Summary
Anopheles gambiae sensu stricto is the most important vector of malaria in Africa although relatively little is known about the density-dependent processes determining its population size.
Mosquito larval density was manipulated under semi-natural conditions using artificial larval breeding sites placed in the field in coastal Kenya; two experiments were conducted: one manipulating the density of a single cohort of larvae across a range of densities and the other employing fewer densities but with the treatments crossed with four treatments manipulating predator access.
In the first experiment, larval survival, development rate and the size of the adult mosquito all decreased with larval density (controlling for block effects between 23% and 31% of the variance in the data could be explained by density).
In the second experiment, the effects of predator manipulation were not significant, but again we observed strong density dependence in larval survival (explaining 30% of the variance).
The results are compared with laboratory studies of A. gambiae larval competition and the few other studies conducted in the field, and the consequences for malaria control are discussed
Keywords: Anopheles gambiae, density dependence, field experiment, mosquito
Introduction
Mosquitoes in the genus Anopheles are the sole vectors of the human malaria pathogen, Plasmodium (Gilles, Warrell & Bruce-Chwatt 1993). Malaria is globally one of the most significant infectious diseases and has been estimated currently to sicken over 200 million people annually and causes about three quarters of a million deaths (WHO 2010) although a very recent study (Murray et al. 2011), using new methodologies, has suggested that the number of deaths are considerably higher: 1·2 (95% uncertainty range, 0·9–1·7) million people in 2010. There has been intensive research on how the toll of malaria can be reduced or eliminated, efforts that have focussed on both targeting the Plasmodium in humans and interventions designed to interrupt transmission by the mosquito (Gilles, Warrell & Bruce-Chwatt 1993). Vector-control measures have included habitat modification to remove larval breeding sites (Ijumba, Mosha & Lindsay 2002; Yohannes et al. 2005), spraying relatively long-lasting insecticides on the walls of huts and houses to kill female mosquitoes where they rest after a blood meal (Pluess et al. 2010), and the use of bed nets, especially those impregnated with insecticide, to protect sleeping people (Lengeler 2004). Recently, there has been growing interest in the possibility of using genetic manipulation to interrupt transmission, either by introducing a construct that reduces mosquito fitness and hence population size or by knocking out a gene that codes for a product essential for malaria transmission (Burt 2003; Sinkins & Gould 2006; Chen et al. 2007). Effective implementation of any mosquito-control strategy requires a good understanding of the vector’s ecology. This article describes a field study designed to help understand population regulation in the most important African vector of malaria.
Malaria burdens are highest in Africa (Snow et al. 2005; Murray et al. 2011) where most transmission is dominated by members of the Anopheles gambiae and Anopheles funestus complexes (Hay et al. 2010; Sinka et al. 2010). A. gambiae mosquitoes are very efficient vectors because of the strong preference of some members of the complex to bite humans. The complex consists of seven morphologically identical species with subtly different ecologies (Coluzzi et al. 1979). The two members responsible for most transmission are A. arabiensis and A. gambiae sensu stricto (henceforth, we use A. gambiae to refer to this form), which tend to be found in dryer and wetter areas, respectively. A. gambiae has a very complex population structure, especially in West Africa, which is still not fully resolved (Riehle et al. 2010).
It is clearly important to understand the population ecology of African Anopheles mosquitoes to design and optimise control measures. Two critical questions are: (i) What are the density-dependent processes that contribute to population regulation in these insects? and (ii) Where in the lifecycle do they occur? Answers to these questions can help determine the strength of interventions required to interrupt transmission and which life-history stage may be the most efficient to target. Most mosquito ecologists have assumed, implicitly or explicitly, that density dependence occurs largely in the larval stage through competition for food. For example, the Ross–McDonald model (Ross 1911; Macdonald 1957) that describes vector-transmitted diseases, and many models based on it, supposes mosquitoes recruit to the adult stage at a constant rate that is equivalent to assuming perfectly compensating pre-adult density dependence. There is limited experimental support for density-dependent larval competition. In laboratory experiments, high larval densities of A. gambiae have been shown both to decrease (Lyimo, Takken & Koella 1992) and to extend (Schneider, Takken & McCall 2000) development times, while their effects on adult size are complex and influenced by temperature (Lyimo, Takken & Koella 1992; Schneider, Takken & McCall 2000). Ng’habi et al. (2005) found evidence that crowding affected male mating success independent of the amount of food provided per larva. Service (1973, 1977) studied natural parasitism and predation in paddy fields on larval A. arabiensis – a member of the A. gambiae complex. Density dependence in A. gambiae has also been inferred from the statistical analysis of time-series data (Russell et al. 2011).
