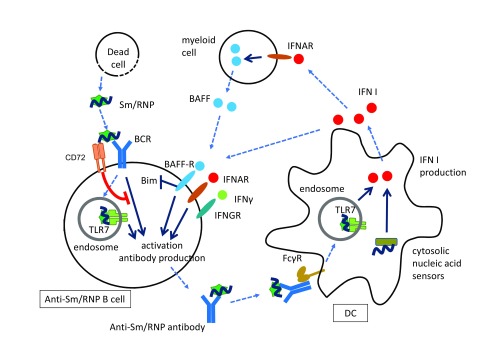
Figure 1. A feed-forward loop consisting of anti-Sm/RNP antibody and type 1 interferons (IFN I) and Sm/RNP-specific B-cell inhibition by CD72.
The nuclear self-antigen Sm/RNP released from dead cells is recognized by Sm/RNP-reactive B cells and generates both B-cell antigen receptor (BCR) signaling and co-stimulatory signaling through TLR7 in the endosome. The combination of these two signaling pathways induces cell activation and production of anti-Sm/RNP antibody. The immune complex consisting of Sm/RNP and anti-Sm/RNP antibody is endocytosed by dendritic cells (DCs) through interaction with Fcγ receptor and is recognized by TLR7 in endosome, resulting in the production of IFN I. IFN I is also produced through recognition of nucleic acids (NAs) by cytosolic NA sensors. IFN I activates B cells through receptor for IFN I (IFNAR) to induce IFN-inducible genes, including TLRs. IFN I also activates B cells indirectly by inducing B cell-activating factor (BAFF) expression in myeloid cells. BAFF inhibits expression of Bim and perturbs B-cell tolerance. IFNγ is also involved in the activation of self-reactive B cells. CD72 recognizes Sm/RNP and specifically inhibits BCR signaling when BCR recognizes Sm/RNP, thereby inhibiting production of anti-Sm/RNP antibody. BAFF-R, B cell-activating factor receptor.
In leukocytes from patients with SLE, expression of genes responsive to type 1 interferons (IFN I) is markedly augmented 25, 26 and this is probably due to augmented IFN I production. The augmented IFN I production in SLE appears to involve TLR7 and TLR9 as signaling through these TLRs induces IFN I expression in innate immune cells 27, especially in plasmacytoid dendritic cells (DCs), which are capable of producing IFN I in large quantity. Upon forming immune complexes with autoantibodies, nuclear self-antigens, such as Sm/RNP 28, and DNA complexed with HMGB1 29 are endocytosed by FcγR in DCs and recognized by TLR7 and TLR9 in endosomes, leading to the production of a large amount of IFN I ( Figure 1). Some of the patients with type I interferonopathies, a set of Mendelian disorders such as Aicardi-Goutières syndrome (AGS) characterized by constitutive IFN I production, and their animal models develop lupus-like autoimmune disease as well as various inflammatory lesions 30. These observations suggest that IFN I perturbs self-tolerance to nuclear self-antigens and induces the development of lupus. Involvement of IFN I in development of lupus is further supported by the finding on a pristane-induced lupus model. Although most of the lupus-prone mice do not show a strong IFN signature, the pristane-induced lupus model shows a strong TLR7-dependent IFN signature 31 and requires the receptor for IFN I (IFNAR) for both autoantibody production and development of lupus 32. IFN I activates the expression of a large number of genes in various immune cells, including genes involved in B-cell activation 33 such as TLRs in B cells 34 and genes for B cell-activating molecules such as B cell-activating factor (BAFF) 35 ( Figure 1). The products of IFN I-inducible genes may collectively abrogate self-tolerance and activate self-reactive B cells. The majority of mutations found in AGS are located in genes involved in the metabolism of cytosolic NAs and their recognition 30. Some of these genes such as TREX1 36 encoding a cytosolic nuclease and IFIH1 37 encoding the cytosolic RNA sensor MDA5 are associated with SLE in humans. Thus, IFN I production caused by augmented responses to cytosolic NAs appears to be involved in SLE as well as AGS. Taken together, autoantibodies to nuclear antigens complexed with self-antigens induce IFN I production in DCs, and IFN I induces production of the autoantibodies in B cells, resulting in a feed-forward loop ( Figure 1). This feed-forward loop may cause a strong IFN signature and massive production of autoantibodies to nuclear self-antigens characteristic of SLE.
