Version Changes
Revised. Amendments from Version 2
At the suggestion of the reviewers, the discussion section regarding the limitations of the study has been extended to emphasize the importance of realistic modeling of neuron morphologies.
Abstract
The detailed biophysical mechanisms through which transcranial magnetic stimulation (TMS) activates cortical circuits are still not fully understood. Here we present a multi-scale computational model to describe and explain the activation of different pyramidal cell types in motor cortex due to TMS. Our model determines precise electric fields based on an individual head model derived from magnetic resonance imaging and calculates how these electric fields activate morphologically detailed models of different neuron types. We predict neural activation patterns for different coil orientations consistent with experimental findings. Beyond this, our model allows us to calculate activation thresholds for individual neurons and precise initiation sites of individual action potentials on the neurons’ complex morphologies. Specifically, our model predicts that cortical layer 3 pyramidal neurons are generally easier to stimulate than layer 5 pyramidal neurons, thereby explaining the lower stimulation thresholds observed for I-waves compared to D-waves. It also shows differences in the regions of activated cortical layer 5 and layer 3 pyramidal cells depending on coil orientation. Finally, it predicts that under standard stimulation conditions, action potentials are mostly generated at the axon initial segment of cortical pyramidal cells, with a much less important activation site being the part of a layer 5 pyramidal cell axon where it crosses the boundary between grey matter and white matter. In conclusion, our computational model offers a detailed account of the mechanisms through which TMS activates different cortical pyramidal cell types, paving the way for more targeted application of TMS based on individual brain morphology in clinical and basic research settings.
Keywords: transcranial magnetic stimulation, computational model, compartmental neuron model, brain stimulation, multi-scale modeling, motor cortex, D-wave, I-wave
Introduction
Transcranial magnetic stimulation (TMS) is a neurostimulation and neuromodulation technique that noninvasively activates neurons in the brain 1, 2. It generates a time varying magnetic field using a coil above the head, which induces an electric field in the brain that can be of sufficient magnitude to depolarize neurons beyond their firing threshold. In recent years, TMS has been widely tested as a tool for diagnosis and treatment for a broad range of neurological and psychiatric disorders 3– 5. Although the efficacy of TMS has been demonstrated, there remains great uncertainty regarding the factors influencing the affected brain areas and relevant circuits.
To provide a better understanding of the biophysical mechanisms behind TMS, several computational studies have been performed to try to reveal the effects of a number of parameters contributing to variable outcomes. The majority of models try to predict the brain regions influenced by TMS exclusively based on stimulus-induced electric fields 6, 7. While early studies utilized spherical models of the human head, in recent years high-resolution volume conduction models of the head have been developed from human magnetic resonance imaging (MRI) to improve the accuracy of calculated electric fields 8– 15. These models have revealed that the geometry of the volume conduction model, such as complex gyral folding patterns, is one of the key parameters determining the induced electric fields. In addition, computational studies have been extended by connecting numerical results with experimental observations to show the agreement of computed electric fields with physiological measurements 16– 18.
Directly monitoring target cells’ activities under stimulation would be immensely valuable for the interpretation of TMS effects, but only a few in vitro studies exist so far 19, 20. However, computational studies can explore the effects of the electromagnetic fields on neural activation in the intact brain by simulating models of neural stimulation in silico. In early computational models, straight axonal fibers were considered numerically and the response of neurons induced by the external field was modeled by means of the cable equation 21, 22. Later models investigated the role of cell morphology using multi-compartmental modeling 23– 25. The responses of cortical neurons vary depending on not only the neuronal morphology but also orientation relative to the induced electric field and stimulus amplitude 6, 26, 27. Therefore, recent models have used anatomical information on cortical folding that induces a wide range of field orientations and have applied the calculated electric field from the head model to the neuronal models 28, 29.
Here, we use an advanced multi-scale modeling approach that combines a high-resolution head model with detailed multi-compartmental neuron models. We construct an anatomically realistic head model based on MRI and calculate the external currents that affect neurons via the TMS-induced electric field with high accuracy. We concentrate on the hand knob area of the motor cortex that is the predominant target of many TMS studies 16, 30. A multitude of layer 5 and layer 3 pyramidal neurons (L5/L3 PNs) is incorporated on the basis that they might be primary activators of the corticospinal tract and provide the main input to the direct pathway 24, 28, 31, 32. We estimate the target area of activation as a function of coil orientation as well as the stimulation intensities required to activate different types of pyramidal neurons. Finally, we predict the precise sites where the neurons initiate their action potentials. We find that action potentials are mostly generated at the axon initial segment of these cells.
Methods
In order to study the cellular effects of TMS in the brain we employed a multi-scale computational modeling approach combining a volume conductor head model with detailed neuronal models of cortical pyramidal neurons. The motor cortex, especially the hand area, was considered as a cortical target location. The volume conductor head model was used to simulate the stimulus-induced electric fields; it was based on the SimNIBS v1.1 software pipeline 14, 33. The precise impact of these fields on different neural targets was evaluated using multi-compartmental models of pyramidal neurons embedded into the head model. This allowed us to predict differences in individual neurons’ susceptibilities to TMS depending on neuron placement and coil orientation.
Volume conductor model
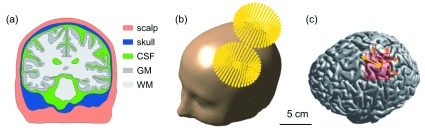
The simulated effects of TMS depend not only on stimulation parameters but also on the anatomical information specified in the volume conductor model. To calculate the precise electric field, a volume conductor head model for TMS that reflected T1-weighted and T2-weighted magnetic resonance (MR) images was constructed using the example dataset provided by SimNIBS v1.1 under the ethical approval 14. Briefly, segmentation of white matter (WM), gray matter (GM), cerebrospinal fluid (CSF), skull and skin was based on FreeSurfer v5.3.0 34, 35, FSL v5.0.0 36 and MeshFix v2.0 37, as shown in Figure 1(a). Then, the head model was constructed by generating an optimized tetrahedral volume mesh using an enhanced resolution in the region of interest (ROI) around the hand knob using Gmsh 38. The total number of tetrahedral elements was approximately 5.6 million. At each layer of the head model, isotropic conductivity was assigned with the following values (in S/m) 13, 39: WM: 0.126; GM: 0.276; CSF: 1.654; skull: 0.01; and skin: 0.465.
Figure 1. The volume conductor model and coil placement.
( a) Cross-section displaying the scalp, skull, cerebrospinal fluid, gray matter and white matter. ( b) The computed coil location is superimposed on the head model. ( c) The yellow dot indicates the location of the center of the TMS coil projected onto the border between gray matter and cerebrospinal fluid. The direction of the primary electric field directly under the coil center is indicated by the yellow arrow. The coil was rotated in steps of 45 degrees (orange colored dotted arrows). The red rectangle represents the pre-selected region of interest covering parts of the precentral and postcentral gyrus.
Field calculations
The TMS-induced electric field was calculated based on the SimNIBS v1.1 pipeline. Briefly, the electric field, consisted of primary and secondary electric fields. The primary electric field was directly determined by the coil geometry and the secondary electric field was caused by charge accumulations at tissue interfaces. Using magnetic dipoles to model the TMS coil, the primary electric field was calculated directly without the volume conductor model and then used as input for the secondary electric field calculation via a finite element method using the GetFEM++ library and MATLAB 33, 40. The Magstim 70 mm figure-8 coil was represented by magnetic dipoles positioned above the hand knob ( Figure 1) and the stimulator output was set to 1 A/μs. The base coil orientation was defined relative to the direction of the central sulcus such that the electric field induced was in the posterior to anterior direction (the yellow arrow in Figure 1(c)). Then, three additional coil orientations were tested by rotating in steps of 45 degrees and reversed orientations were simulated by changing the sign of the current through the coil.