The most important recent study to look at density dependence in African malaria vectors experimentally in the field was undertaken by Gimnig et al. (2002). In two experiments, they manipulated the density of A. gambiae larvae in artificial breeding sites placed in the field in Western Kenya. They found a strong effect of density on development time and adult size but no significant effect on survival. Njunwa (1993; see also White et al. 2011) in an unpublished study manipulated larval densities and found mortality increased and then plateaued as larval densities rose.
Despite the great significance of A. gambiae to human well-being, we believe these studies are the only field experiments to explore larval density dependence. Here, we report a series of experiments in which A. gambiae densities and cohort structure are manipulated in the field in coastal Kenya using experimental methodologies based on Gimnig et al. (2002). We detected strong density dependent in larval competition, although of a form different to the earlier study, and we explore the reasons for these differences and the implications for the control of mosquitoes.
Materials and methods
Study Site
The study was conducted in the village of Jaribuni (03°37·3S; 039° 44·6E) approximately 50 km west of Kilifi in Kilifi County on the Kenyan coast north of Mombasa. The study site is approximately 400 m above sea level and has two wet seasons annually, a long rainy period in April–June and short rains in October–December; mean annual precipitation is 750–1200 mm. Temperatures range from 22 to 30 °C, and the average relative humidity is ~70%. The area around Jaribuni consists of scattered villages and households with subsistence farming and small-scale rearing of cattle, goat and poultry, with some plantations of sisal, coconut and cashew nuts. The rural population mainly lives in stick and mud-walled houses with roofs thatched with coconut leaves. Malaria epidemiology has been studied in this area for many years by workers from the KEMRI-Wellcome Trust Programme. The most common vector is A. gambiae (though with A. arabiensis increasing in frequency) with A. funestus also significant. Our experiments had ethical approval from KEMRI (the Kenyan Medical Research Institute).
Artificial Larval Habitats
Experiments were conducted in artificial larval habitats designed to mimic the natural breeding sites of A. gambiae. Clay pots 35 cm in diameter and 13 cm deep were placed in shallow depressions in the ground approximately 9 cm deep in experimental plots fenced to exclude animals. The plots were unshaded and had good drainage to avoid flooding after heavy rain. Small holes (5 mm diameter) were drilled beneath the rim of the pot and covered with fine gauze net to allow water drainage without loss of mosquito larvae. Locally collected, sterilised mud (1 kg) was smeared around the inner surface of each pot, and five litres of filtered (but not sterilised) water from the River Jaribuni (normally a small stream immediately adjacent to larval breeding sites) was added to prepare the larval habitats. The water depth at the start of the experiment was 10 cm, and further water was added each day to replace that lost to evaporation.
Experimental Mosquitoes
The mosquitoes used in the experiments were obtained from a laboratory colony originally initiated from mosquitoes collected at Kisumu in western Kenya. They were reared in an insectary at the KEMRI-Wellcome trust research laboratory in Kilifi. On the days the experiments were set up, mosquito eggs were allowed to hatch in plastic containers and the first-instar larvae counted into vials, which were transported to the field site.
Experiment 1
In this experiment, the densities of a single cohort of larval mosquitoes were manipulated, and the consequences for development time, survival and adult size assessed. The experiment consisted of seven temporally separated blocks set up between November 2009 and August 2010: three in the dry season and four in the wet season. In each block, larval breeding sites were assigned randomly to five density treatments: initial densities of 32, 64, 128, 256 or 512 first-instar larvae. The total number of larval breeding sites varied across blocks because of variation in the supply of mosquitoes available from the rearing facility and was 25 in two blocks, 20 in three blocks and 15 in two blocks. The pots were covered with fine netting sufficient to exclude predators and oviposition by wild mosquitoes. Each day, the breeding sites were monitored, and any pupae present were removed using a pipette and taken back to the laboratory to be reared. The resulting adults were killed, sexed and then dried over anhydrous calcium sulphate and preserved for their wing length to be measured as an index of size. To do this, a single wing was detached and mounted in xylene-based DPX on a microscope slide. The wing length from the distal end of the alula to the tip of the wing excluding the fringe scales was measured to the nearest 0·01 mm using an ocular micrometer under ×40 magnification.
Experiment 2
The second experiment aimed to look at the combined effects of larval competition and predation. Three larval density levels and four predation manipulation treatments were applied in a factorial experimental design. Experimental larval breeding sites were seeded with either 32, 128 or 512 first-instar larvae in the same manner as Experiment 1. Breeding sites were assigned to four predation treatments. (i) Pots left uncovered so that they could be colonised by any predator. (ii) Pots covered by a coarse wire mesh (5 cm diameter holes) large enough to allow access to any non-vertebrate predators. (iii) Pots covered with finer mesh (1·5–2 cm in diameter holes) allowing colonisation by small but not large invertebrate predators. (iv) Pots covered by mosquito netting excluding all predators as in Experiment 1. The experiment was run six times between April and December 2011, three times each in the dry season and in the wet season. The number of times the 12 treatment combinations were replicated per run depended on the mosquito larvae available in the rearing facility: in four cases, five replicates were set up, and in two runs, there were three replicates. The breeding sites were monitored daily for the presence of pupae and predators. Predator numbers were estimated visually, and representative samples collected and identified morphologically to family level using Merritt & Cummins (1996). Mosquito pupae were collected using a sucking pipette, and their identity confirmed in the laboratory.