Although the pristane-induced lupus model shows a type I IFN signature and requires IFN I for autoantibody production and disease development, IFNγ is overproduced and plays a crucial role in various other mouse models such as MLR-Faslpr/lpr, Sle1b, and Wiskott-Aldrich syndrome (WAS) chimera mice 31, 38–40. In both WAS chimera and Sle1b mice, B cell-specific deletion of IFNγ receptor abrogates spontaneous GC formation, autoantibody production, and the development of lupus-like disease 39, 40, suggesting a crucial role of IFNγ in breach of B-cell tolerance. In spite of the considerable overlap between genes induced by IFN I and those induced by IFNγ, IFNγ-induced gene expression is evident in patients with SLE because these patients overexpress the IFNγ-inducible genes whose expression is genes suppressed by in vivo IFNγ blockade 41. Thus, IFNγ as well as IFN I may play a role in the pathogenesis of human SLE as well as mouse models.
Regulation of central tolerance and clonal anergy by apoptosis and phosphatases
Self-reactive B cells generated in bone marrow by random Ig V gene rearrangements are tolerized by central tolerance such as deletion, anergy and receptor editing. It is already established that Bim, a pro-apoptotic member of the Bcl-2 family, plays a crucial role in the deletion and anergy of self-reactive B cells generated in bone marrow by regulating apoptosis 42– 44. Self-reactive B cells in Bim −/− autoantibody-transgenic mice clearly escape from both deletion and anergy 42, 43. Bim is required for BCR ligation-induced B-cell apoptosis that appears to be involved in the deletion of self-reactive B cells 42. Bim is also involved in premature death of anergic B cells as they are less sensitive to survival signaling generated by BAFF 43 that induces B-cell survival by reducing Bim expression 45. Thus, Bim-mediated apoptosis plays a crucial role in both the deletion and anergy of self-reactive B cells. Breach of deletion and anergy in self-reactive Bim −/− B cells may contribute to the development of lupus-like disease in Bim −/− mice 46.
The lipid phosphatase SHIP-1 and the non-receptor type protein tyrosine phosphatases (PTPs) SHP-1 and LYB/PEP regulate B-cell tolerance and the development of autoimmune diseases 47, 48. A recent study by Getahun et al. 48 demonstrated that inducible deletion of either SHP-1 or SHIP-1 reverses anergy of DNA-reactive B cells and allows spontaneous differentiation of these self-reactive B cells to plasma cells. This result clearly indicates that anergy of self-reactive B cells is reversible and that both SHP-1 and SHIP-1 are required for maintenance of anergy. B cell-specific deletion of SHP-1 or SHIP-1 causes severe lupus-like disease with autoantibody production 12, 13, suggesting that a functional defect in B cells caused by deletion of SHP-1 or SHIP-1 is sufficient to abrogate B-cell tolerance and to develop autoimmune disease.
In B cells, both SHP-1 and SHIP-1 negatively regulate signaling through BCR. SHIP-1 dephosphorylates phosphatidyl inositol 3,4,5-triphosphate (PI(3,4,5)P3), required for phosphatidyl inositol 3-kinase (PI-3K)-mediated activation of AKT, which in turn activates various signaling molecules, including mechanistic target of rapamycin (mTOR), and regulates cell activation processes, including metabolism, proliferation, and cytoskeletal changes 49. The PI-3K pathway as well as the nuclear factor-kappa B (NF-κB) pathway plays a crucial role in BCR and BAFF-R signaling for B-cell survival and activation 50, 51. Thus, SHIP-1 inhibits B-cell survival and activation by negatively regulating the PI-3K pathway. SHP-1 dephosphorylates proximal BCR signaling molecules such as Igα/Igβ and SLP-65/BLNK 52 required for BCR signaling, including the PI-3K pathway. Both SHP-1 and SHIP-1 contain SH2 domains, and their activation requires binding of these SH2 domains to tyrosine-phosphorylated proteins. When BCR interacts with antigens, BCR-associated tyrosine kinases such as Syk and Lyn phosphorylate various cytoplasmic signaling molecules 53. Lyn also phosphorylates B-cell co-receptors, including CD19, CD22, PIR-B, and CD72. Upon phosphorylation, CD19 recruits and activates PI-3K. In contrast, other co-receptors such as CD22, PIR-B, and CD72 recruit SHP-1 at the phosphorylated immuno-receptor tyrosine-based inhibition motifs (ITIMs) in their cytoplasmic regions and activate SHP-1 54 ( Figure 2). Although fully phosphorylated immuno-receptor tyrosine-based activation motifs (ITAMs) in Igα/Igβ recruit the tyrosine kinase Syk, these ITAMs are partially phosphorylated in anergic self-reactive B cells. The partially phosphorylated ITAMs recruit and activate SHIP-1 instead of Syk 47. Probably owing to continuous interaction of BCR with self-antigens in self-reactive B cells, both SHP-1 and SHIP-1 are constitutively activated in anergic B cells and play a crucial role in the maintenance of anergy by suppressing the PI-3K/AKT pathway.