To investigate the TMS-induced cellular effects, we quantified the magnitude of the electric field | | and the orthogonal component of the electric field to the gray matter surface, where is the normal vector for the boundary surface element. The orthogonal component was expected to contribute to TMS-induced brain activation by the theoretical cortical column cosine model of TMS efficacy (C 3 -model) 10, 41.
Multi-compartmental neuronal models
We adapted existing multi-compartmental models of layer 5 and 3 pyramidal neurons (L5/L3 PNs) from cat visual cortex 42 using the NEURON simulation software 43. The electrical properties were unchanged from the original models. Briefly, a high density of fast, inactivating voltage-dependent Na + channels were present in the axon hillock and axon initial segment, and a low density of these channels was present in the soma and dendrites. Slow Ca 2+-dependent K + channels and high threshold Ca 2+ channels were located in the soma and dendrites. Except for the dendrites, fast K + channels were present.
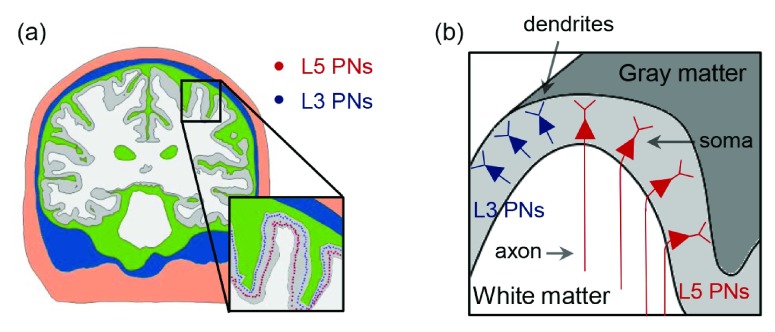
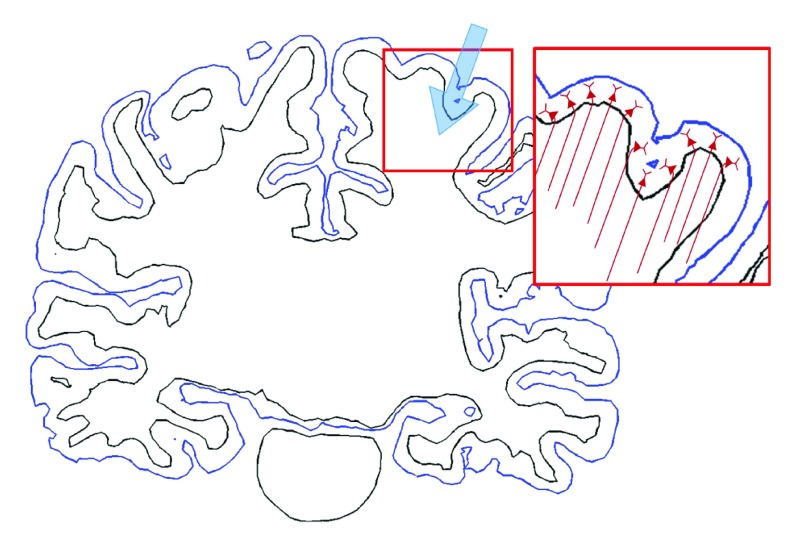
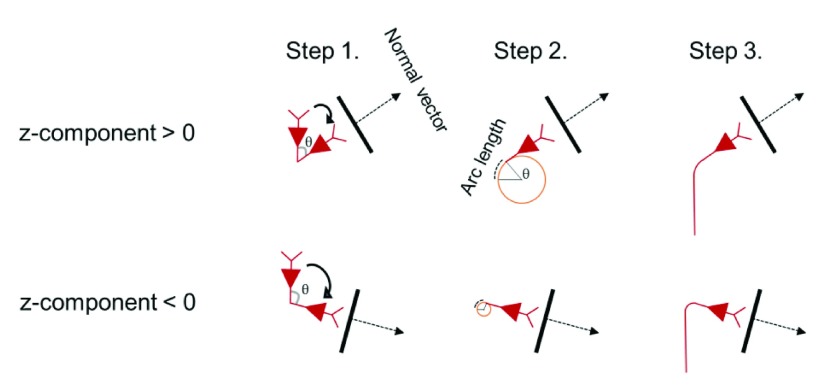
L5/L3 PNs were combined virtually with the head model and modified to accommodate the irregular geometry of the cortex 28, 29, 44– 47, as shown in Figure 2. To reduce superfluous computations, we preselected a region of interest (ROI) of 50 × 50 × 50 mm 3 around the hand knob and then placed L5/L3 PNs in each triangular element comprising the gray matter surface. The multi-compartmental models of PNs consisted of a series of compartments connected by resistors. Each compartment was further discretized into segments of equal length to allow for accurate numerical simulation. The center points of each segment were extracted and used to calculate the necessary changes to neuron geometry, as described below. The dendritic trees were lengthened or shortened by re-scaling the lengths of the compartments according to the local dimensions of the cortex such that dendrites reached layer 1 and the orientation was perpendicular to the cortical surface 46, 48. Since the morphology of the dendritic trees was not symmetric and it might influence the neuronal activation, L5/L3 PNs had randomly rotated dendritic trees at different locations. The axons of L5 PNs were defined to curve beyond the boundary between GM and WM in the direction of the internal capsule ( Supplementary Figure S1) as described previously 45. Further adjustments of L5 PNs’ geometry were as follows (illustrated in Supplementary Figure S2): each dendritic tree was oriented such that its principal axis would align with the normal vector of its associated triangular surface element. The bending part of the axon was calculated according to the normal vector of the surface element. The arc length of the axon bend was set to 0.6 mm when the z-component of the normal vector was positive and otherwise the arc length was 0.3 mm (compare Supplementary Figure S2). Note that when we varied the angle and arc length of the axon bend, it usually did not alter the activation threshold. The axons of L3 PNs were defined to terminate in layer 5/6 within the GM. Altogether, a total of 10,888 L5 PNs and 10,888 L3 PNs was constructed. This process was implemented in MATLAB (MathWorks, Natick, MA, USA).
Figure 2. The placement of L5/L3 PNs in the head model.
( a) The somata of L5/L3 PNs are marked as colored dots (red: L5; blue: L3). ( b) A schematic view of the distribution of the L5/L3 PNs is shown along the cortex folding (gray colored area); note the bending of L5 PN axons after crossing the boundary between gray matter and white matter.
Computation of neuronal activation induced by stimulation
The membrane potentials induced by stimulation were approximated by adding an external current source I ext to the cable model 2, 21, 22, 24, 25:
where r a is the axial resistance per unit length and E 1 represents the component of the electric field that is parallel to each compartment of the PNs. The derivative of the electric field along each compartment was calculated at each center point by where is a unit vector pointing along the direction of the compartment and ∇ E contains the components of the electric field gradient tensor that are estimated by computing the difference of electric fields at neighboring points displaced by ±1 mm along each axis 49.