Statistical Methods
Data were analysed using generalised linear modelling techniques implemented in the R statistical package (R Development Core Team, URL http://www.R-project.org/). The proportion of larvae that pupated per larval breeding site was modelled using a quasi-binomial error structure to account for overdispersion. The error structure of the median time to pupation per breeding site was found to be well described by a quasi-Poisson distribution with the variance proportional to the mean. The mean wing length of the mosquitoes emerging per breeding site was normally distributed. Initial density (either log-transformed or untransformed) was treated as a continuous explanatory variable and season or block (nested within season) as fixed factors. In Experiment 2, the four predation manipulations were treated as fixed factors. Model fits were inspected graphically, and the optimum statistical models investigated by stepwise deletion with significance assessed using the appropriate test (F-test or likelihood ratio) for the assumed error structure.
Results
Experiment 1
There was considerable variation in survival as measured by the probability of successful pupation across the different temporal blocks (Fig. 1a). To explore whether this was due to the effect of the wet vs. the dry season, we first fitted season as a factor and then separate factors for the seven temporal blocks. The addition of both factors was significant (Table 1) with season explaining 11% of the initial deviance (the equivalent in a generalised linear model of the total variance) for one degree of freedom and blocks a further 24% for five degrees of freedom. In the wet season, survival was on average half that in the dry season with the odds of survival decreasing by a factor of 0·50 (SE range, 0·42–0·59). Thus, together, time of year and possibly other block effects explain about a third of the total variation in survival.
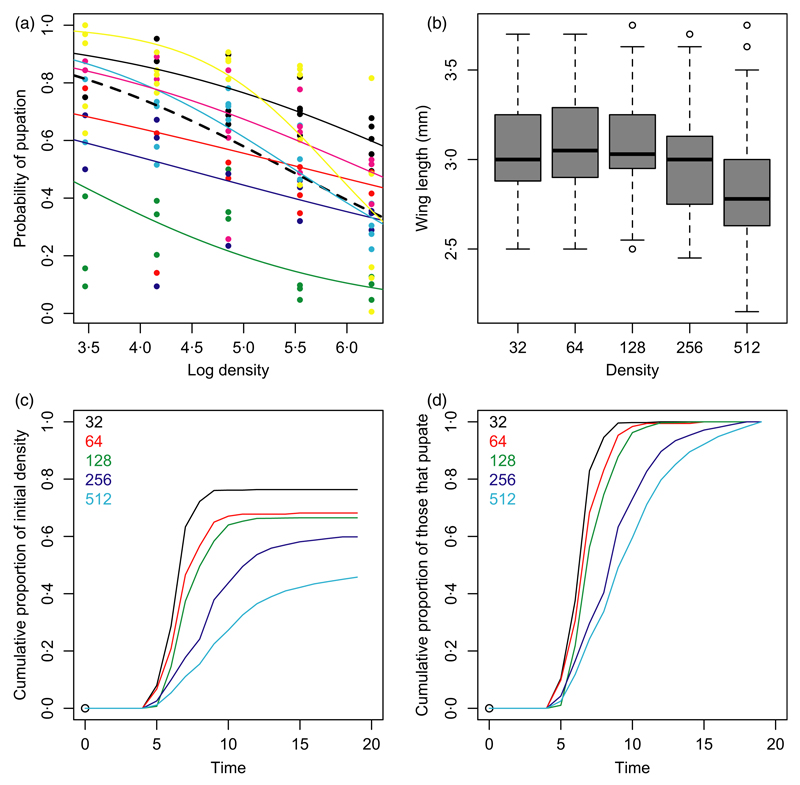
Fig. 1. The effects of larval density on mosquito fitness (Experiment 1).
(a) The probability of pupation as a function of the log of initial density. The raw data are shown with the estimated block mean effects in different colours (dry season: black, red, yellow; wet season: green, blue, cyan, magenta). The overall mean pupation rate as a function of density is represented by the heavy dashed line. (b) Box-whisker plot of wing length as a function of density, all blocks combined. The box shows the inter-quartile range with the median represented by a line; the whiskers show the full range of data except for outliers represented by circles. (c) The cumulative proportion of mosquitoes that pupate in the different density treatments as a function of time after the larvae were added to the experimental breeding sites. (d) As panel c but here the standardised cumulative proportion of all those mosquitoes that will eventually pupate are plotted as a function of time to make visual comparison between the different density treatments easier.