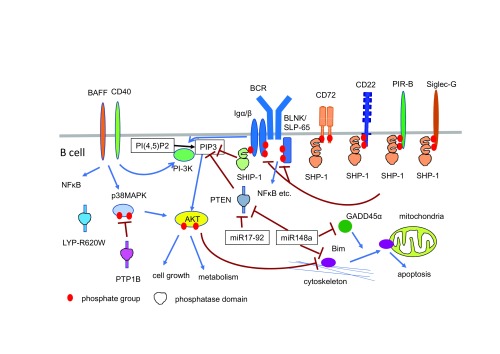
Figure 2. B-cell signaling pathways regulating B-cell tolerance and autoimmunity involve phosphatases and microRNAs.
Phosphatases such as SHP-1, SHIP-1, and LYP-R620W (and also mouse ortholog PEP-R619W) and microRNAs such as miR148 and miR17-92 are demonstrated to reverse central B-cell tolerance, B-cell anergy or both. PTP1B, a protein tyrosine phosphatase, and phosphatase and tensin homolog (PTEN), a lipid phosphatase, are also involved in B-cell tolerance as B cell-specific deletion of these genes causes lupus-like disease 56, 88. SHIP-1 is activated by mono-phosphorylated immuno-receptor tyrosine-based activation motif at Igα/β. Both SHIP-1 and PTEN dephosphorylate PIP3 required for activation of AKT involved in cell activation and survival. SHP-1 is activated by phosphorylated immuno-receptor tyrosine-based inhibition motifs at various inhibitory co-receptors such as CD72, CD22, PIR-B, and Siglec-G and dephosphorylates proximal B-cell antigen receptor (BCR) signaling molecules such as Igα/β and BLNK/SLP-65, thereby reducing BCR signaling, including AKT activation. PTP1B dephosphorylates p38MAPK and negatively regulates AKT activation induced by signaling through CD40 and B cell-activating factor (BAFF) receptor. LYP-R620W and its mouse ortholog PEP-R619W perturb B-cell tolerance. The microRNA miR148a inhibits expression of Bim, GADD45α, involved in Bim translocation to mitochondria, and PTEN, thereby suppressing Bim-mediated B-cell deletion and augmenting AKT activation. PTEN expression is also suppressed by miR17-92. NF-κB, nuclear factor-kappa B; PI-3K, phosphatidyl inositol 3-kinase; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PTP, protein tyrosine phosphatase.
PTP1B is another non-receptor type PTP known to regulate metabolic signaling pathway 55. B cell-specific PTP1B deficiency causes augmented B-cell responses to BAFF, CD40 ligation, and lipopolysaccharide but not BCR ligation and induces lupus-like disease with autoantibody production 56. PTP1B therefore appears to regulate B-cell tolerance as is the case for SHP-1, although PTP1B regulates a signaling pathway distinct from those regulated by SHP-1 ( Figure 2). PTP1B dephosphorylates p38 MAPK as a direct substrate and regulates AKT.
The crucial role of both apoptosis and regulation of the PI-3K pathway in B-cell tolerance is also demonstrated in the studies to isolate microRNAs that inhibit deletion of self-reactive B cells at the immature and mature B-cell stage 57, 58. These studies demonstrated that expression of miR-148a or the miR17-92 family reverses deletion of self-reactive B cells. miR-148a protects B cells from deletion by suppressing Gadd45α, phosphatase and tensin homolog (PTEN), and Bim ( Figure 2). PTEN is a lipid phosphatase that regulates the PI-3K pathway by catalyzing PI(3,4,5)P3. Gadd45α was reported to induce translocation of Bim to mitochondria where Bim inhibits apoptosis 59, and its defect causes lupus-like disease 60. miR17-92 also regulates PTEN 57, 61. Thus, Bim and the PI-3K pathway regulated by PTEN play a crucial role in microRNA-mediated regulation of B-cell tolerance.