We calculated a monophasic pulse that induced a fluctuating magnetic field through an RLC circuit as detailed in 50,
where ω = 30 rad/ms is the angular frequency and τ = 0.08 ms is the decay time. The I ext at each compartment was then multiplied by the normalized time derivative of the monophasic pulse. Finally, we obtained the spatial and temporal membrane potential dynamics. They were used to measure the excitation thresholds, the stimulation site and action potential propagation.
Results
Copyright: © 2017 Seo H et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2017 Seo H et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2017 Seo H et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2017 Seo H et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
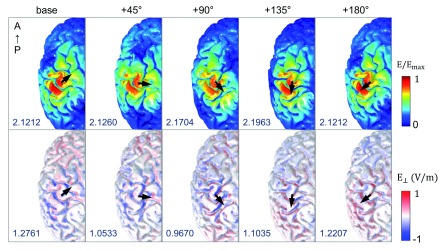
Figure 3 depicts the magnitude of the electric fields (| |, top row) and the orthogonal component of the electric fields ( , bottom row) for different coil orientations. All calculations were performed for a rate of change of the coil current of 1 A/μs. Electric fields had higher magnitudes in the precentral and postcentral gyrus and focused on the top of the gyri, regardless of coil orientations. We observed only slight changes in the absolute field strengths depending on coil orientation. In contrast, the orthogonal component of electric fields ( ) showed different spatial patterns compared to the electric field magnitude. High strengths of were found on the walls of the gyri and strongly depended on coil orientation. Furthermore, while the spatial extent of | | was the same for the standard orientation and the +180 degree orientation, the sign of in the +180 degree orientation was reversed due to the reversed sign of the induced electric fields. Interestingly, the maximum value of | | depended on coil orientation; it was lowest in the base coil orientation and highest at +135 degrees. However, the maximum values of were highest for the standard coil orientation and lowest at +90 degrees.
Figure 3. Effects of coil orientation on the electric fields.
The spatial patterns of the magnitude of electric fields (| |, top row) and their component orthogonal to the gray matter surface ( , bottom row) are visualized; the color scale is adapted for better visualization. The black arrows indicate different coil orientations, and the maximum value of | | and (measured in V/m) are given in the bottom left of each figure. For the orthogonal component of electric fields (bottom row), red color indicates current flowing in the direction from superficial to lower cortical layers and blue color represents currents flowing in the opposite direction.
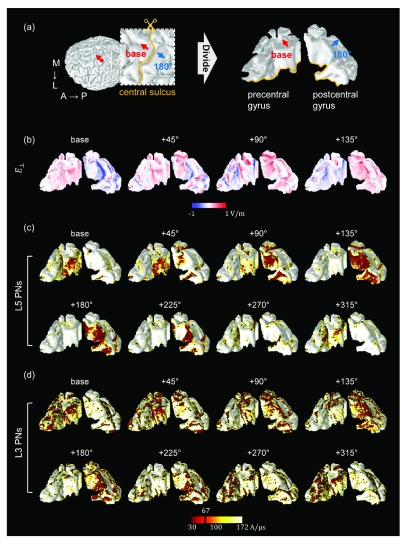
To assess the neuronal activations as a function of coil orientation, we determined the excitation threshold required to cause action potentials of L5/L3 PNs. For each coil orientation, we kept increasing the stimulator output until a neuron generated an action potential or we reached a maximum rate of change of the current defined as 171 A/μs. Our focus is on the excitability for a stimulation intensity corresponding to 67 A/μs, as this value corresponds to the average motor threshold for the Magstim 200 stimulator connected to the coil modeled 18, 29, 49, 51. The excitability of L5/L3 PNs was predicted either by the direct estimation of the electric field ( map in the Figure 4(c)) or by simulating the induced depolarization and firing of the detailed neuronal models (threshold maps in Figure 4(d,e)). The color of the threshold maps represents the stimulator output necessary to activate the corresponding cell and the estimated excitable area in the maps. The blue colored areas indicate an excitability in the opposite direction, because the head model was linear with respect to the electric field. As shown in Figure 4(a), we virtually divided the precentral and postcentral gyrus to better visualize the excitability in the walls of the gyrus.
Figure 4. The spatial extent of predicted excitability based on the orthogonal component of the electric field ( ) and detailed simulations of L5/L3 PNs.
( a) The red dot on the border between GM and CSF indicates the location of the center of the coil. The base orientation is shown as red arrows. The inset represents the region of interest in which PNs were distributed. The blue arrows indicate the opposite coil orientation (+180°). The precentral and postcentral gyri were virtually divided for visualization purposes. The spatial patterns of ( b) and threshold maps of L5 ( c) and L3 ( d) PNs depended on coil orientation as shown. The black and red colored areas in the threshold maps ( c– d) indicate the excitable areas under the stimulator output corresponding to the average motor threshold ( 67 A/μs). The directions of coil orientations in the 2 nd row are the opposite directions of the 1 st row (in the threshold map in ( c and d)) simulated by changing the sign of the current through the coil. Note how the excitable areas strongly depend on the coil orientation.
In L5 PNs, we observed that the predicted excitability depended on coil orientation for both ( Figure 4(b)) and threshold maps ( Figure 4(c)). For the base orientation and +45 degrees, a high excitability was predominantly observed in the wall of the precentral gyrus. In contrast, for orientations +90 degrees to +225 degrees we observed high excitability in the wall of the postcentral gyrus. Comparing these threshold maps to the maps, we see that the excited regions were quite well matched to the results from . Furthermore, in the standard direction, the spatial extents where L5 PNs were activated for stimulation intensities corresponding to the motor threshold seemed to enlarge with increasing coil rotation, while for the opposite direction of the coil current highly excitable areas shrank with increasing coil rotations.
Overall, the excitability of L3 PNs showed behavior comparable to that of L5 PNs ( Figure 4 (c,d)), but notable differences in threshold maps between L5 and L3 PNs were as follows: while L5 PNs in the top of the gyri were never activated, L3 PNs were excited in the top and also the wall of gyri. The excitable areas of L3 PNs caused by the +90 and +135 degree stimulations were relatively focused on the upper parts of the wall of the postcentral gyrus, while L5 PNs placed in the deeper parts of the sulcus were activated. Furthermore in L3 PNs, the excitability in the precentral gyrus and the postcentral gyrus was comparable and a bigger area was affected than for L5 PNs. The discrepancies between L5 and L3 PNs confirmed that the morphology and placement of neuronal models has an important impact beyond the position relative to the coil.