Table 1.
Analysis of deviance of the different components of mosquito fitness measured in Experiment 1. Resid. dev and % dev explained are residual deviance and percentage deviance explained, where deviance is equivalent to sum of squares in an analysis of variance
| d.f. | Resid. dev | F-value | P-value | % dev explained | |
|---|---|---|---|---|---|
| (a) Pupation probability | |||||
| Intercept only | 134 | 7210 | |||
| Add season | 133 | 6401 | 38.0 | < 0.0001 | 11 |
| Add block | 128 | 4643 | 16.5 | < 0.0001 | 24 |
| Add log initial density | 127 | 2940 | 79.9 | < 0.0001 | 24 |
| Add block*density | 121 | 2582 | 2.8 | 0.014 | 5 |
| (b) Time to pupation | |||||
| Intercept only | 134 | 53.4 | |||
| Add season | 133 | 51.5 | 18.8 | < 0.0001 | 4 |
| Add block | 128 | 31.8 | 40 | < 0.0001 | 37 |
| Add initial density | 127 | 15 | 171.3 | < 0.0001 | 31 |
| Add block*density | 121 | 11.7 | 6.5 | < 0.0001 | 6 |
| (c) Wing length, females | |||||
| Intercept only | 72 | 4.05 | |||
| Add season | 71 | 4.05 | 0.01 | 0.91 | <1 |
| Add block | 66 | 3.58 | 2.45 | 0.042 | 12 |
| Add initial density (linear) | 65 | 2.67 | 23.75 | < 0.0001 | (linear and quadratic) 27 |
| Add initial density (quadratic) | 64 | 2.47 | 5.14 | 0.027 | |
| (d) Wing length, males | |||||
| Intercept only | 72 | 3.99 | |||
| Add season | 71 | 3.91 | 2.42 | 0.12 | 2 |
| Add block | 66 | 3.21 | 4.26 | 0.002 | 18 |
| Add initial density (linear) | 65 | 2.42 | 24.07 | < 0.0001 | (linear and quadratic) 26 |
| Add initial density (quadratic) | 64 | 2.17 | 7.57 | 0.008 |
After controlling for block, the addition of log density was highly significant and explained a further 24% of the initial deviance (Fig. 1a; Table 1a). The form of the density dependence was nearly linear (slope, −0·75; SE, 0·09) when the log-odds of survival were plotted against log density. Increasing log density by an amount x results in a reduction in the odds of survival by a multiplicative factor of 0·47x. The form of density dependence did not differ significantly in the dry and wet season although there was a significant block × log density interaction (though explaining only a further 5% of the initial deviance). Inspection of Fig. 1a shows this is due to mortality increasing particularly strongly with density in two blocks, for reasons that we cannot explain.
Higher larval density not only increases mortality but lengthens the time the mosquito requires to reach the pupal stage (Fig. 1c and d). Again there was substantial variation amongst the temporal blocks. The effect of season, though significant, was much less important than in the analysis of survival and explained only 4% of the initial deviance (for one degree of freedom), while block explained a further 37% for five degrees of freedom (Table 1b). After controlling for block, the addition of density significantly improved the fit of the model explaining 31% more of the initial deviance (untransformed rather than log density was used as the relationship was linear on this scale). Across the range of densities used in the experiment, the median time to pupation increased from about 5 to 9 days. There was a significant interaction between block and log density although this only explained 6% of the data for five degrees of freedom.
Mosquitoes emerging from high-density breeding sites tend to be smaller than those from low-density breeding sites. To avoid pseudoreplication, the mean wing length was analysed from mosquitoes emerging from the same larval breeding site. Males and females were analysed separately although the results were similar. Fitting season as a factor had no significant effect although there were significant block effects explaining 12% and 18% of the total variance for females and males, respectively (Table 1c & 1d). Controlling for block effects, the addition of both a linear and quadratic log density term significantly improved the fit of the model explaining together 27% (females) and 26% (males) of the total variance. In Fig. 1b, we plot the wing length of all mosquitoes as a function of density. Mosquitoes emerging from the three lower density treatments were of approximately the same size, but those from the two higher density treatments, and in particular the highest, are smaller.