PEP is a mouse ortholog of human LYP encoded by PTPN22. The PTPN22-C1858T haplotype that encodes LYP-R620W is associated with various autoimmune diseases, including SLE, Graves’ disease, type 1 diabetes, and rheumatoid arthritis 17, 18. PEP/LYP binds to Csk, but this association is disrupted in LYP-R620W 62. Although PEP-deficient mice do not show much phenotype in B cells, mice expressing PEP-R619W carrying the corresponding mutation with human LYP-R620W show hyperactivity of both B and T cells and development of lupus-like disease 63, 64, suggesting that functional properties of LYP acquired by the R620W mutation cause B-cell hyperactivity. In humans, both transitional and mature naïve B cells from individuals with LYP-R620W show much higher frequencies in self-reactive B cells 65, indicating defects in central B-cell tolerance. This defect is corrected by enzymatic inhibitor of LYP 66. Thus, either augmented phosphatase activity or altered functional property due to lack of Csk binding in LYP-R620W may perturb central B-cell tolerance, although its substrates regulating tolerance are not yet clear.
Nuclear antigen-specific tolerance mechanism
Among various B-cell inhibitory co-receptors that activate SHP-1, CD72 plays a unique role in specifically tolerizing B cells reactive to nuclear antigens 67. Recently, Akatsu et al. demonstrated that CD72 specifically binds to Sm/RNP and that CD72-mediated signal inhibition is induced when B cells interact with Sm/RNP through BCR, leading to inhibition of B-cell responses to Sm/RNP ( Figure 1) 67. Sm/RNP may co-ligate Sm/RNP-reactive BCR and CD72, thereby inducing phosphorylation of the CD72 ITIM by Lyn associated with BCR, the event required for SHP-1 activation and signal suppression 54. This finding is consistent with the previous findings that CD72-deficient mice develop lupus-like disease much more severely than mice deficient in other inhibitory receptors such as CD22 and PIR-B 68, 69, although CD72 does not regulate polyclonal BCR signaling induced by anti-IgM antibody 70. By specifically suppressing signaling through BCR reactive to nuclear antigens, CD72 strongly inhibits the development of lupus without affecting polyclonal BCR signaling.
NA sensors, including TLR7, respond to microbial RNA better than endogenous RNA by recognizing the structural features of microbial RNA such as dsRNA and 5′-triphosphate RNA as well as features such as localization of RNA 71, 72. Nonetheless, TLR7 plays a crucial role in autoimmune responses by recognizing the RNA-containing self-antigen Sm/RNP 20, 21. Thus, mechanisms intrinsic to TLR7 may not completely distinguish self-RNA from microbial RNA and CD72 is required for complete suppression of responses to self-RNA. CD72 appears to recognize RNA-related self-antigens but not microbial RNA 67. Microbial RNA is thus distinguished from self-RNA by both mechanisms intrinsic in NA sensors and specific recognition of NA-containing self-antigen by CD72.
B-1 cells are suggested to play a role in autoimmune diseases because (1) self-reactive and poly-reactive B cells are positively selected and accumulated in B-1 cells and (2) B-1 cell expansion is associated with autoimmune diseases in both humans and mice 73. Indeed, B-cell SHP-1 regulates both development of lupus-like disease and B-1 cell expansion 12. However, CD72 regulates the former but not the latter. In contrast, mice deficient in Siglec-10, a SHP-1-recruiting inhibitory receptor abundantly expressed in B-1 cells, show marked expansion of B-1 cells 74 but development of only mild disease in aged mice older than 1 year of age 68. Thus, SHP-1 regulates B-1 cell expansion and development of lupus-like disease through distinct SHP-1-recruiting receptors, Siglec-G and CD72, respectively, and B-1 cell expansion does not necessarily associate with development of autoimmune disease.
Tolerance of germinal center B cells and maturation of self-reactive B cells to plasma cells
Antigen-stimulated B cells differentiate to plasma cells either directly by extrafollicular pathway or through GC reaction, in which B cells undergo Ig diversification by somatic hypermutation in the Ig V region and are selected for production of high-affinity antibody. It is established that somatic mutations of Ig V genes play a role in the generation of self-reactive B cells 75, 76. Comparison of the sequence of autoantibodies and their germline genes demonstrated that many of the autoantibodies are generated from non-self-reactive antibodies and acquire self-reactivity by somatic mutations in Ig V regions, although some autoantibodies are derived from germline-encoded autoantibodies.