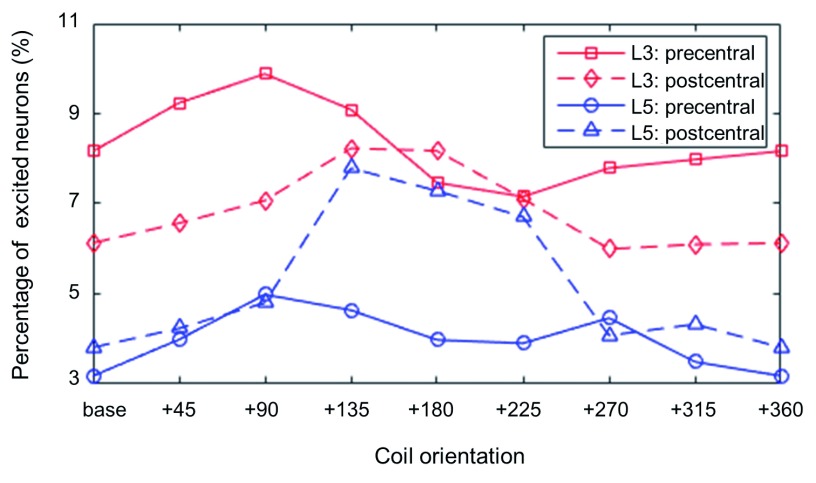
The percentage of excited neurons for a stimulation intensity at the motor threshold is shown in Figure 5. We separately analyzed neurons falling in the precentral and postcentral gyrus. When we focused on PN activations in the precentral gyrus, the highest percentage of excited neurons was observed at +90 degrees rather than the base orientation. For the base orientation PNs in the sulcal wall along the central sulcus were activated. At +90 degrees PNs were activated mostly in the opposite sulcal wall ( Supplementary Figure S3). For the postcentral gyrus, the maximum percentage of activated PNs was observed when the coil was oriented at +135 degrees, which is in agreement with the threshold maps in Figure 4.
Figure 5. The percentage of L5 and L3 PNs that are activated for a stimulation intensity at the motor threshold (67 A/μs) as a function of coil orientation.
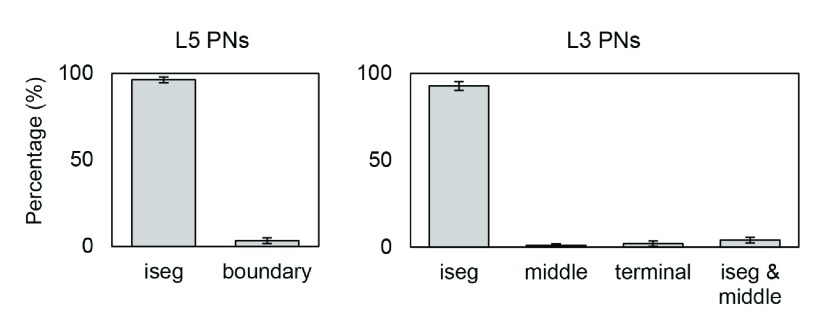
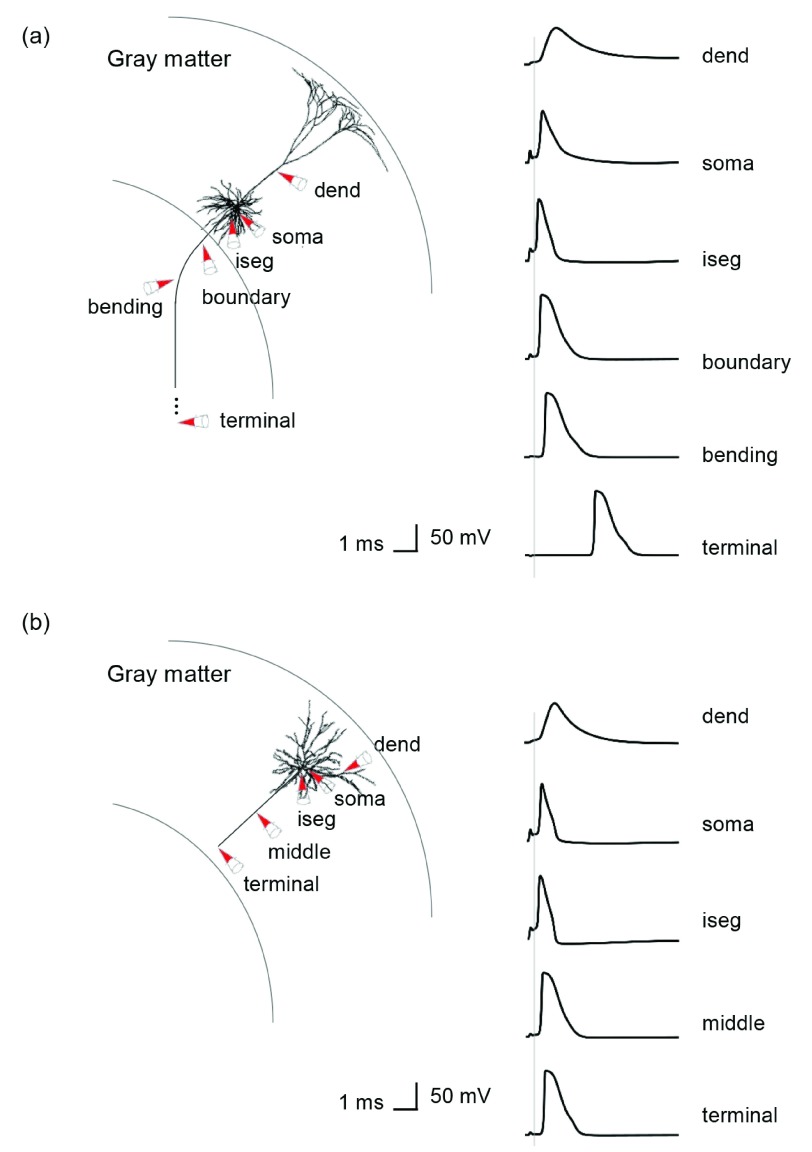
Our multiscale modeling approach also allowed us to identify where on each cell an action potential was initiated. The majority of action potentials were initiated at the axon initial segment and others at the axon near the boundary between GM and WM for L5 PNs and at the middle and terminal points for L3 PNs ( Figure 6). In the base orientation, threshold stimulation elicited action potentials first at the initial segment for 90% of both the L5 and the L3 PNs. This fraction increased with increasing coil rotations up to 97% at +135 degrees in L5 PNs and up to 95% at +90 degrees in L3 PNs. Example plots of membrane potential dynamics induced by the threshold stimulus evoking action potentials are shown in Figure 7. We observe the propagation of the action potentials from the axon initial segment to the more distal parts of the neurons. In both L5 and L3 PNs, following the action potential at the initial segment, the soma was activated next, as it is closest to the initial segment. The terminal points of the axons were activated last as they are most distal from the axon initial segment. Since the axons of the simulated L5 PNs are quite long compared to that of the L3 PNs, the arrival of the action potential at the terminal point was substantially delayed. Similarly, dendrites of L5 PNs showed delayed activation while in the L3 PNs dendrites were occasionally activated early.
Figure 6. The percentage of action potential initiation sites of L5 and L3 PNs for a stimulation intensity corresponding to the motor cortex threshold (67 A/μs) averaged over different coil orientations.
Error bars represent standard deviations. Sites include the axon initial segment (iseg) and the boundary between gray matter and white matter (boundary). Additionally, the terminal part (terminal) and middle point of the axon (middle) for L3 PNs were considered. Most action potentials are first evoked at the axon initial segment of L5 PNs (96.31±1.72%) and L3 PNs (92.76±2.42%). The remaining number of L5 PNs show action potential initiation at the axon near the boundary between gray matter and white matter. Only few L5 PNs (0.49±0.14%) initiate action potentials simultaneously at the axon initial segment and the GM-WM boundary. For L3 PNs, middle (1.05±0.78%) and terminal points (2.08±1.45%) of axons are also activated occasionally.
Figure 7. Virtual recordings of membrane potential dynamics of L5/L3 PNs.
The simulated recordings were performed from dendrites (dend), soma and parts of the axons, as indicated by the red colored cones. ( a) In a L5 PN, the membrane potentials are recorded at the axon initial segment (iseg), the location where the axon crosses the boundary between gray matter and white matter (boundary), bending and terminal points. ( b) Additionally, the middle points of axons of L3 PNs are recorded.