Experiment 2
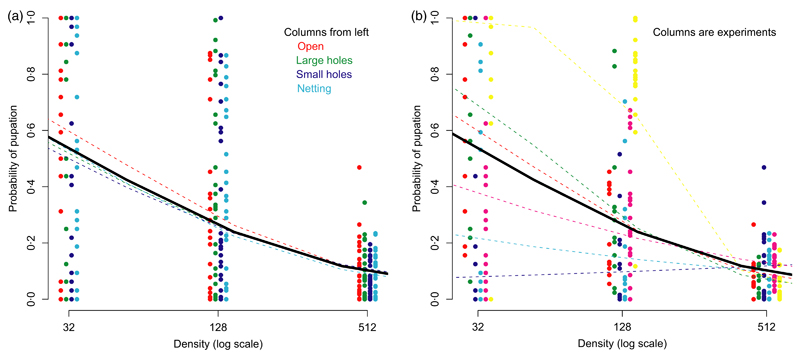
The experiment consisted of three density treatments crossed with four predator treatments replicated in six temporal blocks. Survival varied significantly across blocks, and there was a strong effect of density (Table 2). The average probability of pupating at a density of 32 larvae per breeding site is 0·43 (SE, 0·38–0·49), which falls to 0·32 (SE, 0·29–0·34) at a density of 128 and 0·09 (SE, 0·08–0·10) at the highest density of 512 (Fig. 2). There was a significant block by density treatment interaction (Fig. 2a), and in two blocks, low-density mortality was as high as in the most crowded treatment.
Table 2.
Analysis of deviance of the effects of density and predation treatments in larval mosquito survival in Experiment 2. Column headings as in Table 1
| d.f | Resid. Dev | F-value | P-value | % dev explained | |
|---|---|---|---|---|---|
| Pupation probability | |||||
| Intercept only | 311 | 19584 | |||
| Add block | 306 | 18670 | 6·15 | < 0·0001 | 5 |
| Add density | 304 | 13112 | 93·31 | < 0·0001 | 28 |
| Add density × block | 294 | 8685 | 14·89 | < 0·0001 | 23 |
| Add predation | 291 | 8624 | 0·68 | 0·56 | <1 |
Fig. 2. The probability of pupation as a function of initial population size in the predation experiment. The raw data for different breeding sites are shown as dots.
In (a), the four different predation treatments are shown as separate columns with dotted lines treatments means and the heavy continuous the overall mean. In (b), data from the six replicate experiments are shown in the columns along with experiment and overall fitted means.
There was no significant effect of predator treatment on survival (Table 2, Fig. 2b). Predators did colonise the unenclosed breeding sites but at low densities and with very high variance: most sites had no predators but some had many. There was no significant interaction between the predator and density treatments (Fig. 2b). Survival at low densities in the open (no netting or mesh) treatments was slightly higher than in the others. In this treatment, we found some oviposition by wild Anopheles, which could normally be recognised because the larvae were smaller than the experimental cohort. However, in a few cases, we recorded more pupae than larvae suggesting ovi-position early in the experiment resulting in larvae that could not be separated. The lack of difference across predation treatments in the relationship between survival and larval density shows that this was not a major factor influencing the results.
Discussion
Anopheles gambiae larvae in semi-natural breeding sites in the field experienced substantial density-dependent impairment in fitness. We found that as densities increased survival prospects worsened, the length of time required to reach the pupal stage went up, and there was a reduction in the size of the resulting adult mosquitoes. In the first experiment, we found that ~20–30% of the variation in all three measures could be explained by the density treatment although there were also significant temporal block effects. Exactly, the same thing was found in the second experiment where only survival was recorded. This experiment was designed to explore the effects of different guilds of predation on larval mosquito survival, but none were found. The results are consistent with density dependence being under- rather than over-compensatory.
The main ecological requirements of A. gambiae larvae were established by the 1950s (Muirhead-Thomson 1951) and have been confirmed by a series of recent studies (e.g. Gimnig et al. 2001; Bogh et al. 2003; Klinkenberg et al. 2003; Ye-Ebiyo et al. 2003; Fillinger et al. 2004; Koenraadt, Githeko & Takken 2004; Minakawa et al. 2004, 2005; Mutuku et al. 2006a,b; Tuno et al. 2006). It is found most frequently in small, relatively clean and frequently temporary sunny water bodies, without overhanging vegetation. However, as has been repeatedly stressed (Muirhead-Thomson 1951; Fillinger et al. 2004), A. gambiae has broad habitat tolerances and can be found in many different types of water body, and equally is absent from some apparently suitable pools. The design of the breeding sites used in our experiments, which we refer to as semi-natural, was a compromise between mimicking actual local breeding sites and reducing variance amongst replicates. The containers were thus round, lined with sterilised mud and contained no internal structure such as clods of mud or dead or living vegetation. They were also initiated using unsterilized local river water, mixed to ensure all sites received a similar microbial flora. The breeding sites were sunk into the earth very near to an area where A. gambiae naturally breed, and thus, we believe experienced a very similar microclimate.
Most accounts of the population biology of A. gambiae have assumed that the main source of density dependence affecting population densities is competition for resources at the larval stage, and this may be manifest through increased mortality, increased development time and decreased adult size. The evidence for this comes from laboratory experiments with A. gambiae and a limited number of field experiments.