Involvement of GC reaction in autoantibody production in lupus is further supported by indirect evidence. First, mice that spontaneously develop lupus show spontaneous GC reaction 77, although immunization is required for GC formation in normal mice. Second, GC B cells strongly express Fas, a member of tumor necrosis factor receptor family transmitting apoptotic signaling. B cell-specific deletion of Fas induces the development of lupus-like disease 78, suggesting that Fas-mediated apoptosis of self-reactive GC B cells is involved in self-tolerance for nuclear antigens.
The presence of self-tolerance that tolerizes self-reactive GC B cells generated by somatic mutations was clearly demonstrated by using transgenic mice for anti-hen egg lysozyme (HEL) antibody and mice transgenic for mutated HEL as a surrogate self-antigen. In this elegant experimental system, the mutated HEL is recognized by somatically mutated anti-HEL antibody generated upon affinity maturation but not by un-mutated anti-HEL antibody. Therefore, B cells reactive to the mutated HEL represent self-reactive B cells generated by somatic mutations. This study demonstrated that self-reactive B cells generated by somatic mutations are efficiently eliminated if the reactive self-antigens are present within GCs 79, 80. In the absence of Fas, self-reactive B cells generated by somatic mutations are efficiently eliminated, but GC reaction persists for a prolonged period and generates “rogue GC B cells” that accumulate somatic mutations but are not stringently selected. The “rogue B cells” show a defect in affinity maturation and gain self-reactivity 81. Thus, Fas is not directly required for elimination of self-reactive B cells generated by somatic mutations but is required for the prevention of prolonged GC reaction that generates “rogue GC B cells” due to less stringent selection, thereby indirectly inhibiting generation of self-reactive GC B cells.
Although evidence suggests the involvement of GC reaction in the generation of self-reactive B cells, studies using rheumatoid factor-transgenic mice demonstrated that self-reactive B cells are excluded from GCs and differentiate to plasma cells by the extrafollicular pathway accompanied with somatic mutations of Ig V genes 82, 83. The crucial role of the extrafollicular pathway in autoantibody production is also supported by the deep sequencing analysis of Ig V genes in B cells from patients with SLE 84. This analysis revealed that the Ig V regions of plasma blasts from patients with SLE contain fewer somatic mutations compared with those generated by vaccination and are similar in sequence to those of recently activated B cells but not memory B cells. Thus, involvement of GC reaction and defect in the GC checkpoint in autoantibody production in autoimmune diseases need to be further addressed in the future.
Conclusions
Self-reactive B cells are tolerized by multiple different mechanisms at multiple different B-cell differentiation stages. Autoantibody production therefore requires self-reactive B cells to survive multiple selections at different B-cell differentiation stages. This explains the synergy of genetic defects such as deficiency of Fas and Bim 85– 87 and deficiency of CD72 and Fas 70 in the development of severe autoimmune disease. Nonetheless, various single-gene defects cause autoantibody production and autoimmune diseases. At least some of these genes may breach only a part of the checkpoints, but their defect is sufficient for autoantibody production probably because each checkpoint is not able to completely deplete self-reactive B cells and may become less stringent by aging and environmental factors. Moreover, the presence of multiple mechanisms suggests multiple pathways for autoantibody production, including GC and extrafollicular pathways. Further studies will reveal more precise mechanisms by which self-reactive B cells are generated and differentiate to autoantibody-producing cells in autoimmune disease.