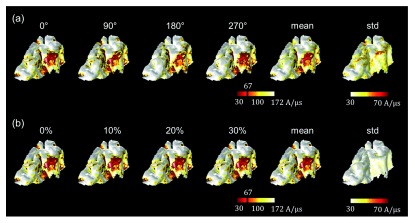
The PNs that were morphologically reconstructed had asymmetric dendritic trees that might affect the neuronal responses. We studied the impact of dendritic trees on threshold maps and the percentage of excited neurons for a stimulation intensity at the motor threshold by rotating them in steps of 90 degrees around the axis defined by their apical dendrite for a fixed base coil orientation. In the threshold maps for L5 PNs as shown in Figure 8(b), the highest variations of the thresholds caused by these rotations were observed in the boundary between the top of the precentral gyrus and the sulcus. However, the dependence on coil orientation in the threshold maps did not change and thus the L5 PNs in the precentral gyrus were activated consistently. Compared to the percentage of excited L5 PNs with randomly rotated dendritic trees (10.53% as shown in Figure 5), the fixed orientation of dendritic trees induced changes in the fraction of activated neurons of up to 2%. The threshold variations in the L3 PNs were hardly noticeable compared to those of the L5 PNs. The percentage of excited L3 PNs was 20.85% with randomly rotated dendritic trees, and when the orientations of dendritic trees were fixed it resulted in changes of at most 0.3%. In addition, the impact of scaling of PNs was investigated by reducing the dimension of the dendritic trees by 10%, 20% or 30%, as shown in Figure 8(b). For this, we scaled the length of all dendritic compartments while keeping their diameters identical 46. We observed consistently activated sites in the threshold maps and only slight changes of the percentage of excited L5 PNs of up to 0.3%. Thus, we found that the rotations of the dendritic trees had a bigger impact on PN excitability than that of scaling the dendritic trees. Overall, morphological changes in dendritic trees did not alter the spatial extent of activated regions much.
Figure 8.
The impact of dendritic tree morphology of L5 PNs was evaluated by ( a) rotating them in steps of 90° and by ( b) reducing their dimensions in steps of 10% for a fixed base coil orientation. The threshold maps according to ( a) the different orientations and ( b) dimensions of dendritic trees and their mean and standard deviation are shown. The map of standard deviations (std) in ( a) indicates that the precise orientation of the dendritic tree can alter activation thresholds in a noticeable fashion in certain situations.
Discussion
The detailed mechanisms through which TMS activates cortical cells and cortical circuits are still not fully understood. In this study, we used multi-scale computational modeling to predict cortical activation as a function of coil orientation in two different ways. First, we simply considered the strength of the component of the TMS-induced electric field that is orthogonal to the gray matter surface as suggested by the C 3-model 10, 41. Second we developed a detailed computational modeling approach that combined an anatomically realistic head model with complex multi-compartment neuronal models of L5/L3 PNs and quantified their stimulation thresholds. A first major finding was the characterization of the induced electric fields and the thresholds of L5/L3 PNs as a function of coil orientations as shown in Figure 4. In addition, threshold variations according to different morphologies of PNs were observed.
The magnitude of the electric field was considered first, because the strength of the electric field is commonly used as a simple way to predict areas of neuronal activation 6, 7, 52. We found that the magnitude of the TMS-induced field is focused on the top of the gyrus, which is in agreement with previous modeling studies 10, 12– 14. However, the electric field magnitude showed little dependency on coil orientation 10. Then, we investigated the directional electric field, specifically the orthogonal component that is perpendicular to the cortical surface, as this has been suggested to contribute most to the TMS-induced activation according to the C 3-model 10, 41, 53. We found a strong dependence of the orthogonal field component on coil orientation, as shown in Figure 3. In contrast to the electric field magnitude, the highest orthogonal field values were found in the sulcal walls and never on the apex (or crown) of the gyrus.
While the analysis of TMS-induced electric fields has been widely addressed in the past, the incorporation of multi-compartment neuronal models has hardly been investigated. To permit a more detailed understanding of the biophysical mechanisms of TMS, a few previous modeling studies employed detailed neuronal models and calculated the membrane potential dynamics generated by the electromagnetic field. However, these attempts had various limitations. First, in early studies no anatomical information on large-scale brain morphology was applied 23– 25. Rather than constructing a finite element head model, these studies applied a uniform electric field to the neuronal model. Even though such investigations achieved reasonable results regarding the neuronal activation, they did not consider the effects of the complex folding patterns of the cortex and the effects of tissue borders such as the borders between GM on the one hand and CSF or WM on the other hand. However, the importance of anatomically realistic head models has been shown convincingly 12– 14. Furthermore, the impact of detailed brain anatomy has been considered in various methods of brain stimulation and substantial differences have been demonstrated by improving anatomical information related to the head model 54– 57. Salvador et al. (2011) investigated neuronal responses using a simplified head model of a cortical sulcus with several types of neurons and found changes of the stimulation threshold depending on the pulse waveform and the coil orientation 29. However, the used head model had an approximated geometry restricted to the motor cortex and a full complex geometry, such as the hook-shaped hand knob, was not considered. Furthermore, the modeled coil orientation was limited to anterior to posterior and its reversal due to the simplified geometry of the head model. Most recently, Goodwin and Butson (2015) proposed a more realistic approach that integrates an anatomically realistic head model derived from MR images with detailed neuronal models 28. They considered the excitability of neurons as a function of coil orientation. However, in contrast to our results, excitability maps hardly showed a systematic dependence on coil orientation and activation thresholds were lower in the gyral crown. We speculate that this might be caused by the different morphology of PNs or the different way in which they calculated the external currents to simulate neuronal responses. Also, we considered two types of L3/L5 PNs spread over a wider region of the cortex. Finally, as another major result, we also established the site of action potential initiation and found that most PNs are activated at the axon initial segment and action potential initiations at other parts of the neuron are comparatively rare.
The threshold maps we calculated demonstrate acute sensitivity to coil orientation, but different spatial extents of activation were observed according to the different morphologies of the PNs. In L5 PNs, activation thresholds were low in the sulcal walls, matching predictions based on the orthogonal component of the electric field. The excitation in the sulcal cortical surface was consistent with the well-established columnar neuronal orientation and functional organization of the cortex and functional imaging studies 41, 58. The L3 PNs had different morphology with shorter axons than the L5 PNs such that they were located completely within the gray matter. Similar to the L5 PNs, the coil orientation had a significant impact on the responses of the L3 PNs, but the precise patterns of the threshold maps differed between the L3 and L5 PNs. As L3 PNs in the gyral crown and the upper parts of the sulcal wall were predominantly activated, as would be expected based on their proximity to the stimulation coil 59.