Larval density dependence in A. gambiae has been demonstrated a number of times in the laboratory. For example, Lyimo, Takken & Koella (1992) showed that at higher larval densities development time increased, and adult size tended to be smaller although this was affected by temperature. Similarly, Takken, Klowden & Chambers (1998) used larval density manipulation to obtain adults of different size, and Schneider, Takken & McCall (2000) showed that at high densities, A. gambiae sibling species competed for food resources. These experiments clearly show the potential for density-dependent larval competition, but their extrapolation to what happens in the field must be made with care. The laboratory environment is relatively benign compared with the field, which may explain why the effects of competition tend to be observed at densities that are high though not unknown in the wild. Perhaps more seriously, larval mosquitoes in the laboratory are typically fed using fish food (to generate a microbial flora), which is clearly different from what mosquitoes experience in the field. Indeed, as Gimnig et al. (2002) stress, we still know relatively little about exactly what microbial organisms A. gambiae most often consume.
There are a small number of experiments that have attempted to manipulate A. gambiae densities in the field of which the most important were conducted by Gimnig et al. (2002). We based some of our experimental methods on this study. In their main experiment, they placed between 20 and 200 first-instar larvae artificial breeding sites containing ~1 L of water, roughly comparable to the densities we used in our slightly bigger containers. They found that adults from high-density treatments were comparatively small and that they took longer to develop. However, unlike in our experiments, they did not observe a decline in survival at high densities. In a further experiment with just two density treatments, they again observed responses in adult size and development time but not survival. In this experiment, they attempted to increase the food resources for the larvae by adding 1 g of cow dung, but this had no effect on mosquito fitness.
Further data on mosquito density dependence in an unpublished PhD thesis (Njunwa 1993) have recently been re-analysed by White et al. (2011). Njunwa placed batches of larvae in artificial breeding sites at five densities. Mortality increased with density but then plateaued (at ~2% survival) in breeding sites with the highest number of larvae. A further experiment explored mortality when first-instar larvae were added not in a single batch, but at staggered individuals; though, the results are harder to interpret.
Our results with the few previous studies that have manipulated larval numbers in the field all point to density dependence at the larval stage being significant in the field. Observed larval densities vary greatly in natural habitats, and although comparisons are difficult to make between natural and experimental breeding sites, they do seem on occasion to reach the highest of our manipulation treatments densities (Fillinger et al. 2004; Mwangangi et al. 2006). It is also clear that larval densities can affect survival, development time and adult size, although it is puzzling that in Gimnig et al.’s (2002) study, mortality was not affected by the number of larvae in the breeding site. While the fitness consequences of increased mortality are obvious, the natural history of A. gambiae suggests that the two other responses may also be highly correlated with fitness. A. gambiae frequently breeds in temporary pools that dry up after rains (Muirhead-Thomson 1951), and so delayed development may indirectly lead to increased mortality (which would not have occurred in our study as we kept the water volumes in the breeding sites constant). Adult longevity in mosquitoes can at least under some circumstances be size-dependent with small individuals having low fitness and, importantly, having reduced probability to live long enough to contract, incubate and transmit pathogens (Lyimo & Koella 1992).
Our attempt to manipulate predation in the field failed. Predators did colonise the breeding sites, but the variance was so great that the statistical power to detect any differences amongst the treatments was negligible. It is possible that there was some aspect of our artificial breeding sites or their location that rendered them unattractive to predators, but we suspect that the nature of A. gambiae breeding sites – small temporary pools – makes their discovery by mosquito natural enemies highly haphazard. Interestingly, another member of the gambiae complex, A. arabiensis breeds in larger water bodies such as rice bodies and field studies by Service (1973, 1977) suggested both parasitism and predation to be measurably important to this species’ dynamics.
For the last 50 years, the dominant conceptual paradigm for studying vector-borne diseases, in particular malaria, has been the Ross–Macdonald model (Ross 1911; Macdonald 1957; Anderson & May 1991; Smith & McKenzie 2004). The equation describing adult mosquito densities assume constant recruitment to the adult stage (or in some extensions seasonally cyclic or rainfall-driven recruitment), which implicitly assumes perfect density dependence in the larval stage. Where vector control is concentrated on killing adult mosquitoes and in particular reducing adult longevity to a level at which few survive long enough to transmit disease, this simplification of the insect’s population dynamics may not be too restrictive. However, there are increasing calls today for integrated vector management (IVM) and for multipronged strategies including larval control and habitat modification (Yohannes et al. 2005; Killeen et al. 2006). There is also an exciting range of new vector-control measures under research employing techniques from modern molecular biology to suppress mosquito populations often by targeting juvenile stages (Burt 2003; Sinkins & Gould 2006). To implement IVM or novel genetic methods efficiently, better models of mosquito-vector population dynamics are required incorporating richer information about larval biology and in particular important ecological processes such as density dependence. Although we have concentrated here on malaria vectors, the same is true of other major mosquito vectors, for example there are only a handful of studies that have looked at density dependence in the field for the yellow fever and dengue virus vector, Aedes aegyptii (Southwood et al. 1972; Dye 1984; Legros et al. 2009; Walsh et al. 2011). Given that A. gambiae can claim to be the most dangerous species of animal on earth (in terms of the scale of deaths and morbidity it causes), and a species whose molecular biology is perhaps more studied than any animal except the classic model organisms, it is salutary and worrying how little we still know about its population ecology.