Acknowledgments
I thank Ji-Yang Wang at Fudan University (Shanghai) for critically reading the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Robert Brink, Immunology Division, Garvan Institute of Medical Research, Darlinghurst, NSW, 2010, Australia; Vincent's Clinical School, UNSW Australia, Darlinghurst, NSW 2010, Australia
Changchun Xiao, Department of Immunology and Microbial Science, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California, 92037, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Wardemann H, Nussenzweig MC: B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. 10.1016/S0065-2776(07)95003-8 [DOI] [PubMed] [Google Scholar]
- 2. Shlomchik MJ: Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28(1):18–28. 10.1016/j.immuni.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 3. Pelanda R, Torres RM: Central B-cell tolerance: where selection begins. Cold Spring Harb Perspect Biol. 2012;4(4):a007146. 10.1101/cshperspect.a007146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cambier JC, Gauld SB, Merrell KT, et al. : B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7(8):633–43. 10.1038/nri2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandik-Nayak L, Racz J, Sleckman BP, et al. : Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J Exp Med. 2006;203(8):1985–98. 10.1084/jem.20060701 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Balázs M, Martin F, Zhou T, et al. : Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–52. 10.1016/S1074-7613(02)00389-8 [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Li H, Weigert M: Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195(2):181–8. 10.1084/jem.20011453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishi Y, Higuchi T, Phoon S, et al. : Apoptotic marginal zone deletion of anti-Sm/ribonucleoprotein B cells. Proc Natl Acad Sci U S A. 2012;109(20):7811–6. 10.1073/pnas.1204509109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suurmond J, Calise J, Malkiel S, et al. : DNA-reactive B cells in lupus. Curr Opin Immunol. 2016;43:1–7. 10.1016/j.coi.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu X, Kim H, Stahl E, et al. : Integrating Autoimmune Risk Loci with Gene-Expression Data Identifies Specific Pathogenic Immune Cell Subsets. Am J Hum Genet. 2011;89(5):682. 10.1016/j.ajhg.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo Y, Orme J, Mohan C: A genopedia of lupus genes - lessons from gene knockouts. Curr Rheumatol Rev. 2013;9(2):90–9. 10.2174/1573397111309020003 [DOI] [PubMed] [Google Scholar]
- 12. Pao LI, Lam K, Henderson JM, et al. : B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27(1):35–48. 10.1016/j.immuni.2007.04.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Maxwell MJ, Duan M, Armes JE, et al. : Genetic segregation of inflammatory lung disease and autoimmune disease severity in SHIP-1 -/- mice. J Immunol. 2011;186(12):7164–75. 10.4049/jimmunol.1004185 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Blasius AL, Beutler B: Intracellular toll-like receptors. Immunity. 2010;32(3):305–15. 10.1016/j.immuni.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 15. Marshak-Rothstein A: Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–35. 10.1038/nri1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crampton SP, Bolland S: Spontaneous activation of RNA-sensing pathways in autoimmune disease. Curr Opin Immunol. 2013;25(6):712–9. 10.1016/j.coi.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harley IT, Kaufman KM, Langefeld CD, et al. : Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10(5):285–90. 10.1038/nrg2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohan C, Putterman C: Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. 2015;11(6):329–41. 10.1038/nrneph.2015.33 [DOI] [PubMed] [Google Scholar]
- 19. Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. : Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7. 10.1038/416603a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Lau CM, Broughton C, Tabor AS, et al. : RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–7. 10.1084/jem.20050630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen SR, Shupe J, Nickerson K, et al. : Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–28. 10.1016/j.immuni.2006.07.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Nickerson KM, Christensen SR, Shupe J, et al. : TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Santiago-Raber M, Dunand-Sauthier I, Wu T, et al. : Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34(4):339–48. 10.1016/j.jaut.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 24. Fukui R, Saitoh S, Kanno A, et al. : Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35(1):69–81. 10.1016/j.immuni.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 25. Baechler EC, Batliwalla FM, Karypis G, et al. : Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Bennett L, Palucka AK, Arce E, et al. : Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uematsu S, Akira S: Toll-like receptors and Type I interferons. J Biol Chem. 2007;282(21):15319–23. 10.1074/jbc.R700009200 [DOI] [PubMed] [Google Scholar]
- 28. Savarese E, Chae OW, Trowitzsch S, et al. : U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107(8):3229–34. 10.1182/blood-2005-07-2650 [DOI] [PubMed] [Google Scholar]
- 29. Tian J, Avalos AM, Mao SY, et al. : Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–96. 10.1038/ni1457 [DOI] [PubMed] [Google Scholar]
- 30. Crow YJ, Manel N: Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15(7):429–40. 10.1038/nri3850 [DOI] [PubMed] [Google Scholar]