The neural response to TMS is composed of a direct (D) and several indirect (I) waves. The D-wave is thought to be produced by direct activation of L5 PNs as we have modeled it here and is followed by I-waves that are thought to be generated by synaptic excitation and/or re-excitation of L5 pyramidal cells with longer latencies 59, 60, presumably via pyramidal cells in superficial cortical layers L2 and L3. According to Di Lazzaro et al. (2004), at the lowest stimulation intensity to evoke neuronal responses, an I-wave is elicited, and with increasing stimulation intensity, the earlier, small D-wave is produced 30. This indicates that thresholds for eliciting I-waves are lower than those for eliciting D-waves 61. In this work, we explored the excitation thresholds of both L3 and L5 PNs and found as another major result that the percentage of excited neurons for a stimulation intensity at the motor threshold was about two times higher for L3 PNs than for L5 PNs. Furthermore, the activation of the L3 PNs was consistently higher than that of the L5 PNs for the full range of stimulation intensities. Mainen and Sejnowski (1996) compared the dendritic structure of L3 and L5 PNs with a common distribution of ion channels and found that a smaller intracellular current injection was necessary to activate L3 PNs compared to L5 PNs 42. Thus, the morphology of L3 PNs might result in their higher excitability in response to TMS. In addition, the lower stimulation thresholds of the L3 PNs are consistent with lower stimulation intensities required to produce I-waves 50, 62, and the higher stimulation intensities required to produce D-waves.
For motor cortex, there is evidence that the base coil orientation induces the highest motor evoked potential 63, 64, while we found that the +90 degrees orientation activates the highest number of PNs across the whole part of the precentral gyrus falling within the ROI ( Figure 5(b)). At +90 degrees PNs were activated mostly in the curved part of the precentral gyrus ( Supplementary Figure S2), and according to the spatial distributions of thresholds ( Figure 4) the sulcal wall along the central sulcus was targeted better at the base coil orientation than for other coil orientations. Thus, the base coil orientation is recommended for M1 by our model, congruent with previous research 63, 64, and +135 degrees should be ideal to stimulate the postcentral gyrus.
The question of the precise initiation site of action potentials is a central issue for understanding the physiological effects of TMS. According to our study, the dominant initiation site leading to action potentials is the axon initial segment in both L5 and L3 PNs. In previous modeling studies, the action potentials were initiated at the axons crossing the boundary between gray matter and white matter, where the conductivity changes abruptly 49 and at the bending parts of the axon due to charge accumulation 29. However Goodwin and Butson (2015) combined a realistic head model with detailed PN models and observed that most action potentials were initiated at or close to the axon hillock just adjacent to the axon initial segment 28. Pashut et al. (2011) have also argued for action potential initiation at the axon initial segment 24. Furthermore, this is consistent with previous studies arguing that action potentials giving rise to the D-wave might be initiated close to the soma and/or axon initial segment 65, 66. The presence of a highly increased density of fast voltage-gated Na + and K + channels leads to high intrinsic excitability of the axon initial segment 67. Additionally, the displaced charges from across the entire dendritic tree can accumulate at the axon initial segment due to the neuronal morphology. This means that the initiation of action potentials in the initial segments is not necessarily a direct effect of TMS at that particular site, but rather the consequence of membrane properties and the morphology of PNs. In our study, L5 PN action potentials were only rarely initiated at the axon where it crosses the boundary between gray matter and white matter, where tissue conductivity changes abruptly 49. It will be important to verify these results with more realistic neuron (in particular: axon) models.
There are several limitations in our modeling study. A first limitation is that we have assumed isotropic conductivity, as is common in computational studies. Opitz et al. (2011) revealed that anisotropy might create hot spots in the WM with increased field strength that might affect neural excitation 12, and Seo et al. (2015) reported that anisotropy affected L5 PNs significantly while it had only minor impact on L3 PNs 45. Thus anisotropic conductivity might have significant effects on L5 PN axons running through the WM.
In this work, the L3 and L5 PNs were taken from cat visual cortex due to the lack of models for most human cortical cell types. Thus, while we lengthened PNs to fit the cortex, the uncertainties regarding morphology of neurons was not fully studied. Wu et al. (2016) incorporated a multitude of PNs with various stimuli 25 and Salvador et al. (2010) constructed various types of neural structures including pyramidal neurons, interneurons, and association fibers 29; they found that the excitability can be shaped by field orientation, pulse wave form, and diameter of neurons. In addition, changes in the electrical properties, such as membrane properties and ion channels had the largest influence on neuronal excitability 25. However, despite the uncertainty with regard to properties of PNs, we produced results matching both experimental studies and other computational studies 23, 25 that incorporated the same models of PNs.
An important limitation of our study is that we have not included realistic axon morphologies. A thorough modeling of the complex morphologies of local axons could reveal additional sites of activation. In particular, axon terminals whose orientations are aligned with the electric field may be particularly easy to stimulate.
While we observed the stimulation of neural activity in the superficial cortex close to the coil, TMS might also affect deep brain areas that cannot be stimulated directly. This can be explained on the basis of the propagation of action potentials along white matter fiber tracts. Recent studies modeled tractography-based white matter fiber tracts using diffusion tensor imaging (DTI) and observed activation of axon bundles 9, 11, 12. Compared to fiber tracts in previous modeling, we modeled straightly stretched axons of L5 PNs inside the WM. Due to such limitations, the axons inside the WM occasionally passed through protruding parts of GM. Notwithstanding the fact that this intersection could affect the neuronal responses such as the action potential initiation or thresholds, most PNs initiated action potentials at the axon initial segment and coil orientation dependency observed in threshold maps was consistent with observations in previous studies. Further developments in tractography may improve detailed neuronal models and may lead to a deeper understanding of the TMS-induced brain activity propagations from the superficial cortex to distant brain regions.
Another limitation of our model is that the reconstructed PNs were synaptically isolated. For the L5 cells this means that we basically studied the generation mechanism of D-waves. The activation of L3 cells could be seen as a proxy for the generation of I-waves. A logical next step is to synaptically couple L3 and L5 cells as done in a recent model for the generation of D and I-waves using L5 PNs that were contacted by a pool of excitatory and inhibitory layer 2 and 3 neurons 50. This model successfully reproduced various characteristics of I-waves and highlighted the importance of the complex morphology of the L5 PNs for the generation of I-waves. An improvement would be to use the anatomical information on the activation of PNs as modeled here, as we found a clear difference in the threshold maps for L5 and L3 PNs based on their morphology. Therefore, in future work, we plan to incorporate synaptic connections between L3 and L5 PNs. We hope that this will bring us one step closer to a detailed understanding of the mechanisms through which TMS activates cortical circuits, paving the way for more precise and effective application of TMS based on individual brain morphology in clinical and basic research settings.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Seo H et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
F1000Research: Dataset 1. Figure 3 input data, 10.5256/f1000research.9277.d132069 68
F1000Research: Dataset 2. Figure 4 input data, 10.5256/f1000research.9277.d132070 69
F1000Research: Dataset 3. Figure 5 raw data, 10.5256/f1000research.9277.d132071 70
F1000Research: Dataset 4. Figure 6 raw data, 10.5256/f1000research.9277.d132072 71
Funding Statement
This work was supported by grant (NRF-2016R1A2B4010897) from the National Research Foundation of Korea. The Lab of JT is supported by a gift from the Quandt foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; referees: 2 approved]
Supplementary Figures
Supplementary Figure S1. Schematic view of L5 PN axons bending toward the internal capsule.
The L5 axons bend towards the internal capsule (direction of the blue arrow) as they cross into the white matter and then run straight in the same direction for a fixed distance (inset).
Supplementary Figure S2. Schematic view of creating L5 PN axon bends according to the normal vector of the corresponding surface element of the cortex.
First, the upper part of L5 PNs is rotated according to normal vector direction (Step 1). Then the axon bend is constructed such that when the z-component of the normal vector is positive we set the arc length to 0.6 mm and when the z-component is negative the arc length is set to 0.3 mm (Step 2). Finally, we attach the remaining straight part of the axon (Step 3).