Acknowledgements
We thank the community members of Jaribuni area, Kilifi County for their kindness and cooperation during this study. We are also grateful to Martha Muturi, Arnold Mramba and Japhet Kahindi from the vector biology team, KEMRI-CGMRC, Kilifi for their tireless effort in the insectary and field work. H.C.J.G. is grateful for help from members of RAPIDD (Research and Policy in Infectious Disease Dynamics Programme of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, NIH).
References
- Anderson RM, May RM. Infectious Diseases of Humans. Oxford University Press; Oxford: 1991. [Google Scholar]
- Bogh C, Clarke SE, Jawara M, Thomas CJ, Lindsay SW. Localized breeding of the Anopheles gambiae complex (Diptera: Culicidae) along the River Gambia, West Africa. Bulletin of Entomological Research. 2003;93:279–287. doi: 10.1079/ber2003239. [DOI] [PubMed] [Google Scholar]
- Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Huang HX, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Dideco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Dye C. Models for the population dynamics of the yellow fever mosquito, Aedes aegypti. Journal of Animal Ecology. 1984;53:247–268. [Google Scholar]
- Fillinger U, Sonye G, Killeen GF, Knols BGJ, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Tropical Medicine & International Health. 2004;9:1274–1289. doi: 10.1111/j.1365-3156.2004.01335.x. [DOI] [PubMed] [Google Scholar]
- Gilles HM, Warrell DA, Bruce-Chwatt LJ. Bruce-Chwatt’s Essential Malariology. Edward Arnold; London: 1993. [Google Scholar]
- Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in western Kenya. Journal of Medical Entomology. 2001;38:282–288. doi: 10.1603/0022-2585-38.2.282. [DOI] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomology. 2002;39:162–172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Hay SI, Sinka ME, Okara RM, Kabaria CW, Mbithi PM, Tago CC, Benz D, Gething PW, Howes RE, Patil AP, Temperley WH, et al. Developing global maps of the dominant Anopheles vectors of human malaria. Plos Medicine. 2010;7:e1000209 1–65. doi: 10.1371/journal.pmed.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijumba JN, Mosha FW, Lindsay SW. Malaria transmission risk variations derived from different agricultural practices in an irrigated area of northern Tanzania. Medical and Veterinary Entomology. 2002;16:28–38. doi: 10.1046/j.0269-283x.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, Fillinger U, de Castro MC. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. American Journal of Tropical Medicine and Hygiene. 2006;74:517–518. [PubMed] [Google Scholar]
- Klinkenberg E, Takken W, Huibers F, Toure YT. The phenology of malaria mosquitoes in irrigated rice fields in Mali. Acta Tropica. 2003;85:71–82. doi: 10.1016/s0001-706x(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Koenraadt CJM, Githeko AK, Takken W. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Tropica. 2004;90:141–153. doi: 10.1016/j.actatropica.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Legros M, Lloyd AL, Huang YX, Gould F. Density dependent intraspecific competition in the larval stage of Aedes aegypti (Diptera: Culicidae): revisiting the current paradigm. Journal of Medical Entomology. 2009;46:409–419. doi: 10.1603/033.046.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bednets and curtains for preveting malaria. Cochrane Database of Systematic Reviews. 2004;(2):1–52. doi: 10.1002/14651858.CD000363. [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Koella JC. Relationship between body size of adult Anopheles gambiae s.1. and infection with the malaria parasite Plasmodium falciparum. Parasitology. 1992;104:233–237. doi: 10.1017/s0031182000061667. [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomologia Experimentalis Et Applicata. 1992;63:265–271. [Google Scholar]
- Macdonald G. The Epidemiology and Control of Malaria. Oxford University Press; Oxford: 1957. [Google Scholar]
- Merritt RW, Cummins KW. An Introduction to the Aquatic Insects of North America. Kendall/Hunt; Dubuque, Iowa: 1996. [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Medical and Veterinary Entomology. 2004;18:301–305. doi: 10.1111/j.0269-283X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou GF, Githeko AK, Yan GY. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. American Journal of Tropical Medicine and Hygiene. 2005;73:157–165. [PubMed] [Google Scholar]
- Muirhead-Thomson RC. Mosquito Behaviour in Relation to Malaria Transmission and Control in the Tropics. Edward Arnold & Co; London: 1951. [Google Scholar]
- Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet. 2011;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Mutuku FM, Alaii JA, Bayoh MN, Gimnig JE, Vulule JM, Walker ED, Kabiru E, Hawley WA. Distribution, description, and local knowledge of larval habitats of Anopheles gambiae s.l. in a village in western Kenya. American Journal of Tropical Medicine and Hygiene. 2006a;74:44–53. [PubMed] [Google Scholar]