- 31. Zhuang H, Szeto C, Han S, et al. : Animal Models of Interferon Signature Positive Lupus. Front Immunol. 2015;6:291. 10.3389/fimmu.2015.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nacionales DC, Kelly-Scumpia KM, Lee PY, et al. : Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56(11):3770–83. 10.1002/art.23023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kiefer K, Oropallo MA, Cancro MP, et al. : Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol. 2012;90(5):498–504. 10.1038/icb.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bekeredjian-Ding IB, Wagner M, Hornung V, et al. : Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174(7):4043–50. 10.4049/jimmunol.174.7.4043 [DOI] [PubMed] [Google Scholar]
- 35. Sjöstrand M, Johansson A, Aqrawi L, et al. : The Expression of BAFF Is Controlled by IRF Transcription Factors. J Immunol. 2016;196(1):91–6. 10.4049/jimmunol.1501061 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Namjou B, Kothari PH, Kelly JA, et al. : Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12(4):270–9. 10.1038/gene.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cunninghame Graham DS, Morris DL, Bhangale TR, et al. : Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011;7(10):e1002341. 10.1371/journal.pgen.1002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balomenos D, Rumold R, Theofilopoulos AN: Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101(2):364–71. 10.1172/JCI750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Domeier PP, Chodisetti SB, Soni C, et al. : IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213(5):715–32. 10.1084/jem.20151722 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Jackson SW, Jacobs HM, Arkatkar T, et al. : B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213(5):733–50. 10.1084/jem.20151724 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Welcher AA, Boedigheimer M, Kivitz AJ, et al. : Blockade of interferon-γ normalizes interferon-regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015;67(10):2713–22. 10.1002/art.39248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Enders A, Bouillet P, Puthalakath H, et al. : Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198(7):1119–26. 10.1084/jem.20030411 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Oliver PM, Vass T, Kappler J, et al. : Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203(3):731–41. 10.1084/jem.20051407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strasser A, Puthalakath H, O'Reilly LA, et al. : What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86(1):57–66. 10.1038/sj.icb.7100141 [DOI] [PubMed] [Google Scholar]
- 45. Craxton A, Draves KE, Gruppi A, et al. : BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202(10):1363–74. 10.1084/jem.20051283 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Bouillet P, Metcalf D, Huang DC, et al. : Proapoptotic Bcl-2 Relative Bim Required for Certain Apoptotic Responses, Leukocyte Homeostasis, and to Preclude Autoimmunity. Science. 1999;286(5445):1735–8. 10.1126/science.286.5445.1735 [DOI] [PubMed] [Google Scholar]
- 47. O'Neill SK, Getahun A, Gauld SB, et al. : Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35(5):746–56. 10.1016/j.immuni.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Getahun A, Beavers NA, Larson SR, et al. : Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med. 2016;213(5):751–69. 10.1084/jem.20150537 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Condé C, Gloire G, Piette J: Enzymatic and non-enzymatic activities of SHIP-1 in signal transduction and cancer. Biochem Pharmacol. 2011;82(10):1320–34. 10.1016/j.bcp.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 50. Jellusova J, Miletic AV, Cato MH, et al. : Context-specific BAFF-R signaling by the NF-κB and PI3K pathways. Cell Rep. 2013;5(4):1022–35. 10.1016/j.celrep.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Srinivasan L, Sasaki Y, Calado DP, et al. : PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–86. 10.1016/j.cell.2009.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tamir I, Dal Porto JM, Cambier JC: Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction. Curr Opin Immunol. 2000;12(3):307–15. 10.1016/S0952-7915(00)00092-3 [DOI] [PubMed] [Google Scholar]
- 53. Niiro H, Clark EA: Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945–56. 10.1038/nri955 [DOI] [PubMed] [Google Scholar]
- 54. Tsubata T: Role of inhibitory BCR co-receptors in immunity. Infect Disord Drug Targets. 2012;12(3):181–90. 10.2174/187152612800564455 [DOI] [PubMed] [Google Scholar]
- 55. Tonks NK: Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–46. 10.1038/nrm2039 [DOI] [PubMed] [Google Scholar]
- 56. Medgyesi D, Hobeika E, Biesen R, et al. : The protein tyrosine phosphatase PTP1B is a negative regulator of CD40 and BAFF-R signaling and controls B cell autoimmunity. J Exp Med. 2014;211(3):427–40. 10.1084/jem.20131196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lai M, Gonzalez-Martin A, Cooper AB, et al. : Regulation of B-cell development and tolerance by different members of the miR-17~92 family microRNAs. Nat Commun. 2016;7:12207. 10.1038/ncomms12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gonzalez-Martin A, Adams BD, Lai M, et al. : The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat Immunol. 2016;17(4):433–40. 10.1038/ni.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tong T, Ji J, Jin S, et al. : Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005;25(11):4488–500. 10.1128/MCB.25.11.4488-4500.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salvador JM, Hollander MC, Nguyen AT, et al. : Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16(4):499–508. 10.1016/S1074-7613(02)00302-3 [DOI] [PubMed] [Google Scholar]