Supplementary Figure S3. The spatial extent of activation thresholds of detailed simulations of L5 PNs as a function of coil orientation. These animations are consistent with Figure 4(c) and allow a better view of the medial part of the hand knob.
.
References
- 1. Barker AT, Freeston IL, Jalinous R, et al. : Clinical evaluation of conduction time measurements in central motor pathways using magnetic stimulation of human brain. Lancet. 1986;1(8493):1325–1326. 10.1016/S0140-6736(86)91243-2 [DOI] [PubMed] [Google Scholar]
- 2. Wagner T, Valero-Cabre A, Pascual-Leone A: Noninvasive Human Brain Stimulation. Annu Rev Biomed Eng. 2007;9:527–565. 10.1146/annurev.bioeng.9.061206.133100 [DOI] [PubMed] [Google Scholar]
- 3. Barker AT, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. 10.1016/S0140-6736(85)92413-4 [DOI] [PubMed] [Google Scholar]
- 4. Di Lazzaro V, Oliviero A, Profice P, et al. : The diagnostic value of motor evoked potentials. Clin Neurophysiol. 1999;110(7):1297–1307. 10.1016/S1388-2457(99)00060-7 [DOI] [PubMed] [Google Scholar]
- 5. Schulz R, Gerloff C, Hummel FC: Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579–587. 10.1016/j.neuropharm.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 6. Radman T, Ramos RL, Brumberg JC, et al. : Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2(4):215–28, 228.e1–3. 10.1016/j.brs.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilmoniemi RJ, Ruohonen J, Karhu J: Transcranial magnetic stimulation--a new tool for functional imaging of the brain. Crit Rev Biomed Eng. 1999;27(3–5):241–284. [PubMed] [Google Scholar]
- 8. De Lucia M, Parker GJ, Embleton K, et al. : Diffusion tensor MRI-based estimation of the influence of brain tissue anisotropy on the effects of transcranial magnetic stimulation. Neuroimage. 2007;36(4):1159–1170. 10.1016/j.neuroimage.2007.03.062 [DOI] [PubMed] [Google Scholar]
- 9. Geeter ND, Dupré L, Crevecoeur G: Modeling transcranial magnetic stimulation from the induced electric fields to the membrane potentials along tractography-based white matter fiber tracts. J Neural Eng. 2016;13(2):026028. 10.1088/1741-2560/13/2/026028 [DOI] [PubMed] [Google Scholar]
- 10. Janssen AM, Oostendorp TF, Stegeman DF: The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J Neuroeng Rehabil. 2015;12:47. 10.1186/s12984-015-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nummenmaa A, McNab JA, Savadjiev P, et al. : Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stimul. 2014;7(1):80–84. 10.1016/j.brs.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Opitz A, Windhoff M, Heidemann RM, et al. : How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;58(3):849–859. 10.1016/j.neuroimage.2011.06.069 [DOI] [PubMed] [Google Scholar]
- 13. Thielscher A, Opitz A, Windhoff M: Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;54(1):234–243. 10.1016/j.neuroimage.2010.07.061 [DOI] [PubMed] [Google Scholar]
- 14. Windhoff M, Opitz A, Thielscher A: Electric field calculations in brain stimulation based on finite elements: An optimized processing pipeline for the generation and usage of accurate individual head models. Hum Brain Mapp. 2013;34(4):923–935. 10.1002/hbm.21479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim D, Jeong J, Jeong S, et al. : Validation of Computational Studies for Electrical Brain Stimulation With Phantom Head Experiments. Brain Stimul. 2015;8(5):914–925. 10.1016/j.brs.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 16. Laakso I, Hirata A, Ugawa Y: Effects of coil orientation on the electric field induced by TMS over the hand motor area. Phys Med Biol. 2014;59(1):203–18. 10.1088/0031-9155/59/1/203 [DOI] [PubMed] [Google Scholar]
- 17. Opitz A, Legon W, Rowlands A, et al. : Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage. 2013;81:253–264. 10.1016/j.neuroimage.2013.04.067 [DOI] [PubMed] [Google Scholar]
- 18. Thielscher A, Kammer T: Linking physics with physiology in TMS: a sphere field model to determine the cortical stimulation site in TMS. Neuroimage. 2002;17(3):1117–1130. 10.1006/nimg.2002.1282 [DOI] [PubMed] [Google Scholar]
- 19. Lenz M, Platschek S, Priesemann V, et al. : Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct Funct. 2015;220(6):3323–3337. 10.1007/s00429-014-0859-9 [DOI] [PubMed] [Google Scholar]
- 20. Lenz M, Galanis C, Müller-Dahlhaus F, et al. : Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. 2016;7:10020. 10.1038/ncomms10020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagarajan SS, Durand DM, Warman EN: Effects of induced electric fields on finite neuronal structures: a simulation study. IEEE Trans Biomed Eng. 1993;40(11):1175–1188. 10.1109/10.245636 [DOI] [PubMed] [Google Scholar]
- 22. Roth BJ, Basser PJ: A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans Biomed Eng. 1990;37(6):588–597. 10.1109/10.55662 [DOI] [PubMed] [Google Scholar]
- 23. Kamitani Y, Bhalodia VM, Kubota Y, et al. : A model of magnetic stimulation of neocortical neurons. Neurocomputing. 2011;38–40:697–703. 10.1016/S0925-2312(01)00447-7 [DOI] [Google Scholar]
- 24. Pashut T, Wolfus S, Friedman A, et al. : Mechanisms of Magnetic Stimulation of Central Nervous System Neurons. PLoS Comput Biol. 2011;7(3):e1002022. 10.1371/journal.pcbi.1002022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu T, Fan J, Lee KS, et al. : Cortical neuron activation induced by electromagnetic stimulation: a quantitative analysis via modelling and simulation. J Comput Neurosci. 2016;40(1):51–64. 10.1007/s10827-015-0585-1 [DOI] [PubMed] [Google Scholar]
- 26. Chan CY, Nicholson C: Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371(1):89–114. 10.1113/jphysiol.1986.sp015963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahman A, Reato D, Arlotti M, et al. : Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591(10):2563–2578. 10.1113/jphysiol.2012.247171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodwin BD, Butson CR: Subject-Specific Multiscale Modeling to Investigate Effects of Transcranial Magnetic Stimulation. Neuromodulation. 2015;18(8):694–704. 10.1111/ner.12296 [DOI] [PubMed] [Google Scholar]
- 29. Salvador R, Silva S, Basser PJ, et al. : Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin Neurophysiol. 2011;122(4):748–758. 10.1016/j.clinph.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Lazzaro V, Oliviero A, Pilato F, et al. : Comparison of descending volleys evoked by transcranial and epidural motor cortex stimulation in a conscious patient with bulbar pain. Clin Neurophysiol. 2004;115(4):834–838. 10.1016/j.clinph.2003.11.026 [DOI] [PubMed] [Google Scholar]
- 31. Gorman AL: Differential patterns of activation of the pyramidal system elicited by surface anodal and cathodal cortical stimulation. J Neurophysiol. 1966;29(4):547–564. [DOI] [PubMed] [Google Scholar]
- 32. Silva S, Basser PJ, Miranda PC: Elucidating the mechanisms and loci of neuronal excitation by transcranial magnetic stimulation using a finite element model of a cortical sulcus. Clin Neurophysiol. 2008;119(10):2405–2413. 10.1016/j.clinph.2008.07.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thielscher A, Antunes A, Saturnino GB: Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? Conf Proc IEEE Eng Med Biol Soc. 2015;2015:222–225. 10.1109/EMBC.2015.7318340 [DOI] [PubMed] [Google Scholar]
- 34. Dale AM, Fischl B, Sereno MI: Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 35. Fischl B, Sereno MI, Dale AM: Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, Jenkinson M, Woolrich WM, et al. : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 37. Attene M: A lightweight approach to repairing digitized polygon meshes. Vis Comput. 2010;26(11):1393–1406. 10.1007/s00371-010-0416-3 [DOI] [Google Scholar]
- 38. Geuzaine C, Remacle JF: Gmsh: A 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int J Numer Methods Eng. 2009;79(11):1309–1331. 10.1002/nme.2579 [DOI] [Google Scholar]
- 39. Wagner TA, Zahn M, Grodzinsky AJ: Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans Biomed Eng. 2004;51(9):1586–1598. 10.1109/TBME.2004.827925 [DOI] [PubMed] [Google Scholar]
- 40. Renard Y, Pommier J: GetFEM++ Homepage — GetFEM++. getfem, A Generic Finite Element Library in C. Documentation2010. Reference Source [Google Scholar]
- 41. Fox PT, Narayana S, Tandon N, et al. : Column-based model of electric field excitation of cerebral cortex. Hum Brain Mapp. 2004;22(1):1–14. 10.1002/hbm.20006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mainen ZF, Sejnowski TJ: Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382(6589):363–366. 10.1038/382363a0 [DOI] [PubMed] [Google Scholar]
- 43. Hines ML, Carnevale NT: The NEURON simulation environment. Neural Comput. 1997;9(6):1179–1209. 10.1162/neco.1997.9.6.1179 [DOI] [PubMed] [Google Scholar]
- 44. Manola L, Holsheimer J, Veltink P, et al. : Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118(2):464–474. 10.1016/j.clinph.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 45. Seo H, Kim D, Jun SC: Computational Study of Subdural Cortical Stimulation: Effects of Simulating Anisotropic Conductivity on Activation of Cortical Neurons. PLoS One. 2015;10(6):e0128590. 10.1371/journal.pone.0128590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wongsarnpigoon A, Grill WM: Computer-based model of epidural motor cortex stimulation: effects of electrode position and geometry on activation of cortical neurons. Clin Neurophysiol. 2012;123(1):160–172. 10.1016/j.clinph.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 47. Zwartjes DG, Heida T, Feirabend HK, et al. : Motor cortex stimulation for Parkinson’s disease: a modelling study. J Neural Eng. 2012;9(5):056005. 10.1088/1741-2560/9/5/056005 [DOI] [PubMed] [Google Scholar]
- 48. DeFelipe J, Alonso-Nanclares L, Arellano JI: Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31(3–5):299–316. 10.1023/A:1024130211265 [DOI] [PubMed] [Google Scholar]
- 49. Miranda PC, Correia L, Salvador R: Tissue heterogeneity as a mechanism for localized neural stimulation by applied electric fields. Phys Med Biol. 2007;52(18):5603–17. 10.1088/0031-9155/52/18/009 [DOI] [PubMed] [Google Scholar]
- 50. Rusu CV, Murakami M, Ziemann U, et al. : A model of TMS-induced I-waves in motor cortex. Brain Stimul. 2014;7(3):401–414. 10.1016/j.brs.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 51. Kammer T, Beck S, Thielscher A, et al. : Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112(2):250–258. 10.1016/S1388-2457(00)00513-7 [DOI] [PubMed] [Google Scholar]
- 52. Bikson M, Rahman A, Datta A, et al. : High-resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation. 2012;15(4):306–315. 10.1111/j.1525-1403.2012.00481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krieg TD, Salinas FS, Narayana S, et al. : Computational and experimental analysis of TMS-induced electric field vectors critical to neuronal activation. J Neural Eng. 2015;12(4):046014. 10.1088/1741-2560/12/4/046014 [DOI] [PubMed] [Google Scholar]
- 54. Grant PF, Lowery MM: Electric field distribution in a finite-volume head model of deep brain stimulation. Med Eng Phys. 2009;31(9):1095–1103. 10.1016/j.medengphy.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 55. Kim D, Seo H, Kim HI, et al. : Computational study on subdural cortical stimulation - the influence of the head geometry, anisotropic conductivity, and electrode configuration. PLoS One. 2014;9(9):e108028. 10.1371/journal.pone.0108028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nummenmaa A, Stenroos M, Ilmoniemi RJ, et al. : Comparison of spherical and realistically shaped boundary element head models for transcranial magnetic stimulation navigation. Clin Neurophysiol. 2013;124(10):1995–2007. 10.1016/j.clinph.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seo H, Kim D, Jun SC: Effect of Anatomically Realistic Full-Head Model on Activation of Cortical Neurons in Subdural Cortical Stimulation-A Computational Study. Sci Rep. 2016;6: 27353. 10.1038/srep27353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krieg TD, Salinas FS, Narayana S, et al. : PET-based confirmation of orientation sensitivity of TMS-induced cortical activation in humans. Brain Stimul. 2013;6(6):898–904. 10.1016/j.brs.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Day BL, Dressler D, Maertens de Noordhout A, et al. : Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412(1):449–473. 10.1113/jphysiol.1989.sp017626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patton HD, Amassian VE: Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17(4):345–363. [DOI] [PubMed] [Google Scholar]
- 61. Hern JE, Landgren S, Phillips CG, et al. : Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon’s motor cortex. J Physiol. 1962;161(1):73–90. 10.1113/jphysiol.1962.sp006874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Di Lazzaro V, Profice P, Ranieri F, et al. : I-wave origin and modulation. Brain Stimul. 2012;5(4):512–525. 10.1016/j.brs.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 63. Brasil-Neto JP, Cohen LG, Panizza M, et al. : Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9(1):132–136. 10.1097/00004691-199201000-00014 [DOI] [PubMed] [Google Scholar]
- 64. Mills KR, Boniface SJ, Schubert M: Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85(1):17–21. 10.1016/0168-5597(92)90096-T [DOI] [PubMed] [Google Scholar]
- 65. Baker SN, Olivier E, Lemon RN: Task-related variation in corticospinal output evoked by transcranial magnetic stimulation in the macaque monkey. J Physiol. 1995;488(Pt 3):795–801. 10.1113/jphysiol.1995.sp021011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Edgley SA, Eyre JA, Lemon RN, et al. : Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990;425(1):301–320. 10.1113/jphysiol.1990.sp018104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kole MH, Ilschner SU, Kampa BM, et al. : Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11(2):178–86. 10.1038/nn2040 [DOI] [PubMed] [Google Scholar]
- 68. Seo H, Schaworonkow N, Jun SC, et al. : Dataset 1 in: A Multi-Scale Computational Model of the effects of TMS on Motor Cortex. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seo H, Schaworonkow N, Jun SC, et al. : Dataset 2 in: A Multi-Scale Computational Model of the effects of TMS on Motor Cortex. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seo H, Schaworonkow N, Jun SC, et al. : Dataset 3 in : A Multi-Scale Computational Model of the effects of TMS on Motor Cortex. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seo H, Schaworonkow N, Jun SC, et al. : Dataset 4 in: A Multi-Scale Computational Model of the effects of TMS on Motor Cortex. F1000Research. 2016. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]