- Mutuku FM, Bayoh MN, Gimnig JE, Vulule JM, Kamau L, Walker ED, Kabiru E, Hawley WA. Pupal habitat productivity of Anopheles gambiae complex mosquitoes in a rural village in western Kenya. American Journal of Tropical Medicine and Hygiene. 2006b;74:54–61. [PubMed] [Google Scholar]
- Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, Mbogo CM, Githure J, Novak R. Survival of immature Anopheles arabiensis (Diptera: Culicidae) in aquatic habitats in Mwea rice irrigation scheme, central Kenya. Malaria Journal. 2006;5:114. doi: 10.1186/1475-2875-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng’habi KR, John B, Nkwengulila G, Knols BGJ, Killeen GF, Ferguson HM. Effect of larval crowding on mating competitiveness of Anopheles gambiae mosquitoes. Malaria Journal. 2005;4:e49, 1–9. doi: 10.1186/1475-2875-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njunwa K. Studies on the productivity of Anopheles breeding sites in relation to adult mosquito density; PhD Thesis; London: London School of Hygiene and Tropical Medicine; 1993. [Google Scholar]
- Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database of Systematic Reviews. 2010;48:1–48. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle MM, Guelbeogo WM, Gneme A, Eiglmeier K, Holm I, Bischoff E, Garnier T, Snyder GM, Li XZ, Markianos K, Sagnon N, Vernick KD. A cryptic subgroup of Anopheles gambiae Is highly susceptible to human malaria parasites. Science. 2010;331:596–598. doi: 10.1126/science.1196759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The Prevention of Malaria. Murray; London: 1911. [Google Scholar]
- Russell TL, Lewtoijera DW, Knols BGJ, Takken W, Killeen GF, Ferguson HM. Linking individual phenotype to density-dependent population growth: the influence of body size on the population dynamics of malaria vectors. Proceedings of the Royal Society B-Biological Sciences. 2011;278:3142–3151. doi: 10.1098/rspb.2011.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Takken W, McCall PJ. Interspecific competition between sibling species larvae of Anopheles arabiensis and An. gambiae. Medical and Veterinary Entomology. 2000;14:165–170. doi: 10.1046/j.1365-2915.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- Service MW. Mortalities of the larvae of the Anopheles gambiae Giles complex and detection of predators by the precipitin test. Bulletin of Entomological Research. 1973;62:359–369. [Google Scholar]
- Service MW. Mortalities of the immature stages of species B of the Anopheles gambiae complex in Kenya: comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. Journal of Medical Entomology. 1977;13:535–545. doi: 10.1093/jmedent/13.4-5.535. [DOI] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Okara RM, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasites & Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nature Reviews Genetics. 2006;7:427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal. 2004;3 doi: 10.1186/1475-2875-3-13. Art. No. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood TRE, Murdie G, Yasuno M, Tonn RJ, Reader PM. Studies on the life budget of A. aegypti in Wat Samphaya, Bangkok, Thailand. Bulletin of the World Health Organistion. 1972;46:211226. [PMC free article] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. Journal of Medical Entomology. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Tuno N, Githeko AK, Nakayama T, Minakawa N, Takagi M, Yan GY. The association between the phytoplankton, Rhopalosolen species (Chlorophyta; Chlorophyceae), and Anopheles gambiae sensu lato (Diptera: Culicidae) larval abundance in western Kenya. Ecological Research. 2006;21:476–482. [Google Scholar]
- Walsh RK, Facchinelli L, Ramsey JM, Bond JG, Gould F. Assessing the impact of density dependence in field populations of Aedes aegypti. Journal of Vector Ecology. 2011;36:300–307. doi: 10.1111/j.1948-7134.2011.00170.x. [DOI] [PubMed] [Google Scholar]
- White MT, Griffin JT, Churcher TS, Ferguson NM, Basanez MG, Ghani AC. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasites & Vectors. 2011;4:e153, 1–14. doi: 10.1186/1756-3305-4-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Malaria Report. WHO; Geneva: 2010. [Google Scholar]
- Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. American Journal of Tropical Medicine and Hygiene. 2003;68:748–752. [PubMed] [Google Scholar]
- Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, Linday SW. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Tropical Medicine &International Health. 2005;10:1274–1285. doi: 10.1111/j.1365-3156.2005.01512.x. [DOI] [PubMed] [Google Scholar]