- 61. Benhamou D, Labi V, Novak R, et al. : A c-Myc/miR17-92/Pten Axis Controls PI3K-Mediated Positive and Negative Selection in B Cell Development and Reconstitutes CD19 Deficiency. Cell Rep. 2016;16(2):419–31. 10.1016/j.celrep.2016.05.084 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Hermiston ML, Zikherman J, Zhu JW: CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228(1):288–311. 10.1111/j.1600-065X.2008.00752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang J, Zahir N, Jiang Q, et al. : The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43(9):902–7. 10.1038/ng.904 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Dai X, James RG, Habib T, et al. : A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123(5):2024–36. 10.1172/JCI66963 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Menard L, Saadoun D, Isnardi I, et al. : The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635–44. 10.1172/JCI45790 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Schickel JN, Kuhny M, Baldo A, et al. : PTPN22 inhibition resets defective human central B cell tolerance. Sci Immunol. 2016;1(1): pii: aaf7153. 10.1126/sciimmunol.aaf7153 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Akatsu C, Shinagawa K, Numoto N, et al. : CD72 negatively regulates B lymphocyte responses to the lupus-related endogenous toll-like receptor 7 ligand Sm/RNP. J Exp Med. 2016;213(12):2691–706. 10.1084/jem.20160560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jellusova J, Wellmann U, Amann K, et al. : CD22 × Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184(7):3618–27. 10.4049/jimmunol.0902711 [DOI] [PubMed] [Google Scholar]
- 69. Takai T, Nakamura A, Endo S: Role of PIR-B in autoimmune glomerulonephritis. J Biomed Biotechnol. 2011;2011:275302. 10.1155/2011/275302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu M, Hou R, Sato-Hayashizaki A, et al. : Cd72 c is a modifier gene that regulates Fas lpr-induced autoimmune disease. J Immunol. 2013;190(11):5436–45. 10.4049/jimmunol.1203576 [DOI] [PubMed] [Google Scholar]
- 71. Schlee M, Hartmann G: Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol. 2016;16(9):566–80. 10.1038/nri.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roers A, Hiller B, Hornung V: Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity. 2016;44(4):739–54. 10.1016/j.immuni.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 73. Duan B, Morel L: Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5(6):403–8. 10.1016/j.autrev.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 74. Hoffmann A, Kerr S, Jellusova J, et al. : Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8(7):695–704. 10.1038/ni1480 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Mietzner B, Tsuiji M, Scheid J, et al. : Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–32. 10.1073/pnas.0803644105 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Detanico T, St Clair JB, Aviszus K, et al. : Somatic mutagenesis in autoimmunity. Autoimmunity. 2013;46(2):102–14. 10.3109/08916934.2012.757597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Luzina IG, Atamas SP, Storrer CE, et al. : Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70(4):578–84. [PubMed] [Google Scholar]
- 78. Hao Z, Duncan GS, Seagal J, et al. : Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29(4):615–27. 10.1016/j.immuni.2008.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Brink R: The imperfect control of self-reactive germinal center B cells. Curr Opin Immunol. 2014;28:97–101. 10.1016/j.coi.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 80. Chan TD, Wood K, Hermes JR, et al. : Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37(5):893–904. 10.1016/j.immuni.2012.07.017 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Butt D, Chan TD, Bourne K, et al. : FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity. 2015;42(5):890–902. 10.1016/j.immuni.2015.04.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. William J, Euler C, Christensen S, et al. : Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297(5589):2066–70. 10.1126/science.1073924 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Herlands RA, William J, Hershberg U, et al. : Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37(12):3339–51. 10.1002/eji.200737752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tipton CM, Fucile CF, Darce J, et al. : Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–65. 10.1038/ni.3175 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Hughes PD, Belz GT, Fortner KA, et al. : Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28(2):197–205. 10.1016/j.immuni.2007.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Hutcheson J, Scatizzi JC, Siddiqui AM, et al. : Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28(2):206–17. 10.1016/j.immuni.2007.12.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Weant AE, Michalek RD, Khan IU, et al. : Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8 + T cell contraction. Immunity. 2008;28(2):218–30. 10.1016/j.immuni.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 88. Suzuki A, Kaisho T, Ohishi M, et al. : Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197(5):657–67. 10.1084/jem.20021101 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation